Abstract
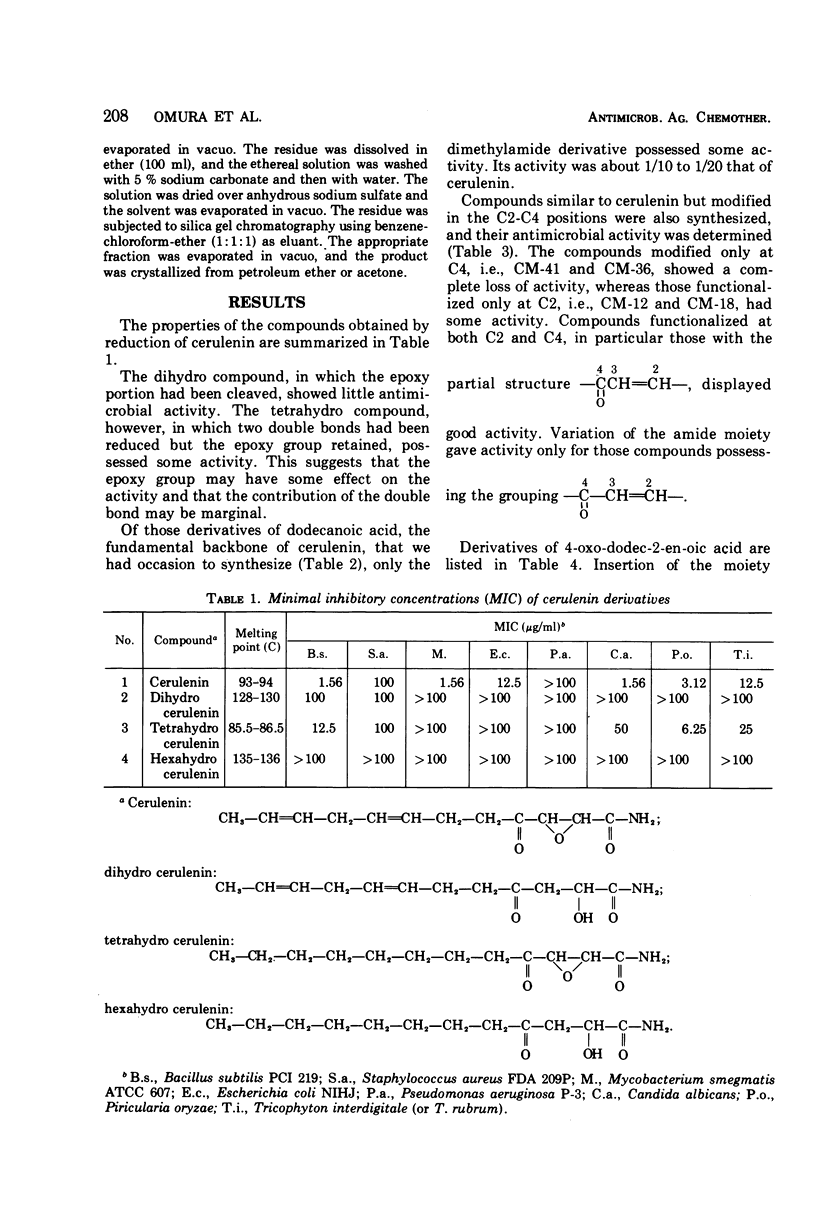
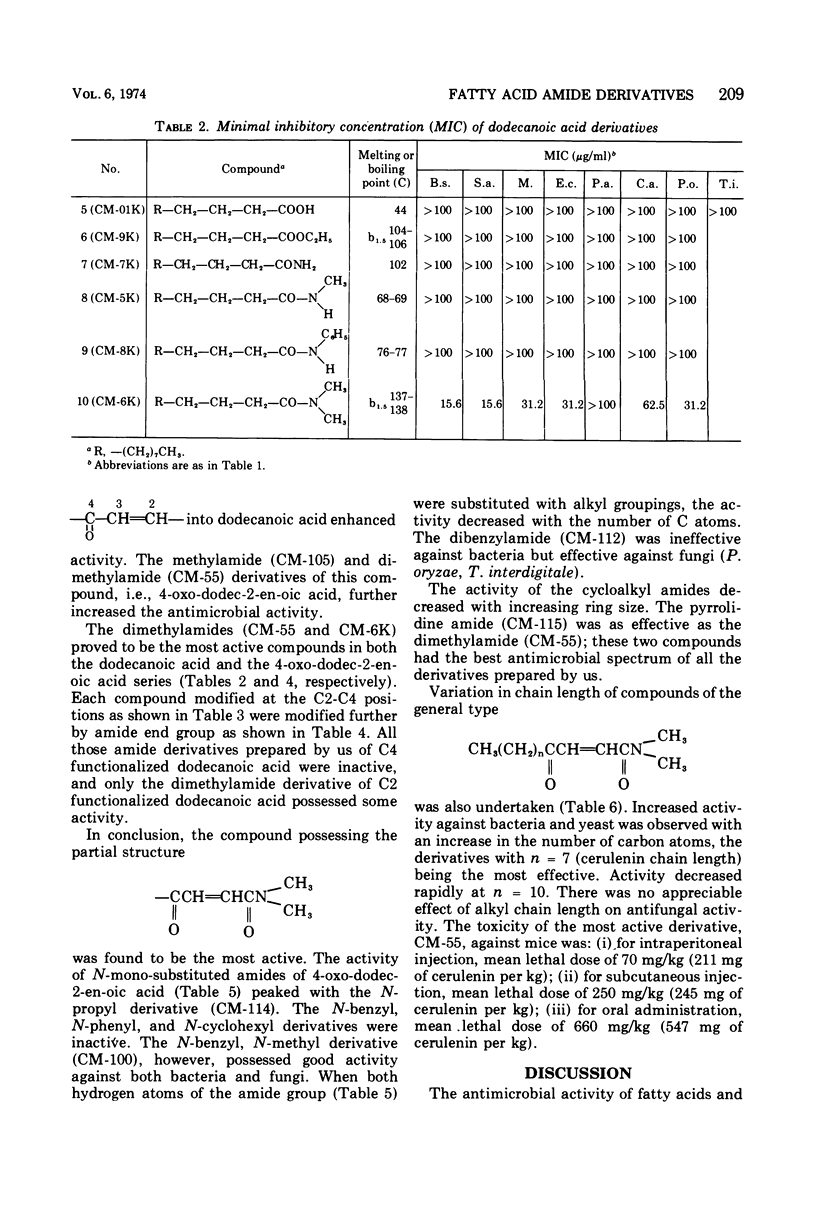
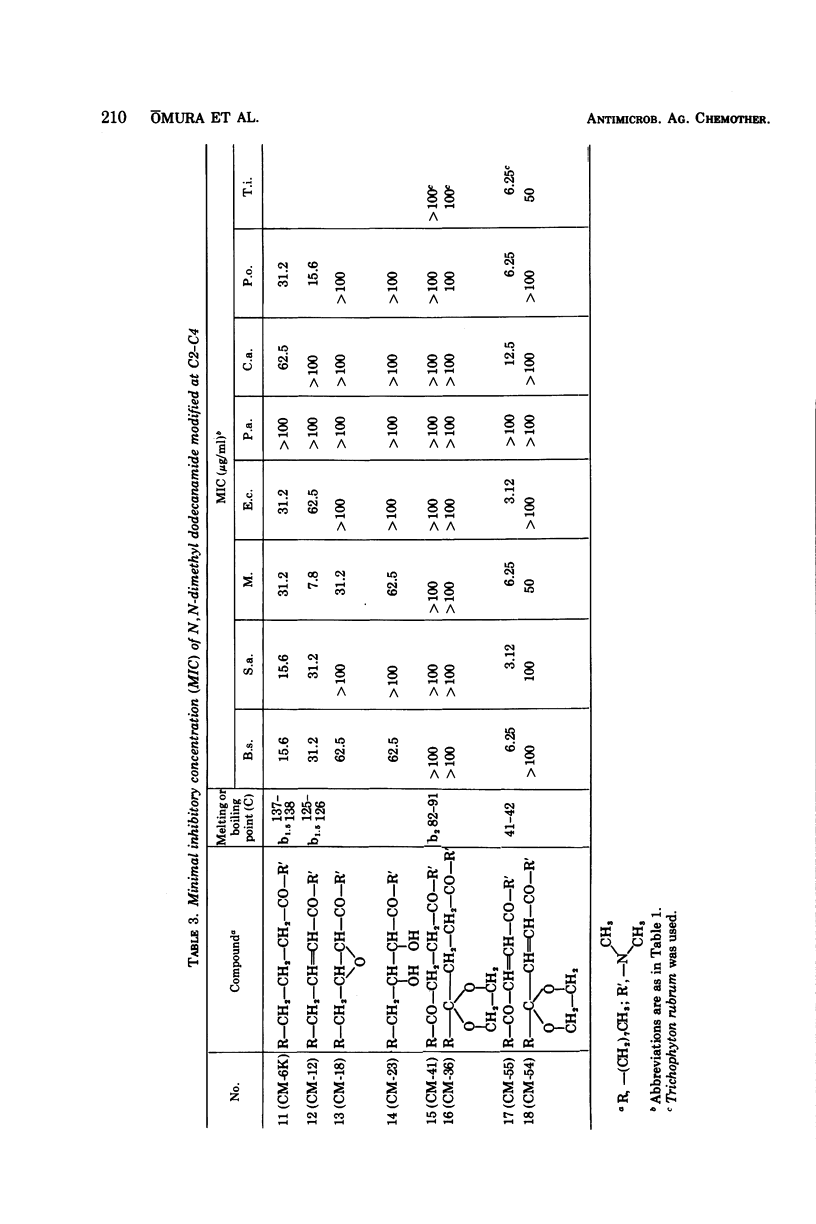
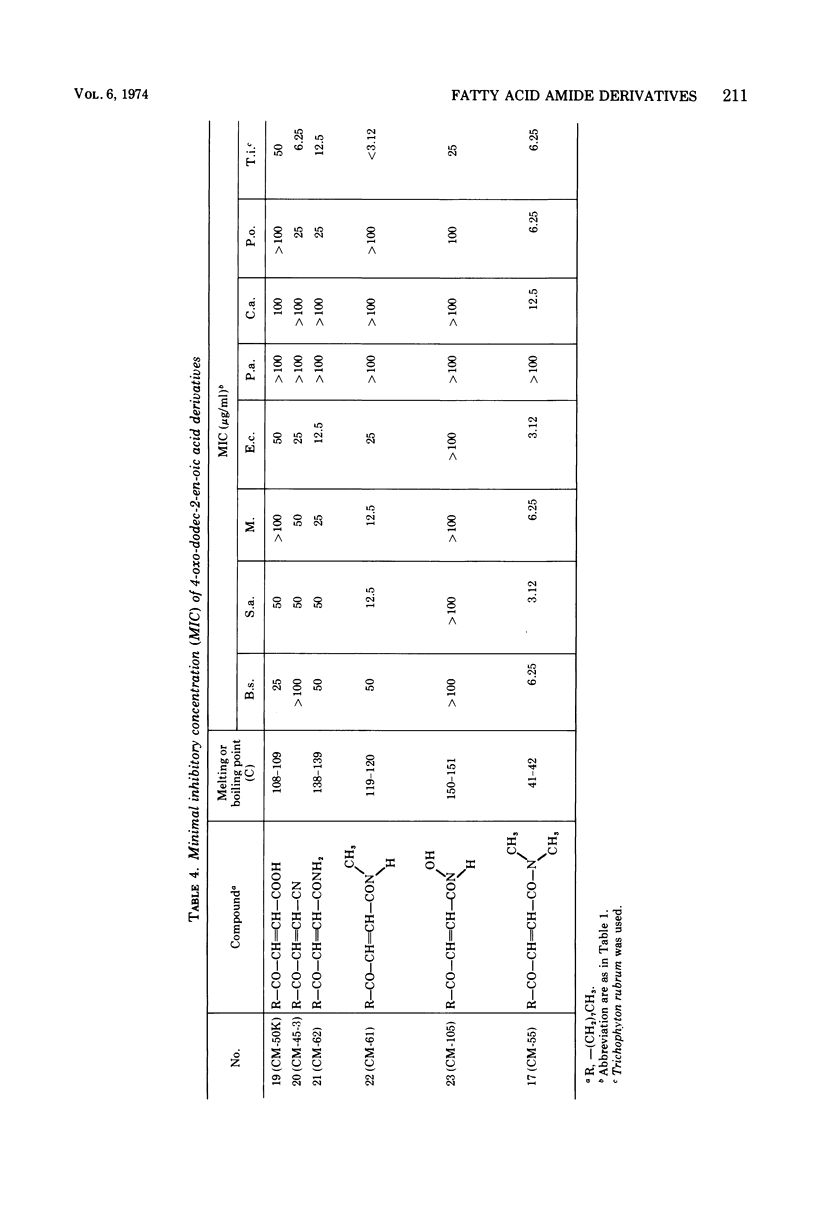
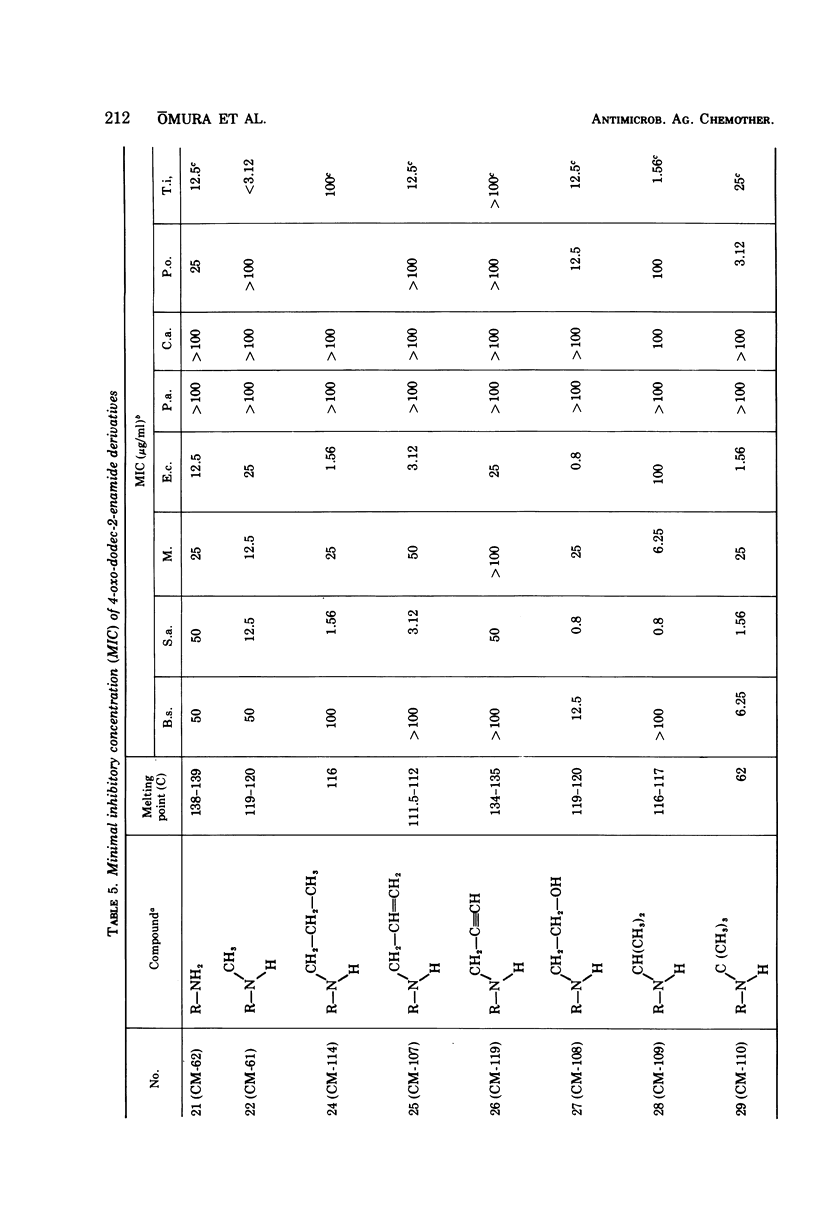
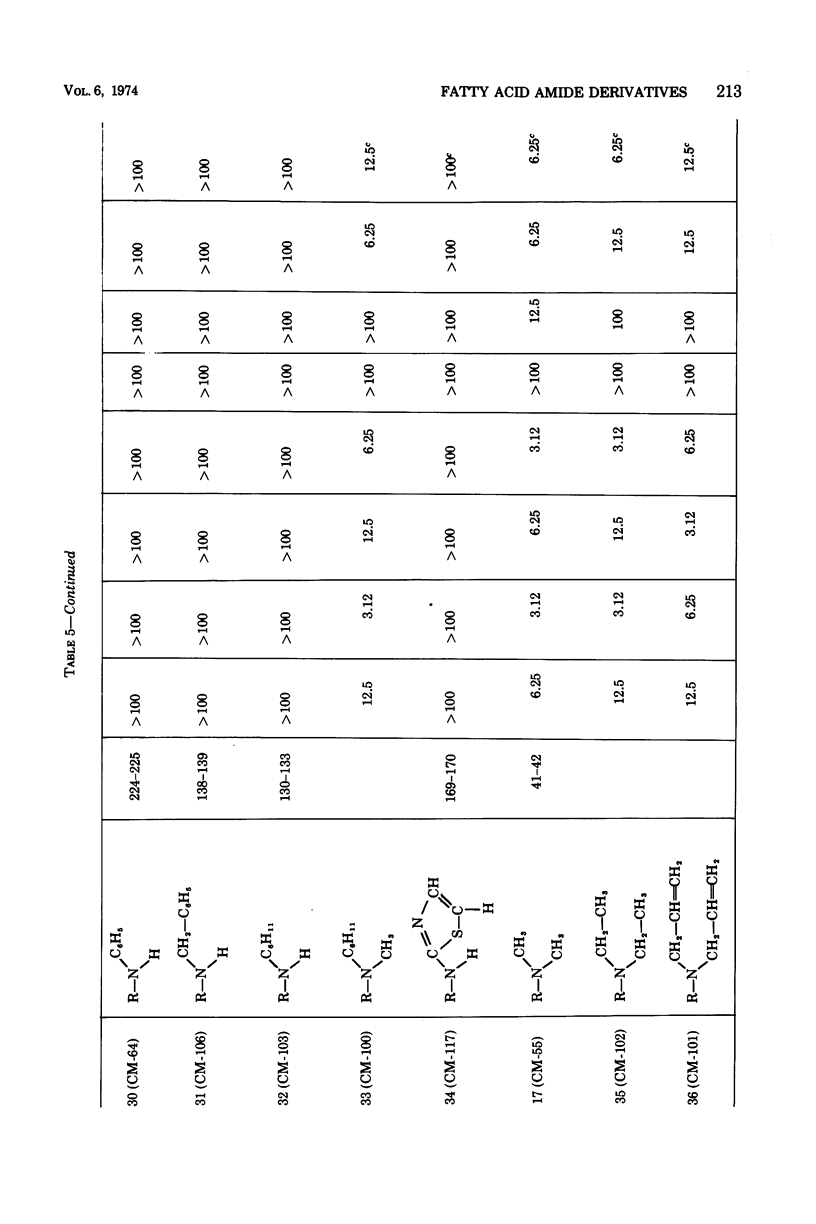
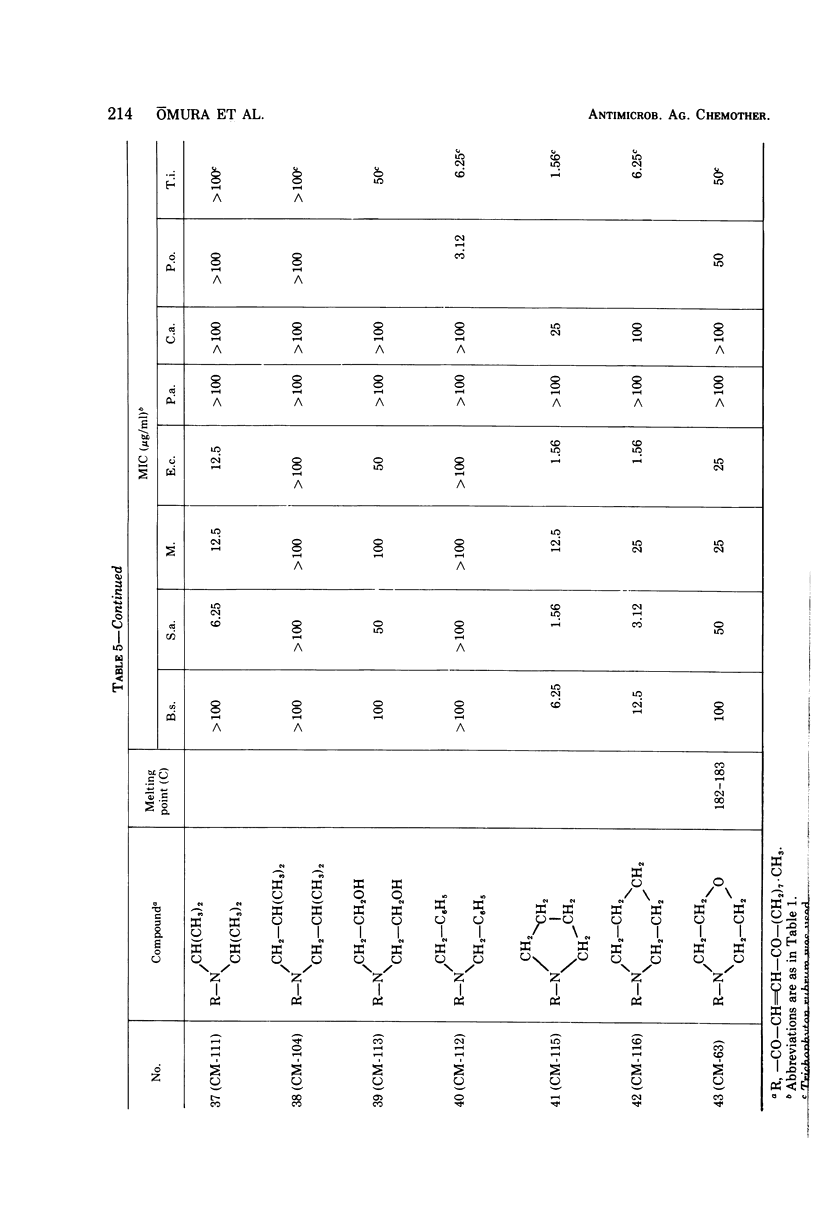
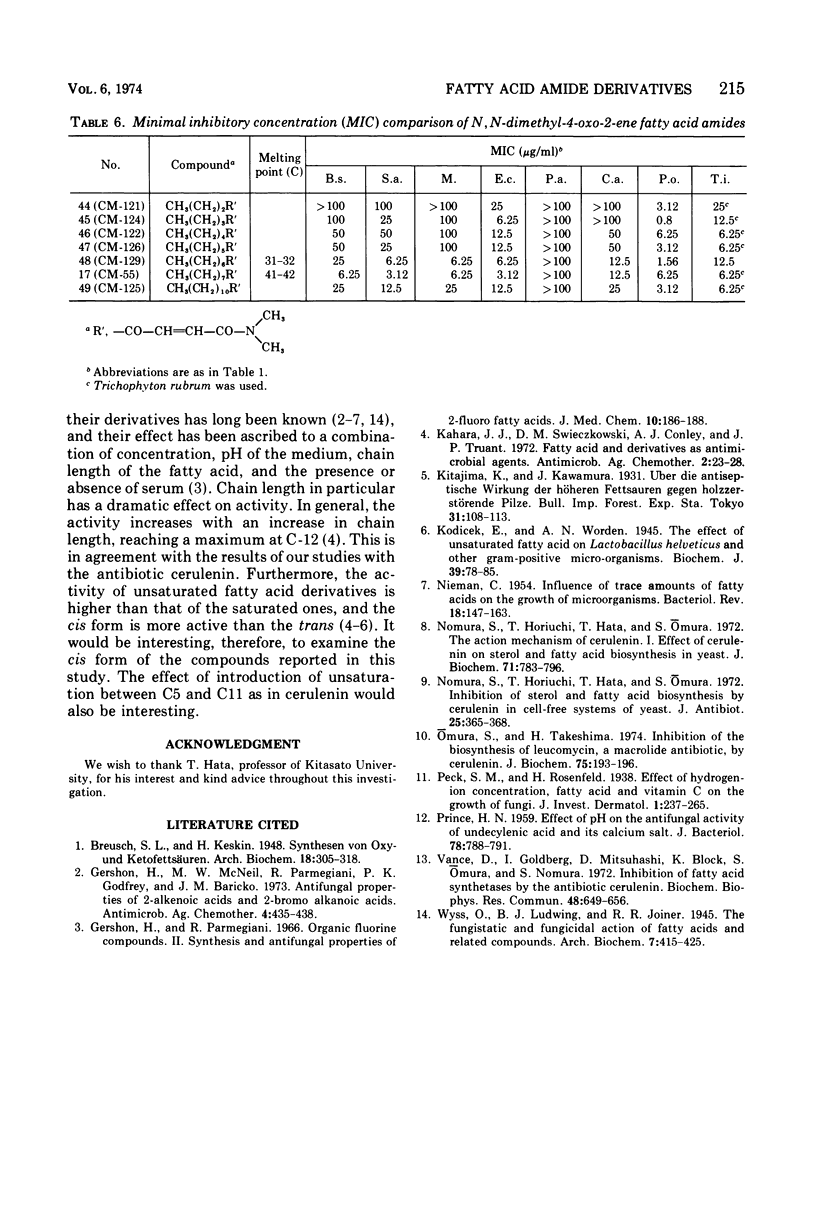
The structure-activity relationships of derivatives of the antibiotic cerulenin were investigated by chemically modifying dodecanoic acid, its skeletal back-bone. The dimethylamide derivatives were active against both gram-positive and -negative bacteria, and fungi. Among the compounds having modified groups at positions C2 and C4, the most active were those with a carbonyl group at C4 and a double bond at C2. The dimethylamide and pyrrolidine amide derivatives of this structure type were the most active. Activity against bacteria and yeast increased with the number of carbon atoms in the skeleton, with the maximum activity being observed at C=12. No significant differences in activity against fungi were observed with change in chain length.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gershon H., McNeil M. W., Parmegiani R., Godfrey P. K., Baricko J. M. Antifungal properties of 2-alkenoic acids and 2-bromo alkanoic acids. Antimicrob Agents Chemother. 1973 Oct;4(4):435–438. doi: 10.1128/aac.4.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon H., Parmegiani R. Organic fluorine compounds. II. Synthesis and antifungal properties of 2-fluoro fatty acids. J Med Chem. 1967 Mar;10(2):186–188. doi: 10.1021/jm00314a013. [DOI] [PubMed] [Google Scholar]
- Kabara J. J., Swieczkowski D. M., Conley A. J., Truant J. P. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972 Jul;2(1):23–28. doi: 10.1128/aac.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodicek E. The effect of unsaturated fatty acids on Lactobacillus helveticus and other Gram-positive micro-organisms. Biochem J. 1945;39(1):78–85. doi: 10.1042/bj0390078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEMAN C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev. 1954 Jun;18(2):147–163. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S., Horiuchi T., Hata T., Omura S. Inhibition of sterol and fatty acid biosyntheses by cerulenin in cell-free systems of yeast. J Antibiot (Tokyo) 1972 Jun;25(6):365–368. doi: 10.7164/antibiotics.25.365. [DOI] [PubMed] [Google Scholar]
- Nomura S., Horiuchi T., Omura S., Hata T. The action mechanism of cerulenin. I. Effect of cerulenin on sterol and fatty acid biosynthesis in yeast. J Biochem. 1972 May;71(5):783–796. doi: 10.1093/oxfordjournals.jbchem.a129827. [DOI] [PubMed] [Google Scholar]
- Omura S., Takeshima H. Inhibition of the biosynthesis of leucomycin, a macrolide antibiotic, by cerulenin. J Biochem. 1974 Jan;75(1):193–195. doi: 10.1093/oxfordjournals.jbchem.a130375. [DOI] [PubMed] [Google Scholar]
- PRINCE H. N. Effect of pH on the antifungal activity of undecylenic acid and its calcium salt. J Bacteriol. 1959 Dec;78:788–791. doi: 10.1128/jb.78.6.788-791.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D., Goldberg I., Mitsuhashi O., Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972 Aug 7;48(3):649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]



