Abstract
Background
Ferumoxytol is an ultrasmall superparamagnetic iron oxide (USPIO) nanoparticle agent used to treat iron deficiency anemia in adults with chronic kidney disease.
Objective
We aim to determine the feasibility of using of ferumoxytol for clinical pediatric cardiac and vascular imaging.
Material and methods
We retrospectively identified 23 consecutive children who underwent MRI with ferumoxytol (11 males; mean age: 7.4 years, range: 3 days–18 years), yielding 12 abdominal MR angiography and 15 cardiac MRI studies. Medical records were reviewed for the clinical indication, ferumoxytol dose, injection rate, sedation and any complication. A two-reader consensus scored the images on a 5-point scale for overall image quality and delineation of various anatomical structures. Signal-to-background ratios for abdominal aorta and inferior vena cava for abdominal cases and blood pool-myocardium contrast ratios for cardiac cases were calculated. The confidence intervals for obtaining a score of 3 or above for each image parameter were calculated by using adjusted Wald method.
Results
For abdominal MR angiography, average scores for overall image quality, as well as delineation of the hepatic artery, superior mesenteric artery, renal artery, and veins were 4.5, 4.3, 4.3, 3.7 and 4.7, respectively. For cardiac exams, the average scores for overall image quality, systemic arteries, pulmonary arteries, pulmonary veins, valves and ventricles were 4.4, 4.6, 4.1, 4.8, 4.1 and 4.7, respectively. For all parameters, lower bound for proportion of cases to have a score of 3 or above was 65%. Signal-to-background ratios for aorta and abdominal veins averaged 86 +/− 74 and 86 +/− 77 for full-dose images, and 23 and 18 for half-dose images, respectively. Mean blood pool to myocardium contrast ratio was 3:3.
Conclusion
Ferumoxytol can provide excellent image quality for pediatric body MR angiography/MR venography at a dose of 1.5 or 3 mg Fe/kg. Further investigation should be directed toward understanding the lowest dose that can be administered.
Keywords: MRI, iron, ferumoxytol, Feraheme, MRA
Introduction
MR angiography, especially for arterial imaging, usually uses bolus-injection of gadolinium-based extracellular agents, which can provide high signal-to-noise, but the enhancement declines rapidly due to leakage of contrast agent into the extravascular space. First-pass extraction and rapid redistribution into the extracellular space within about 11 min limits the window of imaging-enhanced vasculature following gadolinium injection [1]. Venous imaging with this type of agent usually is not optimal unless a very high dose is administered [2]. In contrast, blood pool contrast agents can maintain the signal intensity of arteries and veins for hours, extending the time period available for imaging, which allow longer acquisition times for better SNR and image resolution at lower contrast doses and injection rates [3].
Currently, the most commonly used blood pool contrast agent is a gadolinium chelate, gadofosveset trisodium. In our routine use, we have found that the quality of MR angiography with gadofosveset is somewhat limited after the first pass due to dilution of the agent in the entire blood pool. On the other hand, gadofosveset provides nice venous imaging for about 20 min after administration, at which point images become noticeably noisier.
Ferumoxytol (Feraheme, AMAG Pharmaceuticals, Waltham, MA) is an ultrasmall superparamagnetic iron oxide (USPIO) nanoparticle used for the treatment of iron deficiency anemia in adults with chronic kidney disease. Ferumoxytol has been used as blood pool MR contrast agent in adults with end-stage renal disease [4, 5]. It also has been used in adult body, adult brain and pediatric brain cases [1, 2, 4–12]. The pharmaceutical composition of ferumoxytol (USPIO encased in carbohydrate shell) results in a prolonged circulating half-life (14–15 h) in the intravascular space (blood pool), which can shorten the T1 and T2* relaxation rates of the blood pool for days to months [10]. Investigators have used doses of ferumoxytol as low as 1 mg/kg, with 4 mg/kg commonly used [1, 6, 10]. The highest dose at a bolus infusion is 4 mg (71.6 micromol) iron per kilogram of body weight, and the highest injection rate is 1 mL (537.2 micromol Fe) per s [2, 6].
To our knowledge, the use of ferumoxytol as a blood pool contrast agent for pediatric body imaging is rare. We have recently started using ferumoxytol for pediatric MR angiography and venography, mainly in abdominal and cardiac cases. Our motivation to explore the use of ferumoxytol stems from two circumstances where the agent can enable or improve pediatric vascular and cardiac MRI exams. The first circumstance is in those children with significantly compromised renal function, particularly those with end-stage renal disease on dialysis. For these children, the use of gadolinium-based contrast agents, even macrocyclic and/or ionic agents, is contraindicated.
The second circumstance is cardiac MRI exams, in which the signal-to-noise gains and very long blood pool residence times may enable either higher resolution imaging or an attempt at performing MRI without anesthesia. When using gadolinium-based agents, even blood pool agents, we have found that if a child becomes uncooperative upon injection of contrast, the whole exam is nondiagnostic, and by the time the child becomes cooperative again for another attempt at imaging, the concentration of contrast agent in the blood pool no longer suffices to obtain diagnostic image quality.
Here we describe our experience with ferumoxytol and report preliminary results on the quality of the resultant cardiovascular imaging.
Materials and methods
With IRB approval under waived consent and in HIPAA compliant fashion, we retrospectively identified 23 consecutive children (11 males and 12 females) referred to our clinic for abdominal and cardiac MR angiography at 3T who received ferumoxytol as an off-label use. Medical records were reviewed for the clinical indication, ferumoxytol dose, injection rate, sedation and any complication.
Each subject was scanned on a 3-T MRI scanner (MR750; GE Healthcare, Waukesha, WI) with the following coils: 32-channel cardiac coil (16 cases, 70%), 32-channel torso coil (6 cases, 26%) and 16-channel flexible coil (1 case, 4%), with choice based on patient size. For subsequent image analysis, an axial spoiled gradient echo sequence with intermittent fat suppression was used with parameters of TR (3.8–4.6 ms for abdomen, 3.7–4.6 ms for cardiac), TE (0.9–1.8 ms for abdomen, 1.7–2.1 ms for cardiac), slice thickness (1–3 mm for abdomen, 0.7–1.2 mm for cardiac), matrix (296 × 256 – 416 × 416 for abdomen, 256 × 192 – 416 × 416 for cardiac) and field of view (24–40 cm for abdomen, 26–30 cm for cardiac). Parameters were adjusted to each patient’s anatomy. For cardiac cases, the sequence was ECG-gated, but the sequence was not cardiac gated for abdominal exams. Additionally, for seven cases, a dual echo spoiled gradient echo sequence (echo times of 1.1 and 2.2 ms) was obtained. All images were obtained in steady-state free-breathing, without parallel imaging, and without respiratory gating.
Two readers (S.S.V. and A.H.), with 8 years and 2 years of experience, respectively, interpreting pediatric body MRI scored the images by consensus. Assessments were made for overall image quality, as well as delineation of the hepatic artery, superior mesenteric artery, right renal artery and abdominal veins for abdominal cases, according to criteria detailed in Table 1. Similarly, for cardiac cases, overall image quality and delineation of the systemic arteries, pulmonary arteries, pulmonary veins, heart valves and ventricles were scored according to criteria in Table 2. The confidence intervals for obtaining a score of 3 or above for each image parameter were calculated by using adjusted Wald method.
Table 1.
Criteria for imaging parameters of abdominal MR angiography
| Score | Overall image quality | HA | SMA | RA* | Veins |
|---|---|---|---|---|---|
| 5 | Outstanding; small arteries with high SNR and well-delineated | Second order branches of RHA well seen | Mesenteric arteries (vasa recta) well seen | Arcuate arteries seen | Adrenal vein well seen |
| 4 | Excellent; medium arteries with high SNR and well-delineated | First order branches of RHA well seen | Mesenteric arteries (vasa recta) poorly seen | Interlobar arteries seen | Lumbar and gonadal veins well seen |
| 3 | Good; all medium arteries can be assessed | RHA well seen | Main branches of SMA well seen | All segmental arteries seen | Lumbar or gonadal veins well seen |
| 2 | Diagnostic; all but 1–2 medium arteries can be assessed | Common HA well seen | Main branches of SMA poorly seen | Some segmental arteries seen | Left renal vein well seen |
| 1 | Limited assessment of several medium arteries | Common HA poorly seen | SMA well seen | Right renal artery well seen | IVC well seen |
Used right renal artery
HA hepatic artery, IVC interior vena cava, RA renal artery, RHA right hepatic artery, SNR signal-to-noise ratio, SMA superior mesenteric artery
Table 2.
Criteria for imaging quality parameters of cardiac MRI cases
| Score | Overall image quality | Systemic arteries | Pulmonary arteries | Pulmonary veins | Valves | Ventricles |
|---|---|---|---|---|---|---|
| 5 | Outstanding; small arteries with high SNR and well-delineated | Both coronary origins well seen | Arteries seen to within 1 cm of pleura | Can sharply see entrance to LA for all veins | Sharp on all phases | Sharp trabeculae |
| 4 | Excellent; medium arteries with high SNR and well-delineated | One coronary origin well seen | All segmental arteries well seen | Can sharply see entrance to LA for some veins | Sharp | Trabeculae seen |
| 3 | Good; all medium arteries can be assessed | Arch vessels well seen | Most segmental arteries well seen | Can see entrance to LA of all veins | Blurred | Sharp border |
| 2 | Diagnostic; all but 1–2 medium arteries assessed | Aorta well seen | Lobar arteries well seen | Can see entrance to LA of some veins | Partially seen | Can segment |
| 1 | Limited assessment of several medium arteries | Aorta not well seen | Right and left pulmonary arteries well seen | Cannot see entrance to LA | Not seen | Cannot segment |
LA left atrium, SNR signal-to-noise ratio
Then, signal intensities of the abdominal aorta, inferior vena cava and immediately adjacent non-vascular tissue were measured to calculate signal-to-background ratios for abdominal cases. Measurements of signal intensity in the inferior vena cava between each echo of the dual-echo MR angiography sequences used were also compared to assess T2* effects.
For cardiac cases, signal intensities of the blood pool (measured in the right ventricle adjacent to interventricular septum) and myocardium (measured at mid septum) were measured and the blood pool-myocardium contrast ratios were then calculated.
Results
The clinical indications for the exams were renal transplant evaluation/post-transplant complications (4 cases, 17%), vascular shunts/stenoses/aneurysms (6 cases, 26%), congenital heart disease (10 cases, 44%) and others (3 cases, 13%). Four children underwent both abdominal and cardiac exams, yielding a total of 12 abdominal exams and 15 cardiac exams. None of the patients had suspected iron overload.
Mean age (+/− standard deviation) was 7.4 years +/− 5.7 (range: 3 days–18 years). Sixteen cases (70%) were scanned under general anesthesia and the rest (7 cases, 30%) were scanned without any sedation. Modes of anesthesia used were nasal cannula (seven cases), laryngeal mask airway (five cases) and endotracheal intubation (four cases). All cases were scanned during free breathing (without any breath-holding or control apnea). Among 23 patients, 8 (35%) cases had significantly diminished renal function.
Heart rate, blood pressure and oxygen saturation had been measured prior to, during ferumoxytol administration and for at least 45 min following administration. There were no adverse reactions in any patient. No subject had a decrease in oxygen saturation by more than 1%. Four patients had a measurable decrease in mean arterial pressure: 12 mmHg, 4 mmHg, 4 mmHg and 13 mmHg. Ferumoxytol was injected at a dose of 0.05 mL/kg (1.5 mg Fe/kg, 1 case) and 0.1 mL/kg (3 mg Fe/kg, 22 cases). The average volume of undiluted ferumoxytol injected was 2.7 mL (range: 0.15–8.2 mL). The dose was administered by diluting in sterile normal saline to a volume of 10 mL and injecting at 1 mL/s.
For abdominal MR angiography, average scores of overall image quality, hepatic artery, superior mesenteric artery, renal artery and veins were 4.5, 4.3, 4.3, 3.7 and 4.7, respectively. Excluding the single case with a 0.05 mL/kg dose, the average scores of overall image quality, hepatic artery, superior mesenteric artery, renal artery and veins were 4.4, 4.2, 4.3, 3.7, and 4.7. For cardiac MRIs, the average scores in terms of overall image quality, systemic arteries, pulmonary arteries, pulmonary veins, valves and ventricles were 4.4, 4.6, 4.1, 4.8, 4.1 and 4.7, respectively. One of the cardiac MRI cases had single-dose gadobenate administered 25 min prior to imaging with ferumoxytol; the average scores did not change when this patient was excluded from the analysis. The confidence intervals for obtaining a score of 3 (good) or above for each image parameter are summarized in Table 3 and indicate a good quality is exam is likely to be obtained.
For abdominal MR angiography with a 0.1 mL/kg contrast dose, the mean signal-to-background ratios of artery and veins were 86 (SD 74, max 225.6, min 8.5) and 86 (SD 77, max 235.7, min 9.2), indicative of good vascular enhancement. The mean increase of signal on opposed phase images compared with in-phase images was 26% (max 48%, min 12%), indicating a significant T2* shortening at the administered 0.1 mL/kg dose.
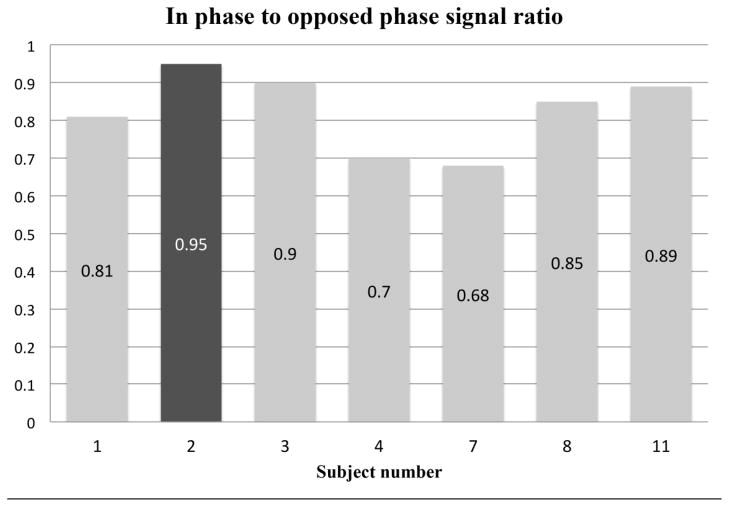
For images obtained in the single subject with a dose of 0.05 mL/kg, signal-to-background ratio of artery and vein were 22.6 and 18.3, which is lower that of any 0.1 mL/kg case. Additionally, for the 0.05 mL/kg dose, signal increase on opposed phase imaging was 5.3%, which is also lower than any of the 0.1 mL/kg dose cases. The vascular signal intensity improvements on out-phase compared to in-phase are summarized in Figure 1. Taken together, these findings of a both increasing signal-to-background and increasing T2* effect when the dose is increased from 0.05 mL/kg to 0.1 mL/kg suggest a 0.1 mL/kg dose is likely to give a good balance between the two competing effects.
Fig. 1.
Signal drop-off at longer echo times on patients with dual echo acquisition. Black bar is from the patient who received the lower dose of 0.05 mL/kg
For cardiac exams, mean blood pool to myocardium contrast ratios was 3.3 (max 4.4, min 2.5). The contrast between myocardium and blood pool permitted easy segmentation of the ventricles. Additionally, valve leaflets were seen well. Of note, one cardiac exam was performed first by the administration of gadobenate for viability assessment, and then after 20 min, ferumoxytol was administered to enable visualization of coronary arteries adjacent to a valve with metallic scaffolding percutaneously delivered into the mitral position. Images in this case were obtained with ferumoxytol 25 minafter gadobenate had been administered. Both sets of images for this subject were evaluated. Blood pool to myocardium contrast ratios of gadobenate and ferumoxytol images were 1.8 and 2.6, respectively. When this patient is excluded, the mean blood pool to myocardial contrast ratio for ferumoxytol increased from 3.3 to 3.4.
Figures 2 through 7 show representative examples. The high vascular signal to background and depiction of minute vessels is evident. Additionally, Figure 8 shows the case with the worst image quality, which has significant blurring from motion. Figure 9 shows the one case in which gadobenate and ferumoxytol were given. Comparing the images to the other patients in the study, the post-ferumoxytol images had poorest contrast between blood-pool and myocardium/valves. This is likely the result of enhancement of the myocardium by the gadobenate, even 25 min after its administration, decreasing the delineation of the myocardial borders.
Fig. 2.
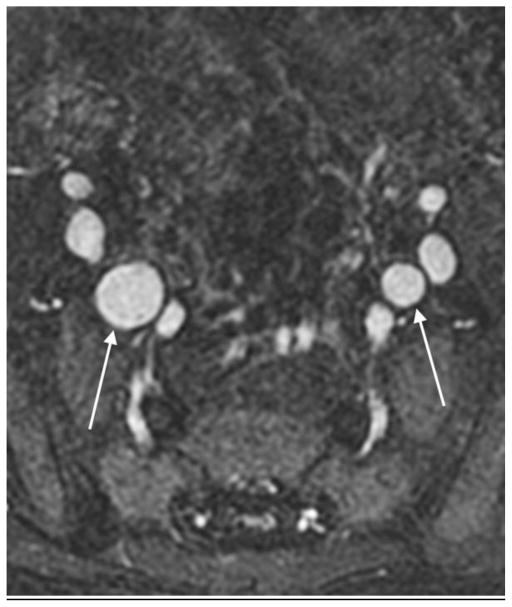
A 2-year-old boy with Kawasaki disease. Axial source (a) and sagittal reformatted (b) images show bilateral internal iliac artery aneurysms (arrows)
Fig. 7.
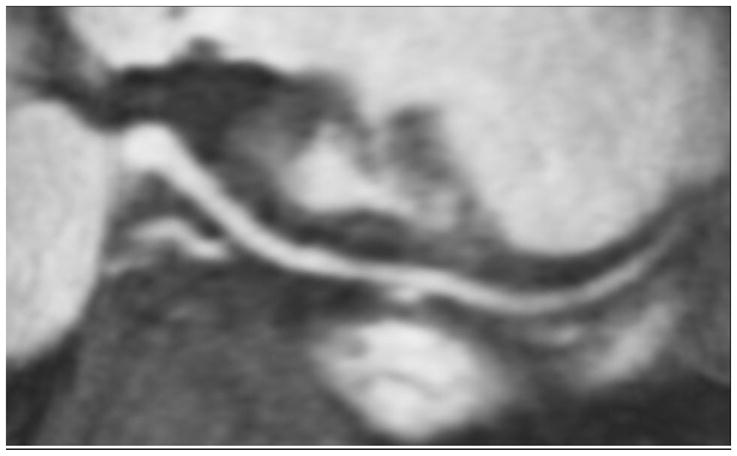
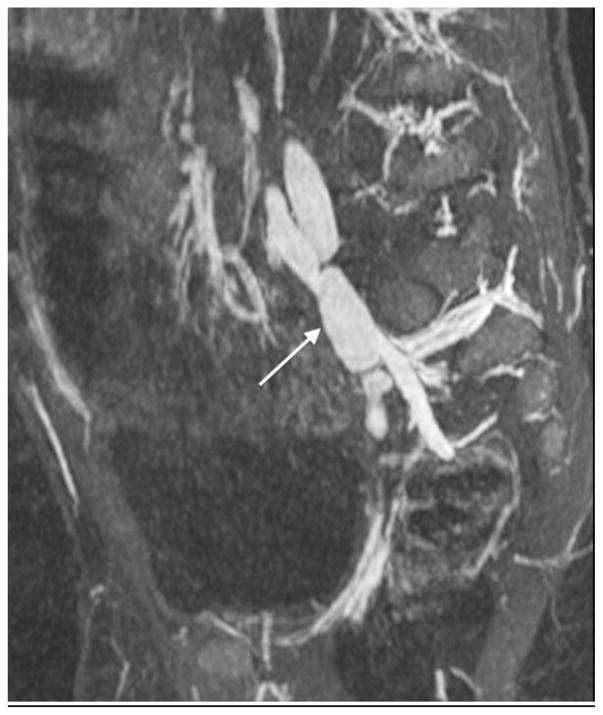
Same patient as in Figure 6. Curved planar reformation shows right coronary artery
Fig. 8.
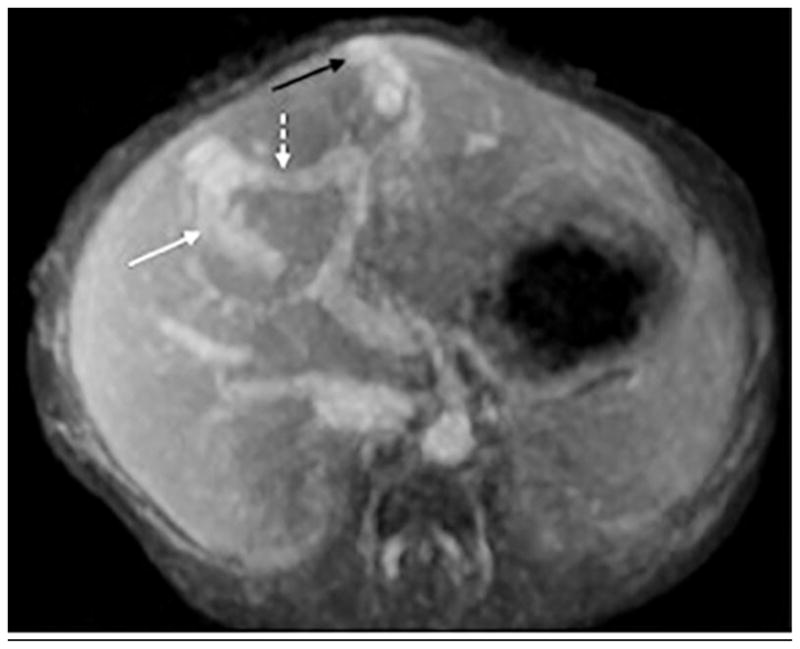
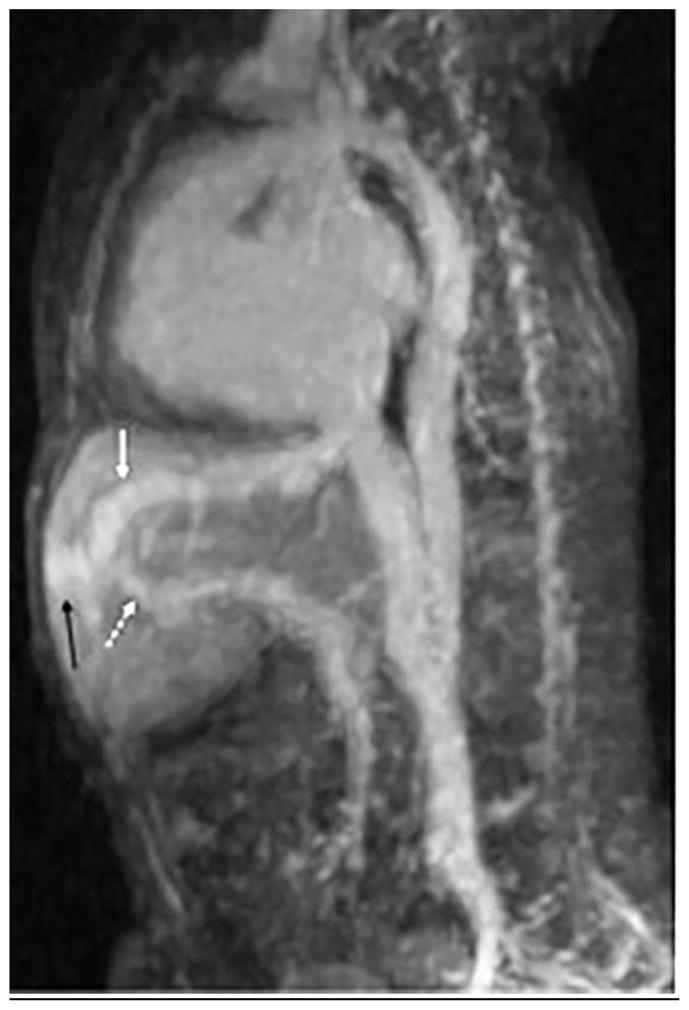
A 3-day-old girl with intrauterine growth restriction and abdominal bruit. The child was agitated during the exam. Note blurring of vessels from motion but image is still diagnostic, detecting Park classification type 3 intrahepatic shunt. a On axial image, note middle hepatic vein (white arrow) draining a branch of the left portal vein (dashed white arrow) via an aneurysmal communication. A similar aneurysmal communication (black arrow) is seen draining via the left hepatic vein. b Sagittal reformatted image shows left hepatic vein (white arrow), aneurysmal communication (black arrow) and branch of left portal vein (dashed white arrow)
Fig. 9.
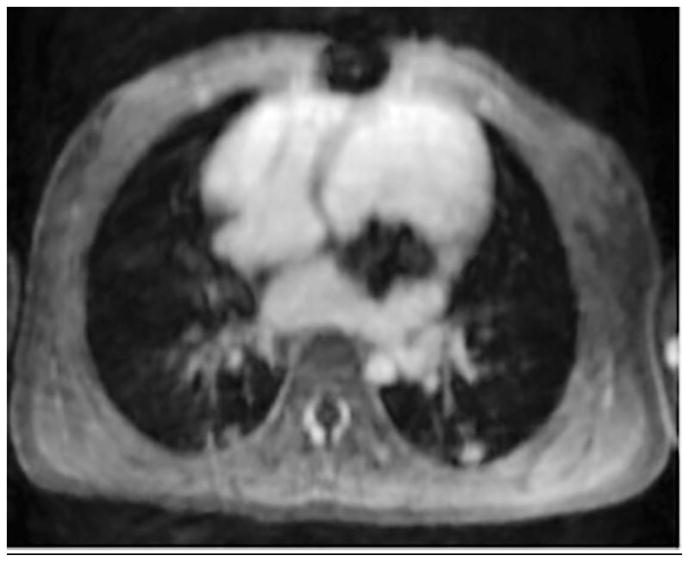
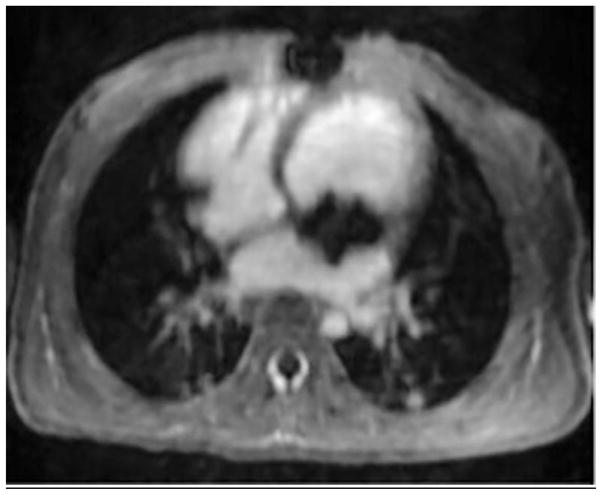
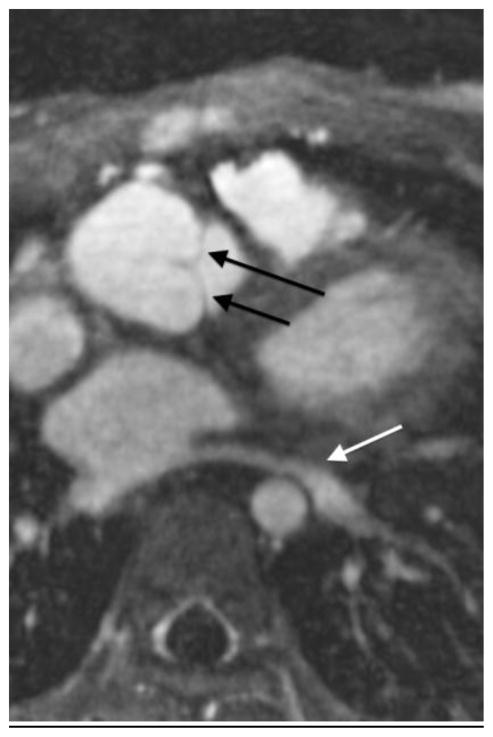
A 2-year-old girl with severe dysplastic mitral valve. Cardiac MRI images after administration of (a) gadobenate and (b) ferumoxytol show metallic scaffolding percutaneously delivered into the mitral position. Note sharper anatomical delineation and better blood pool to myocardium contrast of the ferumoxytol image than the gadobenate image
Discussion
To our knowledge, the use of ferumoxytol as a MR contrast agent in children in a clinical setting is rare. Our study found that ferumoxytol-enhanced MR angiography can provide excellent imaging quality (high SNR with well-delineated medium and some small size vessels), excellent signal to background ratio of arteries and veins for abdominal cases and good blood pool to myocardium contrast ratio for cardiac cases. For dual-echo images, slightly greater vascular signal intensity on lower TE (out-phase) images than higher TE (in-phase) images, likely related to T2* decay on in phase. Additionally, the single case of a 0.05 mL/kg dose administration also produced good vascular enhancement. Thus, it is possible that at the higher dose, 0.1 mL/kg, T2* effects may be adversely impacting vascular signal. It is not immediately clear at what dose optimally balances these competing effects. It is worth noting, for comparison, that the therapeutic dose of ferumoxytol is 0.3 mL/kg.
It is also notable, that since we have established a protocol with ferumoxytol for cardiac and vascular MRI exams at our institution, the workflow for exams performed under anesthesia has been simplified. Since ferumoxytol has such a long residence time, it can be given shortly after induction by the anesthesiologist under controlled monitoring conditions, before entering the MRI suite. This avoids the need to disconnect and reconnect lines supplying intravenous sedatives to give intravenous contrast during the MRI examination. Additionally, because of the long blood pool residence time and relative lack of signal in non-vascular structures, our imaging is now performed in completely free-breathing fashion, which has enabled lighter and shorter anesthesia. This has been the motivation for our use of ferumoxytol in patients who do not have significant renal disease.
Our study has a few limitations. First, we have not performed a direct comparison of image quality between ferumoxytol-enhanced MR angiography and other vascular imaging such as time-of-flight, other gadolinium-based blood pool agents or other imaging modality. Previous studies in adult patients found that ferumoxytol-enhanced MR images had superior image quality compared to pre-contrast time-of-flight images [5, 9], similar image quality to a blood pool gadolinium-based contrast agent [4] and the findings in ferumoxytol MR angiography were consistent with the results of other vascular tests (conventional angiography, gadolinium-enhanced MR angiography, CT angiography and Doppler US) [2]. Here we compared in one subject images with gadobenate and ferumoxytol.
Although we have not performed a direct comparison, based on our experience with other blood pool agent gadofosveset trisodium (Ablavar, Lantheus Medical Imaging, Inc., N. Billerica, MA), which we have administered to more than 100 children, we have qualitatively found that the vascular enhancement with ferumoxytol is noticeably greater. Because the blood pool residence time is quite long, we have been able to obtain free-breathing exams without the use of parallel imaging. These longer acquisitions help to improve the qualitative signal-to-noise ratio and have the effect of motion averaging to reduce artifacts.
Another limitation of the current study is that we have included a relatively small number of patients in our early experience with this agent. We show results primarily at a dose of 0.1 mL/kg, and observed the effect of half this dose in one patient. The lowest, optimal dose of ferumoxytol for vascular imaging requires further investigation. Additionally, we did not systematically optimize the image acquisition parameters nor assess for systemic side effects of iron.
As noted, the major alternative to ferumoxytol for blood pool imaging is gadofosveset, which has United States Food and Drug Administration approval for the evaluation of atherosclerotic aortoiliac disease and has been used in a similar off-label fashion for pediatric MR angiography. It should be noted that although there were no adverse reactions in this small study, it is known that the adverse event rate of ferumoxytol is significantly higher than that of gadolinium-based agents. In particular, hypotension occurs in approximately 0.12% of patients and the anaphylaxis rate is likely ten-fold greater than gadolinium agents, as high as 0.02% [13]. However, it should be noted that these large studies were in adults on hemodialysis, and the severity of hypotension and anaphylaxis were not described. Nonetheless, besides the anaphylaxis rate and hypotension, another consideration in the use of ferumoxytol is that diagnostic MR imaging for non-angiographic indications may have altered contrast for several months [10, 14]. Finally, consideration must be given to the inherent risks of iron toxicity. As a basis for comparison, the tolerable upper limits of daily intake of iron in children is 40 mg [15] vs. an average dose of 81 mg ferumoxytol in our study. Accounting for oral bioavailability, the dose is approximately equivalent to a week of dietary intake. Against these disadvantages, the value of ferumoxytol is that it provides better vascular contrast over a longer period of time.
Thus, three scenarios may benefit from the use of ferumoxytol for pediatric vascular imaging. First, ferumoxytol provides diagnostic MR imaging children with significant renal disease. Second, in situations where very small vessels must be reliably interrogated, ferumoxytol may provide a higher level of confidence. Third, in children who are at the cusp of development to undergo an MRI without anesthesia, ferumoxytol can decouple placement of a peripheral venous catheter and contrast injection from the MR scanning itself and permit several leisurely attempts at contrast-enhanced imaging without anesthesia. In our own practice, our experience with ferumoxytol began with the first group of patients with renal disease. We found in this group markedly improved vascular imaging and thus extended the use of ferumoxytol to challenging vascular exams and have started performing some exams without anesthesia that we would have ordinarily obtained under anesthesia.
Conclusion
Ferumoxytol can provide excellent image quality for pediatric body MR angiography/MR venography at a dose of 0.05 mL/kg or 0.1 mL/kg, particularly in patients with significant renal insufficiency. Further investigation should be directed toward understanding the lowest dose that can be administered to achieve imaging objectives.
Fig. 3.
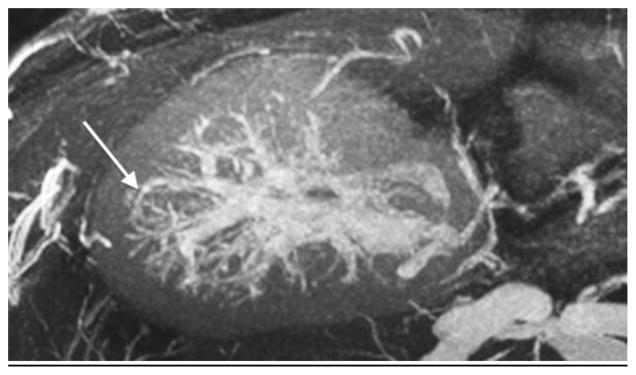
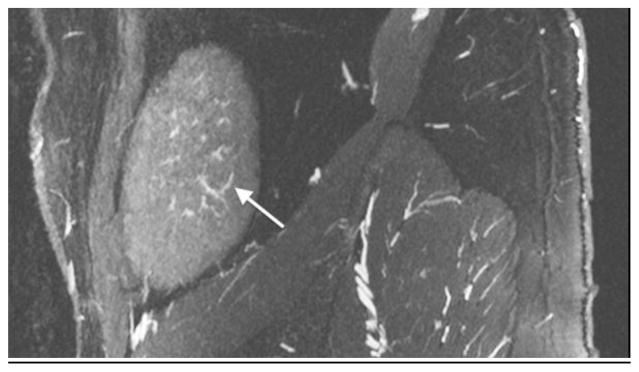
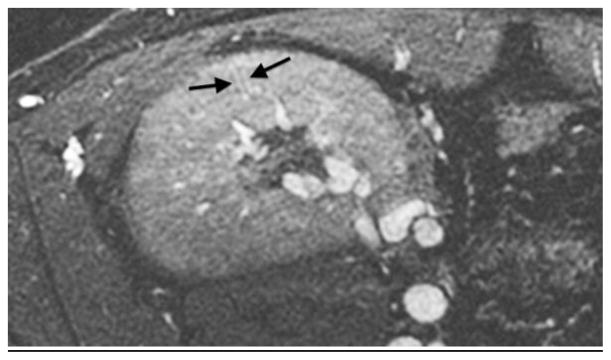
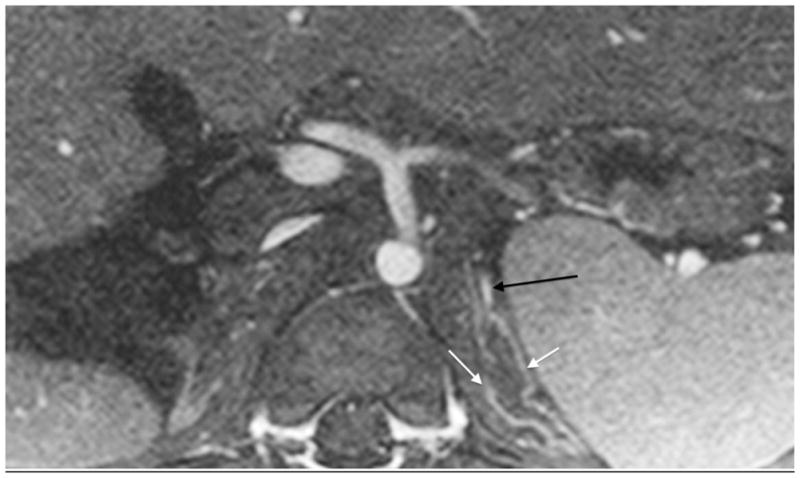
An 18-year-old boy after renal transplantation. White arrows indicate an arcuate artery on axial MIP (a) and sagittal reformatted (b) images. Intralobular artery and vein (c) (black arrows) and transplant renal artery origin (d) off iliac artery (dashed arrow)
Fig. 4.
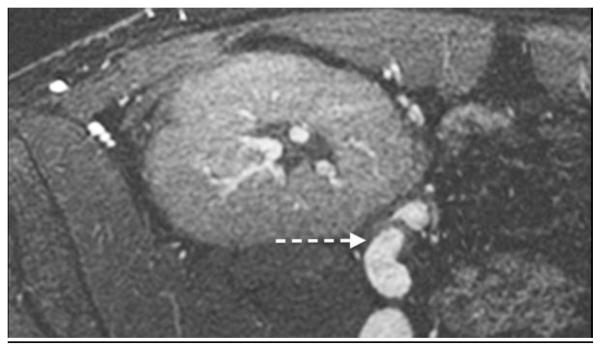
A 1-year-old boy with autosomal recessive polycystic kidney disease after bilateral nephrectomy. MR angiography using ferumoxytol 0.05 mL/kg. Axial image shows good vascular signal intensity despite the lower dose and nicely seen left adrenal vein (black arrow). Also note intensely enhancing medial and lateral limbs of the left adrenal gland (white arrows)
Fig. 5.
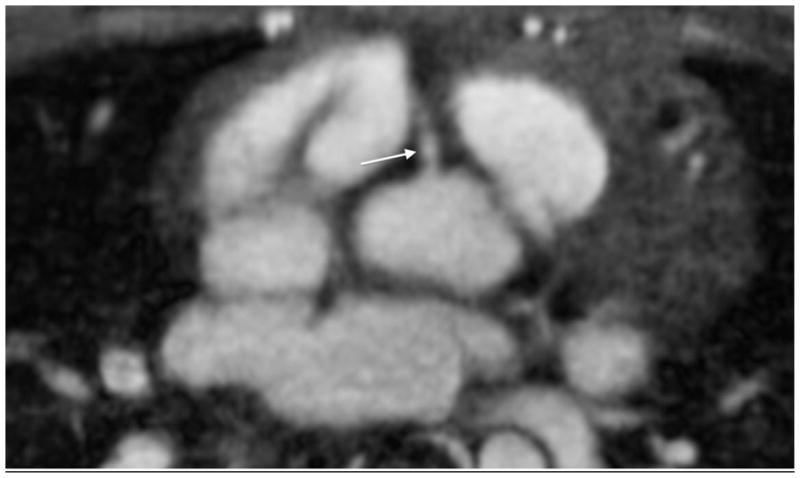
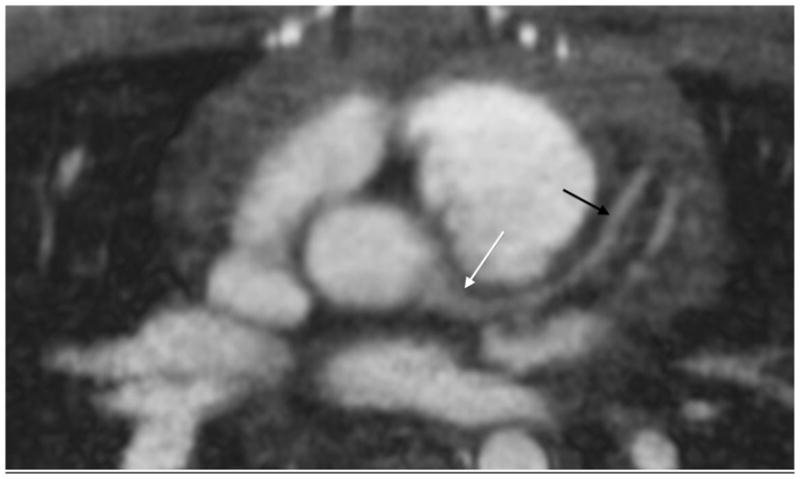
A 10-year-old boy with pulmonary artery stenosis. a Gated axial spoiled gradient echo image at the level of the right coronary artery (arrow) shows its origin. b Axial image at level of the left main coronary artery (white arrow) also shows left anterior descending coronary artery (black arrows). The coronary vessels are not typically seen with spoiled gradient echo sequences with such clarity
Fig. 6.
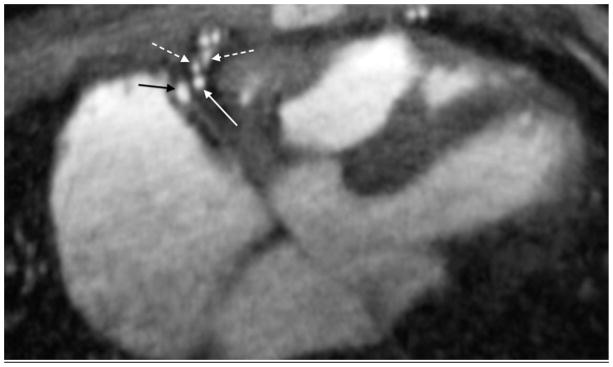
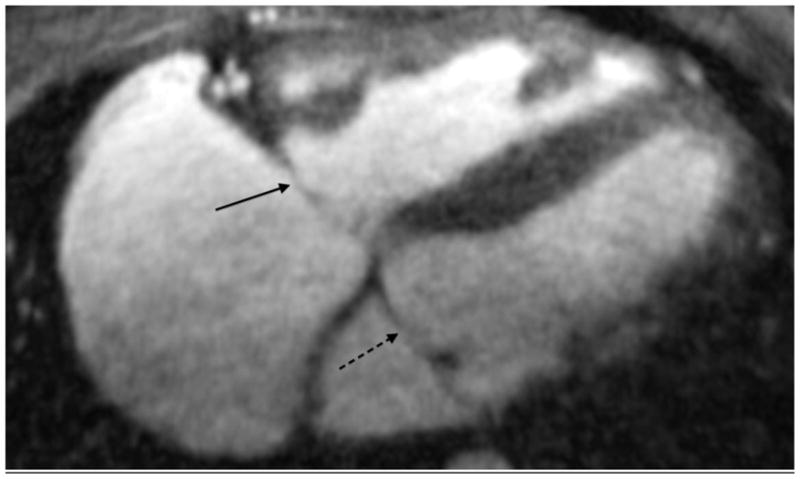

An 11-year-old boy with native anatomy of double outlet right ventricle, now after a Rastelli procedure. (a) Gated axial spoiled gradient echo image shows right coronary artery (white arrow), two acute marginal branches (dashed arrows) and right coronary vein (black arrow). (b) Axial image more inferiorly shows tricuspid (black arrow) and mitral (dashed black arrow) valves. (c) Axial image more superiorly delineates aortic valve leaflets (black arrows) as well as left lower pulmonary vein (white arrow). (d) Oblique sagittal reformat shows all three aortic valve leaflets
Table 3.
Proportion of scores of 3 (good) or above for each parameter with confidence intervals. One abdominal case was performed after bilateral nephrectomies and the another had renal anatomy severely distorted by multicystic dysplastic kidney, preventing scoring of the delineation of the renal arteries. One abdominal case had hepatic artery and adrenal vein passing superior to the field of view, also precluding evaluation
| Image quality feature | Proportion of cases with score ≥3 | 80% confidence interval | |
|---|---|---|---|
| Abdominal | Overall | 11/12 | 0.75–0.98 |
| Hepatic artery | 11/11 | 0.93–1.0 | |
| Superior mesenteric artery | 11/12 | 0.75–0.98 | |
| Renal artery | 9/10 | 0.71–0.98 | |
| Veins | 10/11 | 0.73–0.98 | |
| Cardiac | Overall | 15/15 | 0.95–1.0 |
| Systemic arteries | 15/15 | 0.95–1.0 | |
| Pulmonary arteries | 14/15 | 0.79–0.99 | |
| Pulmonary veins | 15/15 | 0.95–1.0 | |
| Cardiac valves | 14/15 | 0.79–0.99 | |
| Ventricles | 15/15 | 0.95–1.0 |
Acknowledgments
This work is supported by a grant from the National Institutes of Health and the Tashia and John Morgridge Faculty Scholar Fund.
Footnotes
Conflicts of interest None
Contributor Information
Nichanan Ruangwattanapaisarn, Department of Diagnostic and Therapeutic Radiology, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. LPCH Department of Radiology, Stanford University, Stanford, CA 94305, USA.
Albert Hsiao, LPCH Department of Radiology, Stanford University, Stanford, CA 94305, USA.
Shreyas S. Vasanawala, Email: vasanawala@stanford.edu, LPCH Department of Radiology, Stanford University, Stanford, CA 94305, USA
References
- 1.Prince MR, Zhang HL, Chabra SG, et al. A pilot investigation of new superparamagnetic iron oxide (ferumoxytol) as a contrast agent for cardiovascular MRI. J Xray Sci Technol. 2003;11:231–240. [PubMed] [Google Scholar]
- 2.Li W, Tutton S, Vu AT, et al. First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. J Magn Reson Imaging. 2005;21:46–52. doi: 10.1002/jmri.20235. [DOI] [PubMed] [Google Scholar]
- 3.Bremerich J, Bilecen D, Reimer P. MR angiography with blood pool contrast agents. Eur Radiol. 2007;17:3017–3024. doi: 10.1007/s00330-007-0712-0. [DOI] [PubMed] [Google Scholar]
- 4.Bashir MR, Mody R, Neville A, et al. Retrospective assessment of the utility of an iron-based agent for contrast-enhanced magnetic resonance venography in patients with endstage renal diseases. J Magn Reson Imaging. 2014;40:113–118. doi: 10.1002/jmri.24330. [DOI] [PubMed] [Google Scholar]
- 5.Sigovan M, Gasper W, Alley HF, et al. USPIO-enhanced MR angiography of arteriovenous fistulas in patients with renal failure. Radiology. 2012;265:584–590. doi: 10.1148/radiol.12112694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ersoy H, Jacobs P, Kent CK, et al. Blood pool MR angiography of aortic stent-graft endoleak. AJR Am J Roentgenol. 2004;182:1181–1186. doi: 10.2214/ajr.182.5.1821181. [DOI] [PubMed] [Google Scholar]
- 7.Gahramanov S, Muldoon LL, Varallyay CG, et al. Pseudoprogression of glioblastoma after chemo-and radiation therapy: diagnosis by using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology. 2013;266:842–852. doi: 10.1148/radiol.12111472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton BE, Nesbit GM, Dosa E, et al. Comparative analysis of ferumoxytol and gadoteridol enhancement using T1- and T2-weighted MRI in neuroimaging. AJR Am J Roentgenol. 2011;197:981–988. doi: 10.2214/AJR.10.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Salanitri J, Tutton S, et al. Lower extremity deep venous thrombosis: evaluation with ferumoxytol-enhanced MR imaging and dual-contrast mechanism--preliminary experience. Radiology. 2007;242:873–881. doi: 10.1148/radiol.2423052101. [DOI] [PubMed] [Google Scholar]
- 10.McCullough BJ, Kolokythas O, Maki JH, et al. Ferumoxytol in clinical practice: implications for MRI. J Magn Reson Imaging. 2013;37:1476–1479. doi: 10.1002/jmri.23879. [DOI] [PubMed] [Google Scholar]
- 11.Stabi KL, Bendz LM. Ferumoxytol use as an intravenous contrast agent for magnetic resonance angiography. Ann Pharmacother. 2011;45:1571–1575. doi: 10.1345/aph.1Q431. [DOI] [PubMed] [Google Scholar]
- 12.Thompson EM, Guillaume DJ, Dosa E, et al. Dual contrast perfusion MRI in a single imaging session for assessment of pediatric brain tumors. J Neurooncol. 2012;109:105–114. doi: 10.1007/s11060-012-0872-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiller B, Bhat P, Sharma A. Safety and effectiveness of ferumoxytol in hemodialysis patients at 3 dialysis chains in the United States over a 12-month period. Clin Ther. 2013;36:70–83. doi: 10.1016/j.clinthera.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Schieda N. Parenteral ferumoxtol interaction with magnetic resonance imaging: a case report, review of the literature and advisory warning. Insights Imaging. 2013;4:509–512. doi: 10.1007/s13244-013-0262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc: a report of the panel on micronutrients. National Academy Press; Washington, DC: 2011. [Google Scholar]