Abstract
Objective
To determine if a treatment for interepisode bipolar disorder I patients with insomnia improves mood state, sleep, and functioning.
Method
Alongside psychiatric care, interepisode bipolar disorder I participants with insomnia were randomly allocated to a bipolar disorder–specific modification of cognitive behavior therapy for insomnia (CBTI-BP; n = 30) or psychoeducation (PE; n = 28) as a comparison condition. Outcomes were assessed at baseline, the end of 8 sessions of treatment, and 6 months later. This pilot was conducted to determine initial feasibility and generate effect size estimates.
Results
During the 6-month follow-up, the CBTI-BP group had fewer days in a bipolar episode relative to the PE group (3.3 days vs. 25.5 days). The CBTI-BP group also experienced a significantly lower hypomania/mania relapse rate (4.6% vs. 31.6%) and a marginally lower overall mood episode relapse rate (13.6% vs. 42.1%) compared with the PE group. Relative to PE, CBTI-BP reduced insomnia severity and led to higher rates of insomnia remission at posttreatment and marginally higher rates at 6 months. Both CBTI-BP and PE showed statistically significant improvement on selected sleep and functional impairment measures. The effects of treatment were well sustained through follow-up for most outcomes, although some decline on secondary sleep benefits was observed.
Conclusions
CBTI-BP was associated with reduced risk of mood episode relapse and improved sleep and functioning on certain outcomes in bipolar disorder. Hence, sleep disturbance appears to be an important pathway contributing to bipolar disorder. The need to develop bipolar disorder–specific sleep diary scoring standards is highlighted.
Public Health Significance
This study suggests that an intervention to improve sleep and circadian functioning reduces risk of relapse and improves sleep and overall functioning among individuals who meet diagnostic criteria for bipolar disorder.
Keywords: bipolar disorder, cognitive behavior therapy, insomnia, sleep
Bipolar disorder is a common, severe, and chronic disorder (Angst & Sellaro, 2000; Judd et al., 2002). Despite important advances in treatments, new treatment options are urgently needed, particularly for subsyndromal interepisode mood symptoms that impair functioning and increase the risk of relapse (Judd et al., 2002).
Sleep disturbance is among the most prominent correlates of mood episodes and inadequate recovery. Reduced need for sleep is a symptom of mania. During episodes of depression, insomnia or hypersomnia are common (American Psychiatric Association, 2013). Even during the interepisode period, sleep is disturbed; up to 70% of bipolar disorder patients report a clinically significant sleep disturbance (Harvey, Schmidt, Scarna, Semler, & Goodwin, 2005), which is associated with relapse and suicide attempts (Sylvia et al., 2012). Sleep disturbance escalates just before and worsens during episodes (Jackson, Cavanagh, & Scott, 2003) and does not always resolve with medication (Gruber et al., 2009, 2011). In addition, poor sleep efficiency, night-to-night variability, long sleep onset latency (Eidelman, Talbot, Gruber, & Harvey, 2010), and delayed phase (Giglio et al., 2010; Mansour et al., 2005) are also common problems for individuals with bipolar disorder. The heterogeneity of sleep problems in bipolar disorder raise significant management and research challenges.
Multiple lines of evidence suggest that sleep disturbances, such as insomnia, contribute to mood symptoms in bipolar disorder. First, bipolar disorder patients with short compared with longer sleep exhibit more symptoms of mania, depression, anxiety, and irritability (Gruber et al., 2009). Moreover, shorter total sleep time is associated with increased mania and depression severity over 12 months (Gruber et al., 2011). Second, in a 7-day diary study, total wake time was associated with next morning negative mood in bipolar disorder, and evening negative mood was associated with longer total wake time the following night in both bipolar disorder and insomnia (Talbot et al., 2012). Moreover, in an 8-week diary study, there was a tight coupling across days and nights between sleep and negative affect (Gershon et al., 2012). Third, sleep disturbance is a common prodrome, or early warning signal, of relapse (Jackson et al., 2003). Fourth, experimentally induced sleep deprivation is associated with the onset of hypomania or mania in bipolar disorder (e.g., Wehr, Sack, & Rosenthal, 1987). Together, these data raise the possibility that treating sleep disturbance among patients who meet diagnostic criteria for bipolar disorder could improve mood and functioning and alter the course of the disorder.
Despite the clear importance of sleep disturbance in bipolar disorder, very few empirical data specifically address its treatment. We are unaware of any controlled clinical trials for insomnia in bipolar disorder. Zolpidem was the most commonly prescribed medication for insomnia in bipolar disorder in one chart review (Schaffer, Schaffer, Miller, Hang, & Nordahl, 2011), but case reports on benzodiazepines for insomnia in bipolar disorder have not been encouraging (Sattar, Ramaswamy, Bhatia, & Petty, 2003; Weilburg, Sachs, & Falk, 1987). Indeed, drawing from the large sample that participated in the Systematic Treatment Enhancement Program for Bipolar Disorder, Perlis et al. (2010) reported that benzodiazepines were associated with worse outcome. A case report on gabapentin (Egashira et al., 2011) and open trials of medications targeting circadian rhythms (Bersani & Garavini, 2000; Calabrese, Guelfi, Perdrizet-Chevallier, & the Agomelatine Bipolar Study Group, 2007) are more promising but preliminary. Quetiapine was associated with not only relief of bipolar depression but also improved sleep in several studies (e.g., Calabrese et al., 2005; Thase et al., 2006). Existing evidence-based nonpharmacologic interventions for bipolar disorder include psychoeducation (Colom, Vieta, & Scott, 2006), prodrome monitoring (Lam et al., 2003), interpersonal and social rhythm therapy (IPSRT; Frank, 2005), family therapy (Miklowitz & Goldstein, 1997), and cognitive behavior therapy (CBT) administered individually (Lam et al., 2003) or in groups (Patelis-Siotis et al., 2001) as well as combination approaches (Craighead & Craighead, 2001). These treatments, several of which include some attention to sleep disturbances, reduce relapse among adults with bipolar disorder. However, to the best of our knowledge, most of these trials have not had sleep as a primary emphasis or included sleep outcome measures, nor have these treatments incorporated specific insomnia treatment techniques.
In light of the ongoing need for novel, effective, well-tolerated treatments for bipolar disorder, we developed a bipolar disorder– specific modification of CBT for insomnia (CBTI-BP). The modifications were focused on improving safety and targeting the unique features of sleep in bipolar disorder by integrating elements from IPSRT, chronotherapy, and motivational interviewing. CBT for insomnia (CBT-I) was selected as the basis for the treatment because (a) substantial evidence demonstrates the efficacy of CBT-I (Morin et al., 2006), even for insomnia comorbid with psychiatric disorders (Taylor & Pruiksma, 2014), including depression (Manber et al., 2008), posttraumatic stress disorder (Ger-main, Shear, Hall, & Buysse, 2007; Talbot et al., 2014), and schizophrenia (Myers, Startup, & Freeman, 2011); (b) when insomnia is comorbid with another psychiatric disorder, the symptoms associated with the other psychiatric disorder can also improve following CBT-I (Manber et al., 2008; Myers et al., 2011; Talbot et al., 2014); (c) previous treatments for bipolar disorder have not been informed by the principles underlying CBT-I (Eidelman et al., 2010; Harvey, 2008); and (d) CBT-I improves sleep efficiency, reduces night-to-night variability, and improves sleep onset latency, all of which are problematic for patients with bipolar disorder (Eidelman et al., 2010). Elements were added from IPSRT (Frank, 2005) to regularize daily social rhythms, building on regular bed and wake-up times. Promising data on chronotherapy, such as dark therapy and light therapy (Wirz-Justice, Benedetti, & Terman, 2009), were integrated by adding a 30–60 min wind-down period in dim light and exposure to bright light on waking within an individualized brisk wake up. Motivational interviewing (Miller & Rollnick, 2002) was incorporated in every session given the challenges inherent to behavior change and recognizing the rewarding nature of many sleep-incompatible behaviors.
The aim of the present pilot study was to evaluate the efficacy of CBTI-BP to determine initial feasibility and generate effect size estimates. Interepisode adults with insomnia and bipolar disorder Type I were randomly allocated to receive eight sessions of CBTI-BP or eight sessions of psychoeducation (PE). PE controlled for therapeutic attention and expectation of improvement. We hypothesized that CBTI-BP, relative to PE, would be more efficacious for improving mood state, sleep symptoms, and functioning.
Method
Design
Adults with bipolar disorder Type I and insomnia were randomly assigned, in a 1:1 parallel group design, to receive either CBTI-BP or PE. Randomization was stratified by age (≤49 years, ≥50 years), because sleep varies by age (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004). Assessment and therapy teams were blinded to treatment allocation by using sequentially numbered, opaque, sealed envelopes opened by a project coordinator. Only the project coordinator and the assigned therapist knew the treatment allocation of each participant. All other team members were blind. Both treatments were eight sessions. Assessments were conducted at baseline, end of treatment, and 6-month follow-up. The University of California, Berkeley, Committee for the Protection of Human Subjects approved the study. All participants provided written informed consent and were financially compensated for their time and expenses. A data safety and monitoring board reviewed the study every 6 months during the active treatment phase.
Participants
Participants were 58 adults with interepisode bipolar disorder Type I and insomnia recruited between March 2010 and April 2012 through clinicians or advertisements. Individuals considered potentially eligible during a telephone screen were invited for an in-person diagnostic session.
Individuals were eligible if they (a) met Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev. [DSM–IV–TR]; American Psychiatric Association, 2000) criteria for bipolar disorder Type I by the Structured Clinical Interview for DSM–IV (SCID; First, Spitzer, Gibbon, & Williams, 1995); (b) were interepisode as defined by a Young Mania Rating Scale (YMRS; Young, Biggs, Ziegler, & Meyer, 1978) score <12 and an Inventory of Depressive Symptomatology, Clinician Rating (IDS-C; Rush, Carmody, & Reimitz, 2000) score <24 for the past week; (c) met criteria for general insomnia disorder, as defined by the International Classification of Sleep Disorders (2nd ed.; American Academy of Sleep Medicine, 2005), and DSM–IV–TR criteria for primary insomnia but without the exclusion for mental disorders via the Duke Structured Interview for Sleep Disorders (DSISD; Edinger et al., 2004); (d) had a stable medication regimen for the past 4 weeks; (e) had a treating psychiatrist; and (f) were fluent in English.
Exclusion criteria were (a) alcohol and substance abuse/dependence over the past 3 months; (b) current posttraumatic stress disorder; (c) active or progressive physical illness directly related to the onset and course of insomnia; (d) sleep apnea, restless legs syndrome or periodic limb movement disorder on the basis of the DSISD; (e) current suicidal or homicidal risk; (f) pregnancy or current breast-feeding; and (g) overnight shift work in the past 3 months.
The prevalence of delayed sleep phase and hypersomnia features among patients with insomnia and bipolar disorder (Giglio et al., 2010; Kaplan & Harvey, 2009) prompted the addition of elements from chronotherapy and IPSRT. Hence, these disorders were not excluded. We excluded shift work and pregnancy/breast- feeding because techniques for addressing these sleep problems were not included in CBTI-BD. For pregnancy and breast-feeding, there are potential safety concerns with a new treatment, and although delayed sleep phase and hypersomnia can intrinsically be part of bipolar sleep disturbance, shift work and pregnancy/breast-feeding cannot. Also, shift work involves frequently changing sleep patterns that are beyond an individual's control.
Treatments
All treatments were administered by doctoral- or master's-level therapists. Weekly supervision was conducted by a licensed clinical psychologist (Allison G. Harvey) separately for the CBTI-BP and PE therapists. All therapy sessions were tape-recorded, and a randomly selected subset (24 CBTI-BP, 24 PE; 10% of sessions) were closely scrutinized by blind judges. Following previous research in the field (Edinger, Wohlgemuth, Radtke, Marsh, & Quillian, 2001), a checklist of treatment elements specific to CBTI-BP and PE was devised. Each element was rated for presence/absence and whether there was a focus on behavior change (a distinguishing feature of the two treatment conditions). A total of 62 CBTI-BP-specific treatment elements were coded in CBTI-BP sessions, relative to zero CBTI-BP elements in PE sessions. A total of 31 PE-specific treatment elements were coded in PE sessions, relative to one PE element in CBTI-BP sessions. In CBTI-BP sessions, 63 instances of behavior change were present, relative to zero instances in PE sessions.
The elements in common across CBTI-BP and PE were following a structured agenda and setting homework at each session. The specific elements were manualized. The CBTI-BP group did not receive PE, and the PE group did not receive CBTI-BP. The sessions were typically 50–60 min in duration.
CBTI-BP
Session 1 focused on case formulation, goal setting, motivational interviewing, and sleep and circadian education. For subsequent sessions, although all modules were covered for all patients and in the order described here, the “dose” varied on the basis of the case formulation. The behavioral module included (a) stimulus control, strengthening associations between bed and sleep and regularizing sleep and wake times as well as daytime rhythms (e.g., meal and exercise times); (b) sleep restriction to improve sleep efficiency and consolidate sleep; (c) sleep/circadian education, (d) devising a “wind-down” of 30–60 min in which relaxing, sleep-enhancing activities were introduced in dim light conditions; and (e) devising a wake-up routine. The cognitive module included (a) altering unhelpful beliefs about sleep and (b) reducing sleep-related anxiety, bedtime worry, rumination, and vigilance. The module to improve daytime functioning included (a) behavioral experiments to allow the patient to experience the energy-generating effects of activity and (b) identification of energy-sapping and energy-generating activities, with the latter being useful for managing daytime tiredness. Relapse prevention was the focus for the final session. A fuller description of CBTI-BP is available in the online supplemental materials.
PE
PE provided information but did not facilitate or plan for behavior change. Session 1 (S1) introduced a model in which sleep, stress, diet, health, exercise, and mood are interrelated and have reciprocal effects. That and subsequent sessions focused on mood regulation and the etiology of bipolar disorder (S1), symptoms of mania and depression (S2), individualized prodromes (S3), medications (S4), substance use and diet (S5), physical activity and stress management (S6), relaxation and breathing (S7), and self-esteem and sleep in a social context and a summary of all sessions (S8). Patients were asked for a detailed description of their experience with each topic and were provided with general education. PE constituted a conservative test of the benefits of CBTI-BP and controlled for location, experience, and skill level of the therapists, number and quality of handouts, therapist attention, amount and frequency of therapist contact, duration of treatment, and expectations about the efficacy of treatment. Note that the relaxation education differed from the relaxation sometimes included in CBT-I; the total sleep education portion was less than 10 min in duration in PE, and there was no encouragement to try relaxation at home and no resources (e.g., a relaxation tape) or follow-up in subsequent sessions to encourage practice of relaxation.
Measures
Assessors were graduate students in clinical psychology, independent of the therapy team and blind to treatment condition. SCID bipolar disorder diagnosis and interepisode mood state were confirmed by a psychiatrist (Descartes Li). All assessment sessions were tape-recorded, and a random subset (15%) were selected for close scrutiny by raters blind to treatment condition and diagnoses. Interrater reliabilities for the diagnostic measures were very good (insomnia diagnosis: κ = 1.00; bipolar disorder SCID diagnosis and interepisode mood state; κ = 1.00; nonbipolar disorder SCID diagnoses: κ = 0.80). Except where specified, all measures were delivered at baseline, at the end of treatment, and at 6-month follow-up.
Diagnostic outcomes
The SCID (First et al., 1995) was administered to assess for DSM–IV–TR Axis I disorders and to determine the presence or absence of current DSM–IV–TR bipolar episodes (mania, hypomania, depression). The SCID has good reliability. Trained psychology doctoral students and postdoctoral fellows administered the SCID to all participants to assess current and lifetime Axis I disorders. Randomly selected audiotapes of SCID interviews (15%) were rated by a set of independent reviewers in order to check diagnostic reliability. Ratings matched 100% (κ= 1.00) of the primary diagnoses made by the original interviewer. This indicates strong interrater reliability, though the use of a “skip-out” strategy (implemented if initial required criteria for a particular disorder were not met) may have reduced the number of potential disagreements with the original interviewer.
The DSISD (Edinger et al., 2004) is a semistructured interview that assesses whether participants meet criteria for general insomnia disorder as defined by the International Classification of Sleep Disorders (2nd ed.; American Academy of Sleep Medicine, 2005) as well as DSM–IV–TR criteria for primary insomnia but without the exclusion for mental disorders. The DSISD has been shown to have good reliability and validity (Edinger et al., 2009). Randomly selected audiotapes of DSISD interviews (15%) were rated by a set of independent reviewers for diagnostic reliability. Ratings matched 100% (κ= 1.00) of the primary diagnoses made by the original interviewer.
Mood outcomes
Relapse—that is, the emergence of a new syndromal DSM–IV–TR bipolar episode (mania, hypomania, or depression; Frank et al., 1991)—and the number of days spent in bipolar episodes were assessed with the SCID and constituted the primary outcomes.
The YMRS (Young et al., 1978) and the IDS-C (Rush et al., 2000) were secondary mood outcomes. The YMRS is an 11-item measure used to assess the severity of manic symptoms, with each item rated on a five-point scale. It has been shown to have good reliability and validity (Young et al., 1978). The IDS-C is a widely used 30-item instrument assessing depressive symptoms, with each item rated on a four-point scale. The measure has demonstrated good reliability and validity (Rush, Gullion, Basco, Jarrett, & Trivedi, 1996).
Sleep outcomes
Primary sleep outcomes were the Insomnia Severity Index (ISI; Bastien, Vallieres, & Morin, 2001) and insomnia diagnosis as assessed by the DSISD. Though there are no generally accepted criteria for remission in insomnia treatment studies (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006), our two primary outcomes have been established as valid markers of clinically significant change (Buysse et al., 2006; Yang, Morin, Schaefer, & Wallenstein, 2009).
The ISI is a brief seven-item assessment of nighttime variables (difficulties falling asleep, staying asleep, early morning awakenings) and daytime variables (satisfaction with sleep, degree of impairment with daytime functioning, noticeability of impairments, distress). The ISI has adequate internal consistency (Cron-bach's α= .91) and temporal stability (r = .80), has been validated against diary and polysomnographic measures of sleep (Bastien et al., 2001), and is sensitive to therapeutic changes in several treatment studies (e.g., Morin et al., 2009). The following interpretation guidelines are recommended: score of 0–7 (no clinical insomnia), score of 8–14 (subthreshold insomnia), score of 15–21 (insomnia of moderate severity), and score of 22–28 (severe insomnia). The total score and rates of treatment responders (defined as achieving a decrease of greater than 7 points) and remitters (defined as having a final score below 8) were the primary outcome measures for this study.
Following the standard recommendations for sleep research (Buysse et al., 2006; Carney, 2012), the sleep diary included questions to assess time to fall asleep (i.e., sleep onset latency), total length of time awake during the night (i.e., wake after sleep onset), amount of time awake between the final awakening and the time of getting out of bed (i.e., terminal wakefulness), number of awakenings during the night, time spent in bed, total sleep time, bed time, final wake time, and final arising time. The sleep diary has been shown to be a reliable estimate of these sleep parameters (Morin & Espie, 2003). An a priori decision was made to investigate sleep efficiency as the key outcome from the sleep diary because, in interepisode bipolar disorder, lower and more variable sleep efficiency are related to worse illness course and outcome (Eidelman et al., 2010), and sleep efficiency is a common summary metric that incorporates multiple sleep diary parameters. Sleep efficiency is calculated by dividing total sleep time by time in bed and multiplying this value by 100. The secondary sleep outcome measures derived from the diaries were sleep onset latency, wake time after sleep onset, total wake time (summation of sleep onset latency + wake time after sleep onset + terminal wakefulness), time in bed, and total sleep time.
Other secondary sleep outcome measures were the Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) and the Patient-Reported Outcomes Measurement Information System–Sleep Disturbance (PROMIS-SD; Buysse et al., 2010). The PSQI is composed of four open-ended questions and 19 self-rated items (0–3 scale) assessing sleep over a 1-month interval. The global score ranges from 0 to 21. The PSQI has excellent psychometric properties. The PROMIS-SD was developed as part of the National Institutes of Health (NIH) Roadmap initiative. The short form (eight items) was administered, with item scores ranging from 1 (not at all) to 5 (very much). Total raw scores are converted into a T score. This scale has established reliability and validity (convergent and construct; Buysse et al., 2010; Yu, Buysse, Germain, & Moul, 2012).
Functional impairment outcomes
The primary functional impairment outcome was the Sheehan Disability Scale (SDS), which assessed mood- and sleep-related impairment (Sheehan, Harnett-Sheehan, & Raj, 1996). The SDS evaluates the extent to which work/school, social life, and home/family responsibilities are impaired on a 0–10 (not at all to extremely) scale. Its psycho-metrics are well established (Sheehan et al., 1996). The three items were averaged to assess global functional impairment (0 [not impaired] to 10 [highly impaired]).
Secondary measures of functional impairment were the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q-SF; Ritsner, Kurs, Gibel, Ratner, & Endicott, 2005) and the PROMIS– Sleep-Related Impairment (PROMIS-SRI; Buysse et al., 2010). The Q-LES-Q-SF is a brief form (16 items) that maintains the validity and psychometric properties of the 60-item version (Endicott, Nee, Harrison, & Blumenthal, 1993). Scoring is from 1 to 5 (not at all or never to frequently or all the time). Scores are reported as a percentage of the maximum possible score, with higher scores indicating better quality of life. The PROMIS-SRI was developed as part of the NIH Roadmap initiative and designed to improve patient-reported outcomes using state-of-the-art psychometric methods. The short form (eight items) is scored 1 (not at all) to 5 (very much), and total raw scores are converted into a T score. This scale has established reliability and validity (convergent and construct; Buysse et al., 2010; Yu et al., 2012).
Medications
A pharmacotherapy tracking log was completed every visit (assessments and treatment). Assessment of individual differences in response to medications is important for the following reasons: (a) Several medications commonly prescribed for bipolar disorder can be associated with either sedating side effects or alerting side effects (GlaxoSmithKline, 2005; PDR Staff, 2007). (b) Sleep-related side effects are not inevitable for any medication but are reported in 4%–37% of patients (PDR Staff, 2007). (c) Side effects are more likely early in treatment than with continued use (Ketter, 2002). (d) Most patients with bipolar disorder take two or more medications. Studies of multiple medications on sleep have not yet been conducted. For each medication, dose, time of day taken, frequency of use, missed doses, and side effects were assessed. This log was sent to the prescribing physician to verify each month.
Credibility/Expectancy Questionnaire (CEQ; Devilly & Borkovec, 2000)
The CEQ was administered at the end of the first therapy session. After receiving a description of the treatment procedures and their rationale, participants provided ratings (on a five-point scale) of treatment acceptability, treatment plausibility, and expectancies for success. This questionnaire has demonstrated high internal consistency and good test–retest reliability over 1 week.
Safety and tolerability
Given that acute sleep deprivation can result in next-day hypomanic or manic symptoms (Colombo et al., 1999), to minimize risk of relapse as a result of sleep deprivation, time in bed was restricted to no less than 6.5 hr. Adverse outcomes were carefully monitored throughout the treatment phase of the study. Therapists collected information on current manic (YMRS), depressive (Quick Inventory of Depressive Symptoms; QIDS-SR; Rush et al., 2003), and sleepiness (Johns, 2000) symptoms prior to the start of each weekly therapy session. Participant dropout and hospitalizations during treatment were also documented.
Data Analysis
Baseline differences between groups in demographic and clinical characteristics were assessed. An intent-to-treat approach was used. Continuous outcomes evaluated at baseline, posttreatment, and 6-month follow-up were analyzed using hierarchical linear models with restricted maximum likelihood estimation. The fixed part of the model included years of education, an indicator variable for treatment condition (CBTI-BP vs. PE), two indicators for time periods (posttreatment and follow-up, with baseline as the reference), and two Treatment × Period interaction terms. Individual growth trajectories, as a function of time since study entry (in days), could deviate linearly from the group- and age-specific mean growth trajectories with a random intercept and a random slope of time, assumed to have a bivariate normal distribution with zero means and unstructured covariance matrix. Measured outcomes could deviate from the individual growth trajectories by an occasion-specific error term, which was independent of the random intercepts and slopes and assumed to be normally distributed with zero mean and constant variance. The treatment effect of interest was the difference in mean change during the treatment phase (from pre to post) between CBTI-BP and PE. To investigate whether treatment gains were maintained through follow-up, a contrast was used to estimate the change in the treatment-group difference from posttreatment to follow-up. The condition effect on the change during the treatment phase and on the change from baseline through follow-up were also expressed as Cohen's d, obtained by dividing the estimated difference in mean change by the model-implied within-group standard deviation of the changes (for the latter, time was evaluated at the mean for the posttreatment assessment).
Categorical outcomes included mood relapse (depression, mania/hypomania, any episode) and insomnia diagnosis. Chi-square or Fisher's exact tests were used to test differences between CBTI-BP and PE for categorical outcomes at posttreatment and 6-month follow-up. The number needed to treat (NNT) for CBTI-BD relative to PE was also estimated for categorical outcomes. A significance level of .05 was used throughout.
Results
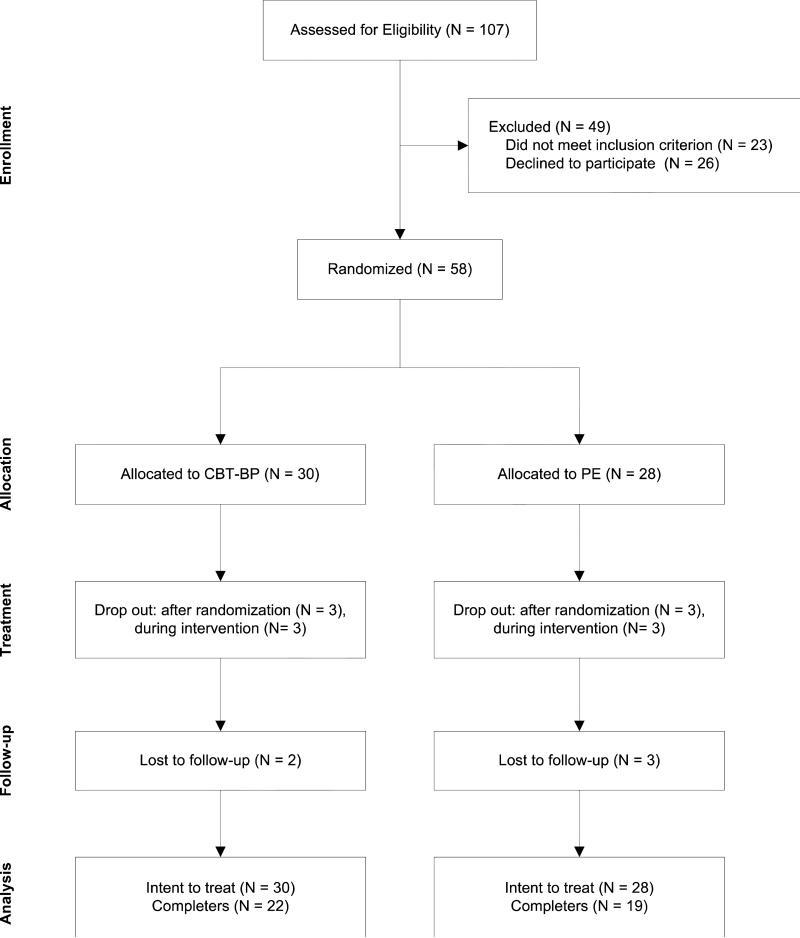
The groups were similar in their demographics and baseline illness characteristics, with the exception of years of education (see Table 1) which was included as a covariate (Senn, 1994). Participant flow is illustrated in Figure 1. Among randomized participants, dropout rates were 10.3% between randomization and Session 1, 11.5% through the posttreatment assessment, and 10.9% through the 6-month follow-up. Attrition rates were not significantly different between treatment groups, χ2(1, N ●●) = 0.02, p = .893. Relative to completers, participants who did not begin treatment or dropped out had a shorter mean duration of bipolar disorder (9.5 vs. 16.1 years), t(56) = 2.12, p = .038. The M ± SD number of therapy sessions attended by participants who initiated treatment was similar for the CBTI-BP (7.3 ± 2.1) and PE (7.3 ± 2.0) groups. There were no significant group differences on the CEQ (all ps > .05).
Table 1.
Baseline Demographic and Clinical Characteristics of Patients Treated With CBTI-BP and PE
| CBTI-BP (n = 30) |
PE (n = 28) |
|||
|---|---|---|---|---|
| Characteristic | n | % | n | % |
| Female | 19 | 63.3 | 17 | 60.7 |
| Ethnicity | ||||
| Hispanic or Latino | 4 | 13.8 | 2 | 7.1 |
| Not Hispanic or Latino | 25 | 86.2 | 26 | 92.9 |
| Race | ||||
| American Indian/Alaska Native | 0 | 0.0 | 1 | 3.6 |
| Asian | 1 | 3.3 | 4 | 14.3 |
| African American | 4 | 13.3 | 3 | 10.7 |
| Caucasian | 18 | 60.0 | 19 | 67.9 |
| Biracial | 5 | 16.7 | 1 | 3.6 |
| Declined to answer | 2 | 6.7 | 0 | 0.0 |
| Marital status | ||||
| Single | 19 | 63.3 | 20 | 71.4 |
| Married/partnered | 7 | 23.3 | 4 | 14.3 |
| Divorced/separated/widowed | 4 | 13.3 | 4 | 14.3 |
| Employment | ||||
| Full-time | 7 | 24.1 | 3 | 11.1 |
| Part-time | 7 | 24.1 | 9 | 33.3 |
| Unemployed | 15 | 51.7 | 15 | 55.6 |
| Annual income | ||||
| < $20,000 | 14 | 46.7 | 11 | 39.3 |
| $20,000-$35,000 | 1 | 3.3 | 3 | 10.7 |
| $35,000-$50,000 | 5 | 16.7 | 6 | 21.4 |
| $50,000-$60,000 | 3 | 10.0 | 1 | 3.6 |
| > $60,000 | 2 | 6.7 | 1 | 3.6 |
| Refused to answer/did not know | 5 | 16.7 | 6 | 21.4 |
| Comorbidity, medical | 19 | 63.3 | 11 | 39.3 |
| Comorbidity, psychiatric | 11 | 36.7 | 11 | 39.3 |
| Mood medication | 21 | 77.8 | 19 | 76.0 |
| Sleep medication | 16 | 59.3 | 16 | 64.0 |
| Prior sleep medication usage | 20 | 74.1 | 18 | 72.0 |
|
M
|
SD
|
M
|
SD
|
|
|---|---|---|---|---|
| Age (years) | 37.7 | 12.4 | 35.5 | 9.3 |
| Education (years) | 16.5 | 2.7 | 14.6 | 3.7 |
| Bipolar disorder duration (years) | 16.2 | 11.9 | 13.1 | 6.8 |
| IDS-C | 11.3 | 8.0 | 13.5 | 6.8 |
| IDS-C (no sleep items) | 7.8 | 7.4 | 9.1 | 6.4 |
| YMRS | 2.4 | 2.5 | 4.0 | 3.5 |
| Insomnia duration (months) | 214.2 | 163.8 | 189.5 | 155.6 |
Note. CBTI-BP = cognitive behavior therapy for insomnia modified for bipolar disorder; PE = psychoeducation; IDS-C = Inventory of Depressive Symptomatology, Clinician Rating; YMRS = Young Mania Rating Scale
Figure 1.
CONSORT diagram illustrating the flow of participants with bipolar disorder Type I and insomnia through the study. Note. CBTI-BP = cognitive behavior therapy for insomnia modified for bipolar disorder; PE = psychoeducation.
Mood Outcomes
Primary outcomes
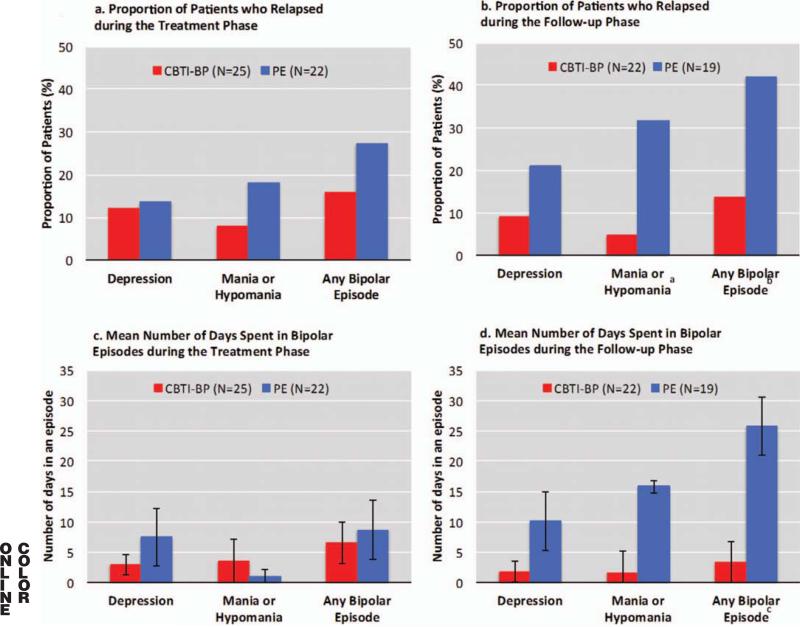
Relapse rates and days spent in bipolar episodes did not differ significantly between PE and CBTI-BP during the acute treatment phase (see Figures 2a and 2c). During the follow-up phase, CBTI-BP yielded a significantly lower mania/ hypomania relapse rates relative to PE (4.6% vs. 31.6%, p = .036; see Figure 2b). The number of patients experiencing depression relapse did not differ significantly between CBTI-BP and PE (9.1% vs. 21.1%, p = .391). Overall relapse rate (i.e., emergence of any type of bipolar mood episode) during the follow-up phase tended to be lower in CBTI-BP compared with PE (13.6% vs. 42.1%, p = .075). The CBTI-BP group spent significantly less time in bipolar episodes relative to the PE group during the follow-up phase (3.3 days vs. 25.5 days, p = .028; see Figure 2d). The estimated NNT to prevent relapse in PE relative to CBTI-BP was 3.7 for mania/hypomania (medium to large effect size), 8.3 for depression (small to medium effect size), and 3.5 (medium to large effect size) for any relapse.
Figure 2.
●●●. CBTI-BP = cognitive behavior therapy for insomnia modified for bipolar disorder; PE = psychoeducation. a Significant difference between treatment groups (Fisher's exact: p = .036). b Marginally significant difference between treatment groups (Fisher's exact: p = .075). c Significant difference between treatment groups, t(39) = 2.28, p = .028.
Exploratory outcomes
There were no significant changes in depressive symptoms on the IDS-C during the treatment phase or from baseline through follow-up for either treatment group (see Tables 2 and 3). Similarly, there were no significant changes in manic symptoms on the YMRS during the treatment phase or from baseline through follow-up for either treatment group (see Tables 2 and 3). The YMRS and IDS-C assess symptoms of mania and depression over the prior week. Because patients were interepisode at study entry, we did not anticipate any major reductions in these symptom ratings. Nonetheless, given the chronic and relapsing course of bipolar disorder, the stability observed on these measures may be interpreted as a positive outcome.
Table 2.
Raw Means and Standard Deviations for Primary and Secondary Outcomes for Cognitive Behavior Therapy for Insomnia Modified for Bipolar Disorder (CBTI-BP) and Psychoeducation (PE) From Baseline Through 6-Month Follow-Up
| CBT-BP (n = 30) |
PE (n = 28) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Posttreatment |
Follow-up |
Baseline |
Posttreatment |
Follow-up |
|||||||
| Outcome | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD |
| Primary | ||||||||||||
| ISI | 17.87 | 4.67 | 6.45 | 5.49 | 6.82 | 6.11 | 18.86 | 3.82 | 13.90 | 5.32 | 12.63 | 5.21 |
| SD-SE (%) | 82.36 | 7.21 | 87.83 | 7.41 | 89.03 | 5.84 | 78.59 | 10.31 | 84.52 | 11.39 | 85.24 | 9.33 |
| SDS-Mood | 3.66 | 2.34 | 3.10 | 2.50 | 2.02 | 2.13 | 4.42 | 2.56 | 2.64 | 2.43 | 3.07 | 2.33 |
| SDS-Sleep | 4.38 | 2.41 | 1.54 | 1.64 | 1.42 | 1.75 | 4.51 | 2.53 | 3.21 | 2.45 | 2.63 | 2.42 |
| Exploratory | ||||||||||||
| IDS-C | 10.93 | 7.44 | 8.38 | 7.60 | 8.27 | 8.13 | 13.86 | 7.11 | 11.55 | 8.84 | 14.05 | 8.84 |
| YMRS | 2.32 | 2.42 | 2.19 | 2.70 | 2.30 | 2.65 | 4.09 | 3.58 | 4.30 | 4.00 | 4.76 | 5.81 |
| PSQI | 11.10 | 3.50 | 5.74 | 3.74 | 7.19 | 4.27 | 11.85 | 3.30 | 8.64 | 3.32 | 8.58 | 4.06 |
| PROMIS-SD | 54.32 | 6.41 | 46.19 | 8.22 | 46.53 | 11.96 | 57.66 | 6.35 | 54.40 | 6.67 | 54.00 | 9.26 |
| SD-SOL | 32.21 | 30.67 | 20.10 | 19.13 | 24.51 | 23.96 | 42.23 | 33.18 | 40.00 | 57.33 | 27.64 | 17.51 |
| SD-WASO | 24.29 | 16.85 | 18.03 | 19.34 | 14.20 | 19.26 | 26.59 | 21.55 | 17.24 | 28.46 | 21.01 | 32.04 |
| SD-TWT | 90.38 | 37.93 | 58.20 | 33.03 | 57.73 | 38.50 | 113.87 | 60.57 | 84.14 | 68.66 | 80.89 | 52.93 |
| SD-TIB | 510.83 | 79.08 | 493.93 | 80.22 | 502.80 | 76.21 | 549.29 | 88.80 | 527.67 | 116.99 | 520.89 | 91.83 |
| SD-TST | 419.92 | 76.99 | 436.00 | 80.19 | 445.84 | 52.23 | 436.61 | 92.32 | 441.67 | 97.39 | 440.16 | 88.88 |
| Q-LES-Q-SF | 57.92 | 16.22 | 60.33 | 15.67 | 65.34 | 19.78 | 55.04 | 15.95 | 60.31 | 14.68 | 60.71 | 18.12 |
| PROMIS-SRI | 59.20 | 6.35 | 50.57 | 8.85 | 50.45 | 12.87 | 62.52 | 6.23 | 59.28 | 6.65 | 58.58 | 9.76 |
Note. ISI = Insomnia Severity Index; SD-SE = sleep diary sleep efficiency; SDS = Sheehan Disability Scale; IDS-C = Inventory of Depressive Symptomatology, Clinician Rating; YMRS = Young Mania Rating Scaled; PSQI = Pittsburgh Sleep Quality Index; PROMIS-SD = Patient-Reported Outcomes Measurement Information System-Sleep Disturbance; SD-SOL = sleep diary sleep onset latency; SD-WASO = sleep diary wake after sleep onset; SD-TWT = sleep diary total wake time; SD-TIB = sleep diary time in bed; SD-TST = sleep diary total sleep time; Q-LES-Q-SF = Quality of Life Enjoyment and Satisfaction Questionnaire; PROMIS-SRI = PROMIS-Sleep-Related Impairment.
Table 3.
Coefficient Estimates From Hierarchical Linear Models of Secondary Outcomes for Cognitive Behavior Therapy for Insomnia Modified for Bipolar Disorder (CBTI-BP; n = 30) and Psychoeducation (PE; n = 28) From Baseline Through 6-Month Follow-up, Controlling for Years of Education
| Treatment condition effect at baseline |
Change from baseline through the treatment phase for the PE group |
Treatment condition effect at baseline on change during the treatment phase |
Change from baseline through follow-up for the PE group |
Treatment effect condition at baseline on change from baseline through follow-up |
Change in condition effect from post to follow-up contrast |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | β | SE | p | β | SE | p | β | SE | p | d | β | SE | p | β | SE | p | d | z | p |
| Primary | |||||||||||||||||||
| ISI | –1.03 | 1.17 | .381 | –4.85 | 0.99 | <.001 | –6.64 | 1.37 | –C001 | –1.42 | –5.47 | 1.32 | <.001 | –5.64 | 1.81 | .002 | –0.93 | 0.63 | .526 |
| SD-SE (%) | 3.38 | 2.45 | .168 | 5.36 | 1.64 | .001 | –0.52 | 2.26 | .817 | –0.07 | 4.57 | 1.91 | .017 | 1.21 | 2.52 | .632 | 0.16 | 0.68 | .500 |
| SDS-Mood | –0.90 | 0.63 | .154 | –1.80 | 0.55 | .001 | 1.18 | 0.77 | .125 | 0.44 | –1.26 | 0.63 | .045 | –0.41 | 0.85 | .627 | –0.15 | –1.90 | .058 |
| SDS-Sleep | –0.17 | 0.61 | 777 | –1.22 | 0.50 | .014 | –1.63 | 0.69 | .081 | –0.67 | –1.73 | 0.63 | .006 | –1.32 | 0.86 | .121 | –0.45 | 0.38 | .704 |
| Exploratory | |||||||||||||||||||
| IDS-C | –2.94 | 1.96 | .133 | –1.90 | 1.83 | .297 | –0.69 | 2.53 | .784 | –0.78 | 0.18 | 2.00 | .929 | –2.68 | 2.73 | .328 | –0.30 | –0.72 | .469 |
| YMRS | –1.72 | 0.85 | .044 | 0.17 | 0.86 | .841 | –0.32 | 1.20 | .789 | –0.08 | 0.66 | 0.97 | .491 | –0.71 | 1.32 | .593 | –.016 | –0.29 | .768 |
| PSQI | –0.68 | 0.92 | .457 | –3.08 | 0.59 | <.001 | –2.19 | 0.83 | .008 | –0.78 | –2.85 | 0.76 | <.001 | –1.14 | 1.05 | .278 | –0.33 | 1.12 | .261 |
| PROMIS-SD | –3.38 | 1.90 | .075 | –3.08 | 1.60 | .055 | –5.61 | 2.23 | .012 | –0.72 | –3.85 | 2.46 | .117 | –5.02 | 3.49 | .150 | –0.42 | 0.21 | .834 |
| SD-SOL | –8.86 | 1.67 | .428 | 1.01 | 5.12 | .844 | –9.73 | 7.01 | .165 | –0.41 | –2.70 | 6.20 | .663 | –0.14 | 8.06 | .987 | –0.01 | 1.18 | .237 |
| SD-WASO | 0.42 | 5.73 | .604 | –9.06 | 5.12 | .077 | 2.09 | 7.04 | .767 | 0.09 | –5.96 | 6.26 | .342 | –5.57 | 8.15 | .495 | –0.23 | –0.93 | .354 |
| SD-TWT | –21.91 | 13.70 | .110 | –25.78 | 9.06 | .004 | –4.44 | 12.43 | .721 | –0.10 | –17.58 | 10.58 | .097 | –11.08 | 13.95 | .427 | –0.26 | –0.47 | .639 |
| SD-TIB | –38.68 | 24.40 | .113 | –17.81 | 16.27 | .274 | –3.53 | 22.32 | .874 | –0.05 | –19.351 | 19.57 | .323 | 7.87 | 25.46 | .758 | 0.10 | 0.44 | .659 |
| SD-TST | –20.18 | 23.65 | .393 | 5.66 | 15.73 | .719 | 4.26 | 21.60 | .844 | 0.06 | 0.86 | 18.77 | .964 | 19.17 | 24.58 | .435 | 0.25 | 0.60 | .549 |
| Q-LES-Q-SF | 2.80 | 4.17 | .504 | 4.04 | 2.99 | .176 | –0.53 | 4.14 | .898 | –0.04 | 4.67 | 4.49 | .298 | 2.34 | 6.19 | .705 | 0.11 | 0.58 | .563 |
| PROMIS-SRI | –3.37 | 1.92 | .079 | –3.08 | 1.69 | .069 | –6.17 | 2.35 | .009 | –0.75 | –4.14 | 2.60 | .111 | –5.72 | 3.69 | .121 | –0.46 | 0.15 | .879 |
Note. All analyses controlled for years of education, d = Cohen's d effect size; ISI = Insomnia Severity Index; SD-SE = sleep diary sleep efficiency; SDS = Sheehan Disability Scale; IDS-C = Inventory of Depressive Symptomatology, Clinician Rating; YMRS = Young Mania Rating Scaled; PSQI = Pittsburgh Sleep Quality Index; PROMIS-SD = Patient-Reported Outcomes Measurement Information System-Sleep Disturbance; SD-SOL = sleep diary sleep onset latency; SD-WASO = sleep diary wake after sleep onset; SD-TWT = sleep diary total wake time; SD-TIB = sleep diary time in bed; SD-TST = sleep diary total sleep time; Q-LES-Q-SF = Quality of Life Enjoyment and Satisfaction /Questionnaire; PROMIS-SRI = PROMIS-Sleep-Related Impairment.
Sleep Outcomes
Primary outcomes
As evident in Tables 2 and 3, although both groups experienced an improvement in ISI scores during the acute treatment phase and from baseline through follow-up, the CBTI-BP group improved to a greater extent than the PE group through posttreatment and 6-month follow-up. Contrasts demonstrated that treatment-group difference did not change significantly from posttreatment to follow-up.
The percentage of participants meeting criteria for response on the ISI was significantly greater for CBTI-BP relative to PE at posttreatment (68.2% vs. 28.6%), χ2(1, N = ●●●) = 6.74, p = .009, and at 6-month follow-up (63.6% vs. 26.3%) χ2(N = ●●●) = 5.71, p = .017. The percentage of participants meeting criteria for insomnia remission on the ISI was significantly greater for CBTI-BP relative to PE at posttreatment (72.7% vs. 14.3%), χ2(1, N = ●●●) = 14.88,, p < .001, and at 6-month follow-up (63.6% vs. 21.1%), χ2(1, N = ●●●) = 7.51, p = .006. To achieve ISI insomnia remission in CBTI-BP relative to the PE group, the estimated NNT through posttreatment was 1.7 (large effect size) and 2.4 (medium to large effect size) through 6-month follow-up.
The percentage of participants no longer meeting diagnostic criteria for insomnia (on the basis of the DSISD) was significantly greater for CBTI-BP relative to PE at posttreatment (73.9% vs. 41.7%), χ2(1, N = ●●●) = 6.65, p = .010, and tended to be greater at 6-month follow-up (73.7% vs. 45.5%), χ2(1, N = ●●●) = 3.35, p = .067. To achieve insomnia diagnosis remission in CBTI-BP relative to the PE group, the estimated NNT through posttreatment was 3.1 (medium to large effect size) and 3.5 (medium to large effect size) through 6-month follow-up.
On the sleep diary, both treatment groups experienced an increase in sleep efficiency during the treatment phase and through follow-up (see Tables 2 and 3). However, no significant Treatment × Period interactions were observed, indicating no between-groups differences through posttreatment or follow-up.
Exploratory outcomes
For the PSQI and PROMIS-SD, both groups reported improvements during the treatment phase, but the CBTI-BP group exhibited greater improvement compared with the PE group on both measures (see Tables 2 and 3). On the sleep diary outcomes, both treatment groups experienced a decrease in total wake time during the treatment phase and through follow-up (see Tables 2 and 3). No significant changes were observed in sleep onset latency, wake after sleep onset, time in bed, or total sleep time (see Tables 2 and 3). For all of these measures, no significant Treatment × Period interactions were observed, indicating no between-groups differences through posttreatment or follow-up.
Functional Impairment Outcomes
Primary outcomes
Both groups showed improvement in mood- and sleep-related impairment on the SDS through post-treatment and follow-up (see Tables 2 and 3). However, no significant Treatment × Period interactions were observed, indicating no between-groups differences through posttreatment or follow-up.
Exploratory outcomes
No significant changes in quality of life, as assessed by the Q-LES-Q-SF, were observed through posttreatment or follow-up (see Tables 2 and 3). On the PROMIS-SRI, the CBTI-BP group exhibited greater improvement compared with the PE group. The rate of improvement through posttreatment and follow-up was not significant, and no group differences were observed through follow-up.
Medications
At study entry, 53 of 58 participants (91.4%) were taking prescription medications to stabilize mood. The M ± SD (median) number of medications per participant was 2.53 ± 1.64 (see the supplemental materials for a full listing). When considering each medication for each participant separately, the doses of 91.9% of mood medications and 88.9% of sleep medications remained stable across the treatment phase. When considering all medications for a particular participant, 88.5% of participants remained on stable doses of all mood medications, and 92.3% remained on stable doses of all sleep medications across the treatment phase.
The percentages of CBTI-BP compared with PE participants taking mood medications were statistically similar at baseline (77.8% vs. 76.0%) and posttreatment (88.5% vs. 86.4%) but significantly lower at the end of the follow-up phase (68.0% vs. 95.2%) χ2(1, N = ●●●) = 5.38, p = .02. There were no significant differences in the percentages of participants discontinuing at least one mood medication at some point during the treatment phase (22.7% vs. 21.1%) or during the follow-up phase (12.5% vs. 30.8%).
The percentages of CBTI-BP and PE participants taking sleep medications were not significantly different at baseline (59.3% vs. 64.0%), posttreatment (38.5% vs. 54.5%), or the end of the follow-up phase (34.6% vs. 58.1%). Likewise, the percentages of CBTI-BP and PE participants who discontinued sleep medications were not significantly different (24.7% vs. 5.9%). The percentage of participants discontinuing at least one sleep medication at some point during the treatment phase was significantly greater in CBTI-BP compared with PE (66.7% vs. 29.4%), χ2(1, N = ●●●) = 4.44, p = .04, though there was no significant difference between groups during the follow-up phase (26.7% vs. 5.9%).
Safety and tolerability
A detailed analysis of safety and tolerability of sleep restriction and stimulus control in the first 15 CBTI-BP participants has been reported elsewhere (Kaplan & Harvey, 2013). Across the entire ITT sample (N = 58), five individuals in CBTI-BP and 12 individuals in PE reported mild to moderate most likely subsyndromal depressive symptoms (QIDSSR = 11–15) at some point during treatment. An additional three CBTI-BP and three PE participants reported depression symptoms in the severe range (QIDS-SR ≥ 16) during treatment. Finally, one individual in CBTI-BP and one in PE reported manic symptoms in the moderate range during treatment (YMRS = 15–24) (Kessler, Merikangas, & Wang, 2007). Of note, sleep restriction was not a factor in the development of moderate manic symptoms in the CBTI-BP individual, as regularizing the sleep schedule between Sessions 1 and 2 was sufficient to bring about positive changes in sleep continuity, and sleep restriction was thus not implemented. Regarding psychiatric hospitalizations during treatment, one individual in CBTI-BP was voluntarily hospitalized during treatment for a transient amnestic episode, and one individual in PE was voluntarily hospitalized during treatment for suicidal ideation.
Discussion
Relative to PE, CBTI-BP was associated with a significantly lower hypomania/mania relapse rate and fewer days in an episode through 6-month follow-up. These findings add to cross-sectional (Gruber et al., 2009), prospective (Gruber et al., 2011; Talbot et al., 2012), and experimental sleep deprivation (e.g., Wehr et al., 1987) evidence pointing to sleep disturbances, such as insomnia, contributing to mood symptoms in bipolar disorder. These findings also highlight insomnia as an important modifiable mechanism in bipolar disorder.
During the active treatment phase, the CBTI-BP group improved to a greater extent, relative to the PE group, on insomnia severity, insomnia response, and insomnia remission as well as on the secondary questionnaire sleep outcomes. Although differences between groups in sleep medication use and discontinuation were not statistically significant, numerical differences suggested the possibility of lower sleep medication use in the CBTI-BP group. Overall, these findings point to greater sleep improvement for CBTI-BP relative to PE. A noteworthy finding is that the results for sleep outcomes were stronger for global, retrospective questionnaires relative to the sleep diary. However, several of effect sizes for CBTI-BP versus PE on the sleep diary at posttreatment (Cohen's d = 0.09–0.41) and 6 months (Cohen's d = 0.01–0.26) provided at least some encouragement, although they were smaller than those found in studies of CBT-I for patients with insomnia without a comorbid severe mental illness. These findings are consistent with the existing evidence that PE is an active bipolar disorder preventive treatment that may even provide at least some sleep benefits (Colom et al., 2006). Regardless, these data will be valuable for planning larger adequately powered studies. Overall, we suggest that the questionnaires provided a fuller assessment of insomnia than the sleep diaries as they included an assessment of both nighttime and daytime features of insomnia. Sleep diary data were also affected by the patients within the sample who exhibited co-occurring features of hypersomnia and delayed sleep phase. Indeed, there is accruing evidence that sleep and circadian problems are not neatly categorized as pure insomnia, particularly in severe mental illness. For example, in bipolar disorder, insomnia can overlap with hypersomnia (Kaplan, Gruber, Eidelman, Talbot, & Harvey, 2011; Kaplan & Harvey, 2009), delayed sleep phase (Giglio et al., 2010; Mansour et al., 2005), and irregular sleep– wake schedules (Gruber et al., 2009). As such, there may be a need to develop bipolar disorder–specific sleep diary scoring standards that take these complexities into account.
Although the improvement observed in both treatment groups in sleep was generally sustained to 6 months, the statistically significant advantage of CBTI-BD over PE observed at posttreatment was no longer significant for insomnia diagnosis. On the one hand, the percentages of patients no longer meeting ISI criteria for insomnia at 6 months were 63.6% for CBTI-BP and 21.1% for PE, suggesting the possibility of a clinically significant sleep advantage for CBTI-BP. On the other hand, our nonsignificant findings may reflect the relatively short treatment (eight sessions), treatment resistance as a result of advance-stage bipolar disorder (the patients had had bipolar disorder for an average of 1.5 decades), the small sample size, and/or the need for “booster” sessions to ensure gains were maintained.
Although several results indicate a clear advantage for CBTI-BP over PE, other measures highlight positive effects from PE. Specifically, both treatments improved on ISI, SDS-Mood, and SDS-Sleep as well as several secondary outcomes. These findings extend previous research highlighting the benefits of PE (Colom et al., 2006). However, the (nonsignificant) direction of the mean values favored CBTI-BP, and several of the effect sizes were at least moderate in size, suggesting that significant group differences could emerge with a larger sample. In any event, our study suggests that a study with a larger sample size is warranted.
We emphasize that substantial caution is necessary when interpreting the findings, given the small sample size (Kraemer, Mintz, Noda, Tinklenberg, & Yesavage, 2006). A larger study is needed to determine whether our results can be replicated, to conduct subgroup analyses to determine whether the results differ by pertinent variables (sex, illness duration, etc.), and to determine potential moderators and mediators of treatment response. Also, 10.3% of participants dropped out between randomization and Session 1, 11.5% dropped out through the posttreatment assessment, and 10.9% dropped out through the 6-month follow-up. We carefully documented the reasons for each dropout, which included significant life events (e.g., moved to another state to care for sick mother, moved to another country, seriously injured in a bike accident), scheduling issues (e.g., got a time-consuming new job, school schedule changed), and declining to complete the paperwork associated with the study. Although our dropout rate was low compared with those in longer term bipolar disorder pharmaco-therapy trials, we cannot rule out the possibility that this loss of data may have biased outcomes. Nonetheless, this information is likely to be important for powering future studies.
Our study had several other limitations. First, we included a 6-month follow-up. Although 6 months is a substantive period of time, longer follow-up would be an important inclusion in future research, particularly to assess the need for booster sessions after the acute treatment phase. The decision as to whether the therapists were crossed across treatments or nested within one treatment is a challenging one. After careful consideration, we decided to nest therapists within one treatment condition. Our rationale was that in this first pilot test of CBTI-BD, we wanted to prioritize delivering “pure” treatments and reduce the risk of contamination. Of course, a limitation to this approach is that we cannot rule out therapist effects. However, we note that the therapists had equivalent levels of training and supervision, the same qualifications, and scored similarly on the CEQ.
This study deviated from the recommendations made by Buysse et al. (2006) by not including polysomnography (PSG). However, Buysse et al. (2006) noted that their “recommendations for the 4 types of research studies should not be taken in overly literal terms; hybrid studies should include a reasonable mix of assessments described for each prototype” (p. 1168). The present study was part of a treatment development process, not a fully powered randomized controlled trial. Also, many patients cannot tolerate multiple nights of PSG and, given the cost of PSG, the results observed from PSG in previous randomized controlled trials have been underwhelming. Nonetheless, we recognize that not having PSG was a limitation of the current study.
In sum, this first study comparing the effects of two psychosocial treatments for bipolar disorder patients with insomnia adds to emerging evidence that treating insomnia comorbid with another psychiatric disorder can improve not only the insomnia but also the symptoms associated with the comorbid psychiatric disorder (e.g., Manber et al., 2008; Myers et al., 2011). The results also underscore the value of treatments that aim to improve sleep in bipolar disorder and support the proposal that disturbed sleep constitutes a mechanism that substantively contributes to bipolar disorder.
Supplementary Material
Acknowledgments
Terence A. Ketter's affiliations with the following could be perceived as real or apparent conflicts of interest: grant support (Agency for Healthcare Research and Quality, AstraZeneca Pharmaceuticals LP, Cephalon Inc., Eli Lilly and Company, National Institute of Mental Health, Pfizer Inc., and Sunovion Pharmaceuticals); consultancy (Allergan, Inc.; Avanir Pharmaceuticals; Bristol-Myers Squibb Company; Cephalon Inc.; Forest Pharmaceuticals; Janssen Pharmaceutica Products LP; Merck & Co., Inc.; Pro-Phase; Sunovion Pharmaceuticals; and Teva Pharmaceuticals); lecture honoraria (Abbott Laboratories, Inc.; Astra Zeneca Pharmaceuticals LP; GlaxoSmithKline; Otsuka Pharmaceuticals; and Pfizer Pharmaceuticals); and royalties (American Psychiatric Publishing, Inc.). In addition, Ketter's spouse, Nzeera Ketter, and her affiliation with Janssen Pharmaceuticals as an employee and through stock, could be seen as a conflict of interest. Daniel J. Buysse 's relationship with Merck Pharm, Purdue Pharm, Philips Respironics, Medscape, Emmi Solutions, General Sleep Corp., Eisai, CME Outfitters, and Otsuka as consultant and with Servier and Astellas as a one-time speaker could be perceived as real or apparent conflicts of interest.
Footnotes
Supplemental materials: http://dx.doi.org/10.1037/a0038655.supp
Contributor Information
Allison G. Harvey, Department of Psychology, University of California, Berkeley
Adriane M. Soehner, Department of Psychology, University of California, Berkeley
Kate A. Kaplan, Department of Psychology, University of California, Berkeley
Kerrie Hein, Department of Psychology, University of California, Berkeley.
Jason Lee, Department of Psychology, University of California, Berkeley.
Jennifer Kanady, Department of Psychology, University of California, Berkeley.
Sophia Rabe-Hesketh, Graduate School of Education, University of California, Berkeley.
Thomas C. Neylan, Department of Psychiatry, School of Medicine, University of California, San Francisco
Descartes Li, Department of Psychiatry, School of Medicine, University of California, San Francisco.
Terence A. Ketter, Department of Psychiatry and Behavioral Sciences, School of Medicine, Stanford University
Daniel J. Buysse, Department of Psychiatry, University of Pittsburgh.
References
- American Academy of Sleep Medicine . International classification of sleep disorders: Diagnostic and coding manual. 2nd ed. Author; Westchester, IL: 2005. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Author; Washington, DC: 2000. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Author; Washington, DC: 2013. [Google Scholar]
- Angst J, Sellaro R. Historical perspectives and natural history of bipolar disorder. Biological Psychiatry. 2000;48:445–457. doi: 10.1016/s0006-3223(00)00909-4. http://dx.doi.org/10.1016/S0006-3223(00)00909-4. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. http://dx.doi.org/10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Bersani G, Garavini A. Melatonin add-on in manic patients with treatment resistant insomnia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2000;24:185–191. doi: 10.1016/s0278-5846(99)00097-4. http://dx.doi.org/10.1016/S0278-5846(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Guelfi JD, Perdrizet-Chevallier C, the Agomelatine Bipolar Study Group Agomelatine adjunctive therapy for acute bipolar depression: Preliminary open data. Bipolar Disorders. 2007;9:628–635. doi: 10.1111/j.1399-5618.2007.00507.x. http://dx.doi.org/10.1111/j.1399-5618.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Keck PE, Jr., Macfadden W, Minkwitz M, Ketter TA, Weisler RH, Mullen J. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. The American Journal of Psychiatry. 2005;162:1351–1360. doi: 10.1176/appi.ajp.162.7.1351. http://dx.doi.org/10.1176/appi.ajp.162.7.1351. [DOI] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, Morin CM. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom F, Vieta E, Scott J. Psychoeducation manual for bipolar disorder. Cambridge University Press; New York: 2006. http://dx.doi.org/10.1017/CBO9780511543685. [Google Scholar]
- Craighead WE, Craighead LW. The role of psychotherapy in treating psychiatric disorders. Medical Clinics of North America. 2001;85:617–629. doi: 10.1016/s0025-7125(05)70332-1. http://dx.doi.org/10.1016/S0025-7125(05)70332-1. [DOI] [PubMed] [Google Scholar]
- Devilly GJ, Borkovec TD. Psychometric properties of the Credibility/Expectancy Questionnaire. Journal of Behavior Therapy and Experimental Psychiatry. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. http://dx.doi.org/10.1016/S0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Stepanski EJ. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: A randomized controlled trial. JAMA. 2001;285:1856–1864. doi: 10.1001/jama.285.14.1856. http://dx.doi.org/10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wyatt JK, Olsen MK, Stechuchak KM, Carney CE, Chiang A, Radtke RA. Reliability and validity of the Duke Structured Interview for Sleep Disorders for insomnia screening.. Paper presented at the 23rd Annual Meeting of the Associated Professional Sleep Societies; Seattle, WA.. 2009. [Google Scholar]
- Egashira T, Inoue T, Shirai Y, Iwata K, Honma J, Koyama T. Adjunctive gabapentin for treatment-resistant insomnia of bipolar disorder: A case report. Clinical Neuropharmacology. 2011;34:129–130. doi: 10.1097/WNF.0b013e318213b9cd. http://dx.doi.org/10.1097/WNF.0b013e318213b9cd. [DOI] [PubMed] [Google Scholar]
- Eidelman P, Talbot LS, Gruber J, Harvey AG. Sleep, illness course, and concurrent symptoms in inter-episode bipolar disorder. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41:145–149. doi: 10.1016/j.jbtep.2009.11.007. http://dx.doi.org/10.1016/j.jbtep.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer MB, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders–patient edition (SCID-I/P, Version 2.0) Biometrics Research, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Frank E. Treating bipolar disorder: A clinician’s guide to interpersonal and social rhythm therapy. Guilford Press; New York: 2005. [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Archives of General Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. http://dx.doi.org/10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Germain A, Shear MK, Hall M, Buysse DJ. Effects of a brief behavioral treatment for PTSD-related sleep disturbances: A pilot study. Behaviour Research and Therapy. 2007;45:627–632. doi: 10.1016/j.brat.2006.04.009. http://dx.doi.org/10.1016/j.brat.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Gershon A, Thompson WK, Eidelman P, McGlinchey EL, Kaplan KA, Harvey AG. Restless pillow, ruffled mind: Sleep and affect coupling in interepisode bipolar disorder. Journal of Abnormal Psychology. 2012;121:863–873. doi: 10.1037/a0028233. http://dx.doi.org/10.1037/a0028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio LM, Magalhães PV, Andersen ML, Walz JC, Jakobson L, Kapczinski F. Circadian preference in bipolar disorder. Sleep and Breathing. 2010;14:153–155. doi: 10.1007/s11325-009-0301-3. http://dx.doi.org/10.1007/s11325-009-0301-3. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline . PDR psychotropic prescribing guide (Physicians’ Desk Reference PDR 2005) 8th ed. Thomson Healthcare; Montvale, NJ: 2005. [Google Scholar]
- Gruber J, Harvey AG, Wang PW, Brooks JO, III, Thase ME, Sachs GS, Ketter TA. Sleep functioning in relation to mood, function, and quality of life at entry to the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Journal of Affective Disorders. 2009;114:41–49. doi: 10.1016/j.jad.2008.06.028. http://dx.doi.org/10.1016/j.jad.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Miklowitz DJ, Harvey AG, Frank E, Kupfer D, Thase ME, Ketter TA. Sleep matters: Sleep functioning and course of illness in bipolar disorder. Journal of Affective Disorders. 2011;134:416–420. doi: 10.1016/j.jad.2011.05.016. http://dx.doi.org/10.1016/j.jad.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG. Sleep and circadian rhythms in bipolar disorder: Seeking synchrony, harmony, and regulation. American Journal of Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. http://dx.doi.org/10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Schmidt DA, Scarnà A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. American Journal of Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. http://dx.doi.org/10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. Journal of Affective Disorders. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. http://dx.doi.org/10.1016/S0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Johns MW. Sensitivity and specificity of the Multiple Sleep Latency Test (MSLT), the Maintenance of Wakefulness Test and the Epworth Sleepiness Scale: Failure of the MSLT as a gold standard. Journal of Sleep Research. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. http://dx.doi.org/10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, Keller MB. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Archives of General Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. http://dx.doi.org/10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- Kaplan KA, Gruber J, Eidelman P, Talbot LS, Harvey AG. Hypersomnia in inter-episode bipolar disorder: Does it have prognostic significance? Journal of Affective Disorders. 2011;132:438–444. doi: 10.1016/j.jad.2011.03.013. http://dx.doi.org/10.1016/j.jad.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan KA, Harvey AG. Hypersomnia across mood disorders: A review and synthesis. Sleep Medicine Reviews. 2009;13:275–285. doi: 10.1016/j.smrv.2008.09.001. http://dx.doi.org/10.1016/j.smrv.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Kaplan KA, Harvey AG. Behavioral treatment of insomnia in bipolar disorder. American Journal of Psychiatry. 2013;170:716–720. doi: 10.1176/appi.ajp.2013.12050708. http://dx.doi.org/10.1176/appi.ajp.2013.12050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annual Review of Clinical Psychology. 2007;3:137–158. doi: 10.1146/annurev.clinpsy.3.022806.091444. http://dx.doi.org/10.1146/annurev .clinpsy.3.022806.091444. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Wang PW. Psychotropic medications in bipolar disorder: Pharmacodynamics, pharmacokinetics, drug interactions, adverse effects and their management. In: Yatham LM, Kusumakar V, editors. Bipolar disorder: A clinician's guide to biological treatments. Brunner-Routledge; London: 2002. pp. 265–307. [Google Scholar]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Archives of General Psychiatry. 2006;63:484–489. doi: 10.1001/archpsyc.63.5.484. http://dx.doi.org/10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- Lam DH, Watkins ER, Hayward P, Bright J, Wright K, Kerr N, Sham P. A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder: Outcome of the first year. Archives of General Psychiatry. 2003;60:145–152. doi: 10.1001/archpsyc.60.2.145. http://dx.doi.org/ 10.1001/archpsyc.60.2.145. [DOI] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour HA, Wood J, Chowdari KV, Dayal M, Thase ME, Kupfer DJ, Nimgaonkar VL. Circadian phase variation in bipolar I disorder. Chronobiology International. 2005;22:571–584. doi: 10.1081/CBI-200062413. http://dx.doi.org/10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Goldstein MJ. Bipolar disorder: A family-focused treatment approach. Guilford Press; New York: 1997. [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. Guilford Press; New York: 2002. [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998–2004). Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- Morin CM, Espie CA. Insomnia: A clinical guide to assessment and treatment. Kluwer Academic/Plenum Publishers; New York: 2003. http://dx.doi.org/10.1002/0471264385.wei0914. [Google Scholar]
- Morin CM, Vallières A, Guay B, Ivers H, Savard J, Mérette C, Baillargeon L. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: A randomized controlled trial. JAMA. 2009;301:2005–2015. doi: 10.1001/jama.2009.682. http://dx.doi.org/10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers E, Startup H, Freeman D. Cognitive behavioural treatment of insomnia in individuals with persistent persecutory delusions: A pilot trial. Journal of Behavior Therapy and Experimental Psychiatry. 2011;42:330–336. doi: 10.1016/j.jbtep.2011.02.004. http://dx.doi.org/10.1016/j.jbtep.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Patelis-Siotis I, Young LT, Robb JC, Marriott M, Bieling PJ, Cox LC, Joffe RT. Group cognitive behavioral therapy for bipolar disorder: A feasibility and effectiveness study. Journal of Affective Disorders. 2001;65:145–153. doi: 10.1016/s0165-0327(00)00277-9. http://dx.doi.org/10.1016/S0165-0327(00)00277-9. [DOI] [PubMed] [Google Scholar]
- PDR Staff . Physicians’ desk reference 2008: Hospital/library version. 62nd ed. Thomson Healthcare; Stamford, CT: 2007. [Google Scholar]
- Perlis RH, Ostacher MJ, Miklowitz DJ, Smoller JW, Dennehy EB, Cowperthwait C. Benzodiazepine use and risk of recurrence in bipolar disorder: A STEP-BD report. Journal of Clinical Psychiatry. 2010;71:194–200. doi: 10.4088/JCP.09m05019yel. http://dx.doi.org/10.4088/JCP .09m05019yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Carmody T, Reimitz PE. The Inventory of Depressive Symptomatology (IDS): Clinician (IDS-C) and self-report (IDS-SR) ratings of depressive symptoms. International Journal of Methods in Psychiatric Research. 2000;9:45–59. http://dx.doi.org/10.1002/mpr.79. [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. http://dx.doi.org/10.1016/S0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Sattar SP, Ramaswamy S, Bhatia SC, Petty F. Somnambulism due to probable interaction of valproic acid and zolpidem. Annals of Pharmacotherapy. 2003;37:1429–1433. doi: 10.1345/aph.1C500. http://dx.doi.org/10.1345/aph.1C500. [DOI] [PubMed] [Google Scholar]
- Schaffer CB, Schaffer LC, Miller AR, Hang E, Nordahl TE. Efficacy and safety of nonbenzodiazepine hypnotics for chronic insomnia in patients with bipolar disorder. Journal of Affective Disorders. 2011;128:305–308. doi: 10.1016/j.jad.2010.07.018. http://dx.doi.org/10.1016/j.jad.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Senn S. Testing for baseline balance in clinical trials. Statistics in Medicine. 1994;13:1715–1726. doi: 10.1002/sim.4780131703. http://dx.doi.org/10.1002/sim.4780131703. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. International Clinical Psychopharmacology. 1996;11(Suppl. 3):89–95. doi: 10.1097/00004850-199606003-00015. http://dx.doi.org/10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- Sylvia LG, Dupuy JM, Ostacher MJ, Cowperthwait CM, Hay AC, Sachs GS, Perlis RH. Sleep disturbance in euthymic bipolar patients. Journal of Psychopharmacology. 2012;26:1108–1112. doi: 10.1177/0269881111421973. http://dx.doi.org/10.1177/0269881111421973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot LS, Maguen S, Metzler TJ, Schmitz M, McCaslin SE, Richards A, Neylan TC. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: A randomized controlled trial. Sleep. 2014;37:327–341. doi: 10.5665/sleep.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot LS, Stone S, Gruber J, Hairston IS, Eidelman P, Harvey AG. A test of the bidirectional association between sleep and mood in bipolar disorder and insomnia. Journal of Abnormal Psychology. 2012;121:39–50. doi: 10.1037/a0024946. http://dx.doi.org/10.1037/a0024946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Macfadden W, Weisler RH, Chang W, Paulsson B, Khan A, Calabrese JR. Efficacy of quetiapine mono-therapy in bipolar I and II depression: A double-blind, placebo-controlled study (the BOLDER II Study). Journal of Clinical Psychopharmacology. 2006;26:600–609. doi: 10.1097/01.jcp.0000248603.76231.b7. http://dx.doi.org/10.1097/01.jcp.0000248603.76231.b7. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Sack DA, Rosenthal NE. Sleep reduction as a final common pathway in the genesis of mania. American Journal of Psychiatry. 1987;144:201–204. doi: 10.1176/ajp.144.2.201. [DOI] [PubMed] [Google Scholar]
- Weilburg JB, Sachs G, Falk WE. Triazolam-induced brief episodes of secondary mania in a depressed patient. Journal of Clinical Psychiatry. 1987;48:492–493. [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for affective disorders: A clinician's manual for light and wake therapy. Karger; Basel, Switzerland: 2009. http://dx.doi.org/10.1159/isbn.978-3-8055-9121-8. [Google Scholar]
- Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the Insomnia Severity Index: Using health-related outcomes to define the minimally important difference. Current Medical Research and Opinion. 2009;25:2487–2494. doi: 10.1185/03007990903167415. http://dx.doi.org/10.1185/03007990903167415. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. http://dx.doi.org/10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.