Abstract
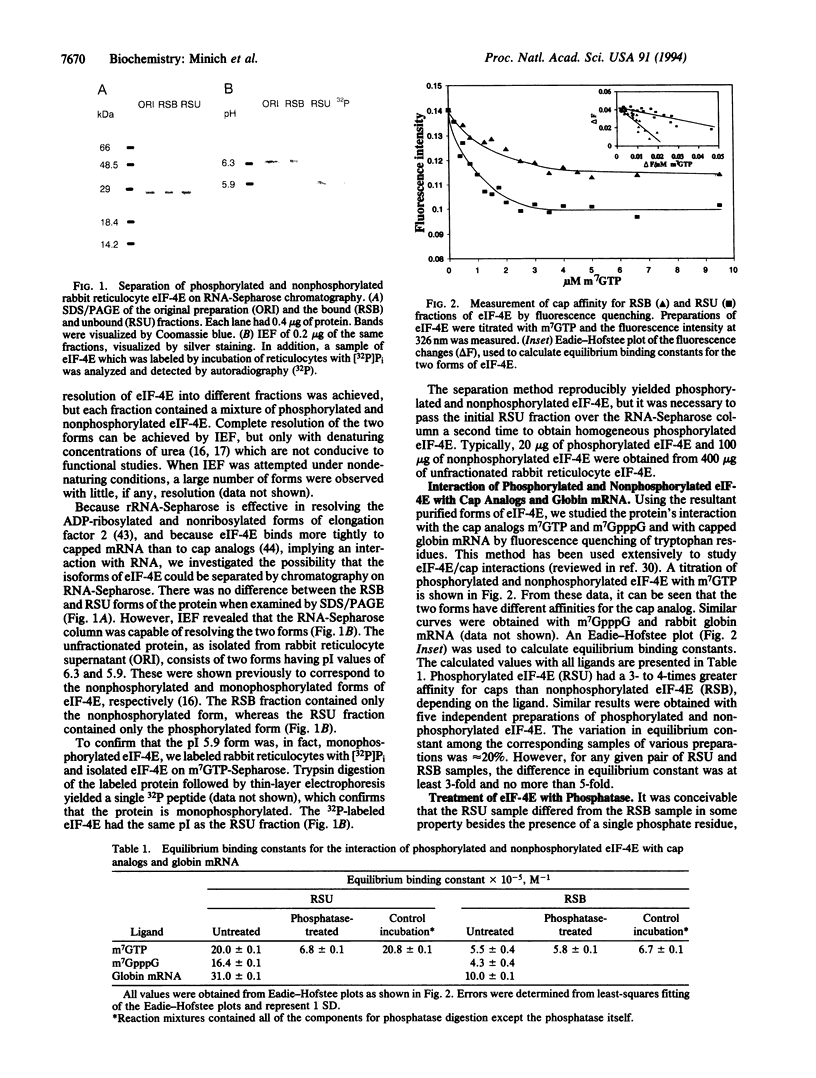
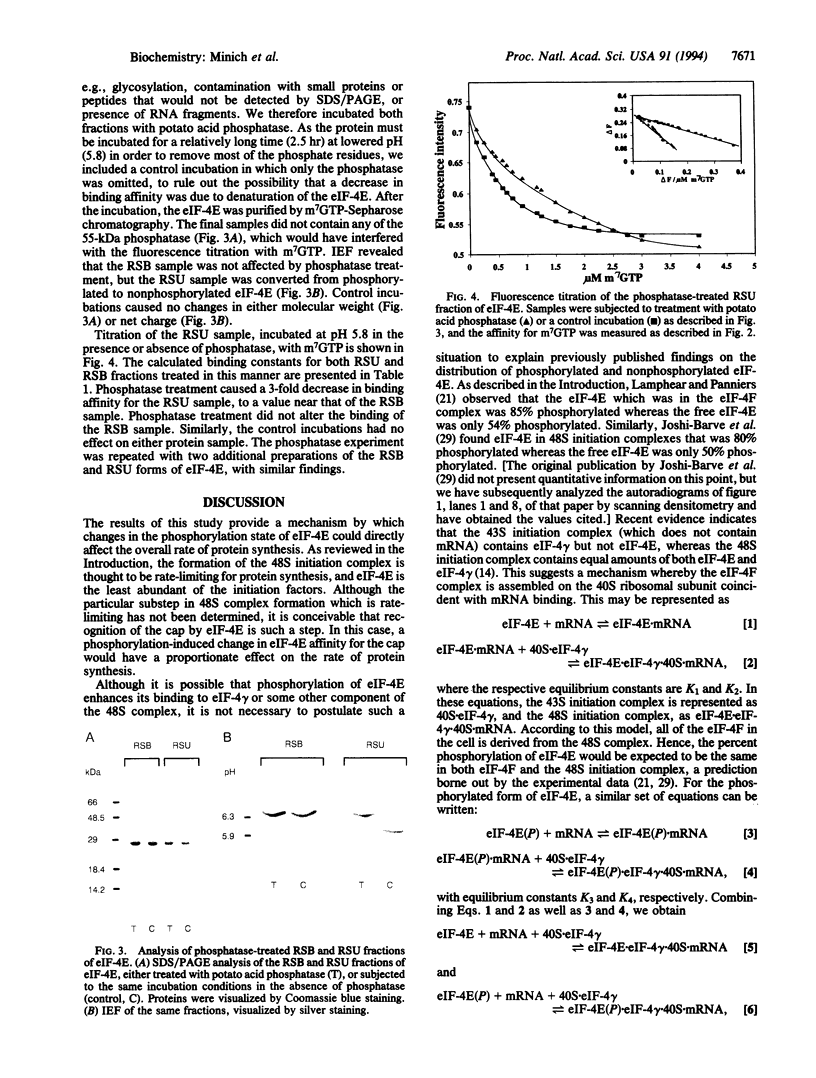
Eukaryotic translation initiation factor eIF-4E plays a central role in the recognition of the 7-methylguanosine-containing cap structure of mRNA and the formation of initiation complexes during protein synthesis. eIF-4E exists in both phosphorylated and nonphosphorylated forms, and the primary site of phosphorylation has been identified. Previous studies have suggested that eIF-4E phosphorylation facilitates its participation in protein synthesis. However, the biochemical basis for the functional difference between the two forms of eIF-4E is unknown. To address this directly, we have developed a method for the separation of phosphorylated and nonphosphorylated eIF-4E from rabbit reticulocytes by chromatography on rRNA-Sepharose. Using the resultant purified forms, we have studied the protein's interaction with the cap analogs m7GTP and m7GpppG and with the cap of globin mRNA by fluorescence quenching of tryptophan residues. It was found that phosphorylated eIF-4E had 3- to 4-fold greater affinity for cap analogs and mRNA than nonphosphorylated eIF-4E. The equilibrium binding constants (x 10(5), expressed as M-1) for the interaction of phosphorylated eIF-4E with m7GTP, m7GpppG, and globin mRNA were 20.0 +/- 0.1, 16.4 +/- 0.1, and 31.0 +/- 0.1, respectively, whereas those for the nonphosphorylated form were 5.5 +/- 0.4, 4.3 +/- 0.4, and 10.0 +/- 0.1, respectively. Treatment with potato acid phosphatase converted the phosphorylated form to the nonphosphorylated form and decreased the binding constant for m7GTP by a factor of 3. The increased affinity for mRNA caps may account for the in vivo and in vitro correlations between eIF-4E phosphorylation and accelerated protein synthesis and cell growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Herbert E., Godchaux W. Factors affecting the rate of protein synthesis in lysate systems from reticulocytes. Arch Biochem Biophys. 1968 May;125(2):671–683. doi: 10.1016/0003-9861(68)90625-5. [DOI] [PubMed] [Google Scholar]
- Amick G. D., Damuni Z. Protamine kinase phosphorylates eukaryotic protein synthesis initiation factor 4E. Biochem Biophys Res Commun. 1992 Mar 16;183(2):431–437. doi: 10.1016/0006-291x(92)90499-b. [DOI] [PubMed] [Google Scholar]
- Bonneau A. M., Sonenberg N. Involvement of the 24-kDa cap-binding protein in regulation of protein synthesis in mitosis. J Biol Chem. 1987 Aug 15;262(23):11134–11139. [PubMed] [Google Scholar]
- Browning K. S., Lax S. R., Ravel J. M. Identification of two messenger RNA cap binding proteins in wheat germ. Evidence that the 28-kDa subunit of eIF-4B and the 26-kDa subunit of eIF-4F are antigenically distinct polypeptides. J Biol Chem. 1987 Aug 15;262(23):11228–11232. [PubMed] [Google Scholar]
- Buckley B., Ehrenfeld E. The cap-binding protein complex in uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1987 Oct 5;262(28):13599–13606. [PubMed] [Google Scholar]
- Carberry S. E., Darzynkiewicz E., Stepinski J., Tahara S. M., Rhoads R. E., Goss D. J. A spectroscopic study of the binding of N-7-substituted cap analogues to human protein synthesis initiation factor 4E. Biochemistry. 1990 Apr 3;29(13):3337–3341. doi: 10.1021/bi00465a027. [DOI] [PubMed] [Google Scholar]
- Carberry S. E., Rhoads R. E., Goss D. J. A spectroscopic study of the binding of m7GTP and m7GpppG to human protein synthesis initiation factor 4E. Biochemistry. 1989 Oct 3;28(20):8078–8083. doi: 10.1021/bi00446a017. [DOI] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Rhoads R. E. Overexpression of eukaryotic protein synthesis initiation factor 4E in HeLa cells results in aberrant growth and morphology. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8212–8216. doi: 10.1073/pnas.87.21.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Milburn S. C., Hershey J. W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987 Jan 5;262(1):380–388. [PubMed] [Google Scholar]
- Edery I., Hümbelin M., Darveau A., Lee K. A., Milburn S., Hershey J. W., Trachsel H., Sonenberg N. Involvement of eukaryotic initiation factor 4A in the cap recognition process. J Biol Chem. 1983 Sep 25;258(18):11398–11403. [PubMed] [Google Scholar]
- Etchison D., Milburn S. Separation of protein synthesis initiation factor eIF4A from a p220-associated cap binding complex activity. Mol Cell Biochem. 1987 Jul;76(1):15–25. doi: 10.1007/BF00219394. [DOI] [PubMed] [Google Scholar]
- Goss D. J., Carberry S. E., Dever T. E., Merrick W. C., Rhoads R. E. Fluorescence study of the binding of m7GpppG and rabbit globin mRNA to protein synthesis initiation factors 4A, 4E, and 4F. Biochemistry. 1990 May 29;29(21):5008–5012. doi: 10.1021/bi00473a002. [DOI] [PubMed] [Google Scholar]
- Goyer C., Altmann M., Trachsel H., Sonenberg N. Identification and characterization of cap-binding proteins from yeast. J Biol Chem. 1989 May 5;264(13):7603–7610. [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Morgan M. A., Shatkin A. J., Merrick W. C. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983 May 10;258(9):5804–5810. [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Hiremath L. S., Hiremath S. T., Rychlik W., Joshi S., Domier L. L., Rhoads R. E. In vitro synthesis, phosphorylation, and localization on 48 S initiation complexes of human protein synthesis initiation factor 4E. J Biol Chem. 1989 Jan 15;264(2):1132–1138. [PubMed] [Google Scholar]
- Jagus R., Huang W., Hiremath L. S., Stern B. D., Rhoads R. E. Mechanism of action of developmentally regulated sea urchin inhibitor of eIF-4. Dev Genet. 1993;14(6):412–423. doi: 10.1002/dvg.1020140603. [DOI] [PubMed] [Google Scholar]
- Joshi-Barve S., Rychlik W., Rhoads R. E. Alteration of the major phosphorylation site of eukaryotic protein synthesis initiation factor 4E prevents its association with the 48 S initiation complex. J Biol Chem. 1990 Feb 15;265(5):2979–2983. [PubMed] [Google Scholar]
- Joshi B., Yan R., Rhoads R. E. In vitro synthesis of human protein synthesis initiation factor 4 gamma and its localization on 43 and 48 S initiation complexes. J Biol Chem. 1994 Jan 21;269(3):2048–2055. [PubMed] [Google Scholar]
- Kaufman R. J., Murtha-Riel P., Pittman D. D., Davies M. V. Characterization of wild-type and Ser53 mutant eukaryotic initiation factor 4E overexpression in mammalian cells. J Biol Chem. 1993 Jun 5;268(16):11902–11909. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamphear B. J., Panniers R. Cap binding protein complex that restores protein synthesis in heat-shocked Ehrlich cell lysates contains highly phosphorylated eIF-4E. J Biol Chem. 1990 Apr 5;265(10):5333–5336. [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Montine K. S., Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990 Jun 7;345(6275):544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Marcus A. Tobacco mosaic virus ribonucleic acid-dependent amino acid incorporation in a wheat embryo system in vitro. Analysis of the rate-limiting reaction. J Biol Chem. 1970 Mar 10;245(5):955–961. [PubMed] [Google Scholar]
- McMullin E. L., Haas D. W., Abramson R. D., Thach R. E., Merrick W. C., Hagedorn C. H. Identification of a protein kinase activity in rabbit reticulocytes that phosphorylates the mRNA cap binding protein. Biochem Biophys Res Commun. 1988 May 31;153(1):340–346. doi: 10.1016/s0006-291x(88)81228-2. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992 Jun;56(2):291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov L. P., Avanesov A. T., Seriakova T. A., Alzhanova A. T., Radzhabov H. M. A comparison of the RNA-binding proteins with the proteins of polyribosomal messenger ribonucleoproteins in rabbit reticulocytes. Eur J Biochem. 1978 Oct 16;90(3):527–535. doi: 10.1111/j.1432-1033.1978.tb12632.x. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., Joshi-Barve S., Rinker-Schaeffer C. Mechanism of action and regulation of protein synthesis initiation factor 4E: effects on mRNA discrimination, cellular growth rate, and oncogenesis. Prog Nucleic Acid Res Mol Biol. 1993;46:183–219. doi: 10.1016/s0079-6603(08)61022-3. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E. Regulation of eukaryotic protein synthesis by initiation factors. J Biol Chem. 1993 Feb 15;268(5):3017–3020. [PubMed] [Google Scholar]
- Rinker-Schaeffer C. W., Austin V., Zimmer S., Rhoads R. E. Ras transformation of cloned rat embryo fibroblasts results in increased rates of protein synthesis and phosphorylation of eukaryotic initiation factor 4E. J Biol Chem. 1992 May 25;267(15):10659–10664. [PubMed] [Google Scholar]
- Rychlik W., Gardner P. R., Vanaman T. C., Rhoads R. E. Structural analysis of the messenger RNA cap-binding protein. Presence of phosphate, sulfhydryl, and disulfide groups. J Biol Chem. 1986 Jan 5;261(1):71–75. [PubMed] [Google Scholar]
- Rychlik W., Rush J. S., Rhoads R. E., Waechter C. J. Increased rate of phosphorylation-dephosphorylation of the translational initiation factor eIF-4E correlates with the induction of protein and glycoprotein biosynthesis in activated B lymphocytes. J Biol Chem. 1990 Nov 15;265(32):19467–19471. [PubMed] [Google Scholar]
- Rychlik W., Russ M. A., Rhoads R. E. Phosphorylation site of eukaryotic initiation factor 4E. J Biol Chem. 1987 Aug 5;262(22):10434–10437. [PubMed] [Google Scholar]
- Safer B., Kemper W., Jagus R. Identification of a 48 S preinitiation complex in reticulocyte lysate. J Biol Chem. 1978 May 25;253(10):3384–3386. [PubMed] [Google Scholar]
- Sitikov A. S., Davydova E. K., Bezlepkina T. A., Ovchinnikov L. P., Spirin A. S. Eukaryotic elongation factor 2 loses its non-specific affinity for RNA and leaves polyribosomes as a result of ADP-ribosylation. FEBS Lett. 1984 Oct 29;176(2):406–410. doi: 10.1016/0014-5793(84)81207-7. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Jaramillo M., Liu Y. L., Dever T. E., Merrick W. C., Kung H. F., Sonenberg N. Translation initiation factors induce DNA synthesis and transform NIH 3T3 cells. New Biol. 1990 Jul;2(7):648–654. [PubMed] [Google Scholar]
- Tuazon P. T., Merrick W. C., Traugh J. A. Comparative analysis of phosphorylation of translational initiation and elongation factors by seven protein kinases. J Biol Chem. 1989 Feb 15;264(5):2773–2777. [PubMed] [Google Scholar]
- Tuazon P. T., Morley S. J., Dever T. E., Merrick W. C., Rhoads R. E., Traugh J. A. Association of initiation factor eIF-4E in a cap binding protein complex (eIF-4F) is critical for and enhances phosphorylation by protein kinase C. J Biol Chem. 1990 Jun 25;265(18):10617–10621. [PubMed] [Google Scholar]
- Webb N. R., Chari R. V., DePillis G., Kozarich J. W., Rhoads R. E. Purification of the messenger RNA cap-binding protein using a new affinity medium. Biochemistry. 1984 Jan 17;23(2):177–181. doi: 10.1021/bi00297a001. [DOI] [PubMed] [Google Scholar]





