Abstract
Modern Arctic Siberia provides a wealth of resources for archaeological, geological, and paleontological research to investigate the population dynamics of faunal communities from the Pleistocene, particularly as the faunal material coming from permafrost has proven suitable for genetic studies. In order to examine the history of the Canid species in the Siberian Arctic, we carried out genetic analysis of fourteen canid remains from various sites, including the well-documented Upper Paleolithic Yana RHS and Early Holocene Zhokhov Island sites. Estimated age of samples range from as recent as 1,700 years before present (YBP) to at least 360,000 YBP for the remains of the extinct wolf, Canis cf. variabilis. In order to examine the genetic affinities of ancient Siberian canids species to the domestic dog and modern wolves, we obtained mitochondrial DNA control region sequences and compared them to published ancient and modern canid sequences. The older canid specimens illustrate affinities with pre-domestic dog/wolf lineages while others appear in the major phylogenetic clades of domestic dogs. Our results suggest a European origin of domestic dog may not be conclusive and illustrates an emerging complexity of genetic contribution of regional wolf breeds to the modern Canis gene pool.
Introduction
It is widely accepted that the domestic dog (Canis lupus familiaris) descended from the gray wolf (Canis lupus), but the process of domestication as well as geographical origin and approximate date of first domestication is still debated [1,2,3]. Genetic studies of modern dog and wolf populations have shown divergent views, from a single origin in East/South Asia [4,5] or the Near East [6] to multiple areas of domestication and/or hybridization with regional wolf breeds [6,7]. Furthermore, the possibility of admixture with other canid species has also been previously suggested [8,9]. On the other hand, recent mitochondrial genome analysis of ancient canids has suggested a European origin of domestic dogs [10]. Archaeological evidence is not always straightforward for the morphological identification of domestic dogs, especially as the earliest dogs were essentially the same size as wolves [11,12,13], but advanced morphometric analyses have improved the efforts [14,15,16]. The oldest archaeological evidence of domestic dog has been identified in western Europe and the Near East, dating to at least 14,000 cal BC [17,18]. Some have argued that domesticated dogs were present prior to the Last Glacial Maximum, but this is currently disputed [13,19,20,21,22].
Archaeological and paleontological research conducted in the Arctic Siberia within past couple of decades have yielded a large amount of bone material suitable for genetic studies, as they mostly come from permafrost deposits that are common in the area. Many ancient DNA studies have focused on extinct Pleistocene or wild species that occupied Siberia [23,24,25,26], but here we focus on the oldest domesticated species Canis. Different Canidae species, such as the arctic fox and wolf, were among the Pleistocene arctic fauna that continued into the present [27,28]. Within the region, studies have claimed the presence of dogs in the Russian Plain and Kamchatka by 13,000 cal BC [29,30]. A recent study has suggested the presence of a domestic dog in southern Siberia dated to ca. 33,300 cal BC, which predates the oldest evidence from western Europe and the Near East [22]. However, the Siberian canid remain was morphologically most similar to dogs from Greenland and unlike ancient and modern wolves and putative dogs from central Russia [22]. Sablin and Khlopachev [29] have argued that the presence of Pleistocene dog in the central Russian plain at Eliseevichi I dated to 13,000–17,000 cal BC was the result of domestication in situ from local northern wolves. Therefore, the possibility that Late Pleistocene/Early Holocene wolves may have contributed to the regional dog breed remains open.
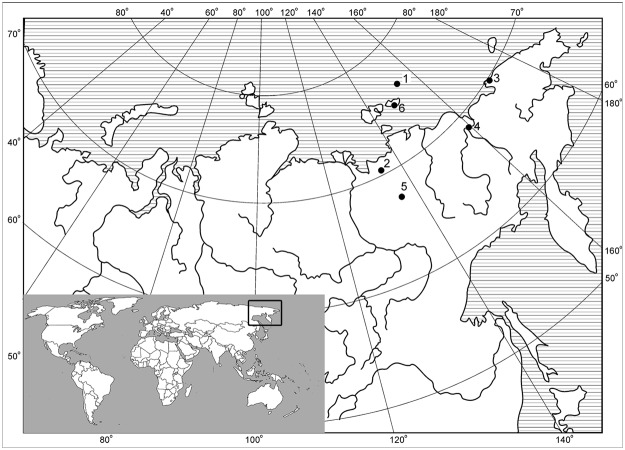
We examined thirteen prehistoric canid remains and one contemporary wolf sample from the Siberian Arctic: Ulakhan-Sullar, Duvanny Yar, Yana RHS, Zhokhov Island, and Aachim Lighthouse (Fig 1 and Table 1). The oldest specimens come from the exposures of Quaternary deposits (locality #1, Canis cf. variabilis) and from Duvanny Yar exposure (locality #2), Details of each site are provided in the next section. Mitochondrial DNA (mtDNA) analysis of the canid specimens was carried out to infer the phylogenetic relationships of these ancient canids with modern canid species, with particular focus on the Canis cf. variabilis specimen from Ulakhan-Sullar that may provide clues to the origin of domestic dogs.
Fig 1. Location of the sites studied.
Corresponding numbers and information are provided in Table 1.
Table 1. Description of the canid specimens analyzed in this study.
| ID | Location | Morphological Classification | Age (YBP) a | Nr. on the map b |
|---|---|---|---|---|
| S809 | Ulakhan-Sullar, Adycha River | Canis cf. variabilis | 360,000–400,000 | 5 |
| S503 | DuvanyYar, Lower Kolyma River | Canis lupus | >47,000 | 4 |
| S504 | DuvanyYar, Lower Kolyma River | Canis lupus | >47,000 | 4 |
| S501 | Yana RHS, Lower Yana River | Canis lupus | 28,520 ± 240 | 2 |
| S601 | Yana RHS, Lower Yana River | Canis lupus | 27,840 ± 220 | 2 |
| S805 | Yana RHS, Lower Yana River | Canis lupus | na | 2 |
| S806 | Yana RHS, Lower Yana River | Canis lupus | na | 2 |
| S502 | Aachim, East Siberian Sea Coast | Canis sp. | 1,740 ± 40 | 3 |
| S603 | Aachim, East Siberian Sea Coast | Canis sp. | 1,760 ± 40 | 3 |
| S602 | Zhokhov Island, New Siberian Islands | Canis sp. | 8,710 ± 50 | 1 |
| S902 | Zhokhov Island, New Siberian Islands | Canis sp. | na | 1 |
| S903 | Zhokhov Island, New Siberian Islands | Canis sp. | na | 1 |
| S904 | Zhokhov Island, New Siberian Islands | Canis sp. | na | 1 |
| S905 | New Siberia Islands | Canis lupus | Contemporary | 6 |
Information of the specimens analyzed in this study, including the location, morphological classification, and estimated age.
aAge of specimens are based on 14C AMS dates (see Supplemental Tables for further details).
bRefer to the map at Fig 1 for the site location.
Materials and Methods
Sample Information
Our data set consists of five groups of fossils collected in several areas of Arctic Siberia, that cover the area from Zhokhov Island to middle Yana River in latitudinal direction (from 76° 06’ N to 67° 50’ N, or a distance of almost 1000 km) and from low Yana River to Aachim Peninsula, Western Chukotka in the longitude one (from 135° 25’ E to 173° 30’ E, or a distance of 1500 km). The contemporary wolf specimen was collected as a reference sample from the New Siberian Islands [31].
Specimens analyzed in this study have been collected during field excavations (1999–2007) that were conducted under the permits issued by the government agency to Vladimir V. Pitulko, Senior Research Scientist of the Institute for the History of Material Culture, RAS (Zhokhov-2000 project leader): 1) 1999: Credential Letter №123 (form 2—survey), issued by the Field Committee on May 7, 1999, for survey and excavations in Western Chukotka, Pevek district; 2) 2000: Credential Letter №307 (form 2—survey), issued by the Field Committee on May 29, 2000, for survey in New Siberian islands and excavations in Zhokhov island; 3) 2001: Credential Letter №381 (form 1—excavations), issued by the Field Committee on June 8, 2001, for survey in New Siberian islands, excavations in Zhokhov island, and survey in Northern Yana Indighirka lowland; 4) 2002: Credential Letter №638 (form 1—excavations), issued by the Field Committee on June 28, 2002; 5) 2003: Credential Letter №525 (form 1—excavations), issued by the Field Committee on June 16, 2003, for excavations in Zhokhov island, survey in Northern Yana Indighirka lowland, and excavations in Yana site; 6) 2004: Credential Letter №87 (form 4—salvage excavations), issued by the Field Committee on April 16, 2004, for excavations in Zhokhov island and excavations in Yana site; 7) 2005: Credential Letter №506 (form 4—salvage excavations), issued by the Field Committee on June 10, 2005, for excavations in Zhokhov island and excavations in Yana site; 8) 2006: Credential Letter №308 (form 4—salvage excavations), issued by the Field Committee on May 26, 2006, for excavations in Yana site; 9) 2007: Credential Letter №99 and 100 (form 1, excavations, and form 2, survey), issued by the Field Committee on April 13, 2007, excavations in Yana site and survey in Northern Yana Indighirka lowland. All information can be verified through the Field Committee, Institute of Archaeology, Russian Academy of Sciences, at opiiaran@yandex.ru.
Location and Site Information
Ulakhan-Sullar
The mandible of Canis cf. variabilis (S809) was obtained from Layer 2 dated to 360,000 ± 17,000 years ago based on ESR measurements of bivalve shells found in the same layer [32]. The site is a 65 m-high and 1.2 km-long bluff located at the right bank of low Adycha River (Yana River basin) at 67°41’N, 135°44’E. The species Canis cf. variabilis was characterized by Teilhard de Chardin and Pei from specimens of small Middle Pleistocene wolves in Asia [33]. Often grouped together with Canis mosbachensis in Europe referred to as the C. mosbachensis-variabilis group, the species shows morphological features including a relatively small body size, elongated and slender snout, and a relatively long metastyle P4.
Duvanny Yar
Duvanny Yar is a key section of Upper Pleistocene deposits of Western Beringia, situated on the right bank of the lower Kolyma River in Northeastern Yakutia (68°38’N; 159°03’E) [34,35]. Four stratigraphic units have been characterized at the site, especially Unit III dated to between 45,000 and 13,500 14C years before present (YBP) and has revealed numerous remains of mammals and plant remains [36,37,38,39]. Two canid remains were recovered from the lowermost portion of Unit III, for which radiocarbon dates suggest a date of at least 47,000 14C YBP (S1 Table).
Yana RHS
Four specimens of Canis lupus were obtained from the site dated to 28,500–27,000 14C YBP [40,41,42]. Located in the low Yana River, the Yana RHS site has yielded rich layers of cultural artifacts and faunal remains, which have been described in detail in previous studies [41,42,43]. Three of four samples have individual 14C AMS date (S2 Table), and all together they have a firm age estimation of around 28,000 radiocarbon years BP. Excavations of Yana RHS yielded a number of possible wolf remains, with heavily worn teeth.
Zhokhov
The Zhokhov site refers to the Zhokhov Island situated beneath 76°N latitude and belongs to the New Siberian island chain, which constitutes the natural boundary between the Laptev and the East Siberian Seas. Dated to 7,800–8,000 YBP, this is one of the northern most archaeological sites in the Arctic that harbors abundant artifacts and faunal remains, including numerous microprismatic cores and microblades, pieces of hunting equipment, and wooden artifacts [44,45,46]. Canid remains were found among a large artifact assemblage that included faunal remains of polar bear and reindeer, approximating 150 identifiable bones of at least nine animals. Four canid specimens (S602, S902, S904, S903) were included for this study (S3 Table).
Aachim Lighthouse
The site is located in the northern part of Aachim Peninsula on the East Siberian Sea coast [47]. Two canid mandibles from this site (S4 Table) are dated to 1,760 ± 40 BP (Beta-231449) and 1,740 ± 40 (Beta-231444), and based on the potential marine reservoir effect the appropriate age accepted would be around 1,700 YBP or a little younger [46]. Despite the age, these specimens predate recent contact, therefore would represent indigenous canid breeds. Apart from the two canid remains recovered that we analyze in this study, the site includes numerous sea mammal remains as well as stone flake and artifacts [47].
Sample Preparation
All remains except for specimens collected at Aachim remained in permafrost conditions shortly after the deposition event until excavation. Specimens were selected to ensure separate animals (skulls or skull fragments) in order to avoid sampling duplication. Teeth were obtained from cranial and mandibular remains. Remains for a reference material was obtained from a wolf that died of natural causes in New Siberia Island in 2003.
Experimental Methods
All experimental procedures for DNA analysis were carried out in a dedicated sterile facility for ancient DNA research, which include separate rooms dedicated to drilling, extracting, and preparing samples for PCR. The facility is equipped with high efficiency particulate (HEPA) filtered air, has UV lights over all surfaces, and airflow between the rooms is limited by magnetic interlocks on the doors and by maintaining a serial positive pressure. General practices include the use of disposable garments and gloves, as well as sterilizing exposed surfaces with bleach before each procedure.
Claws and teeth were obtained for DNA extraction. First, specimens were decontaminated with bleach before drilling to produce fine bone powder ranging from 0.45g to 5.45g. Samples were drilled and extracted in groups of five, plus one negative control. Detailed extraction procedures have previously been described [48]. Briefly, samples were first decalcification in EDTA, followed by incubation with proteinase K, then purified using a modified silica-based spin-column protocol followed by centrifugation.
PCR was carried out for the mitochondrial DNA control region using three overlapping primers spanning nucleotide positions (np) 15424 to 15837 (413 basepairs). We used two different primers for the first segment between np 15424 and 15580 as noted in S5 Table. Amplification was carried out multiple times to verify the authenticity. Multiple extracts were used when available and consistent sequences from multiple amplifications were determined reliable. Successfully amplified PCR products were verified by agarose gel electrophoresis and amplicons were purified on a 96-well Multiscreen HTS plate (Millipore, Billerica, MA) to remove single strand DNA and primers. Amplicons were prepared for sequencing using the BigDye Terminator v.3.1 (Applied Biosystems, Foster City, CA). After standard alcohol precipitation, samples were directly sequenced on an ABI PRISM 377XL DNA Sequencer (Applied Biosystems, Foster City, CA).
Sequences were compiled for ancient DNA canid data as well as a representative spectrum of modern dog and wolf samples from the literature and GenBank [4,10,49,50,51,52,53,54,55,56,57,58,59,60,61]. Sequences were aligned using ClustalX [62] and MEGA 5.2.2 [63] and we incorporated our data in three separate median-joining networks that comprised of: 1) ancient DNA canid sequences (N = 33), 2) modern dog sequences (N = 290), and 3) modern wolf sequences (N = 101). Median-joining networks were calculated in Network 4.6 [64].
Results and Discussion
We successfully obtained endogenous mtDNA control region sequences for all fourteen canid samples and identified nine haplotypes. The two Aachim Lighthouse specimens shared one haplotype and the four remains from Zhokhov Island shared another haplotype. Among the four Yana RHS canid specimens, two shared the same haplotype. Only the Aachim and Zhokhov haplotypes matched other published canid sequences while sequences from Yana RHS, Ulakhan-Sullar, and Duvanny Yar were unique. Sequences can be found under GenBank accession numbers KJ909851-KJ909864.
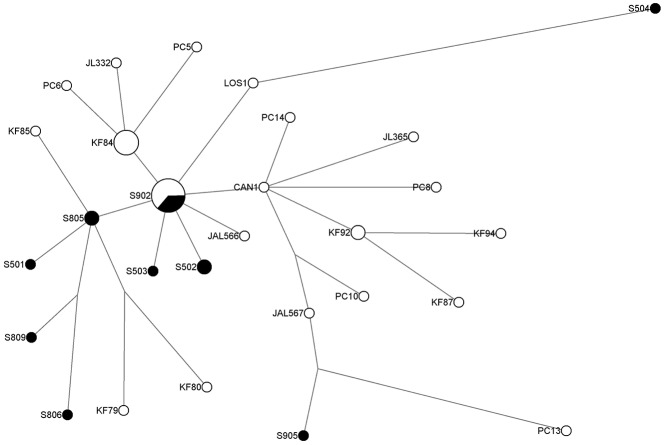
Direct analysis of whole mitogenomes from ancient canid specimens have recently suggested a European origin of domestic dogs based on the phylogenetic arrangement of three canids from Belgium dated to 26,000–36,000 YBP [10], which is in contrast to an Asian or Near East origin based on modern specimens [5,6]. Based on mtDNA control region sequences, the canid specimens from Yana RHS and Ulakhan-Sullar from our study appear as divergent as the ancient European specimens that were used as evidence of a European origin for domestic dogs. Therefore in light of our results, a European origin of domestic dogs may not be conclusive. The median joining network for ancient canid sequences (Fig 2) shows a Yana haplotype (S805) that is one step away from a Zhokhov haplotype (S902) that represents one of the main phylogenetic clades among canids (Clade A). Several ancient canid haplotypes are oriented around the Yana S805 including some of the oldest canid haplotypes reported to date, including the Ulakhan-Sullar specimen (Canis cf. variabilis, S809) from our study, in addition to ancient canid haplotypes from Belgium dated to 30,000 YBP and 36,000 YBP and a canid haplotype from Kostenki, Russia dated to 22,000 YBP [10]. The ancient canid specimens in this cluster may represent possible progenitors of the domestic dog, as coalescence time for the dog-wolf divergence is thought to have occurred by around 10,000 years ago [4,65] though some have suggested as early as 32,000 years ago [66]. Based on the position of the Yana S805 haplotype, it may potentially represent a direct link from the putative progenitor (including Canis cf. variabilis) to the domestic dog and modern wolf lineages.
Fig 2. Median-joining network of ancient canid specimens for the mitochondrial DNA control region.
Black circles indicate samples from our study (see Table 1 and S6 Table for sample ID). Sequences analyzed in the network span nucleotide positions 15561–15789.
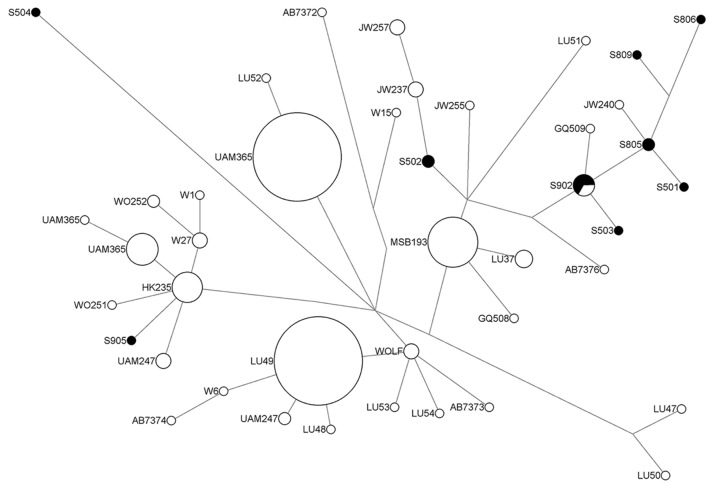
Overall, most of the Siberian canids from our study are phylogenetically clustered with other wolves from Asia and Russia (Fig 3). One branch from the Yana haplotype (S805) consists of a modern wolf haplotype. Interestingly, this haplotype is from a now-extinct Japanese wolf specimen (Canis lupus hodophilax) dated from the 14th-18th century [54]. The Japanese wolf is reported to have been fairly widely distributed in the main islands of Japan since the Jomon period (10,000–250 B.C.) until as recent as the early 1900s, but it is unclear where they originate from [54,67]. While it is largely thought that the gray wolf (Canis lupus) is directly ancestral to the domestic dog, the phylogenetic relationship between the gray wolf and Canis cf. variabilis is still a subject of debate. Canis cf. variabilis is thought to have been widespread in Eurasia until around 300,000 YBP and does not appear to overlap with the earliest occurrence of the morphologically distinctive gray wolf [68,69]. Our study is the first to report DNA results from Canis cf. variabilis and our results suggest variabilis as another possible source of genetic contribution to the domestic dog.
Fig 3. Median-joining network of wolf specimens for the mitochondrial DNA control region.
Black circles indicate samples from our study. Sequences analyzed in the network span nucleotide positions 15547–15792. See Table 1 for the sample IDs from this study. Notable haplotypes include GQ509 (GenBank Accession No. GQ376509; Ural mountains of Russia [59]), JW237, 240, 255, 257 (GenBank Accession No. AB480736-AB480742, AB500700; Canis lupus hodophilax, Japan [54]), and LU51 (GenBank Accession No. AY812735; New Mexico, USA [57]).
One interesting feature in the phylogenetic group of ancient canids surrounding the Yana haplotype (S805) is a Duvanny Yar canid (S504), which is dated to more than 47,000 14C YBP but is separated from the other ancient canid haplotypes and appears to be connected to domestic dogs but not wolves. In both ancient canid and domestic dog median-joining networks (Figs 2 and 4), S504 is connected by dog haplotypes that are a couple of mutations away from Clade A but the wolf network shows S504 genetically divergent from any other haplotypes (Fig 3). This may suggest that the Duvanny Yar canid lineage has a distinct phylogeny from wolves but yet possibly genetically ancestral to domestic dogs. While it is possible that the Duvanny Yar lineage simply did not play a major role in the domestication of modern dog lineages, it may represent a localized genetic source for modern dog lineages.
Fig 4. Median-joining network of dog specimens for the mitochondrial DNA control region.
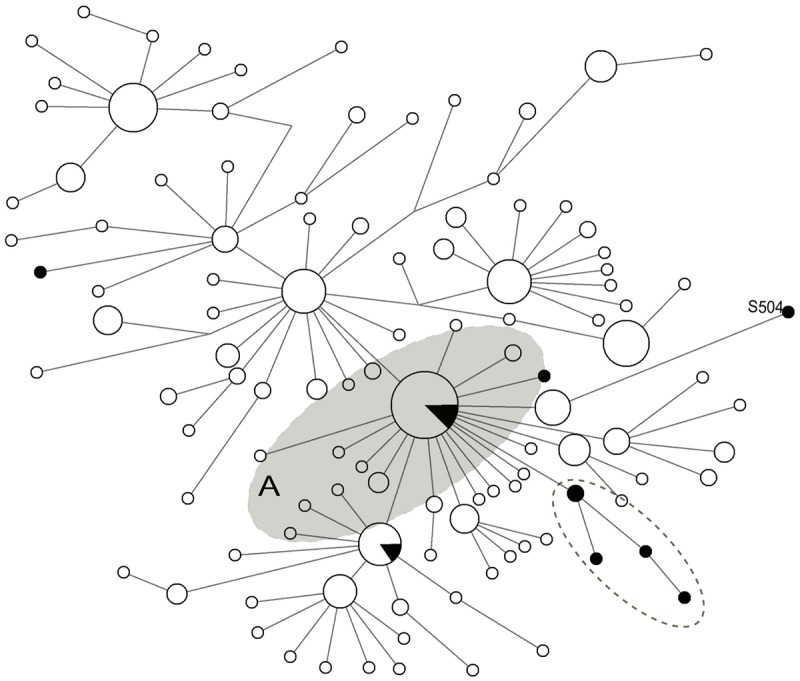
Black circles indicate samples from our study. Sequences analyzed in the network span nucleotide positions 15547–15705. Clade A is highlighted in light gray and the Duvanny Yar sample S504 is noted in the network. The bottom-right cluster highlighted with a dashed line circle includes the samples S805, S806, S809, and S501.
The phylogenetic network with domestic dog sequences show the cluster oriented around the Yana haplotype (S805) is retained from the wolf network (Fig 4). Both the Zhokhov and Aachim haplotypes situated in Clade A are shared with multiple domestic dogs from a wide geographical distribution [4,53,55]. This major phylogenetic group known as Clade A, along with Clades B, C and D, have been characterized from previously canid studies that includes the majority of domestic dog lineages [53,70]. Both Zhokhov and Aachim haplotypes appear to be genetically indistinguishable from domestic dogs, which may suggest that domestic dogs were in Arctic Siberia by at least 8,000 14C YBP. On the other hand, the Zhokhov and Aachim canids show genetic affinity with Asiatic wolves from Fig 3. In particular, C. lupus chanco and C. lupus hodophilax have either shared haplotypes or are separated by one mutation from the Siberian canids, including one Yana canid. While a recent genome-wide analysis of Chinese indigenous dogs showed a closer genetic affinity to domestic dogs [66], another explanation is that the Siberian canids retained a genetic signature from admixture with local breeds through geographical isolation, which has been suggested in other ancient dogs breeds [71].
Conclusions
Overall, our data suggests a genetic contribution from regional sources of wolves, including possibly Canis cf. variabilis, to the modern dog lineage and highlights the importance of further exploring the genetic contribution of regional wolf breeds to the domestic dog gene pool. Furthermore, our results are consistent with recent studies suggesting that that dog domestication occurred through a complex process of admixture among diverse breeds integrated with isolation of regional breeds throughout history. Future studies may further illuminate the phylogenetic relationship between the different canid species.
Supporting Information
The specimen was obtained from Layer 2 at Ulakhan-Sullar and described as Canis cf. variabilis.
(TIF)
Morphologically identified as Canis lupus, the specimen was obtained from the Kolyma River downstream.
(TIF)
Information for the two specimens from Duvanny Yar including their field code, description of remains, location, and details of radiocarbon dating.
(DOCX)
Information for the four specimens from Yana RHS including their field code, description of remains, location, and details of radiocarbon dating.
(DOCX)
Information for the four specimens from Zhokhov including their field code, description of remains, location, and details of radiocarbon dating.
(DOCX)
Information for the two specimens from Aachim including their field code, description of remains, location, and details of radiocarbon dating.
(DOCX)
Three primer pairs were designed for the study and one primer pair was used from a previous study [64].
(DOCX)
The ID codes in Fig 2 correspond to the indicated GenBank Accession numbers and reference.
(DOCX)
Acknowledgments
We thank Fedor Shidlovsky for his support in the Duvanny Yar investigation. We also thank Stacy McGrath, Jennifer Luedtke Kennedy, Elena Kouneski Rapu, Lisa Mandarino, and Julia Frecessa for their help with the molecular procedures.
Data Availability
All sequence files are available from GenBank (accession numbers KJ909851-KJ909864).
Funding Statement
This experimental procedures and excavations for Zhokhov, Yana RHS, and New Siberian Islands were supported by the Rock Foundation. The Ford Motor Company aided in the research of the Aachim Lighthouse site. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Clutton-Brock J (1995) Origin of the dog: domestication and early history In: Serpell J, editor. The Domestic Dog: Its evolution, behaviour and interaction with people. Cambridge: Cambridge University Press; pp. 7–20. [Google Scholar]
- 2. Crockford SJ (2000) A commentary on dog evolution: regional variation, breed development and hybridisation with wolves In: Crockford SJ, editor. Dogs through Time: an archaeological perspective. Oxford: Archaeopress; pp. 295–312. [Google Scholar]
- 3. Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, et al. (2005) Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438: 803–819. [DOI] [PubMed] [Google Scholar]
- 4. Pang JF, Kluetsch C, Zou XJ, Zhang AB, Luo LY, Angleby H, et al. (2009) mtDNA data indicate a single origin for dogs south of Yangtze River, less than 16,300 years ago, from numerous wolves. Molecular Biology and Evolution 26: 2849–2864. 10.1093/molbev/msp195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding ZL, Oskarsson M, Ardalan A, Angleby H, Dahlgren LG, Tepeli C, et al. (2012) Origins of domestic dog in southern East Asia is supported by analysis of Y-chromosome DNA. Heredity 108: 507–514. 10.1038/hdy.2011.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. vonHoldt BM, Pollinger JP, Lohmueller KE, Han E, Parker HG, Quignon P, et al. (2010) Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature 464: 898–902. 10.1038/nature08837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gray MM, Sutter NB, Ostrander EA, Wayne RK (2010) The IGF1 small dog haplotype is derived from Middle Eastern grey wolves. BMC Biol 8: 16 10.1186/1741-7007-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adams JR, Leonard JA, Waits LP (2003) Widespread occurrence of a domestic dog mitochondrial DNA haplotype in southeastern US coyotes. Mol Ecol 12: 541–546. [DOI] [PubMed] [Google Scholar]
- 9. Wayne RK, Roy MS, Gittleman JL (1998) Origin of the Red Wolf: Response to Nowak and Federoff and Gardener. Conservation Biology 12: 726–729. [Google Scholar]
- 10. Thalmann O, Shapiro B, Cui P, Schuenemann VJ, Sawyer SK, Greenfield DL, et al. (2013) Complete mitochondrial genomes of ancient canids suggest a European origin of domestic dogs. Science 342: 871–874. 10.1126/science.1243650 [DOI] [PubMed] [Google Scholar]
- 11. Musil R (1984) The first known domestication of wolves in central Europe In: Grigson C, Clutton-Brock J, editors. Animals and Archaeology. Oxford: British Archaeological Reports International Series; 227 pp. 23–25. [Google Scholar]
- 12.Nobis G (1986) Die Wildaugetiere in der Umwelt des Menschen von Oberkassel bei Bonn und das Domestikationsproblem von Wolfen in Jungpaläolithikum. Bonner Jahrbucher 186.
- 13. Germonpré M, Sablin MV, Stevens RE, Hedges REM, Hofreiter M, Stiller M, et al. (2009) Fossil dogs and wolves from Palaeolithic sites in Belgium, the Ukraine and Russia: osteometry, ancient DNA and stable isotopes. Journal of Archaeological Science 36: 473–490. [Google Scholar]
- 14. MacDonald DW, Sillero-Zubiri C, editors (2004) The Biology and Conservation of Wild Canids. Oxford: Oxford University Press. [Google Scholar]
- 15. Pionnier-Capitan M, Bemilli C, Bodu P, Célérier G, Ferrié J-G, Fosse P, et al. (2011) New evidence for Upper Palaeolithic small domestic dogs in South-Western Europe. Journal of Archaeological Science 38: 2123–2140. [Google Scholar]
- 16. Drake AG, Coquerelle M, Colombeau G (2015) 3D morphometric analysis of fossil canid skulls contradicts the suggested domestication of dogs during the late Paleolithic. Sci Rep 5: 8299 10.1038/srep08299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davis SJM, Valla FR (1978) Evidence for domestication of the dog 12,000 years ago in the Natufian of Israel. Nature 276: 608–610. [Google Scholar]
- 18. Napierala H, Uerpmann H-P (2012) A ‘new’ palaeolithic dog from central Europe. International Journal of Osteoarchaeology 22: 127–137. [Google Scholar]
- 19. Crockford SJ, Kuzmin YV (2012) Comments on Germonpré et al. , Journal of Archaeological Science 36, 2009 "Fossil dogs and wolves from Palaeolithic sites in Belgium, the Ukraine and Russia: osteometry, ancient DNA and stable isotopes", and Germonpré, Lázkičková-Galetová, and Sablin, Journal of Archaeological Science 39, 2012 "Palaeolithic dog skulls at the Gravettian Předmostí site, the Czech Republic". Journal of Archaeological Science 39: 2797–2801. [Google Scholar]
- 20. Germonpré M, Sablin MV, Després V, Hofreiter M, Lázničková-Galetová M, Stevens RE, et al. (2013) Palaeolithic dogs and the early domestication of the wolf: a reply to the comments of Crockford and Kuzmin (2012). Journal of Archaeological Science 40: 786–792. [Google Scholar]
- 21. Crockford SJ (2006) Rhythms of Life: thyroid hormone and the origin of species. Victoria, B.C.: Trafford; 272 p. [Google Scholar]
- 22. Ovodov ND, Crockford SJ, Kuzmin YV, Higham TF, Hodgins GW, van der Plicht J (2011) A 33,000-year-old incipient dog from the Altai Mountains of Siberia: evidence of the earliest domestication disrupted by the Last Glacial Maximum. PLoS ONE 6: e22821 10.1371/journal.pone.0022821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shapiro B, Drummond AJ, Rambaut A, Wilson MC, Matheus PE, Sher AV, et al. (2004) Rise and fall of the Beringian steppe bison. Science 306: 1561–1565. [DOI] [PubMed] [Google Scholar]
- 24. Barnes I, Shapiro B, Lister A, Kuznetsova T, Sher A, Guthrie D, et al. (2007) Genetic structure and extinction of the woolly mammoth, Mammuthus primigenius. Current Biology 17: 1072–1075. [DOI] [PubMed] [Google Scholar]
- 25. Barnett R, Shapiro B, Barnes I, Ho SY, Burger J, Yamaguchi N, et al. (2009) Phylogeography of lions (Panthera leo ssp.) reveals three distinct taxa and a late Pleistocene reduction in genetic diversity. Mol Ecol 18: 1668–1677. 10.1111/j.1365-294X.2009.04134.x [DOI] [PubMed] [Google Scholar]
- 26. Campos PF, Willerslev E, Sher A, Orlando L, Axelsson E, Tikhonov A, et al. (2010) Ancient DNA analyses exclude humans as the driving force behind late Pleistocene musk ox (Ovibos moschatus) population dynamics. Proc Natl Acad Sci U S A 107: 5675–5680. 10.1073/pnas.0907189107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kurtén B, Anderson E (1980) Pleistocene Mammals of North America. New York: Columbia University Press. [Google Scholar]
- 28. Carmichael LE, Krizan J, Nagy JA, Fuglei E, Dumond M, Johnson D, et al. (2007) Historical and ecological determinants of genetic structure in arctic canids. Mol Ecol 16: 3466–3483. [DOI] [PubMed] [Google Scholar]
- 29. Sablin MV, Khlopachev GA (2002) The Earliest Ice Age Dogs: Evidence from Eliseevichi 1. Current Anthropology 43: 795–799. [Google Scholar]
- 30. Dikov NN (1996) The Ushki sites, Kamchatka Peninsula In: West FH, editor. American Beginnings The Prehistory and Paleoecology of Beringia. Chicago: University of Chicago Press; pp. 244–250. [Google Scholar]
- 31. Pitulko VV, Anisimov MA, Basilyan AE, Giria EY, Nikolskiy PA, Odess DP, et al. (2003) Making a New Step: Zhokhov 2000 project, Expedition of 2001 Proceedings of the Arctic System Science Program All-Hands Workshop 2002 Fairbanks, Alaska: Arctic Research Consortium of the United States; pp. 189. [Google Scholar]
- 32. Nikolskiy PA (2010) Systematics and stratigraphic significance of Late Cenozoic elk/moose (Alcini, Cervidae, Mammalia) from Eurasia and North America. [PhD Dissertation]. Moscow: Geological Institute of the Russian Academy of Sciences; 258 p. [Google Scholar]
- 33. Teilhard de Chardin P, Pei WC (1941) The fossil mammals of locality 13 in Choukoutien. Palaeontologia Sinica 11: 1–105. [Google Scholar]
- 34. Kaplina TN, Giterman RE, Lakhtina OV, Abrashov BA, Kiselyov SV, Sher AV (1978) Duvanny Cliff—key section of the late pleistocene deposits of the Kolyma lowland. Bulletin of Quarternary Commission 48: 49–65. [Google Scholar]
- 35. Sher AV, Kaplina TN, Giterman RE, Lozhkin AV, Arkhangelov AA, Kiselyov SV, et al. (1979) Late Cenozoic of the Kolyma Lowland. XIV Pacific Science Congress, Tour Guide XI, Khabarovsk August 1979. Moscow, Academy of Sciences of the USSR: XIV Pacific Science Congress; pp. 1–116. [Google Scholar]
- 36. Giterman RE, Sher AV, Matthews JV Jr (1982) Comparison of the development of tundra-steppe environments in west and east Beringia: pollen and macrofossil evidence from key sections. In: Hopkins DM, Matthews JV, Schweger CE, Young SB, editors. Paleoecology of Beringia: Academic Press; pp. 43–73. [Google Scholar]
- 37. Zanina OG, Gubin SV, Kuzmina SA, Maximovich SV, Lopatina DA (2011) Late-Pleistocene (MIS 3–2) palaeoenvironments as recorded by sediments, palaeosols, and ground-squirrel nests at Duvanny Yar, Kolyma lowland, northeast Siberia. Quaternary Science Reviews 30: 2107–2123. [Google Scholar]
- 38. Vasil'chuk YK, Vasil'chuk AC, Rank D, Kutschera W, Kim JC (2001) Radiocarbon dating of δ18O-δD plots in late Pleistocene ice-wedges of the Duvanny Yar (lower Kolyma River, northern Yakutia). Radiocarbon 43: 541–553. [Google Scholar]
- 39. Yashina S, Gubin S, Maksimovich S, Yashina A, Gakhova E, Gilichinsky D (2012) Regeneration of whole fertile plants from 30,000-y-old fruit tissue buried in Siberian permafrost. Proc Natl Acad Sci U S A 109: 4008–4013. 10.1073/pnas.1118386109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pitulko VV, Nikolsky PA, Girya EY, Basilyan AE, Tumskoy VE, Koulakov SA, et al. (2004) The Yana RHS site: humans in the Arctic before the last glacial maximum. Science 303: 52–56. [DOI] [PubMed] [Google Scholar]
- 41. Pitulko VV, Pavlova EY (2010) Geoarchaeology and Radiocarbon Chronology of the Stone Age of the North-East Asia. St. Petersburg: Nauka; 263 p. [Google Scholar]
- 42. Pitulko VV, Pavlova EY, Kuzmina SA, Nikolskiy PA, Basilyan AE, Tumskoy VE, et al. (2007) Natural-Climatic changes in the Yana-Indigirka Lowland during the Terminal Kargino Time and Habitat of Late Pleistocene man in Northern part of East Siberia. Doklady Earth Sciences 417: 1256–1260. [Google Scholar]
- 43. Pitulko VV (2010) Late Pleistocene habitation and adaptations of the ancient man across North-East Asia In: Derevianko AB, Kudelin AB, Tishkov VA, editors. Adaptations of peoples and cultures to natural changes, social and man-caused transformations. Moscow: Russian Academy of Sciences; pp. 38–46. [Google Scholar]
- 44. Pitulko VV, Kasparov AK (1996) Ancient Arctic Hunters: Material Culture and Survival Strategy. Arctic Anthropology 33: 1–36. [Google Scholar]
- 45. Pitulko VV (2003) The Bear-Hunters of the Zhokhov Island, East Russian Arctic In: Habu J, Savelle S, Koyama S, Hongo H, editors. Hunter-gatherers of the North Pacific Rim. Osaka: National Museum of Ethnology; pp. 141–152. [Google Scholar]
- 46. Pitulko VV (2006) Notes on the radiocarbon chronology of the Holocene Stone Age of North-East Asia In: Lebedintsev AI, editor. Neolithic and Paleo-Metal of the Northern Far East. Magadan: NEISRI FEB RAS; pp. 50–66. [Google Scholar]
- 47. Pitulko VV (2001) Maritime adaptations in North-East Asia In: Lebedintsev AI, Gogoleva TY, editors. Magadan: North-East Scientific Center; pp. 64–70. [Google Scholar]
- 48. Lee EJ, Anderson LM, Dale V, Merriwether DA (2009) MtDNA origins of an enslaved labor force from the 18th century Schuyler Flatts Burial Ground in colonial Albany, NY: Africans, Native Americans, and Malagasy? Journal of Archaeological Science 36: 2805–2810. [Google Scholar]
- 49. Losey RJ, Garvie-Lok S, Leonard JA, Katzenberg MA, Germonpré M, Nomokonova T, et al. (2013) Burying dogs in ancient Cis-Baikal, Siberia: temporal trends and relationships with human diet and subsistence practices. PLoS ONE 8: e63740 10.1371/journal.pone.0063740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Druzhkova AS, Thalmann O, Trifonov VA, Leonard JA, Vorobieva NV, Ovodov ND, et al. (2013) Ancient DNA analysis affirms the canid from Altai as a primitive dog. PLoS ONE 8: e57754 10.1371/journal.pone.0057754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leonard JA, Wayne RK, Wheeler J, Valadez R, Guillen S, Vila C (2002) Ancient DNA evidence for Old World origin of New World dogs. Science 298: 1613–1616. [DOI] [PubMed] [Google Scholar]
- 52. Losey RJ, Bazaliiskii VI, Garvie-Lok S, Germonpré M, Leonard JA, Allen AL, et al. (2011) Canids as persons: Early Neolithic dog and wolf burials, Cis-Baikal, Siberia. Journal of Anthropological Archaeology 30: 174–189. [Google Scholar]
- 53. Vilà C, Savolainen P, Maldonado JE, Amorim IR, Rice JE, Honeycutt RL, et al. (1997) Multiple and ancient origins of the domestic dog. Science 276: 1687–1689. [DOI] [PubMed] [Google Scholar]
- 54. Ishiguro N, Inoshima Y, Shigehara N (2009) Mitochondrial DNA analysis of the Japanese wolf (Canis lupus hodophilax Temminck, 1839) and comparison with representative wolf and domestic dog haplotypes. Zoolog Sci 26: 765–770. 10.2108/zsj.26.765 [DOI] [PubMed] [Google Scholar]
- 55. Tsuda K, Kikkawa Y, Yonekawa H, Tanabe Y (1997) Extensive interbreeding occurred among multiple matriarchal ancestors during the domestication of dogs: evidence from inter- and intraspecies polymorphisms in the D-loop region of mitochondrial DNA between dogs and wolves. Genes Genet Syst 72: 229–238. [DOI] [PubMed] [Google Scholar]
- 56. Ishiguro N, Inoshima Y, Shigehara N, Ichikawa H, Kato M (2010) Osteological and genetic analysis of the extinct Ezo wolf (Canis lupus hattai) from Hokkaido Island, Japan. Zoolog Sci 27: 320–324. 10.2108/zsj.27.320 [DOI] [PubMed] [Google Scholar]
- 57. Leonard JA, Vila C, Wayne RK (2005) Legacy lost: genetic variability and population size of extirpated US grey wolves (Canis lupus). Mol Ecol 14: 9–17. [DOI] [PubMed] [Google Scholar]
- 58. Weckworth BV, Dawson NG, Talbot SL, Flamme MJ, Cook JA (2011) Going coastal: shared evolutionary history between coastal British Columbia and Southeast Alaska wolves (Canis lupus). PLoS ONE 6: e19582 10.1371/journal.pone.0019582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weckworth BV, Talbot SL, Cook JA (2010) Phylogeography of wolves (Canis lupus) in the Pacific Northwest. J Mammal 91: 363–375. [Google Scholar]
- 60. Verginelli F, Capelli C, Coia V, Musiani M, Falchetti M, Ottini L, et al. (2005) Mitochondrial DNA from prehistoric canids highlights relationships between dogs and South-East European wolves. Molecular Biology and Evolution 22: 2541–2551. [DOI] [PubMed] [Google Scholar]
- 61. Klutsch CF, Seppala EH, Fall T, Uhlen M, Hedhammar A, Lohi H, et al. (2010) Regional occurrence, high frequency but low diversity of mitochondrial DNA haplogroup d1 suggests a recent dog-wolf hybridization in Scandinavia. Anim Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with Clustal X. Trends Biochem Sci 23: 403–405. [DOI] [PubMed] [Google Scholar]
- 63. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bandelt HJ, Forster P, Rohl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16: 37–48. [DOI] [PubMed] [Google Scholar]
- 65. Skoglund P, Gotherstrom A, Jakobsson M (2011) Estimation of population divergence times from non-overlapping genomic sequences: examples from dogs and wolves. Molecular Biology and Evolution 28: 1505–1517. 10.1093/molbev/msq342 [DOI] [PubMed] [Google Scholar]
- 66. Wang GD, Zhai W, Yang HC, Fan RX, Cao X, Zhong L, et al. (2013) The genomics of selection in dogs and the parallel evolution between dogs and humans. Nat Commun 4: 1860 10.1038/ncomms2814 [DOI] [PubMed] [Google Scholar]
- 67. Miyao T, Nishizawa T, Suzuki S (1980) Mammalian Remains of the Earliest Jomon Period at the Rockshelter Site of Tochibara, Nagano Prefecture I. Fauna of the Mammalian Remains. Journal of the Mammalogical Society of Japan 8: 181–188. [Google Scholar]
- 68. Tedford RH, Wang X, Taylor BE (2009) Phylogenetic systematics of the North American fossil Caninae (Carnivora: Canidae) AMNH Bulletin No 325. New York, NY: American Museum of Natural History—Scientific Publications; pp. 218. [Google Scholar]
- 69. Sotnikova M, Rook L (2010) Dispersal of the Canini (Mammalia, Canidae: Caninae) across Eurasia during the Late Miocene to Early Pleistocene. Quaternary International 212: 86–97. [Google Scholar]
- 70. Savolainen P, Zhang YP, Luo J, Lundeberg J, Leitner T (2002) Genetic evidence for an East Asian origin of domestic dogs. Science 298: 1610–1613. [DOI] [PubMed] [Google Scholar]
- 71. Larson G, Karlsson EK, Perri A, Webster MT, Ho SY, Peters J, et al. (2012) Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc Natl Acad Sci U S A 109: 8878–8883. 10.1073/pnas.1203005109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The specimen was obtained from Layer 2 at Ulakhan-Sullar and described as Canis cf. variabilis.
(TIF)
Morphologically identified as Canis lupus, the specimen was obtained from the Kolyma River downstream.
(TIF)
Information for the two specimens from Duvanny Yar including their field code, description of remains, location, and details of radiocarbon dating.
(DOCX)
Information for the four specimens from Yana RHS including their field code, description of remains, location, and details of radiocarbon dating.
(DOCX)
Information for the four specimens from Zhokhov including their field code, description of remains, location, and details of radiocarbon dating.
(DOCX)
Information for the two specimens from Aachim including their field code, description of remains, location, and details of radiocarbon dating.
(DOCX)
Three primer pairs were designed for the study and one primer pair was used from a previous study [64].
(DOCX)
The ID codes in Fig 2 correspond to the indicated GenBank Accession numbers and reference.
(DOCX)
Data Availability Statement
All sequence files are available from GenBank (accession numbers KJ909851-KJ909864).