Abstract
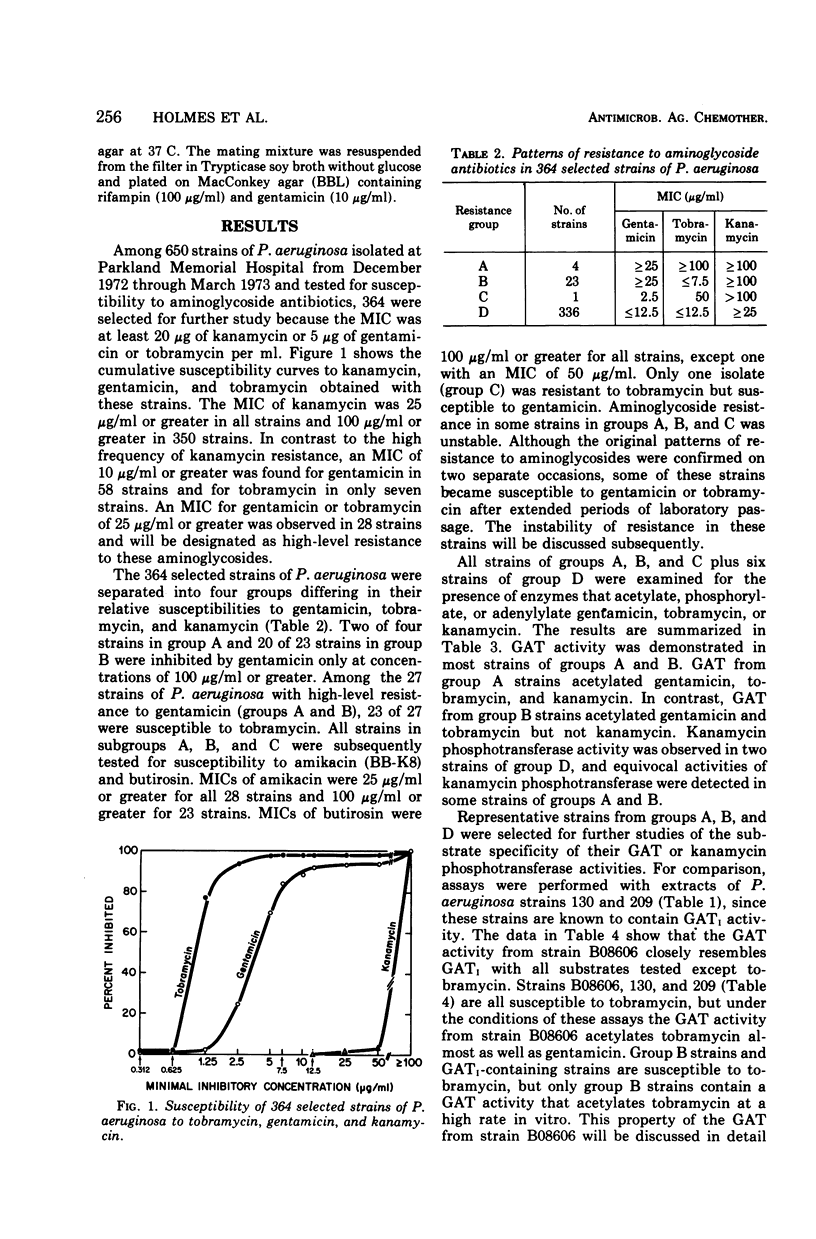
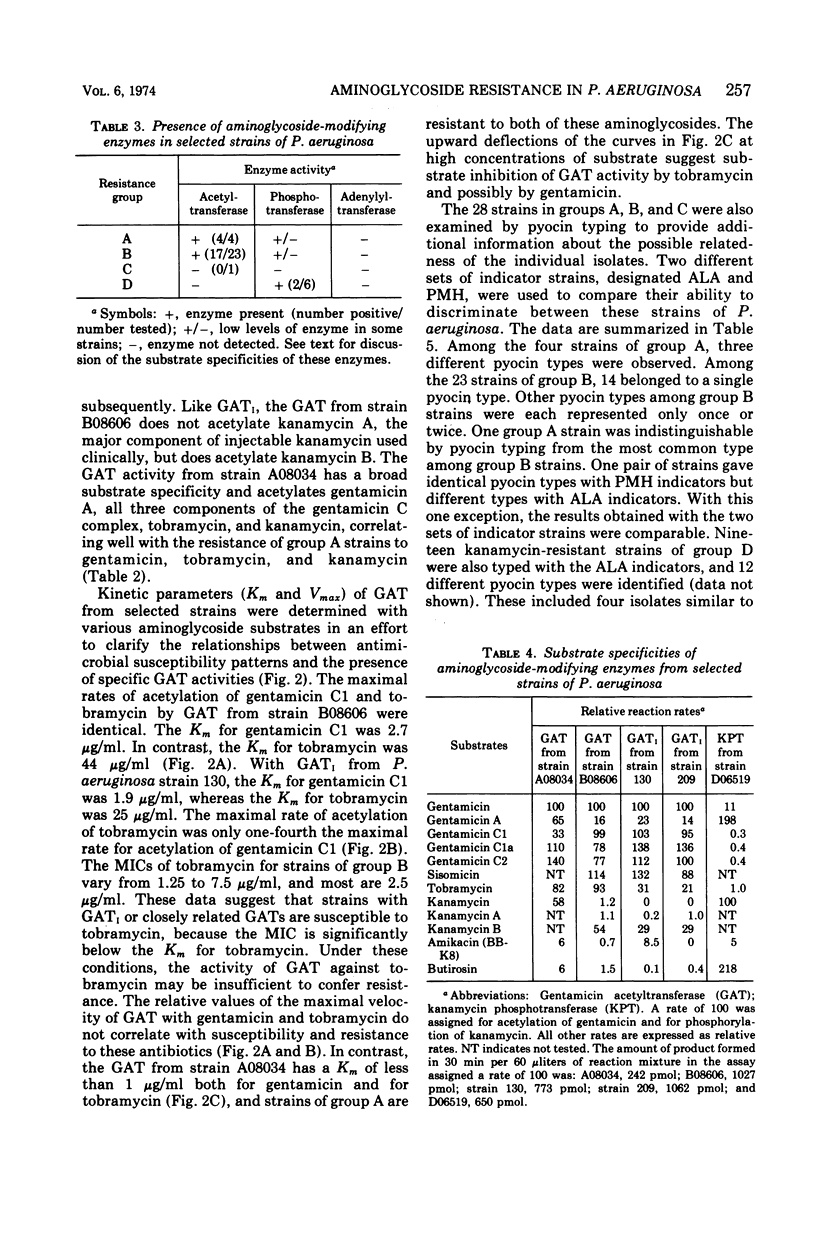
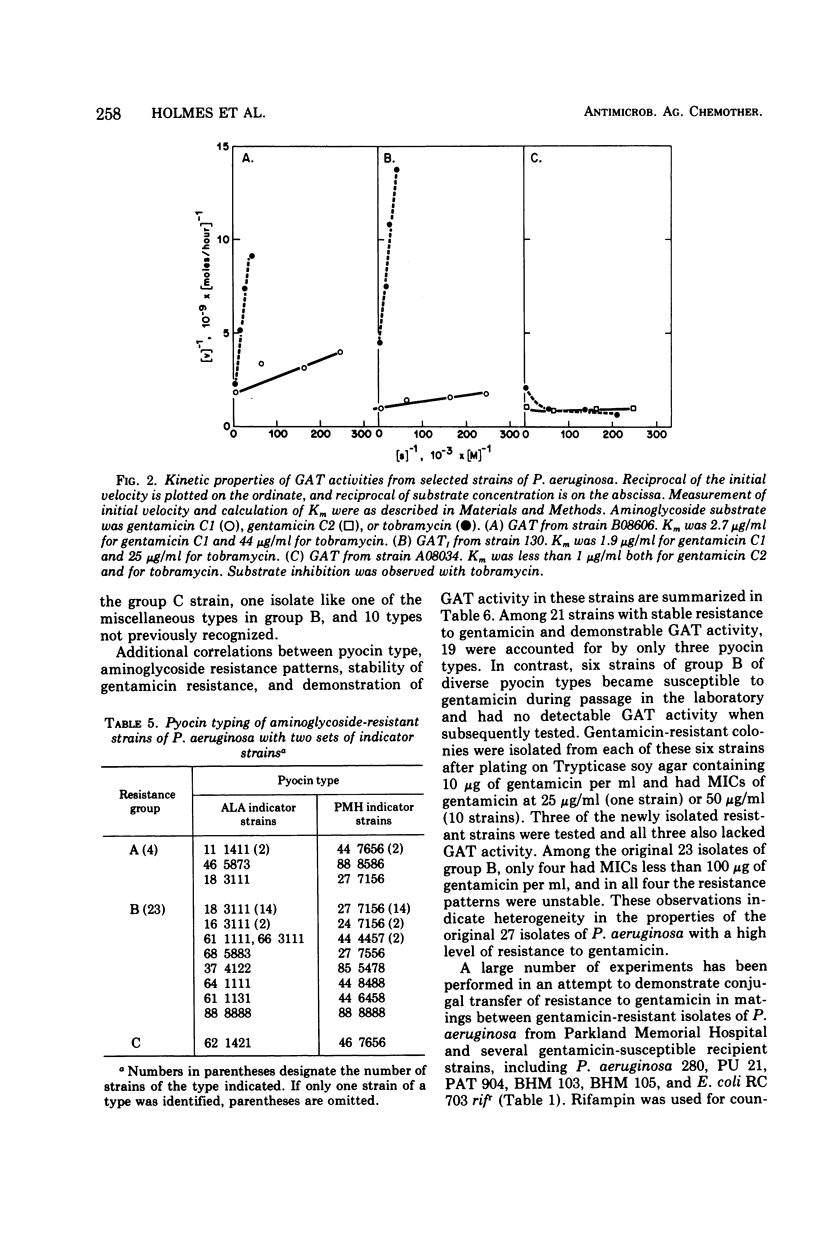
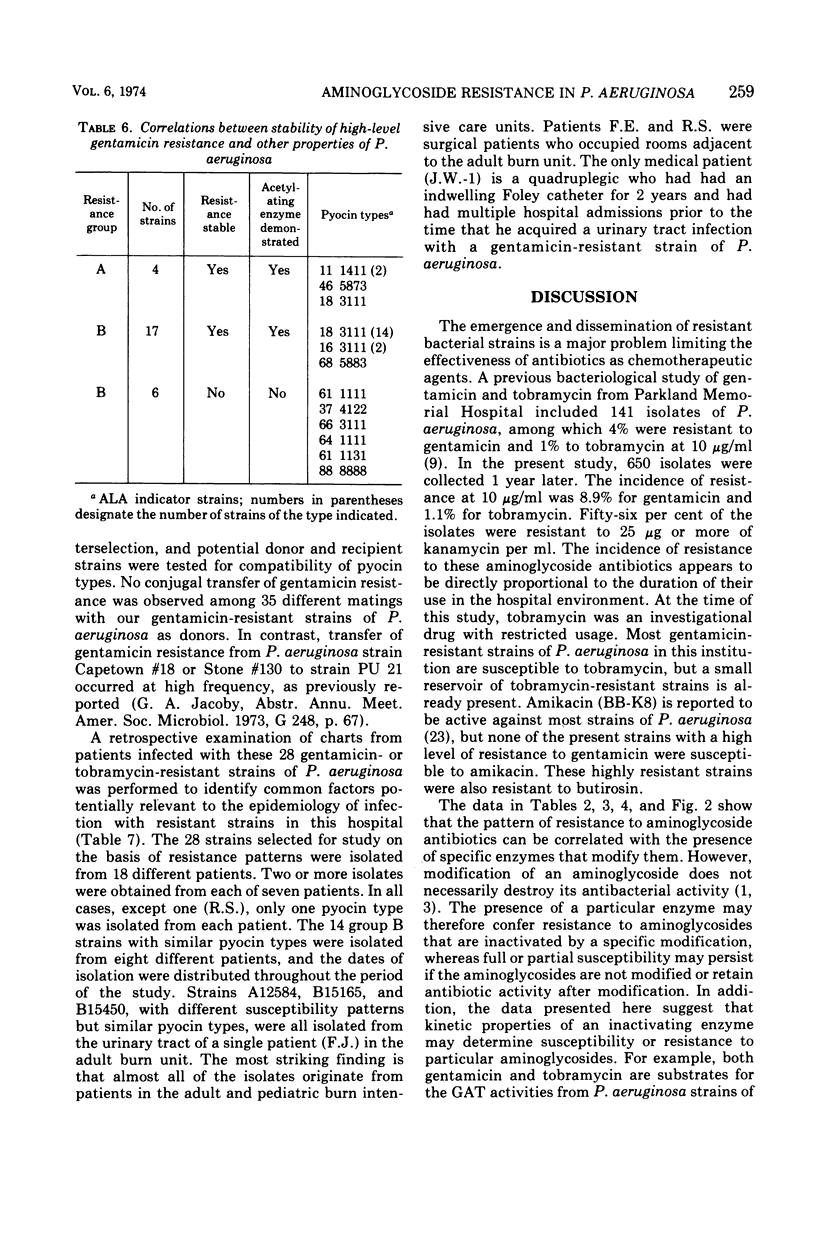
This study was undertaken to investigate biochemical, genetic, and epidemiological aspects of resistance to aminoglycoside antibiotics among 650 consecutive isolates of Pseudomonas aeruginosa from Parkland Memorial Hospital, Dallas, Tex. In 364 strains, minimal inhibitory concentrations were 25 μg/ml or greater for gentamicin (G), tobramycin (T) or kanamycin (K). Four patterns of resistance were noted: (A) G, T, K (four strains), (B) G, K (23 strains), (C) T, K (one strain), and (D) K (336 strains). Gentamicin acetyltransferase (GAT) activities were associated with resistance to gentamicin in strains of groups A and B, whereas kanamycin phosphotransferase activity was found in strains of group D. The GAT from group B strains acetylates both gentamicin and tobramycin. Resistance to gentamicin and susceptibility to tobramycin may reflect the fact that the Km's for tobramycin (25 to 44 μg/ml) of GAT activities in these group B strains are much greater than the Km's for gentamicin (1.9 to 2.7 μg/ml) and exceed the minimal inhibitory concentrations for tobramycin (1.25 to 7.5 μg/ml). GAT from strains of group A was associated with resistance to G, T, and K. Gentamicin acetyltransferases can be distinguished by their specificities for aminoglycoside substrates. The substrate specificity of GAT from group B strains is similar to that reported for GATI, but the specificity of GAT from group A strains differs from those described for GATI and GATII. Conjugal transfer of gentamicin or tobramycin resistance from our strains of P. aeruginosa to various potential recipient strains was not observed. Pyocin typing showed that many, but not all, of the strains resistant to gentamicin were similar, and retrospective epidemiological investigation revealed that these strains were isolated almost exclusively from patients in the adult and pediatric burn intensive care units and geographically continguous areas of the hospital.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Enzymatic acetylation of aminoglycoside antibiotics by Escherichia coli carrying an R factor. Biochemistry. 1971 May 11;10(10):1787–1796. doi: 10.1021/bi00786a009. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. R-factor mediated gentamicin resistance: A new enzyme which modifies aminoglycoside antibiotics. FEBS Lett. 1971 May 20;14(5):293–296. doi: 10.1016/0014-5793(71)80282-x. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Yamada T., Davies J. Enzymatic Adenylylation of Streptomycin and Spectinomycin by R-Factor-Resistant Escherichia coli. Infect Immun. 1970 Jan;1(1):109–119. doi: 10.1128/iai.1.1.109-119.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Semaka S. D., Van den Elzen H. M., Kinnear J. E., Whitehouse R. L. Characteristics of R931 and other Pseudomonas aeruginosa R factors. Antimicrob Agents Chemother. 1973 May;3(5):625–637. doi: 10.1128/aac.3.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M., Tseng J. T. Transferable drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1972 Jan;1(1):22–29. doi: 10.1128/aac.1.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinska M., Benveniste R., Davies J., Daniels P. J., Weinstein J. Gentamicin resistance in strains of Pseudomonas aeruginosa mediated by enzymatic N-acetylation of the deoxystreptamine moiety. Biochemistry. 1972 Feb 29;11(5):761–765. doi: 10.1021/bi00755a013. [DOI] [PubMed] [Google Scholar]
- Brzezinska M., Davies J. Two enzymes which phosphorylate neomycin and kanamycin in Escherichia coli strains carrying R factors. Antimicrob Agents Chemother. 1973 Feb;3(2):266–269. doi: 10.1128/aac.3.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger L. M., Sanford J. P., Zweighaft T. Tobramycin: bacteriological evaluation. Am J Med Sci. 1973 Feb;265(2):135–142. [PubMed] [Google Scholar]
- Davies J. E., Rownd R. Transmissible multiple drug resistance in Enterobacteriaceae. Science. 1972 May 19;176(4036):758–768. doi: 10.1126/science.176.4036.758. [DOI] [PubMed] [Google Scholar]
- Hewitt W. L. Second international symposium on gentamicin, an aminoglycoside antibiotic. Symposium perspectives. J Infect Dis. 1971 Dec;124 (Suppl):S297–S300. doi: 10.1093/infdis/124.supplement_1.s297. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Sanford J. P. Enzymatic assay for gentamicin and related aminoglycoside antibiotics. J Infect Dis. 1974 May;129(5):519–527. doi: 10.1093/infdis/129.5.519. [DOI] [PubMed] [Google Scholar]
- Jones L. F., Zakanycz J. P., Thomas E. T., Farmer J. J., 3rd Pyocin typing of Pseudomonas aeruginosa: a simplified method. Appl Microbiol. 1974 Feb;27(2):400–406. doi: 10.1128/am.27.2.400-406.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutit J. S., Pearce L. E., Marinus M. G. Investigation of the mating system of Pseudomonas aeruginosa strain 1. I. Kinetic studies. Genet Res. 1968 Aug;12(1):29–36. doi: 10.1017/s0016672300011587. [DOI] [PubMed] [Google Scholar]
- MATNEY T. S., GOLDSCHMIDT E. P. New uses for membrane filters II. Isolation of auxotrophic mutants. J Bacteriol. 1962 Oct;84:874–874. [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S., Kobayashi F., Yamaguchi M. Enzymatic inactivation of gentamicin C components by cell-free extract from Pseudomonas aeruginosa. J Antibiot (Tokyo) 1971 Jun;24(6):400–401. doi: 10.7164/antibiotics.24.400. [DOI] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Olsen R. H., Shipley P. Host range and properties of the Pseudomonas aeruginosa R factor R1822. J Bacteriol. 1973 Feb;113(2):772–780. doi: 10.1128/jb.113.2.772-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B., Benveniste R., Tipper D., Davies J. Aminoglycoside antibiotics: inactivation by phosphorylation in Escherichia coli carrying R factors. J Bacteriol. 1969 Nov;100(2):1144–1146. doi: 10.1128/jb.100.2.1144-1146.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K. E., Pursiano T. A., DeFuria M. D. Activity of BB-K8 (amikacin) against clinical isolates resistant to one or more aminoglycoside antibiotics. Antimicrob Agents Chemother. 1974 Feb;5(2):143–152. doi: 10.1128/aac.5.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone H. H., Kolb L. D. The evolution and spread of gentamicin-resistant Pseudomonads. J Trauma. 1971 Jul;11(7):586–589. doi: 10.1097/00005373-197107000-00009. [DOI] [PubMed] [Google Scholar]
- Weinstein M. J., Drube C. G., Moss E. L., Jr, Waitz J. A. Microbiologic studies related to bacterial resistance to gentamicin. J Infect Dis. 1971 Dec;124 (Suppl):S11–S17. doi: 10.1093/infdis/124.supplement_1.s11. [DOI] [PubMed] [Google Scholar]
- Witchitz J. L., Chabbert Y. A. High level transferable resistance to gentamicin. J Antibiot (Tokyo) 1971 Feb;24(2):137–139. doi: 10.7164/antibiotics.24.137. [DOI] [PubMed] [Google Scholar]



