Abstract
Objective
The hippocampus is crucial for paired-associate learning. Obesity is associated with increased MR activity in peripheral and possibly central tissues, decreased hippocampal size in humans and impaired hippocampal learning in rodents. The MR is expressed in hippocampal neurons and MR blockade improves hippocampal learning in obese animals. We sought to determine whether MR blockade would modulate paired-associate learning in obese men and women.
Design and Methods
Men and women ages 20-61 years with BMIs between 30-45 kg/m2 were randomly assigned to placebo (n=11; 7 women) or 50 mg spironolactone daily (n=12; 7 women) for six weeks. At baseline and post treatment, subjects underwent a clinical and hormonal evaluation. They also underwent a computerized task that assesses paired-associate learning and has been shown by functional magnetic resonance imaging to activate the hippocampus.
Results
In an ANCOVA model that adjusted for baseline paired-associate learning, age, and race, spironolactone treatment was associated with a significant (p=0.043) improvement in hippocampal memory as compared to placebo treatment.
Conclusions
Our findings demonstrate, for the first time, that blocking MR with chronic, low-dose spironolactone treatment improves paired-associate learning in obese individuals, suggesting that MR activation contributes to hippocampal memory modulation in humans.
Keywords: Obesity, Neurochemistry, Cardiovascular Drugs
Introduction
The hippocampus is critical for formation of relational memories, or memories in which novel associations are formed between at least two previously unrelated items.1 In humans, the hippocampus is also necessary for performing paired-associate learning tasks2–4 for example, a task in which a person is presented with combinations of people and places and is later required to remember the combinations they were presented. Interestingly, obesity (defined as BMI≥30 mg/kg2)5 and increased waist to hip ratios have been associated with decreased hippocampal volumes.6 In animal studies, experimentally induced obesity has a detrimental effect on hippocampus-dependent learning. For example, Zucker obese rats subjected to a Morris water maze cross the target platform significantly less (p<0.05) than lean controls, and exhibit higher go/no-go latency ratios on delayed alternation tasks when faced with longer intertrial intervals (suggesting impairment of hippocampal-dependent memory).7,8 Obesity is associated with increased mineralocorticoid receptor (MR) activity in peripheral tissues and possibly in central tissues as well.9–14
The MR has similar affinity for aldosterone and cortisol. Due to the 100 to 1000-fold excess concentration of cortisol versus aldosterone,15,16, it is primarily occupied by cortisol except in those cells expressing the enzyme 11-β-hydroxysteroid-dehydrogenase-2 (11-βHSD-2), which converts active cortisol to inactive cortisone.17,18 The MR is widely expressed in the hippocampus.19 In conjunction with the glucocorticoid receptor (GR) (which is also found in selectively higher concentrations in the hippocampus18), the MR modulates learning and memory, including that mediated by the hippocampus. The hippocampal MR is involved in cognitive aspects of the initial stress response, in selection of behavioral responses to novel situations, in maintenance of a stable excitatory tone, and in regulation of limbic neuronal proliferation and apoptosis in response to corticosteroid exposure. Meanwhile the GR dampens the initial stress reaction and facilitates storage of information for future use.15,18,20
In C57BL/6 lean, inbred mice, MR blockade reduces impairments in hippocampal function (measured by performance on a delayed alternation task) induced by exposure to supraphysiological doses of glucocorticoids (administration of glucocorticoids in the form of corticosterone conjugated to bovine serum albumin decreases alternation by ∼40%, while MR antagonism with RU-28318 improves alternation 10% beyond baseline).21 Studies of obese, diabetic KKAy mice additionally suggest that MR activation is an important determinant of the hippocampal dysfunction associated with obesity, as administration of spironolactone to such rodents improves their performance in a Morris Water Maze task (as measured by escape latency and time spent in the platform quadrant).14 However, the relationship of MR activation to hippocampal function in humans is unclear. Therefore, we assessed paired-associate learning in obese humans who were treated with placebo or the MR antagonist spironolactone for six weeks.
Methods
Study Design
This was a double blind, randomized controlled study (ClinicalTrials.gov identifier NCT01406015) that enrolled subjects of both genders aged 20-61 years who had body mass indices (BMI)>30 kg/m2 and ≤45 kg/m2 and were otherwise in good health as evidenced by history, physical examination and blood chemistries.22 Subjects were recruited via advertisements on clinical research websites, hospital and clinic bulletin boards, newspapers, and email postings. Exclusion criteria included: medical illnesses other than treated hypothyroidism; blood pressures >135/85 mm Hg or systolic blood pressures <90 mm Hg; hepatic disease (transaminases >3 times normal); renal impairment (creatinine clearance <60 mL/min); baseline serum potassium levels of >5.0 mmol/L; history of drug or alcohol abuse; allergies to spironolactone; participation in any other concurrent clinical trials; use of oral contraceptives within the last three months or use of any other prescription medications. The Partners HealthCare Institutional Review Board approved the study protocol and all participants provided written informed consent. Details of this clinical study were described previously.22
A baseline assessment was performed during an inpatient stay at the Brigham and Women's Hospital Center for Clinical Investigation. Participants had consumed an isocaloric, controlled nutrient diet (2,500 mL of fluid, 200 mEq Na, 100 mEq K, and 1000 mg Ca, 50-55% carbohydrates, 15-20% protein, and 25-30% fat) for 5 days prior to admission. Participants were kept supine and fasted after midnight and blood samples for assessment of cortisol and aldosterone were collected in the morning. The hippocampal memory task (described below) was then administered. Participants were subsequently provided with a meal and discharged.
After the baseline studies, subjects were randomized to spironolactone 50 mg daily or placebo daily for six weeks. A second admission occurred six weeks after randomization to placebo or spironolactone. Randomization was performed by the Investigational Drug Service at Brigham and Women's Hospital using a random number generator on the website randomization.com. The placebo and active drug administered in the study were visually indistinguishable. The protocol for this post treatment assessment was identical to that described for baseline assessment. Subjects started taking the study drug on the day of discharge from the first study visit, and took the last dose the evening before the second admission to the Center for Clinical Investigation.
Hippocampal Memory Test
Hippocampal function is crucial for establishing novel relational memories like those assessed in paired-associate learning tasks.23 Thus, we used a paired-associate learning task previously described by Sheridan et al.24 The encoding phase of this task is associated with hippocampal activation on functional magnetic resonance imaging (fMRI) in humans24, replicating results seen with similar tasks.25,26 In this task, participants viewed 18 pictures depicting smiling faces alone (items), 18 pictures of the insides of houses alone (items), and 36 pictures of smiling faces superimposed on pictures of the insides of houses (pairs) for a total of 72 encoded stimuli (Figure 1). Task order (i.e. whether items or pairs were shown first) was fixed between participants. Subjects were instructed to remember the people without houses, the houses without people, and the people and houses they lived in. During two encoding runs, each picture was presented centrally on a laptop computer for 1.5 seconds, and participants viewed each stimulus 7 times randomly distributed across the entire duration of encoding runs. Pictures were presented in blocks of either items or pairs, with the order of presentation fixed between participants. Directly following the encoding period, a recognition test was administered. During the test, subjects were shown subject-house pairs previously presented during the encoding period, as well as three types of pair foils: novel pairs where neither the houses nor faces had been seen before, re-paired items where houses and faces previously presented alone were presented in combination with each other; and rearranged pairs where person A, previously seen in house A, was now presented in house B. Subjects were shown randomly ordered interleaved old pairs and pair foils for two seconds each. They were instructed to press a button only to indicate the occurrence of an old pair (“press a button if you see someone in their house”). Stimuli presented at visit 2 were distinct from those presented at visit 1.
Figure 1. Example images from hippocampal memory task.
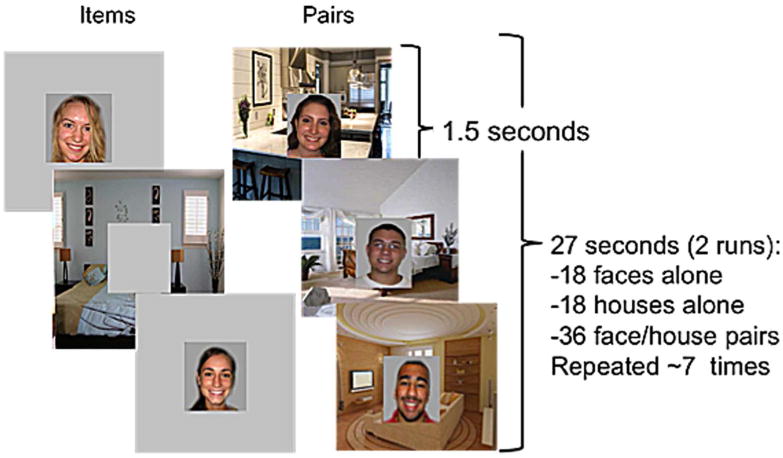
During the encoding portion of the task, subjects were presented with “items” (such as the faces or houses alone seen above) or “pairs” (combinations of faces and houses) over the course of two runs. Subjects were instructed to remember faces presented alone, houses presented alone, and pairs of faces and houses. Each image was presented 7 times per encoding run, for 1.5 seconds each. A recognition test was administered after the encoding period during which subjects were asked to differentiate pairs they had seen during encoding from several types of foils. Figure adapted from Sheridan et al., 2013.
To assess performance, each subject's responses to correct, old picture pairs (hit rate) and responses to incorrect, new picture pairs (false alarms) were normalized and subtracted from each other (d-prime). In this analysis, d-prime was calculated separately comparing memory for previously learned (old) pairs to each of the three types of foils: novel stimuli, newly paired items, and re-arranged pairs. For the purposes of specifically assessing hippocampal memory, we report on d-prime for previously viewed pairs relative to re-arranged pairs. This metric is the strictest assessment of hippocampal memory because correctly rejecting previously re-arranged pairs specifically requires binding together of two previously unrelated items2. Hereafter in this paper, we refer to this metric simply as “hippocampal memory.” However, d-prime calculated in this fashion may also capture variance related to memory that is unrelated to hippocampal function, such as alertness, working memory, or attention. Therefore we additionally examined the effect of treatment on a variable that assessed non-hippocampal specific memory processes: d-prime for previously viewed pairs relative to completely novel stimuli. If treatment affected non-hippocampal memory processes, we would expect to see an effect on both our hippocampal memory and non-hippocampal memory metrics.
The maximal hippocampal memory d-prime attainable with our test was 6.2, while the minimum attainable score was -6.2. If a subject correctly pressed a button every time a previously viewed pair appeared during the test, and additionally took no action when viewing re-arranged pairs, they would have received the maximum score. If a subject failed to take any action when a previously viewed pair appeared during the test, and additionally pressed a button every time they saw a re-arranged pair, they would have received the minimum score. A score of 0 would have represented a subject taking absolutely no action during the test or pressing a button for every single stimulus they saw. Similarly, the maximal non-hippocampal memory d-prime attainable with our test was 6.2, while the minimum attainable score was -6.2.
Statistical Methods
Group data were summarized as means ± SD, unless noted otherwise. Normality of variables was assessed via the Kolmogorov-Smironov normality test. Comparisons of treatment arms for demographic and other baseline variables were performed using independent samples t-tests (for normally distributed variables: baseline age, hippocampal memory, cortisol, mean arterial pressure, and BMI), independent samples Mann-Whitney tests (for non-normally distributed variables: baseline aldosterone, potassium, and non-hippocampal memory) or chi-square tests (for gender and race).
The primary outcome of this study was change in hippocampal memory between visits 1 and 2. Consistent with the approach to analyzing change proposed by Fitzmaurice et al27, this metric was evaluated using a repeated measures analysis of covariance (ANCOVA) model covering the baseline and six-week visit data. Variables considered for inclusion in the ANCOVA model were age, baseline hippocampal memory, race, and treatment status (spironolactone versus placebo). Age was included in the model because normal aging is known to be associated with deterioration in hippocampal-dependent cognition.28 Race was included in the model because in the setting of heart failure, African Americans have been shown to respond differently to spironolactone treatment than whites and thus a differential effect of spironolactone on hippocampal memory by race is feasible.29 Spironolactone versus placebo was considered in the primary design. Comparisons of between-visit changes across groups for all others values were made via independent samples t-tests for normally distributed variables (serum cortisol, BMI, and MAP) and independent samples Mann-Whitney tests for non-normally distributed variables (serum aldosterone and potassium).
Nominal p-values are reported. All statistical analyses were performed with SPSS Version 22.
Results
Subject Characteristics
Baseline subject characteristics are summarized in Table 1 (clinical parameters) and Table 2 (memory task results) for the 23 subjects who met inclusion criteria and had both pre and post treatment hippocampal memory data available for analysis. There were no significant differences (at a level of p<0.05) between placebo and spironolactone treated patients in terms of baseline hippocampal memory or any other relevant study parameter (age, race, BMI, mean arterial pressure, serum cortisol, aldosterone, potassium, gender distribution, race distribution, or non-hippocampal memory), consistent with our random assignment approach.
Table 1. Baseline demographic and clinical parameters and between visit changes in clinical parameters displayed by treatment group.
| Placebo Group | Spironolactone Group | |||
|---|---|---|---|---|
| Baseline | Between-Visit Change | Baseline | Between-Visit Change | |
| N | 11 | - | 12 | - |
| Mean Age, yr | 41 ± 14 | - | 47 ± 11 | - |
| Female, n (%) | 7 (64%) | - | 7 (58%) | - |
| Premenopausal | 6 (86%) | 3 (43%) | ||
| Race, n (%) | - | - | ||
| Caucasian | 6 (55%) | - | 9 (75%) | - |
| African American | 5 (45%) | - | 3 (25%) | - |
| Mean Arterial Pressure (mmHg) | 88.2 ± 9.9 | 1.9 ± 7.4 | 90.6 ± 6.5 | -4.2 ± 5.6* |
| BMI (kg/m2) | 35.7 ± 5.4 | 0.4 ± 1.3 | 38.5 ± 6.9 | 0.2 ± 0.4 |
| Serum Aldosterone (ng/dL) | 3.2 ± 1.2 | -0.3 ± 1.3 | 3.4 ± 1.5 | 5.2 ± 7.4* |
| Serum Cortisol (mcg/dL) | 7.3 ± 4.1 | 2.4 ± 4.1 | 8.9 ± 3.2 | 0.9 ± 3.7 |
| Serum Potassium (mmol/L) | 4.3 ± 0.5 | 0.0 ± 0.4 | 4.2 ± 0.2 | 0.1 ± 0.4 |
Data are expressed as mean ± SD unless stated otherwise. Changes are calculated as parameter post-treatment minus parameter at baseline for each group. There were no significant clinical differences between treatment and placebo groups pre-randomization; however between-visit changes in serum aldosterone and mean arterial pressure were significantly different across treatment groups.
Significant (p<0.05) difference between treatment and placebo groups.
Table 2. Hippocampal memory and non-hippocampal memory scores, by visit and treatment group.
| Visit 1 | Visit 2 | Between-Visit Change | |||||
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | ||
| Hippocampal Memory | Placebo | 1.37 ± 1.60§ | 0.96 (-0.35, 4.70) | 1.09 ± 0.77 | 1.02 (0, 2.24) | -0.28 ± 0.96 | 0.14 (-2.64, 0.64) |
| Spironolactone | 0.83 ± 0.58 | 0.81 (0.05, 1.68) | 1.16 ± 0.90 | 1.17 (-0.20, 2.71) | 0.34 ± 0.53 | 0.28 (-0.28, 1.35) | |
| Non-Hippocampal Memory | Placebo | 2.66 ± 1.44 | 2.99 (0.48, 4.70) | 2.43 ± 1.38 | 2.21 (0, 4.33) | -0.23 ± 0.94 | -0.27 (-1.46, 2.02) |
| Spironolactone | 2.70 ± 0.94 | 2.99 (0.57, 3.70) | 2.62 ± 1.05 | 2.46 (1.20, 4.49) | -0.09 ± 0.95 | 0.19 (-1.72, 1.57) | |
There were no significant differences in hippocampal memory or non-hippocampal memory scores between groups at baseline. Between-visit changes are calculated as parameter post-treatment minus parameter at baseline for each group.
The variance of the two groups' hippocampal memory scores differed significantly at baseline, but not at visit 2. Variances of the two groups' non-hippocampal memory scores did not differ significantly at either visit.
Mean Arterial Pressure and Serum Aldosterone
Treatment with spironolactone significantly increased serum aldosterone levels (U(2,21)=25.0, p=0.01) and decreased mean arterial pressure (t(2,21)=-2.26, p=0.04) from visit 1 to visit 2 (Table 1). Spironolactone did not significantly affect BMI, serum cortisol, or serum potassium levels.
Hippocampal Memory
As depicted in Table 2, which shows measures of hippocampal memory and non-hippocampal memory at visits 1 and 2, hippocampal memory scores ranged from -0.2 to 4.7 in this study. These scores are in the range of values previously reported for this task by Sheridan et al.24 There was a significant negative correlation between age and baseline hippocampal memory (p=0.03), and a significant positive correlation between female gender and baseline hippocampal memory when analyzing all subjects (p=0.03), but these associations did not reach significance within individual groups. Baseline hippocampal memory did not correlate with BMI, mean arterial pressure, race, or aldosterone or cortisol levels.
Average changes in hippocampal memory between visits 1 and 2 were −0.28 ± 0.96 in the placebo group and 0.34 ± 0.53 in the spironolactone group.
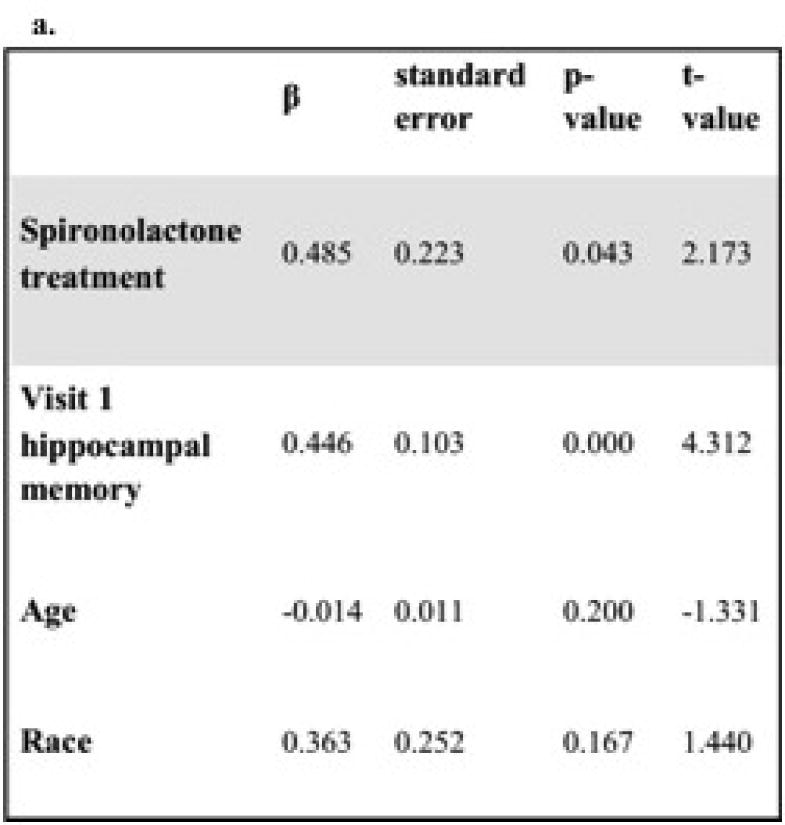
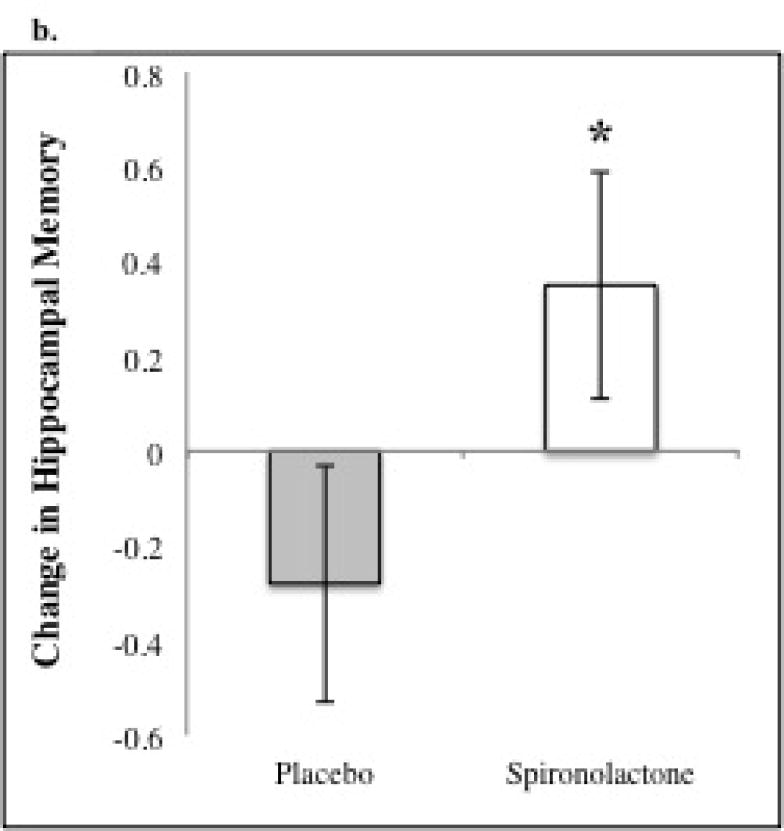
An ANCOVA model predicting between-visit change in hippocampal memory revealed a significant, positive effect of spironolactone treatment (β=0.49, p=0.04) when adjusting for hippocampal memory at visit 1, age and race (see Figure 2a). Factors that did not additionally contribute to the model and thus were not included were gender, serum cortisol, serum aldosterone, serum potassium, mean arterial pressure, and BMI at visits 1 or 2, and changes in the aforementioned variables between visits. As depicted in Figure 2b, predicted adjusted post-treatment change in hippocampal memory (± SE of the estimate) was +0.28 ± 0.15 with spironolactone, and -0.21 ± 0.16 with placebo. This beneficial effect of treatment appeared to be specific for hippocampal-dependent memory since an ANCOVA model adjusting for visit 1 non-hippocampal memory, age and race showed no effect of treatment on predicting the between-visit change in non-hippocampal memory.
Figure 2. Analysis of covariance model predicting between visit change in hippocampal memory.
a. Model adjusts for race, baseline hippocampal memory, and age. b. Spironolactone treatment improved hippocampal memory as compared with placebo (* p= 0.043). The predicted adjusted between-visit change in hippocampal memory (± SE of the estimate) was +0.28 ± 0.15 with spironolactone, and -0.21 ± 0.16 with placebo.
Discussion
In this randomized controlled pilot study, six weeks of treatment with the MR antagonist spironolactone improved hippocampal memory as compared with placebo. Since spironolactone treatment had no effect on non-hippocampal memory, we conclude that the effects observed are due to spironolactone's effect on hippocampal function rather than on alertness, working memory, attention, or other non-hippocampal functions. These results suggest that MR modulates hippocampal function in human obesity.
Our results are consistent with preclinical data showing that MR activation mediates hippocampal dysfunction in obese rodents. Numerous studies, in which hippocampal MR activity was manipulated through use of pharmacological and transgenic approaches, have shown that the MR regulates hippocampal neuronal activity and hippocampal associated learning.30,21 For example, in a study by Dorey et al., hippocampal administration of the MR antagonist RU-28318 fifteen minutes before hippocampal corticosterone injection significantly improved glucocorticoid-induced memory impairment.21
Differing effects of MR blockade in obese versus lean rodents provide further insight into the role of excess MR activity in hippocampal memory modulation. When female, type 2 diabetic, obese mice were treated with spironolactone (50 mg/kg per day in chow) their spatial memory (as measured by time spent in the platform quadrant during a Water Maze Test) improved.14 A similar effect was not observed in healthy rodents.31
Obesity is associated with increases in MR activity in peripheral tissues. The increase in MR activation appears to be mediated by multiple mechanisms, including increased MR levels11, altered 11-βHSD-1 activity leading to tissue-specific increases in cortisol levels12, increased aldosterone levels9, and increased activity of Rac1 GTP (which induces the actions of MR independently of ligand-binding).10,13 While not clearly established, it is possible that obesity is also associated with increased MR action in the in the brain, where cortisol, not aldosterone, is the primary ligand for MR. This could be via increased MR levels11, increased activity of Rac1 GTP10 (which mediates the rapid, nongenomic actions of MR [as assessed by the slope of the field excitatory postsynaptic potentials] in rat hippocampal neurons13), or altered activity of 11-βHSD-1 activity resulting in increased cortisol availability.12 MR blockade additionally reduces obesity-related cardiometabolic abnormalities such as insulin resistance, inflammation, lipid disturbances and excess hepatic fat.32
The finding that MR blockade improved hippocampal function only in obese rodents in the aforementioned studies suggests that there is increased activity of the MR in obesity (but not the lean state) possibly mediated through effects on neurons, vasculature, and/or the neuronal microenvironment, and that this activation mediates hippocampal dysfunction in animals. We show for the first time that the relationship between MR activation and hippocampal function also holds in humans with obesity.
Our results lend support to a growing body of evidence regarding the cognitive benefits of MR antagonism in disease states characterized by increased basal MR activation. In a recent study of hypertensive subjects, increased plasma aldosterone concentration was associated with significantly worse performance on a mini-mental state examination (MMSE; which was used as a measure of general cognitive function). Notably, treatment with the MR antagonists spironolactone and eplerenone improved MMSE scores.33 Our findings supplement the latter results by suggesting that MR blockade counteracts the deleterious cognitive effects of excess MR activation specifically in the hippocampus. Meanwhile, a 2006 observational study of almost 3,300 patients age 65 years and older showed that use of potassium-sparing diuretics, including spironolactone, was associated with a significantly lower incidence of developing Alzheimer's, a disease characterized by hippocampal dysfunction.34 We provide further support for the concept that MR activation affects brain function by demonstrating the hippocampal memory benefits of MR antagonism in a randomized, controlled setting.
A few previous studies in humans failed to show a positive effect of MR antagonist treatment on cognitive function, but these studies differed significantly from the current study as they did not specifically assess hippocampal memory, used large doses of spironolactone, and examined acute, not chronic, effects of spironolactone. Specifically, Otte et al. reported that administration of 900 mg spironolactone over 5.5 hours impairs selective attention, visuospatial memory, and mental flexibility/set shifting in healthy young men.35 Cornelisse et al. showed that a single dose of 400 mg spironolactone impairs selective attention in a trial of 64 young, healthy men.36 Rimmele et al. demonstrated that administration of 400 mg of spironolactone (split across two 200 mg doses) to young healthy men in the nine hours before testing impaired free recall of texts and pictures.37 The aforementioned studies also differed from ours in that they were conducted in healthy subjects who are not thought to have increased MR activation. In contrast we studied obese subjects, and obesity is thought to be associated with increased peripheral and possibly central MR activity.9–14
Our study has several strengths. We used spironolactone doses (50 mg daily) that are similar to the clinically relevant doses used in studies showing beneficial effects of spironolactone on hypertension.38 Blood pressure and aldosterone were measured under conditions in which environmental factors that could affect these parameters (posture, dietary sodium, stress) were carefully controlled. The decreases in blood pressure and increases in aldosterone seen in our study suggest that subjects successfully took their medications, and that spironolactone effectively blocked the MR in those subjects assigned to it. Finally, the hippocampal memory task we used, a paired-associate learning task, has been shown to selectively activate the hippocampus in fMRI studies.24,25 One potential drawback of our study is that the variance of our groups' baseline hippocampal memory scores differed significantly. This difference in variance was attributable to one subject in the placebo group whose baseline performance was two standard deviations above the mean. Concerned by whether this subject was driving our significant effect, we excluded him from the analysis for exploratory purposes. We found that when this subject was excluded, the variance of baseline hippocampal memory no longer differed significantly between groups. Moreover, our model (in which treatment significantly predicts the between-visit change in hippocampal memory when adjusting for hippocampal memory at visit 1, age, and race) still holds, suggesting that the effect of treatment on hippocampal memory is a true one not driven by outliers.
There are also limitations to the applicability of our findings. Firstly, we studied healthy, obese subjects, so our results cannot be extrapolated to other populations, for example those with diabetes or hypertension. Similarly, our study population was relatively young so the results may not apply to an elderly population. Because our study looked at the effects of six weeks of spironolactone treatment, we cannot determine whether longer treatment would or would not have resulted in further improvements in hippocampal memory. Since cortisol occupies both the MR and GR, it is possible that the effects in our study are due to a change in the relative amounts of MR versus GR activation in the setting of MR blockade (prior studies have shown that decreasing the functional balance between MR and GR in the cerebral cortex and hippocampus by inhibiting MR and/or increasing GR activity leads to deleterious changes in memory and cognition, among other parameters).16 Additionally, spironolactone is not selective for the MR, but can block the progesterone and androgen receptors as well (although much less effectively than the MR), with possible associated effects on memory; both androgen and progesterone receptors are expressed in the hippocampus.39 Thus, future larger studies should stratify results by gender and use a more selective MR antagonist. Although our data does indicate a selective effect of treatment on hippocampal vs. non-hippocampal function, since we did not use a comprehensive cognitive battery we cannot specifically assess which non-hippocampal functions (working memory, attention) were unaffected by MR blockade. Furthermore, given that MR blockade is known to improve cerebral vascular remodeling40, it is possible that the beneficial memory effects of MR antagonism seen in our study involve beneficial effects on brain vasculature. Finally, we tested the effects of spironolactone on hippocampal memory in a relatively small number of subjects. However, our treatment groups were well matched at baseline and we observed a significant effect of spironolactone treatment even after adjusting for baseline characteristics, which argues for the strength of our effect.
Overall, we have shown that chronic, low-dose MR blockade with spironolactone improves hippocampal memory in obese subjects. Our findings suggest that MR activation modulates hippocampal memory in humans, and thus represent an important advancement in the understanding of the role of the MR in human hippocampal memory regulation.
What is already known about this subject?
The mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) are expressed in the hippocampus and modulate hippocampal function.
Obesity is associated with excess MR activity, decreased hippocampal size in humans and impaired hippocampal learning in rodents.
Rodent studies suggest that MR activation is an important determinant of the hippocampal dysfunction associated with obesity.
What this study adds
In obese subjects, chronic (6 week) blockade of the MR with low-dose spironolactone treatment improves paired-associate learning – a hippocampal-dependent function.
Consistent with previous rodent work, this result suggests that MR activation modulates hippocampal memory in humans.
Acknowledgments
GA, MS, and RG conceived of and carried out the study. LR, MS, and GA analyzed data and crafted the publication's conclusions. While LR had primary responsibility for manuscript writing, all authors were involved in writing the paper and had final approval of the submitted and published versions.
This work was supported by grants from the National Institutes of Health (K01 5K01MH092555; K24 HL103845; UL1 RR025758) and by the Robert Wood Johnson Health and Society Scholar Program, Harvard. The authors would additionally like to thank Maria Baimas-George for her assistance with study coordination and execution.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
References
- 1.Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behav Brain Res. 1999;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 2.Bunsey M, Eichenbaum H. Critical role of the parahippocampal region for paired-associate learning in rats. Behav Neurosci. 1993;107:740–747. doi: 10.1037//0735-7044.107.5.740. [DOI] [PubMed] [Google Scholar]
- 3.Henke K, Weber B, Kneifel S, Wieser HG, Buck A. Human hippocampus associates information in memory. Proc Natl Acad Sci USA. 1999;96:5884–5889. doi: 10.1073/pnas.96.10.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holdstock JS, Crane J, Bachorowski JA, Milner B. Equivalent activation of the hippocampus by face-face and face-laugh paired associate learning and recognition. Neuropsychologia. 2010;48:3757–3771. doi: 10.1016/j.neuropsychologia.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005;62:1545–1548. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- 7.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 8.Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, et al. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behav Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- 9.Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92:4472–4475. doi: 10.1210/jc.2007-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun M, Huang X, Yan Y, Chen J, Wang Z, Xie M, et al. Rac1 Is a Possible Link Between Obesity and Oxidative Stress in Chinese Overweight Adolescents. Obesity. 2012;20:2233–2240. doi: 10.1038/oby.2012.63. [DOI] [PubMed] [Google Scholar]
- 11.Schäfer N, Lohmann C, Winnik S, van Tits L, Miranda M, Vergopolous A, et al. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J. 2013;34:3515–24. doi: 10.1093/eurheartj/eht095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morton NM. Obesity and corticosteroids: 11beta-hydroxysteroid type 1 as a cause and therapeutic target in metabolic disease. Mol Cell Endocrinol. 2010;316:154–164. doi: 10.1016/j.mce.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami-Mori F, Shimosawa T, Mu S, Wang H, Ogura S, Yatomi Y, et al. NADPH oxidase-mediated Rac1 GTP activity is necessary for nongenomic actions of the mineralocorticoid receptor in the CA1 region of the rat hippocampus. AJP Endocrinol Metab. 2012;302:E425–E432. doi: 10.1152/ajpendo.00227.2011. [DOI] [PubMed] [Google Scholar]
- 14.Sakata A, Mogi M, Iwanami J, Tsukuda K, Min L, Jing F, et al. Improvement of cognitive impairment in female type 2 diabetes mellitus mice by spironolactone. J Renin-Angiotensin-Aldosterone Syst. 2012;13:84–90. doi: 10.1177/1470320311412810. [DOI] [PubMed] [Google Scholar]
- 15.De Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Sanchez E. Brain mineralocorticoid receptors in cognition and cardiovascular homeostasis. Steroids. 2014;91:20–31. doi: 10.1016/j.steroids.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seckl JR, Walker BR. Minireview: 11beta-hydroxysteroid dehydrogenase type 1 - A tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142:1371–1376. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- 18.De Kloet ER, Van Acker SABE, Sibug RM, Sibug RM, Oitzl MS, Meijer OC, Rahmouni K, et al. Brain mineralocorticoid receptors and centrally regulated functions. Kidney International. 2000;57:1329–1336. doi: 10.1046/j.1523-1755.2000.00971.x. [DOI] [PubMed] [Google Scholar]
- 19.Van Eekelen JA, Jiang W, De Kloet ER, Bohn MC. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J Neurosci Res. 1988;21:88–94. doi: 10.1002/jnr.490210113. [DOI] [PubMed] [Google Scholar]
- 20.Joels M, Karst H, DeRijk R, de Kloet ER. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008;31:1–7. doi: 10.1016/j.tins.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Dorey R, Piérard C, Shinkaruk S, Tronche C, Chaveau F, Baudonnat M, et al. Membrane mineralocorticoid but not glucocorticoid receptors of the dorsal hippocampus mediate the rapid effects of corticosterone on memory retrieval. Neuropsychopharmacology. 2011;36(13):2639–49. doi: 10.1038/npp.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg R, Kneen L, Williams GH, Adler GK. Effect of Mineralocorticoid Receptor Antagonist on Insulin Resistance and Endothelial Function in Obese Subjects. Diabetes Obes Metab. 2014;16(3):268–272. doi: 10.1111/dom.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eichenbaum H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Sheridan MA, How J, Araujo M, Schamberg MA, Nelson CA. What are the links between maternal social status, hippocampal function, and HPA axis function in children? Dev Sci. 2013;16:665–675. doi: 10.1111/desc.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Achim AM, Bertrand MC, Montoya A, Malla AK, Lepage M. Medial temporal lobe activations during associative memory encoding for arbitrary and semantically related object pairs. Brain Res. 2007;1161:46–55. doi: 10.1016/j.brainres.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 27.Fitzmaurice G. A conundrum in the analysis of change. Nutrition. 2001;17:360–361. doi: 10.1016/s0899-9007(00)00593-1. [DOI] [PubMed] [Google Scholar]
- 28.Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RM, et al. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- 29.Vardeny O, Cavallari LH, Claggett B, Desai AS, Anand I, Rossignol P, et al. Race influences the safety and efficacy of spironolactone in severe heart failure. Circ Heart Fail. 2013;6:970–976. doi: 10.1161/CIRCHEARTFAILURE.113.000530. [DOI] [PubMed] [Google Scholar]
- 30.Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yau JLW, Noble J, Seckl JR. Continuous blockade of brain mineralocorticoid receptors impairs spatial learning in rats. Neurosci Lett. 1999;277:45–48. doi: 10.1016/S0304-3940(99)00858-7. [DOI] [PubMed] [Google Scholar]
- 32.Tirosh A, Garg R, Adler GK. Mineralocorticoid receptor antagonists and the metabolic syndrome. Curr Hypertens Rep. 2010;12:252–257. doi: 10.1007/s11906-010-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yagi S, Akaike M, Aihara K, Iwase T, Yoshida S, Sumitomo-Ueda Y, et al. High plasma aldosterone concentration is a novel risk factor of cognitive impairment in patients with hypertension. Hypertens Res. 2011;34:74–78. doi: 10.1038/hr.2010.179. [DOI] [PubMed] [Google Scholar]
- 34.Khachaturian AS, Zandi PP, Lyketsos CG, Hayden K, Skoog I, Norton M. Antihypertensive medication use and incident Alzheimer disease: the Cache County study. Archives of Neurology. 2006;63:686–692. doi: 10.1001/archneur.63.5.noc60013. [DOI] [PubMed] [Google Scholar]
- 35.Otte C, Moritz S, Yassouridis A, Koope M, Madrischewski AM, Wiedemann K. Blockade of the mineralocorticoid receptor in healthy men: effects on experimentally induced panic symptoms, stress hormones, and cognition. Neuropsychopharmacology. 2007;32:232–238. doi: 10.1038/sj.npp.1301217. [DOI] [PubMed] [Google Scholar]
- 36.Cornelisse S, Joëls M, Smeets T. A randomized trial on mineralocorticoid receptor blockade in men: effects on stress responses, selective attention, and memory. Neuropsychopharmacology. 2011;36:2720–8. doi: 10.1038/npp.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rimmele U, Besedovsky L, Lange T, Born J. Blocking mineralocorticoid receptors impairs, blocking glucocorticoid receptors enhances memory retrieval in humans. Neuropsychopharmacology. 2013;38:884–94. doi: 10.1038/npp.2012.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batterink J, Stabler S, Tejani A, Fowkes C. Spironolactone for hypertension. Cochrane Database Syst Rev. 2010;8 doi: 10.1002/14651858.CD008169.pub2. [DOI] [PubMed] [Google Scholar]
- 39.McEwen BS, Milner TA. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rigsby CS, Ergul A, Portik Dobos V, Pollock DM, Dorrance AM. Effects of spironolactone on cerebral vessel structure in rats with sustained hypertension. Am J Hypertens. 2011;24:708–715. doi: 10.1038/ajh.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]