Abstract
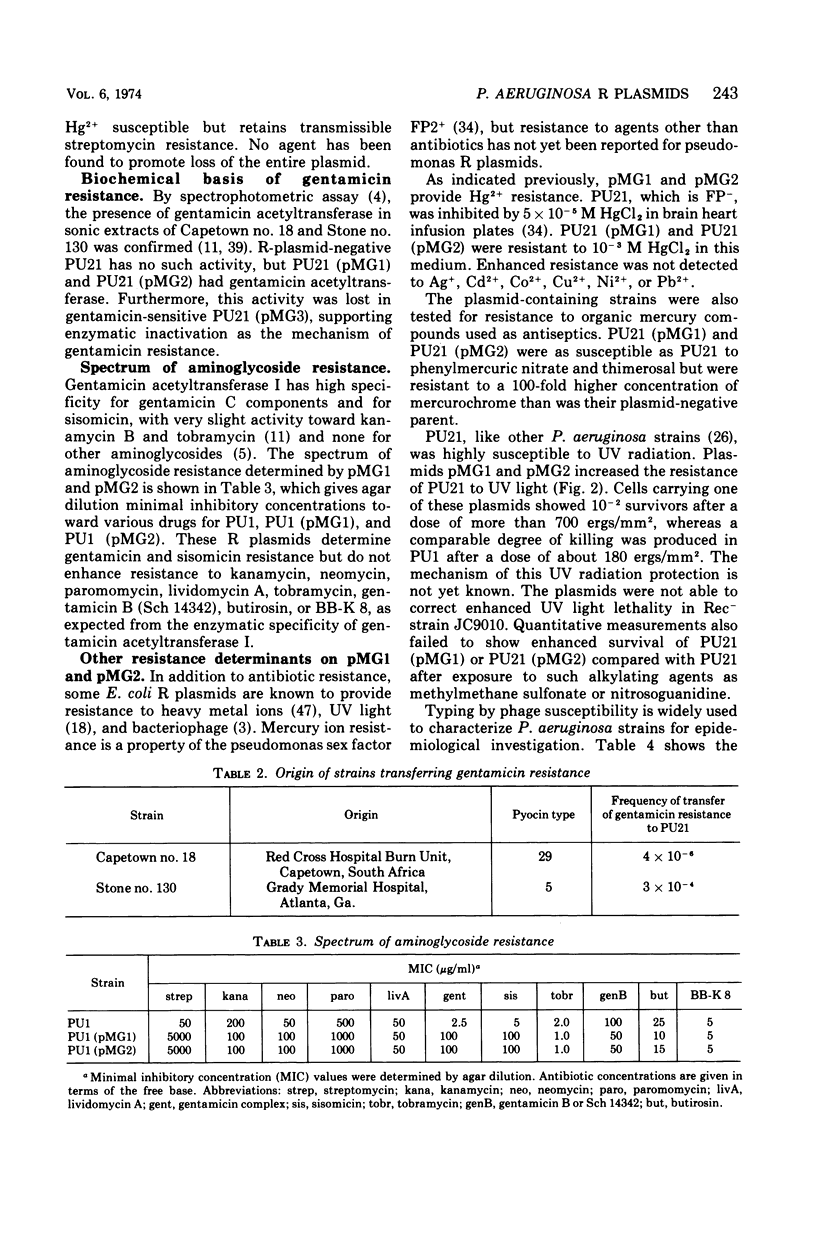
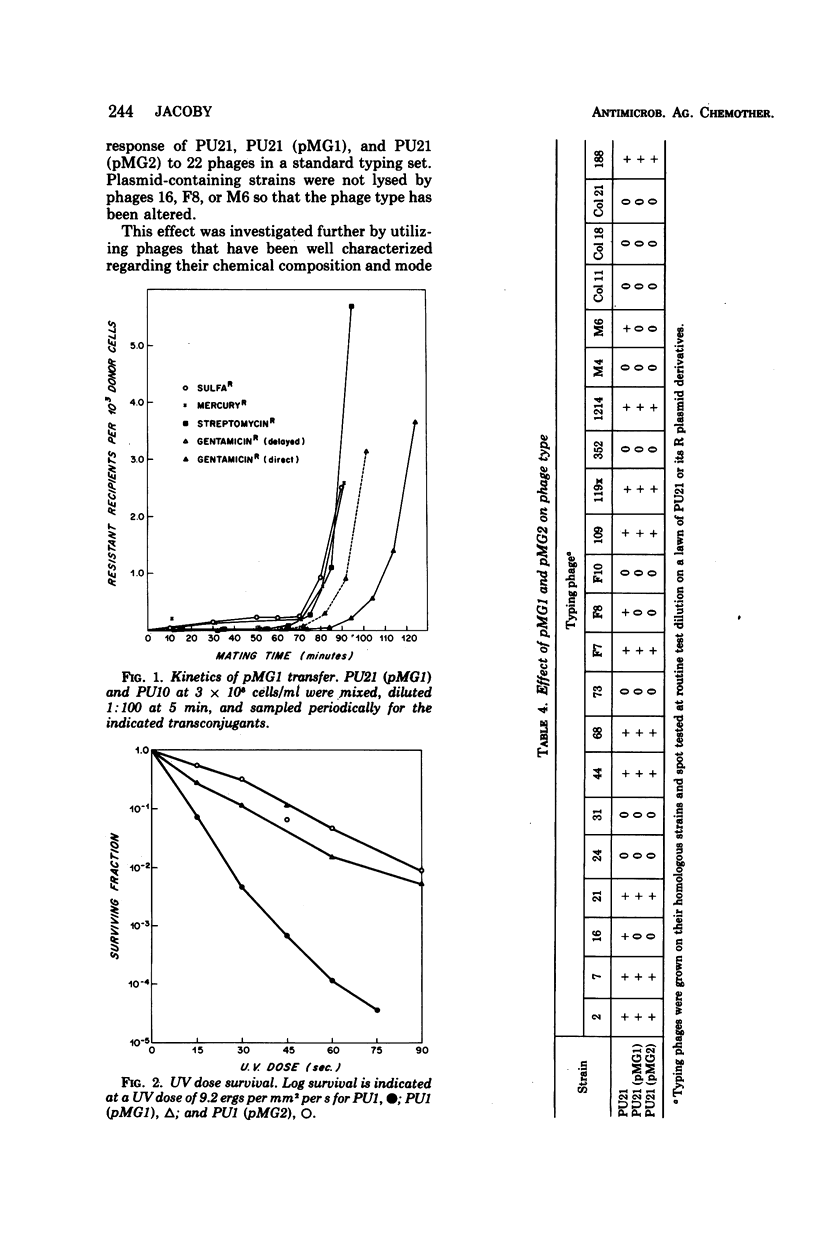
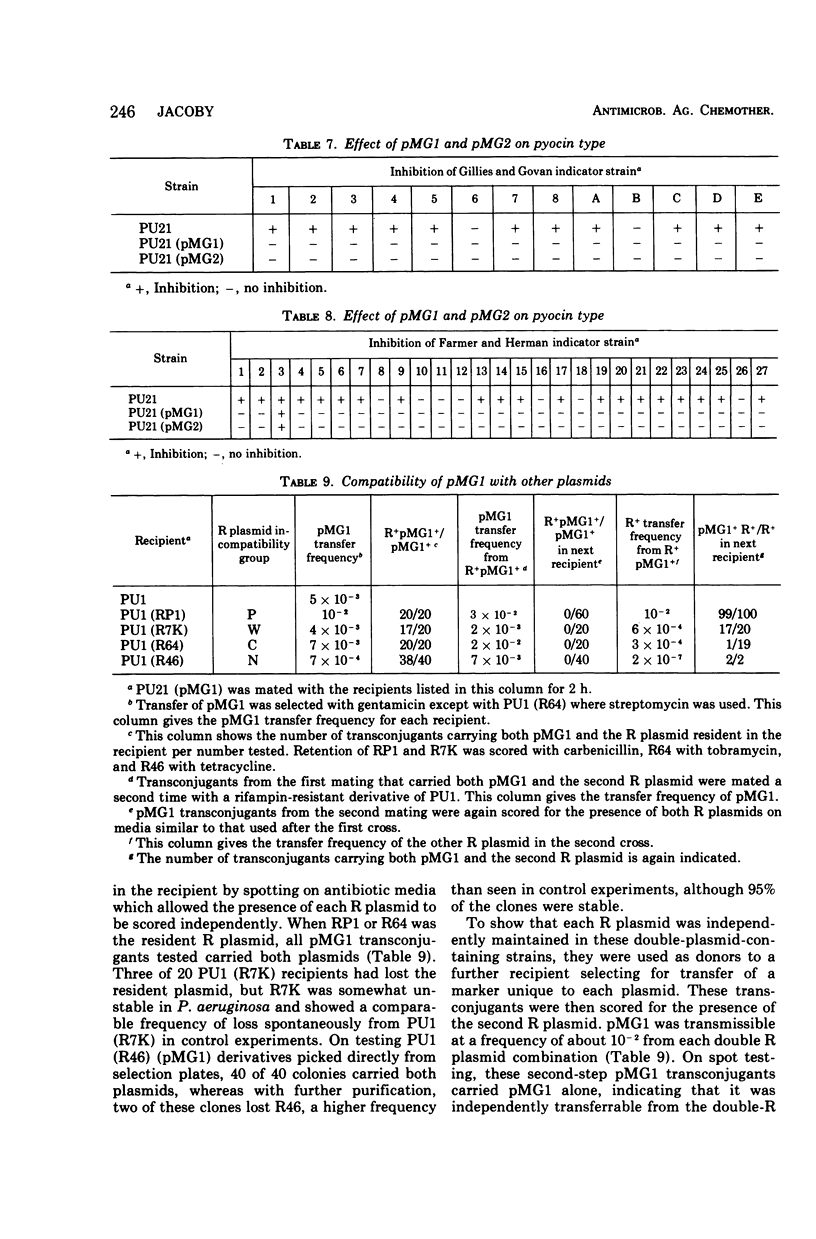
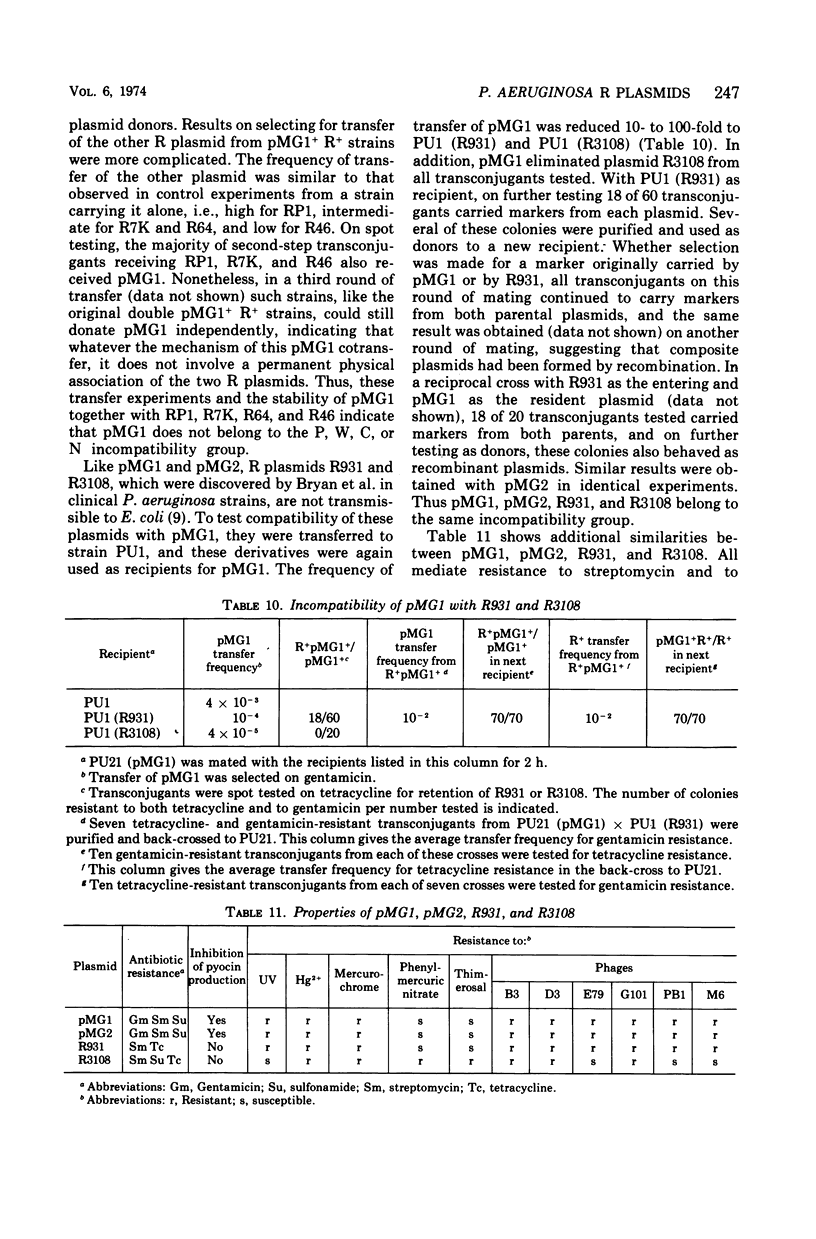
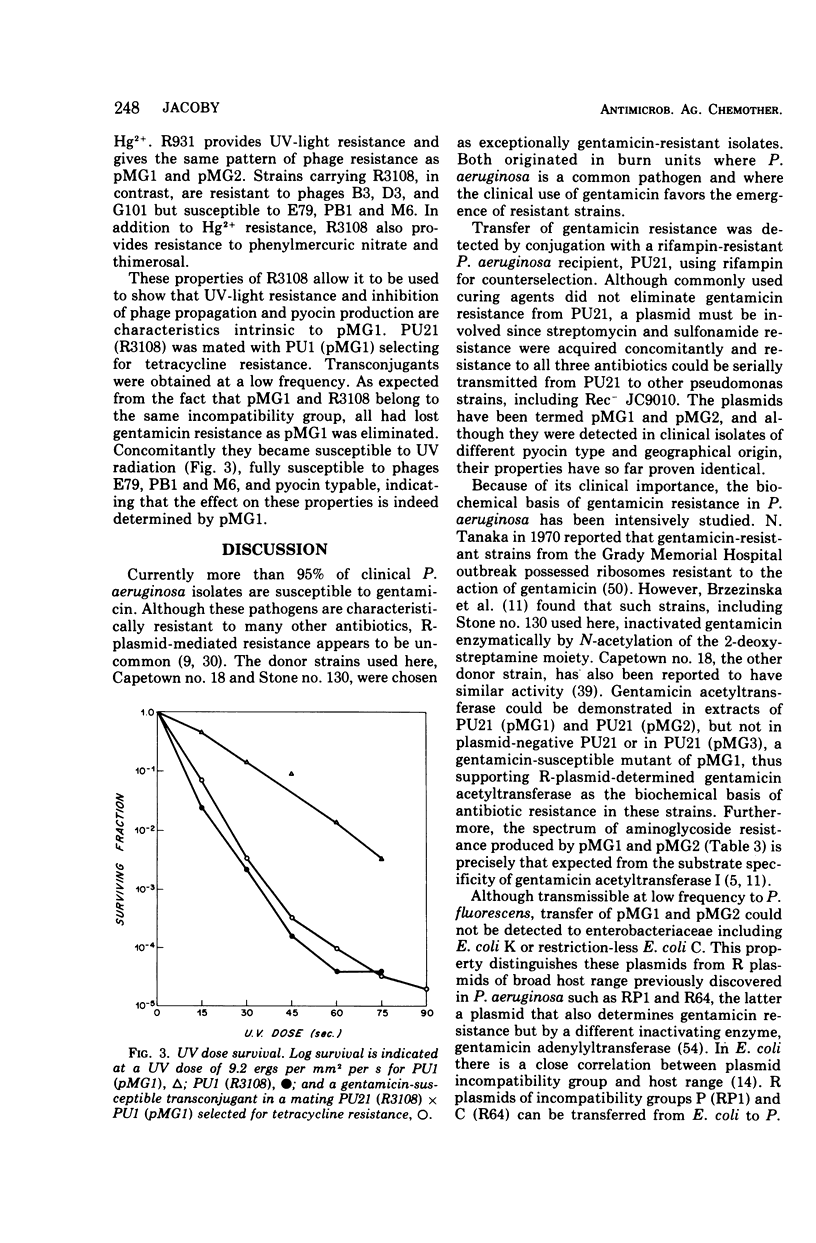
Two clinical isolates of Pseudomonas aeruginosa, one a pyocin type 5 strain from Atlanta, could transfer gentamicin resistance by conjugation. Donor and recipient strains inactivated gentamicin by acetylation. The R plasmids, pMG1 and pMG2, also determined resistance to sisomicin, another substrate of gentamicin acetyltransferase I, sulfonamides, and streptomycin, but not resistance to kanamycin, neomycin, tobramycin, butirosin, or BB-K 8. They were transmissible to many strains of P. aeruginosa, including a Rec− strain, but not to Escherichia coli or other enterobacteriaceae. These R plasmids were compatible with R plasmids transmissible to P. aeruginosa from E. coli, including members of C, N, P, and W incompatibility groups. From a strain carrying pMG1 and a compatible plasmid, pMG1 was transferred independently but transfer of the second plasmid often resulted in cotransfer of pMG1. In contrast, pMG1 and pMG2 were incompatible with pseudomonas R plasmids R931 and R3108, and with R931 they readily formed recombinant plasmids. The four plasmids in this incompatibility group determine additional biological properties, including resistance to inorganic and organic mercury compounds, to ultraviolet light, and to certain deoxyribonucleic acid phages. pMG1 and pMG2 also phenotypically inhibited pyocin production. Consequently such R plasmids alter the phage and pyocin types of their host strains.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi H., Nakano M., Inuzuka M., Tomoeda M. Specific role of sex pili in the effective eliminatory action of sodium dodecyl sulfate on sex and drug resistance factors in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1114–1124. doi: 10.1128/jb.109.3.1114-1124.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister D., Glover S. W. Restriction and modification of bacteriophages by R+ strains of Escherichia coli K12. Biochem Biophys Res Commun. 1968 Mar 27;30(6):735–738. doi: 10.1016/0006-291x(68)90575-5. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Enzymatic acetylation of aminoglycoside antibiotics by Escherichia coli carrying an R factor. Biochemistry. 1971 May 11;10(10):1787–1796. doi: 10.1021/bi00786a009. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Basic characterization of a Pseudomonas aeruginosa pilus-dependent bacteriophage with a long noncontractile tail. J Virol. 1973 Nov;12(5):1139–1148. doi: 10.1128/jvi.12.5.1139-1148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E., Robertson D. The structure and infective process of a contractile Pseudomonas aeruginosa bacteriophage. J Gen Virol. 1968 Sep;3(2):247–254. doi: 10.1099/0022-1317-3-2-247. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Semaka S. D., Van den Elzen H. M., Kinnear J. E., Whitehouse R. L. Characteristics of R931 and other Pseudomonas aeruginosa R factors. Antimicrob Agents Chemother. 1973 May;3(5):625–637. doi: 10.1128/aac.3.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinska M., Benveniste R., Davies J., Daniels P. J., Weinstein J. Gentamicin resistance in strains of Pseudomonas aeruginosa mediated by enzymatic N-acetylation of the deoxystreptamine moiety. Biochemistry. 1972 Feb 29;11(5):761–765. doi: 10.1021/bi00755a013. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Compatibility groups among fi - R factors. Nature. 1971 Nov 26;234(5326):222–223. doi: 10.1038/234222a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Host ranges of R factors. J Gen Microbiol. 1972 May;70(3):453–460. doi: 10.1099/00221287-70-3-453. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. R factors identified in Paris, some conferring gentamicin resistance, constitute a new compatibility group. Ann Inst Pasteur (Paris) 1972 Dec;123(6):849–852. [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabble W. T., Stocker B. A. R (transmissible drug-resistance) factors in Salmonella typhimurium: pattern of transduction by phage P22 and ultraviolet-protection effect. J Gen Microbiol. 1968 Aug;53(1):109–123. doi: 10.1099/00221287-53-1-109. [DOI] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Herman L. G. Epidemiological fingerprinting of Pseudomonas aeruginosa by the production of and sensitivity of pyocin and bacteriophage. Appl Microbiol. 1969 Nov;18(5):760–765. doi: 10.1128/am.18.5.760-765.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies R. R., Govan J. R. Typing of Pseudomonas pyocyanea by pyocine production. J Pathol Bacteriol. 1966 Apr;91(2):339–345. doi: 10.1002/path.1700910207. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Gillies R. R. Further studies in the pyocine typing of Pseudomonas pyocyanea. J Med Microbiol. 1969 Feb;2(1):17–25. doi: 10.1099/00222615-2-1-17. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. H., Smith D. H. Phenotypic suppression by streptomycin-adenlate and bluensomycin-adenylate. Genet Res. 1969 Dec;14(3):259–273. doi: 10.1017/s0016672300002093. [DOI] [PubMed] [Google Scholar]
- Holloway B. W. Genetics of Pseudomonas. Bacteriol Rev. 1969 Sep;33(3):419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Stanisich V. Pseudomonas genetics. Annu Rev Genet. 1971;5:425–446. doi: 10.1146/annurev.ge.05.120171.002233. [DOI] [PubMed] [Google Scholar]
- Jones L. F., Pinto B. V., Thomas E. T., Farmer J. J., 3rd Simplified method for producing pyocins from Pseudomonas aeruginosa. Appl Microbiol. 1973 Jul;26(1):120–121. doi: 10.1128/am.26.1.120-121.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Mikoshiba F., Nagasaki S., Matsumoto H., Tazaki T. Prevalence of Pseudomonas aeruginosa strains possessing R factor in a hospital. J Antibiot (Tokyo) 1972 Oct;25(10):607–609. doi: 10.7164/antibiotics.25.607. [DOI] [PubMed] [Google Scholar]
- Kung A. H., Lee B. T. The isolation and survival characterization of radiation and chemical-mutagen sensitive mutants of Pseudomonas aeruginosa. Mutat Res. 1973 Nov;20(2):175–190. doi: 10.1016/0027-5107(73)90187-5. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- Loutit J. S. Investigation of the mating system of Pseudomonas aeruginosa strain 1. V. The effect of N-methyl-N'-nitro-N-nitrosoguanidine on a donor strain. Genet Res. 1969 Oct;14(2):103–109. doi: 10.1017/s0016672300001932. [DOI] [PubMed] [Google Scholar]
- Loutit J. S. Investigation of the mating system of Pseudomonas aeruginosa strain I. VI. Mercury resistance associated with the sex factor (FP). Genet Res. 1970 Oct 2;16(2):179–184. doi: 10.1017/s0016672300002408. [DOI] [PubMed] [Google Scholar]
- Loutit J. S., Pearce L. E., Marinus M. G. Investigation of the mating system of Pseudomonas aeruginosa strain 1. I. Kinetic studies. Genet Res. 1968 Aug;12(1):29–36. doi: 10.1017/s0016672300011587. [DOI] [PubMed] [Google Scholar]
- Lowbury E. J., Lilly H. A., Kidson A., Ayliffe G. A., Jones R. J. Sensitivity of Pseudomonas aeruginosa to antibiotics: emergence of strains highly resistant to carbenicillin. Lancet. 1969 Aug 30;2(7618):448–452. doi: 10.1016/s0140-6736(69)90163-9. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G. Effect of an R factor on resistance of Salmonella typhimurium to radiation and chemical treatment. Mutat Res. 1972 Apr;14(4):450–453. doi: 10.1016/0027-5107(72)90146-7. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi S., Kobayashi F., Yamaguchi M. Enzymatic inactivation of gentamicin C components by cell-free extract from Pseudomonas aeruginosa. J Antibiot (Tokyo) 1971 Jun;24(6):400–401. doi: 10.7164/antibiotics.24.400. [DOI] [PubMed] [Google Scholar]
- Olsen R. H., Shipley P. Host range and properties of the Pseudomonas aeruginosa R factor R1822. J Bacteriol. 1973 Feb;113(2):772–780. doi: 10.1128/jb.113.2.772-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M. F116: a DNA bacteriophage specific for the pili of Pseudomonas aeruginosa strain PAO. Virology. 1973 Oct;55(2):558–560. doi: 10.1016/0042-6822(73)90203-1. [DOI] [PubMed] [Google Scholar]
- Pemberton J. M., Holloway B. W. Chromosome mapping in Pseudomonas aeruginosa. Genet Res. 1972 Jun;19(3):251–260. doi: 10.1017/s0016672300014518. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Chakrabarty A. M., Gunsalus I. C. A transmissible plasmid controlling camphor oxidation in Pseudomonas putida. Proc Natl Acad Sci U S A. 1973 Mar;70(3):885–889. doi: 10.1073/pnas.70.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- Shooter R. A., Walker K. A., Williams V. R., Horgan G. M., Parker M. T., Asheshov E. H., Bullimore J. F. Faecal carriage of Pseudomonas aeruginosa in hospital patients. Possible spread from patient to patient. Lancet. 1966 Dec 17;2(7477):1331–1334. doi: 10.1016/s0140-6736(66)92082-4. [DOI] [PubMed] [Google Scholar]
- Shulman J. A., Terry P. M., Hough C. E. Colonization with gentamicin-resistant Pseudomonas aeruginosa, pyocine type 5, in a burn unit. J Infect Dis. 1971 Dec;124 (Suppl):S18–S23. doi: 10.1093/infdis/124.supplement_1.s18. [DOI] [PubMed] [Google Scholar]
- Smith D. H. R factors mediate resistance to mercury, nickel, and cobalt. Science. 1967 May 26;156(3778):1114–1116. doi: 10.1126/science.156.3778.1114. [DOI] [PubMed] [Google Scholar]
- Stone H. H., Kolb L. D. The evolution and spread of gentamicin-resistant Pseudomonads. J Trauma. 1971 Jul;11(7):586–589. doi: 10.1097/00005373-197107000-00009. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Lewis E. Volatilization of mercuric chloride by mercury-resistant plasmid-bearing strains of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. J Bacteriol. 1973 Feb;113(2):1070–1072. doi: 10.1128/jb.113.2.1070-1072.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N. Biochemical studies on gentamicin resistance. J Antibiot (Tokyo) 1970 Sep;23(9):469–471. doi: 10.7164/antibiotics.23.469. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Yagisawa M., Matsuhashi Y., Naganawa H., Yamamoto H. Letter: Gentamicin acetyltransferase in Escherichia coli carrying R factor. J Antibiot (Tokyo) 1973 Oct;26(10):612–614. doi: 10.7164/antibiotics.26.612. [DOI] [PubMed] [Google Scholar]
- Weinstein M. J., Drube C. G., Moss E. L., Jr, Waitz J. A. Microbiologic studies related to bacterial resistance to gentamicin. J Infect Dis. 1971 Dec;124 (Suppl):S11–S17. doi: 10.1093/infdis/124.supplement_1.s11. [DOI] [PubMed] [Google Scholar]
- Weppelman R. M., Brinton C. C., Jr Infection of Pseudomonas aeruginosa spheroplasts by RNA from a pilus phage. Virology. 1970 May;41(1):116–134. doi: 10.1016/0042-6822(70)90060-7. [DOI] [PubMed] [Google Scholar]
- Witchitz J. L., Chabbert Y. A. Résistance transférable à la gentamicine I. Expression du caractère de résistance. Ann Inst Pasteur (Paris) 1971 Dec;121(6):733–742. [PubMed] [Google Scholar]
- Yoshikawa M. Selective enrichment of R- segregants as the main mechanism of "curing" of the R factor by acridine dyes. Genet Res. 1971 Feb;17(1):9–16. doi: 10.1017/s001667230001199x. [DOI] [PubMed] [Google Scholar]



