Abstract
Adipose-derived mesenchymal stem cells (ASCs) hold promise for use in cell-based therapies. Their intrinsic anti-inflammatory properties are potentially useful for treatments of inflammatory conditions such as uveitis, while their ability to differentiate along multiple cell lineages suggests use in regenerating damaged or degenerated tissue. However, how ASCs will respond to the intraocular environment is poorly studied. We have recently reported that aqueous humor (AH), the fluid that nourishes the anterior segment of the eye, potently increases alkaline phosphatase (ALP) activity of ASCs, indicating osteogenic differentiation. Here, we expand on our previous findings to better define the nature of this response. To this end, we cultured ASCs in the presence of 0, 5, 10, and 20% AH and assayed them for ALP activity. We found ALP activity correlates with increasing AH concentrations from 5 to 20%, and that longer treatments result in increased ALP activity. By using serum free media and pretreating AH with dextran-coated charcoal, we found that serum and charcoal-adsorbable AH components augment but are not required for this response. Further, by heat-treating the AH, we established that thermally labile components are required for the osteogenic response. Finally, we showed myocilin, a protein present in AH, could induce ALP activity in ASCs. However, this was to a lesser extent than untreated 5% AH, and myocilin could only partially rescue the effect after heat treatment, documenting there were additional thermally labile constituents of AH involved in the osteogenic response. Our work adds to the understanding of the induction of ALP in ASCs following exposure to AH, providing important insight in how ASCs will be influenced by the ocular environment. In conclusion, increased osteogenic potential upon exposure to AH represents a potential challenge to developing ASC cell-based therapies directed at the eye.
Keywords: aqueous humor, mesenchymal stem cells, osteogenic potential, myocilin, alkaline phosphatase
1. Introduction
Mesenchymal Stromal/Stem Cells (MSCs) have been derived from a variety of tissue sources including adipose tissue (Bourin et al., 2013). MSCs are characterized by expression of CD73, CD90, CD105, CD146 surface markers, lack expression of CD31, CD34, or CD45 surface markers, and are capable of tri-lineage differentiation into adipocytes, osteocytes, chondrocytes (Dominici et al., 2006; Horwitz et al., 2005). Additionally, MSCs do not express MHC II, making them ideal for allogenic as well as autologous cell therapies. Low immunogenicity and their differentiation potential positions MSCs for regenerative medicine applications including bone and tendon repair (Caplan, 2013). Furthermore, MSCs have also been shown to be immunomodulatory and have been investigated in clinical trials for the treatment of immune mediated diseases (Nauta and Fibbe, 2007; Voswinkel et al., 2013). Adipose-derived MSCs (ASCs) are an especially promising source for use in regenerative medicine, as they can be acquired in high numbers from minimally invasive lipectomy procedures (Zuk et al., 2001).
The promise of cell therapies in general and ASC therapy specifically is no less apparent in the eye (Joe and Gregory-Evans, 2010; Rajashekhar, 2014). Degenerative ocular diseases such as diabetic retinopathy, age-related macular degeneration (AMD), and glaucoma are candidates for cell replacement therapies. Additionally, these diseases have known immune components in their progression, which could potentially be ameliorated by the immunosuppressive effects of ASCs. Several recent publications have highlighted the potential for cell therapy in the eye. MSCs have been successfully differentiated in retinal progenitor cells (Moviglia et al., 2012), keratocyte-like cells (Arnalich-Montiel et al., 2008; Liu et al., 2012; Park et al., 2012), corneal endothelial-like cells (Joyce et al., 2012), retinal pigmented epithelium cells (Vossmerbaeumer et al., 2009), and photorecptors (Kicic et al., 2003). Further, MSCs have been used to treat models of glaucoma (Johnson et al., 2010; Manuguerra-Gagne et al., 2013), retinopathy (Chung et al., 2011; Inoue et al., 2007; Jiang et al., 2014; Machalinska et al., 2013), autoimmune uveoretinitis (Li et al., 2013; Zhang et al., 2011), and corneal wounds (Arnalich-Montiel et al., 2008; Jia et al., 2012; Oh et al., 2008; Yao et al., 2012). The bulk of these studies focus on how MSCs influence the ocular environment and limited consideration is given to how the ocular environment influences the behavior of MSCs.
We have recently reported that aqueous humor (AH), the fluid that nourishes the tissues adjoining the anterior segment of the eye, potently stimulates osteogenic potential in ASCs (Morgan et al., 2014). AH is a complex mixture of proteins, lipids, salts, and other small molecules (Cousins et al., 1991; De Berardinis et al., 1965; Duan et al., 2010; Edwards et al., 2014; Greiner et al., 1991; Iyer et al., 2012; Knisely et al., 1994; Lee et al., 1977; Rao et al., 2000; Russell et al., 2001; Tripathi et al., 1989). Several of these solutes have been previously implicated in osteogenesis, including ascorbate and glucocorticoids (Bellows et al., 1987; Bellows et al., 1986; Herbertson and Aubin, 1995; Maniatopoulos et al., 1988; Tenenbaum and Heersche, 1982), growth-factor like lipids such as lysophosphatidic acid (LPA) (Liu et al., 2010), and the protein myocilin (Kwon et al., 2013). Understanding this effect is important to ensuring the safety and efficacy of ASC administration to the eye. Further, illuminating the osteogenic potential of AH may provide insight into the calcification of anterior chamber tissues such as the trabecular meshwork, believed to play a role in the progression of glaucoma (Borras and Comes, 2009; Gonzalez et al., 2004; Gonzalez et al., 2000; Vittitow and Borras, 2004; Xue et al., 2007; Xue et al., 2006). In this study, we expanded on our previous results to define the effects of AH dose and treatment duration. We further identified the importance of thermally labile and charcoal adsorbable components in AH to the differentiation process. Finally, we showed myocilin by itself can increase osteogenic potential, although this induction was not as robust as that of complete AH.
2. Methods
2.1 Preparation of AH and myocilin
AH was extracted using a 25-gauge needle from enucleated bovine eyes shipped overnight on ice (Pel-freez, Rogers, AR). AH was sterile filtered, aliquoted, and stored at −20°C until use. Some AH was heat treated (AH/HT) to denature protein components by heating it to 90°C for 30 min. AH was also treated with dextran-coated charcoal (AH/DCC) to reduce the concentration of hormones and lipids (Herbert et al., 1965; Keane et al., 1968; Lee et al., 1998). Briefly, 20 mg DCC (Sigma-Aldrich, St. Louis, MO) was added per 1 mL AH and incubated with rocking overnight at 4°C. The sample was then centrifuged at 5,000g for 15 min and sterile filtered to remove the DCC.
Full length human MYOCILIN cDNA was cloned into the pCS2-FLAG vector as described (Kwon et al., 2009) and used for transient transfection of HEK293 cells. Transfection was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Serum-containing medium was replaced by serum-free medium 14-16 h after transfection, and cells were incubated for 48 h. Conditioned medium was collected and myocilin-FLAG protein was purified using anti-FLAG M2 agarose beads according to the manufacturer’s instructions (Sigma, St. Louis, MO). Myocilin was further purified by ion-exchange chromatography using HiTrap-SP FF 1-ml columns (GE Healthcare). The purity of the isolated myocilin was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Two closely migrated bands with mobilities corresponding to myocilin were observed after Coomassie blue staining of the gel, similar to shown in Fig. 1 of (Kwon et al., 2009).
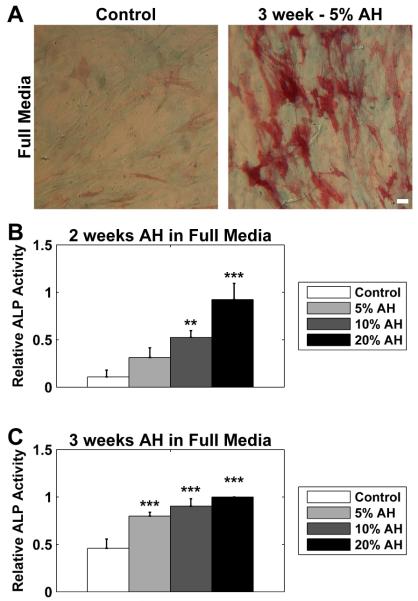
Figure 1. ASCs exhibited a time and dose dependent response to aqueous humor.
ASCs were cultured in full media (serum supplemented DMEM) in the presence of 5, 10, or 20% AH. (A) Cells were stained for alkaline phosphatase (ALP) activity (red) and with Alcian blue (blue). Some ALP activity was observed in untreated cells (Control), but cells treated with AH exhibit dramatic increase in ALP activity, exemplified by cells cultured for 3 weeks in the presence of 5% AH (3 week - 5% AH). There was apparently less effect of AH on Alcian blue staining. (B) At 2 weeks, ALP activity was increased in a dose dependent manner, with 10% and 20% eliciting a significant response when compared to control cells. (C) At 3 weeks, all doses resulted in a significant increase in ALP activity compared to control. All data were normalized to the response of 3 week treatment with 20% AH. Data are mean ± SEM (n = 3 donors). * p < 0.05; ** p < 0.01; *** p < 0.001. Scale bar 50 μm.
2.2 Cell Culture
Primary cultures of ASCs were cultured from human donor adipose tissue as previously described (Chung et al., 2012; Morgan et al., 2014; Toupadakis et al., 2010; Wood et al., 2012). Briefly, 10–13 g of fat was minced and incubated with rocking 2 h at 37°C in 50 mL of PBS (Invitrogen, Carlsbad, CA) with 0.1% collagenase/1% bovine serum albumin (Worthington, Lakewood, NJ). The tissue was then centrifuged to remove the lipid layer and repeatedly washed with PBS. Cell pellets were re-suspended with low glucose DMEM supplemented with 10% FBS and 1% penicillin/streptomycin (Life Technologies, Carlsbad, CA), plated, and incubated at 37°C, 5% CO2. Cells were passaged at 70% confluence and maintained in the supplemented DMEM, henceforth referred to as full media.
For experiments, cells were plated at 50,000 cells per well in a 24-well plate in full media and allowed to attach overnight. Cells were rinsed with PBS and placed in either full or serum free DMEM with AH or myocilin supplements. To avoid disrupting the cell monolayer, half-volume media exchanges were performed twice weekly. At either 2 or 3 weeks, the cells were briefly fixed in 4% formaldehyde and rinsed in PBS.
2.3 Staining and imaging of cells
Immediately after fixation, cells were stained for ALP activity as previously described (Morgan et al., 2014). Briefly, they were stained for 15 min with 0.1% naphthol AS-MX phosphate (Sigma) and 0.1% fast red violet LB (Sigma) dissolved in 56mM 2-amino-2-methyl-1,3-propanediol (pH 9.9; Sigma). In the initial dose response experiments, cells were costained for the presence of sulphated acid proteoglycans using Alcian blue after ALP staining (Asahina et al., 1993). Briefly, the cells were rinsed in 0.1N HCl (pH 1.0), stained for 15 min with 1% w/v Alcian Blue 8GX (Sigma) in 0.1N HCl, and rinsed with 0.1N HCl to remove non-specific staining. Following staining, the coverslips were rinsed in PBS and mounted on slides for imaging. Coverslips were imaged using a Nikon DS-Fi1 color camera attached to a Nikon Diaphot inverted microscope. Six random 0.89 mm2 fields were taken of each coverslip. ALP activity was quantified and averaged across the six images using custom analysis programs written in the MATLAB (Mathworks, Natick, MA) software package.
2.4 Statistics
All experiments were performed on ASCs isolated from three donors. Data for each donor were normalized. For each experiment, significance was assessed on the normalized values by one way ANOVA and Fisher’s post-hoc test. Levels of significance are denoted throughout the manuscript by *** = p<0.001, ** = p<0.01, and * = p<0.05. All bar graphs are shown as mean ± SEM.
3 Results
3.1 Dose and time dependence of aqueous humor effect on ASCs
We cultured human ASCs in serum supplemented DMEM with AH added at concentrations of 5, 10, and 20%. Control cells were cultured similarly without the addition of AH. At 2 and 3 weeks, the ASCs were fixed and stained for ALP activity and Alcian blue, indicating osteogenic and chondrogenic potential, respectively (Asahina et al., 1993). Control cells showed weak ALP activity (Figure 1A; left panel; red stain) and similarly exhibited little Alcian blue staining (Figure 1A; left panel; blue stain). However, after treatment with 5% aqueous humor for 3 weeks, ASCs exhibited a dramatic increase in ALP activity (Figure 1A; right panel; red stain) with a lesser effect on Alcian blue staining. We therefore focused on ALP activity for further characterization of the dose and duration response to AH.
Using cells isolated from 3 donors, we quantified the ALP activity of ASCs treated with 5, 10 and 20% AH at both 2 and 3 weeks. To control for variability between donors, we normalized staining intensity for a donor to the 20% addition of AH for 3 weeks. At 2 weeks (Figure 1B), there was a clear dose response, with ALP activity with correlating with AH concentration, resulting in significant increases at both 10 and 20%. At 3 weeks (Figure 1C), all three concentrations resulted in significant increases in ALP activity and little concentration dependence could be discerned at 3 weeks. It is also important to note that 2 weeks of treatment was sufficient for the 20% to induce close to maximal activation (black bars of Figures 1B and 1C).
3.2 Effect of serum free culture aqueous humor effect on ASCs
As serum contains numerous growth factors and other signaling molecules, we considered the possibility that serum was providing an essential component to the osteogenic differentiation process. To test this possibility, we also performed the above experiments in serum free DMEM. Similar to the results with serum, AH resulted in an increase in ALP activity (Figure 2A). However, the increase in ALP activity was substantially reduced compared to cells cultured with AH in the presence of serum. Similar to the serum containing experiments, we found a lesser effect on Alcian blue activity and focused on ALP activity.
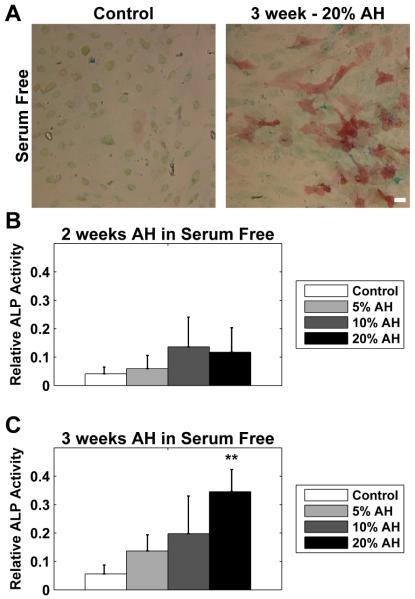
Figure 2. Serum was not required for the osteogenic effect of aqueous humor on ASC.
ASCs were cultured serum free (un-supplemented DMEM) in the presence of 5, 10, or 20% AH. (A) Cells were stained for ALP activity (red) and with Alcian blue (blue). ALP activity was virtually absent in untreated cells (Control), but cells treated with AH exhibited noticeable ALP activity, exemplified by cells cultured for 3 weeks in the presence of 20% AH (3 week - 20% AH). There was less effect of AH on Alcian blue staining. (B) At 2 weeks, ALP activity was not significantly increased at any dose, although there was an apparent trend towards increase. (C) At 3 weeks, there was a dose response, and cells treated with 20% exhibited a significant increase over control. All data were normalized to the response of 3 week treatment with 20% AH in serum containing media. Note the smaller Y-axis when compared to Figure 1. Data are mean ± SEM (n = 3 donors). * p < 0.05; ** p < 0.01; *** p < 0.001. Scale bar 50 μm.
We similarly tested the effect of time and dose in the absence of serum for cells. All values for a donor were normalized to the 20%/3 week values for the serum-containing cultures. This allowed direct comparison between the ALP activity in the presence and absence of serum. At 2 weeks (Figure 2B), there was a trend towards increasing ALP activity, although it was less compared to the AH plus serum data (Figure 1B, note the altered Y-axis) and did not reach statistical significance. At 3 weeks (Figure 2C), there was a more apparent dose response, although only exposure to 20% AH reached significance (but was still far reduced compared to the cells treated with equivalent AH in serum containing media-Figure 1C, note the altered Y-axis).
3.3 Deactivation of aqueous humor by charcoal stripping and heat
To gain insight into which components of AH may contribute to the increased ALP activity; we utilized dextran-coated charcoal stripping (AH/DCC) to remove hormones and lipids (Herbert et al., 1965; Keane et al., 1968; Lee et al., 1998) and heating (AH/HT) to denature protein components. As our previous results indicated a stronger overall response with serum-containing media, and little effect of dose at 3 weeks, we tested AH/DCC and AH/HT at 5% AH in full media. As before, there was a dramatic increase in ALP activity after treatment with AH, but this was reduced with AH/DCC and eliminated with AH/HT (Figure 3A).
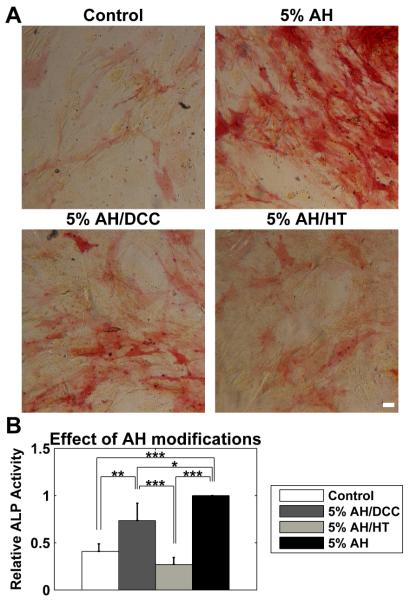
Figure 3. The effect of aqueous humor on the osteogenic potential of ASCs was dependent on thermally labile and charcoal-adsorbable components.
ASCs were cultured for 3 weeks in full media (serum supplemented DMEM) in the presence of 5% AH. The AH was either untreated, incubated with dextran-coated charcoal (DCC), or heat treated. (A) Both DCC (5% AH/DCC) and HT (5% AH/HT) treatments reduced ALP activity when compared to untreated AH (5% AH), with HT reducing activity to the level seen in untreated cells (Control). (B) Both DCC and HT significantly reduced the ALP induction caused by AH. The effect of DCC was partial, and was still significantly elevated compared to control cells, while HT resulted in complete loss of AH osteogenic activity. All data normalized to the response of 5% AH case. Data are mean ± SEM (n = 3 donors). * p < 0.05; ** p < 0.01; *** p < 0.001. Scale bar 50 μm.
We again performed this study on three donors, and normalized the ALP activity to the response to 5% AH (Figure 3B). Treatment with 5% AH/DCC significantly increased ALP activity compared to control cells, but was significantly lower than the unmodified AH. ALP activity after 5% AH/HT was not different than control, but was significantly lower than both unmodified AH and AH/DCC.
3.4 Effect of myocilin on ALP activity of ASCs
The aforementioned results demonstrated a requirement for a thermally labile component of the AH for promotion of osteogenic differentiation. The AH component protein myocilin (Rao et al., 2000; Russell et al., 2001) has recently been shown to accelerate osteogenesis in bone-marrow derived MSCs cultured in osteogenic media (Kwon et al., 2013). However, it was not shown if myocilin would have a similar effect in normal culture media, or if it would rescue the loss of ALP induction in AH/HT. To this end, we treated cells with 3 μg/mL myocilin in full media, or full media supplemented with 5% AH/HT. This dose was selected as it elicited maximal response when used with osteogenic media in a previous study (Kwon et al., 2013).
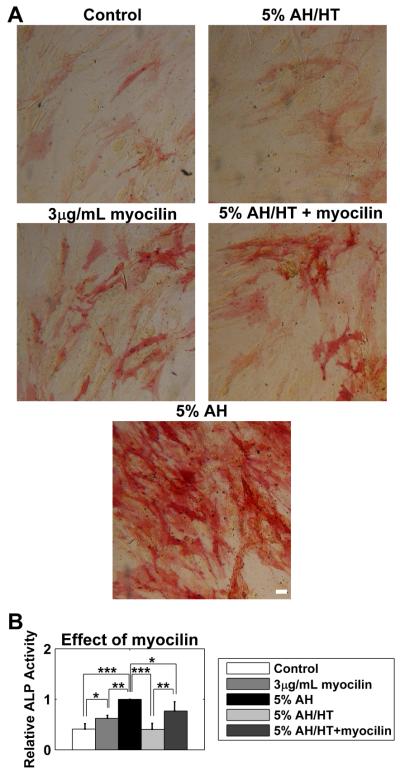
Exogenous myocilin induced a subtle though significant increase in ALP activity, both in the presence or absence of 5% AH/HT (Figure 4A, second row) when compared to the appropriate control (Figure 4A, first row). However, it was not as dramatic as unmodified 5% AH (Figure 4A, third row). Similar to the previous experiments, we quantified this for three donors, normalizing to the 5% AH response (Figure 4B). Myocilin treatment significantly increased ALP activity compared to control cells, although not to the levels observed in AH treated cells. Similar results were observed in cells treated with AH/HT. Myocilin treatment significantly elevated ALP activity in these cells, but again not to the level of unmodified AH.
Figure 4. Myocilin partially rescued the loss of activity induced by HT.
ASCs were cultured for 3 weeks in full media (serum supplemented DMEM) in the presence of 3 μg/mL myocilin (3 μg/mL myocilin), 5% aqueous humor (5% AH), heat treated 5% AH (5% AH/HT), or myocilin and 5% AH/HT (5% AH/HT + myocilin). (A) Myocilin, both separately and in conjunction with AH/HT, increased ALP activity. (B) Quantification of ALP activity. All data normalized to the response of 5% AH case. Data are mean ± SEM (n = 3 donors). * p < 0.05; ** p < 0.01; *** p < 0.001. Scale bar 50 μm.
4. Discussion
The results of this study expand on our previous finding that AH potently increases the osteogenic potential of ASCs, even in the absence of traditional osteogenic media (Morgan et al., 2014). In this study, we initially considered chondrogenic potential as well, using Alcian blue staining. However, we found the effect of AH on Alcian blue staining was less pronounced than the induction of ALP activity. Therefore, we chose to focus on the ALP promoting activity of AH when investigating the dose and time dependant effects of AH treatment.
When cultured for 2 weeks, there was a clear trend with increasing dose of AH (Figure 1B). However, after 3 weeks, that trend had diminished, with 5% treatment resulting in roughly equivalent ALP activity as supplementation of 10 and 20% AH (Figure 1C). Additionally, there was little difference between 20% treatment at 2 weeks and the 5, 10 and 20% treatments at 3 weeks (compare Figure 1B and 1C). These results suggest that the ultimate osteogenic potential of AH on the ASC population is not directly influenced by AH dose per se, but that lower doses (e.g. 5 and 10%) just take a longer time to reach this maximal level of activation. This may indicate subpopulations of responsive and non-responsive cells, which would be consistent with previous reports that have shown distinct subpopulations in bone-marrow derived MSC cultures with varying osteogenic potential (Kuznetsov et al., 1997; Liu et al., 2009).
AH has only a fraction (~0.4%) of the protein content of serum (Dernouchamps, 1982). It is possible that the osteogenic effect of AH depends on the presence of serum as a rich source of additional mitogens and other signaling molecules. To study this, we performed serum free experiments in parallel, finding that serum amplified, but was not required for, the increase in ALP activity following AH treatment (Figure 2). The effect of AH was much reduced without serum; however, extended treatments at 20% AH in serum free media resulting in a significant increase in ALP activity.
AH is known to possess chemical factors important in osteogenesis, including lipids such as LPA and steroid hormones (Bellows et al., 1987; Bellows et al., 1986; Herbertson and Aubin, 1995; Iyer et al., 2012; Knisely et al., 1994; Maniatopoulos et al., 1988; Tenenbaum and Heersche, 1982). To test the role of the lipids and steroids known to be present in AH, we incubated AH with dextran-coated charcoal (DCC), known to strip lipids and steroids (Dang and Lowik, 2005; Herbert et al., 1965; Keane et al., 1968; Lee et al., 1998). DCC pretreatment significantly reduced, but did not eliminate, ALP activity following AH treatment. This demonstrates that there is a charcoal adsorbable component of AH involved in the osteogenic response. Since AH is known to contain LPA and steroids, those are likely constituents involved in the osteogenic activation. It further demonstrates that there are additional ALP inducing components that were not adsorbed by charcoal, such as proteins.
To determine the effect of thermally labile components, including proteins, we denatured the AH by heating it to 90°C for 30 min (AH/HT) and found the induction of ALP activity completely abolished (Figure 3). A prominent protein component of normal AH is myocilin, which has previously been shown to increase osteogenic potential of MSCs treated cultured in osteogenic media (Kwon et al., 2013; Rao et al., 2000; Russell et al., 2001). Complete loss of ALP induction by AH/HT would be consistent with myocilin being a key contributor to osteogenic potential of AH to ASCs.
To further support this hypothesis, we treated cells with recombinant myocilin and found that myocilin alone is sufficient to induce ALP activity in ASCs, even in the absence of osteogenic media (Figure 4). This result expands previous findings of the effect of myocilin, tested in the context of osteogenic media (Kwon et al., 2013). However, myocilin alone was not sufficient to fully explain the effects of AH. Indeed, further experiments on the heat treated AH revealed that the addition of myocilin only partially rescued the loss of induction caused by heat treatment. Our results show conclusively that thermally labile components in AH are essential for the induction of osteogenic differentiation of ASCs. Myocilin, known to be present in AH, is one of these components.
These findings may also provide insight into the pathogenesis of glaucoma. The primary outflow pathway of AH is the trabecular meshwork (Gottanka et al., 1997; Johnson, 2006; Lutjen-Drecoll, 2005; Rohen et al., 1993), calcification of which has been implicated in glaucoma (Borras and Comes, 2009; Gonzalez et al., 2004; Gonzalez et al., 2000; Vittitow and Borras, 2004; Xue et al., 2007; Xue et al., 2006). This is consistent with glaucoma-associated increased AH expression of molecules linked to calcification, such as growth factor like lipids (Iyer et al., 2012; Liu et al., 2010), myocilin (Howell et al., 2010; Jacobson et al., 2001; Menaa et al., 2011; Nguyen et al., 1998; Polansky et al., 1997; Rao et al., 2000; Russell et al., 2001; Tamm, 2002), and connective tissue growth factor (Browne et al., 2011; Ho et al., 2005; Junglas et al., 2012; Junglas et al., 2009). In healthy meshwork, the activity of these stimulators of calcification is likely inhibited by the high expression of proteins such as matrix Gla protein (Gonzalez et al., 2004; Gonzalez et al., 2000; Tomarev et al., 2003; Vittitow and Borras, 2004). Indeed, matrix Gla inhibits the upregulation of ALP activity in cultured trabecular meshwork cells and likely plays a similar role in vivo (Xue et al., 2007; Xue et al., 2006). Taken with these studies showing the role of calcification in glaucoma, our findings offer further evidence that AH continuously bathes the anterior chamber with osteogenic stimuli.
While AH alone and myocilin in serum containing media did both increase the osteogenic potential of ASCs, additional serum components greatly augmented this process. Further, maximal osteogenic induction involved thermally labile components of AH, one of which was myocilin. Additional components of AH that adsorb to charcoal and ones present in serum greatly enhanced the osteogenic signals. In aggregate, these data add to the understanding of the induction of ALP in ASCs following AH treatment and provide important insight in how ASCs will be influenced by the ocular environment. The promotion of osteogenic potential for ASC’s upon exposure to AH indicates the need for careful evaluation of safety for ASCs grafted into the intraocular environment.
Aqueous humor induces alkaline phosphatase activity in mesenchymal stem cells
There is both a dose and temporal dependence on the response
This is mediated by both charcoal adsorbable and thermally labile components
Exogenous myocilin, a component of aqueous humor, has a similar effect
Myocilin partially reverses the effects of heat treatment
Acknowledgement
This work was funded by grants from the National Institutes of Health R01EY019475 and R01EY019970, and an unrestricted grant from Research to Prevent Blindness, and the Intramural Research Program of the National Eye Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnalich-Montiel F, Pastor S, Blazquez-Martinez A, Fernandez-Delgado J, Nistal M, Alio JL, De Miguel MP. Adipose-derived stem cells are a source for cell therapy of the corneal stroma. Stem Cells. 2008;26:570–579. doi: 10.1634/stemcells.2007-0653. [DOI] [PubMed] [Google Scholar]
- Asahina I, Sampath TK, Nishimura I, Hauschka PV. Human osteogenic protein-1 induces both chondroblastic and osteoblastic differentiation of osteoprogenitor cells derived from newborn rat calvaria. J Cell Biol. 1993;123:921–933. doi: 10.1083/jcb.123.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellows CG, Aubin JE, Heersche JN. Physiological concentrations of glucocorticoids stimulate formation of bone nodules from isolated rat calvaria cells in vitro. Endocrinology. 1987;121:1985–1992. doi: 10.1210/endo-121-6-1985. [DOI] [PubMed] [Google Scholar]
- Bellows CG, Aubin JE, Heersche JN, Antosz ME. Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int. 1986;38:143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- Borras T, Comes N. Evidence for a calcification process in the trabecular meshwork. Exp Eye Res. 2009;88:738–746. doi: 10.1016/j.exer.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne JG, Ho SL, Kane R, Oliver N, Clark AF, O’Brien CJ, Crean JK. Connective tissue growth factor is increased in pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2011;52:3660–3666. doi: 10.1167/iovs.10-5209. [DOI] [PubMed] [Google Scholar]
- Caplan AI. MSCs as Therapeutics. In: Hematti P, Keating A, editors. Mesenchymal Stromal Cells. Springer; New York: 2013. pp. 79–90. [Google Scholar]
- Chung DJ, Hayashi K, Toupadakis CA, Wong A, Yellowley CE. Osteogenic proliferation and differentiation of canine bone marrow and adipose tissue derived mesenchymal stromal cells and the influence of hypoxia. Res Vet Sci. 2012;92:66–75. doi: 10.1016/j.rvsc.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Chung JK, Park TK, Ohn YH, Park SK, Hong DS. Modulation of retinal wound healing by systemically administered bone marrow-derived mesenchymal stem cells. Korean J Ophthalmol. 2011;25:268–274. doi: 10.3341/kjo.2011.25.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201–2211. [PubMed] [Google Scholar]
- Dang ZC, Lowik CW. Removal of serum factors by charcoal treatment promotes adipogenesis via a MAPK-dependent pathway. Mol Cell Biochem. 2005;268:159–167. doi: 10.1007/s11010-005-3857-7. [DOI] [PubMed] [Google Scholar]
- De Berardinis E, Tieri O, Polzella A, Iuglio N. The chemical composition of the human aqueous humour in normal and pathological conditions. Exp Eye Res. 1965;4:179–186. doi: 10.1016/s0014-4835(65)80030-6. [DOI] [PubMed] [Google Scholar]
- Dernouchamps JP. The proteins of the aqueous humour. Documenta ophthalmologica. Advances in ophthalmology. 1982;53:193–248. doi: 10.1007/BF00140422. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Duan X, Xue P, Wang N, Dong Z, Lu Q, Yang F. Proteomic analysis of aqueous humor from patients with primary open angle glaucoma. Mol Vis. 2010;16:2839–2846. [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Aribindi K, Guerra Y, Bhattacharya SK. Sphingolipids and ceramides of mouse aqueous humor: Comparative profiles from normotensive and hypertensive DBA/2J mice. Biochimie. 2014 doi: 10.1016/j.biochi.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez P, Caballero M, Liton PB, Stamer WD, Epstein DL. Expression analysis of the matrix GLA protein and VE-cadherin gene promoters in the outflow pathway. Invest Ophthalmol Vis Sci. 2004;45:1389–1395. doi: 10.1167/iovs.03-0537. [DOI] [PubMed] [Google Scholar]
- Gonzalez P, Epstein DL, Borras T. Characterization of gene expression in human trabecular meshwork using single-pass sequencing of 1060 clones. Invest Ophthalmol Vis Sci. 2000;41:3678–3693. [PubMed] [Google Scholar]
- Gottanka J, Johnson DH, Martus P, Lutjen-Drecoll E. Severity of optic nerve damage in eyes with POAG is correlated with changes in the trabecular meshwork. Journal of glaucoma. 1997;6:123–132. [PubMed] [Google Scholar]
- Greiner JV, Chanes LA, Glonek T. Comparison of phosphate metabolites of the ocular humors. Ophthalmic research. 1991;23:92–97. doi: 10.1159/000267095. [DOI] [PubMed] [Google Scholar]
- Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25:1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Herbertson A, Aubin JE. Dexamethasone alters the subpopulation make-up of rat bone marrow stromal cell cultures. J Bone Miner Res. 1995;10:285–294. doi: 10.1002/jbmr.5650100216. [DOI] [PubMed] [Google Scholar]
- Ho SL, Dogar GF, Wang J, Crean J, Wu QD, Oliver N, Weitz S, Murray A, Cleary PE, O’Brien C. Elevated aqueous humour tissue inhibitor of matrix metalloproteinase-1 and connective tissue growth factor in pseudoexfoliation syndrome. Br J Ophthalmol. 2005;89:169–173. doi: 10.1136/bjo.2004.044685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- Howell KG, Vrabel AM, Chowdhury UR, Stamer WD, Fautsch MP. Myocilin levels in primary open-angle glaucoma and pseudoexfoliation glaucoma human aqueous humor. Journal of glaucoma. 2010;19:569–575. doi: 10.1097/IJG.0b013e3181d13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Iriyama A, Ueno S, Takahashi H, Kondo M, Tamaki Y, Araie M, Yanagi Y. Subretinal transplantation of bone marrow mesenchymal stem cells delays retinal degeneration in the RCS rat model of retinal degeneration. Exp Eye Res. 2007;85:234–241. doi: 10.1016/j.exer.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Iyer P, Lalane R, 3rd, Morris C, Challa P, Vann R, Rao PV. Autotaxin lysophosphatidic acid axis is a novel molecular target for lowering intraocular pressure. PLoS One. 2012;7:e42627. doi: 10.1371/journal.pone.0042627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001;10:117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- Jia Z, Jiao C, Zhao S, Li X, Ren X, Zhang L, Han ZC, Zhang X. Immunomodulatory effects of mesenchymal stem cells in a rat corneal allograft rejection model. Exp Eye Res. 2012;102:44–49. doi: 10.1016/j.exer.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhang Y, Zhang L, Wang M, Zhang X, Li X. Therapeutic effect of bone marrow mesenchymal stem cells on laser-induced retinal injury in mice. International journal of molecular sciences. 2014;15:9372–9385. doi: 10.3390/ijms15069372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe AW, Gregory-Evans K. Mesenchymal stem cells and potential applications in treating ocular disease. Curr Eye Res. 2010;35:941–952. doi: 10.3109/02713683.2010.516466. [DOI] [PubMed] [Google Scholar]
- Johnson M. What controls aqueous humour outflow resistance? Exp Eye Res. 2006;82:545–557. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce NC, Harris DL, Markov V, Zhang Z, Saitta B. Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol Vis. 2012;18:547–564. [PMC free article] [PubMed] [Google Scholar]
- Junglas B, Kuespert S, Seleem AA, Struller T, Ullmann S, Bosl M, Bosserhoff A, Kostler J, Wagner R, Tamm ER, Fuchshofer R. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol. 2012;180:2386–2403. doi: 10.1016/j.ajpath.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Junglas B, Yu AH, Welge-Lussen U, Tamm ER, Fuchshofer R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp Eye Res. 2009;88:1065–1075. doi: 10.1016/j.exer.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Keane PM, Pearson J, Walker WH. Dextran-coated charcoal immunoassay of insulin. Diabetologia. 1968;4:339–344. doi: 10.1007/BF01211769. [DOI] [PubMed] [Google Scholar]
- Kicic A, Shen WY, Wilson AS, Constable IJ, Robertson T, Rakoczy PE. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci. 2003;23:7742–7749. doi: 10.1523/JNEUROSCI.23-21-07742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisely TL, Hosoi J, Nazareno R, Granstein RD. The presence of biologically significant concentrations of glucocorticoids but little or no cortisol binding globulin within aqueous humor: relevance to immune privilege in the anterior chamber of the eye. Invest Ophthalmol Vis Sci. 1994;35:3711–3723. [PubMed] [Google Scholar]
- Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, Robey PG. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- Kwon HS, Johnson TV, Tomarev SI. Myocilin stimulates osteogenic differentiation of mesenchymal stem cells through mitogen-activated protein kinase signaling. J Biol Chem. 2013;288:16882–16894. doi: 10.1074/jbc.M112.422972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HS, Lee HS, Ji Y, Rubin JS, Tomarev SI. Myocilin is a modulator of Wnt signaling. Mol Cell Biol. 2009;29:2139–2154. doi: 10.1128/MCB.01274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Lee P, Lam KW, Lai M. Aqueous humor ascorbate concentration and open-angle glaucoma. Arch Ophthalmol. 1977;95:308–310. doi: 10.1001/archopht.1977.04450020109018. [DOI] [PubMed] [Google Scholar]
- Li G, Yuan L, Ren X, Nian H, Zhang L, Han ZC, Li X, Zhang X. The effect of mesenchymal stem cells on dynamic changes of T cell subsets in experimental autoimmune uveoretinitis. Clin Exp Immunol. 2013;173:28–37. doi: 10.1111/cei.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Toh WS, Lu K, MacAry PA, Kemeny DM, Cao T. A subpopulation of mesenchymal stromal cells with high osteogenic potential. J Cell Mol Med. 2009;13:2436–2447. doi: 10.1111/j.1582-4934.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang J, Liu CY, Hayashi Y, Kao WW. Bone marrow mesenchymal stem cells can differentiate and assume corneal keratocyte phenotype. J Cell Mol Med. 2012;16:1114–1124. doi: 10.1111/j.1582-4934.2011.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YB, Kharode Y, Bodine PV, Yaworsky PJ, Robinson JA, Billiard J. LPA induces osteoblast differentiation through interplay of two receptors: LPA1 and LPA4. J Cell Biochem. 2010;109:794–800. doi: 10.1002/jcb.22471. [DOI] [PubMed] [Google Scholar]
- Lutjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res. 2005;81:1–4. doi: 10.1016/j.exer.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Machalinska A, Kawa M, Pius-Sadowska E, Stepniewski J, Nowak W, Roginska D, Kaczynska K, Baumert B, Wiszniewska B, Jozkowicz A, Dulak J, Machalinski B. Long-term neuroprotective effects of NT-4-engineered mesenchymal stem cells injected intravitreally in a mouse model of acute retinal injury. Invest Ophthalmol Vis Sci. 2013;54:8292–8305. doi: 10.1167/iovs.13-12221. [DOI] [PubMed] [Google Scholar]
- Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254:317–330. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- Manuguerra-Gagne R, Boulos PR, Ammar A, Leblond FA, Krosl G, Pichette V, Lesk MR, Roy DC. Transplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment. Stem Cells. 2013;31:1136–1148. doi: 10.1002/stem.1364. [DOI] [PubMed] [Google Scholar]
- Menaa F, Braghini CA, Vasconcellos JP, Menaa B, Costa VP, Figueiredo ES, Melo MB. Keeping an eye on myocilin: a complex molecule associated with primary open-angle glaucoma susceptibility. Molecules. 2011;16:5402–5421. doi: 10.3390/molecules16075402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Wood JA, Walker NJ, Raghunathan VK, Borjesson DL, Murphy CJ, Russell P. Human Trabecular Meshwork Cells Exhibit Several Characteristics of, but Are Distinct from, Adipose-Derived Mesenchymal Stem Cells. J Ocul Pharmacol Ther. 2014;30:254–266. doi: 10.1089/jop.2013.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moviglia GA, Blasetti N, Zarate JO, Pelayes DE. In vitro differentiation of adult adipose mesenchymal stem cells into retinal progenitor cells. Ophthalmic research. 2012;48(Suppl 1):1–5. doi: 10.1159/000339839. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- Park SH, Kim KW, Chun YS, Kim JC. Human mesenchymal stem cells differentiate into keratocyte-like cells in keratocyte-conditioned medium. Exp Eye Res. 2012;101:16–26. doi: 10.1016/j.exer.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Chen P, Chen H, Lutjen-Drecoll E, Johnson D, Kurtz RM, Ma ZD, Bloom E, Nguyen TD. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. 1997;211:126–139. doi: 10.1159/000310780. [DOI] [PubMed] [Google Scholar]
- Rajashekhar G. Mesenchymal stem cells: new players in retinopathy therapy. Frontiers in endocrinology. 2014;5:59. doi: 10.3389/fendo.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PV, Allingham RR, Epstein DL. TIGR/myocilin in human aqueous humor. Exp Eye Res. 2000;71:637–641. doi: 10.1006/exer.2000.0920. [DOI] [PubMed] [Google Scholar]
- Rohen JW, Lutjen-Drecoll E, Flugel C, Meyer M, Grierson I. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG) Exp Eye Res. 1993;56:683–692. doi: 10.1006/exer.1993.1085. [DOI] [PubMed] [Google Scholar]
- Russell P, Tamm ER, Grehn FJ, Picht G, Johnson M. The presence and properties of myocilin in the aqueous humor. Invest Ophthalmol Vis Sci. 2001;42:983–986. [PubMed] [Google Scholar]
- Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002;21:395–428. doi: 10.1016/s1350-9462(02)00010-1. [DOI] [PubMed] [Google Scholar]
- Tenenbaum HC, Heersche JN. Differentiation of osteoblasts and formation of mineralized bone in vitro. Calcif Tissue Int. 1982;34:76–79. doi: 10.1007/BF02411212. [DOI] [PubMed] [Google Scholar]
- Tomarev SI, Wistow G, Raymond V, Dubois S, Malyukova I. Gene expression profile of the human trabecular meshwork: NEIBank sequence tag analysis. Invest Ophthalmol Vis Sci. 2003;44:2588–2596. doi: 10.1167/iovs.02-1099. [DOI] [PubMed] [Google Scholar]
- Toupadakis CA, Wong A, Genetos DC, Cheung WK, Borjesson DL, Ferraro GL, Galuppo LD, Leach JK, Owens SD, Yellowley CE. Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am J Vet Res. 2010;71:1237–1245. doi: 10.2460/ajvr.71.10.1237. [DOI] [PubMed] [Google Scholar]
- Tripathi RC, Millard CB, Tripathi BJ. Protein composition of human aqueous humor: SDS-PAGE analysis of surgical and post-mortem samples. Exp Eye Res. 1989;48:117–130. doi: 10.1016/0014-4835(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Vittitow J, Borras T. Genes expressed in the human trabecular meshwork during pressure-induced homeostatic response. J Cell Physiol. 2004;201:126–137. doi: 10.1002/jcp.20030. [DOI] [PubMed] [Google Scholar]
- Vossmerbaeumer U, Ohnesorge S, Kuehl S, Haapalahti M, Kluter H, Jonas JB, Thierse HJ, Bieback K. Retinal pigment epithelial phenotype induced in human adipose tissue-derived mesenchymal stromal cells. Cytotherapy. 2009;11:177–188. doi: 10.1080/14653240802714819. [DOI] [PubMed] [Google Scholar]
- Voswinkel J, Francois S, Simon JM, Benderitter M, Gorin NC, Mohty M, Fouillard L, Chapel A. Use of Mesenchymal Stem Cells (MSC) in Chronic Inflammatory Fistulizing and Fibrotic Diseases: a Comprehensive Review. Clinical reviews in allergy & immunology. 2013 doi: 10.1007/s12016-012-8347-6. [DOI] [PubMed] [Google Scholar]
- Wood JA, Chung DJ, Park SA, Zwingenberger AL, Reilly CM, Ly I, Walker NJ, Vernau W, Hayashi K, Wisner ER, Cannon MS, Kass PH, Cherry SR, Borjesson DL, Russell P, Murphy CJ. Periocular and intra-articular injection of canine adipose-derived mesenchymal stem cells: an in vivo imaging and migration study. J Ocul Pharmacol Ther. 2012;28:307–317. doi: 10.1089/jop.2011.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Comes N, Borras T. Presence of an established calcification marker in trabecular meshwork tissue of glaucoma donors. Invest Ophthalmol Vis Sci. 2007;48:3184–3194. doi: 10.1167/iovs.06-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Wallin R, Olmsted-Davis EA, Borras T. Matrix GLA protein function in human trabecular meshwork cells: inhibition of BMP2-induced calcification process. Invest Ophthalmol Vis Sci. 2006;47:997–1007. doi: 10.1167/iovs.05-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Li ZR, Su WR, Li YP, Lin ML, Zhang WX, Liu Y, Wan Q, Liang D. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One. 2012;7:e30842. doi: 10.1371/journal.pone.0030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ren X, Li G, Jiao C, Zhang L, Zhao S, Wang J, Han ZC, Li X. Mesenchymal stem cells ameliorate experimental autoimmune uveoretinitis by comprehensive modulation of systemic autoimmunity. Invest Ophthalmol Vis Sci. 2011;52:3143–3152. doi: 10.1167/iovs.10-6334. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]