Abstract
Aim: Myocardial infarction (MI) due to sudden occlusion of a major coronary artery leads to a complex series of events that result in left ventricle (LV) impairment eventual heart failure. Therapeutic options are limited to reverse such trends post MI. The aim of this study was to compare the acute cardioprotective effects of the antioxidants, resveratrol (RES) and coenzyme Q10 (CoQ10), either individually or in combination, on infracts size, LV hemodynamics, inflammation and oxidative stress markers in rats with experimentally induced MI. Methods: Male Wistar rats were randomly divided into six groups: control without surgery, sham without occlusion, MI without antioxidants, RES pre-treated then MI (20 mg/kg, orally), CoQ10 then MI (20 mg/kg, intramuscular.), and combined RES and CoQ10 then MI with (each group n = 10). Pretreatment commenced 7 days prior to the permanent occlusion of the left anterior descending (LAD) coronary artery. Infarct area, hemodynamics, inflammation and oxidative stress markers were assessed 24 hours post-MI. Results: Compared to RES alone, CoQ10 pre-administration either by itself or in combination with RES, significantly reduced LV infarct area (57%), and normalized LV hemodynamic parameters like LVEDP (100%), LVSP (95.4%), LV +dp/dt and -dp/dt (102 and 73.1%, respectively). CoQ10 also decreased serum levels of brain natriuretic peptide (70%), and various circulating inflammatory markers like TNF-α (83.2%) and IL-6 (83.2%). Regarding oxidative stress, TBARS scores were lowered with a concurrent increase in both superoxide dismutase and glutathione peroxidase activities with CoQ10 alone or in combination with RES. Conclusion: Coenzyme Q10 protects against the acute sequelae of myocardial infarction. It profoundly reduced infarct area, inflammation and oxidative stress while normalizing LV hemodynamics post MI.
Keywords: Myocardial infarction, coenzyme Q10, resveratrol, hemodynamics, apoptosis, oxidative stress
Introduction
Acute myocardial infarction (MI) due to coronary artery occlusion represents a major cause of morbidity and mortality in humans [1]. MI-related complications such as heart failure are of great socioeconomic burdens to society and health care systems. MI leads to irreversible loss of cardiomyocytes accompanied by the deterioration of contractile functioning and arrhythmias. Even though patients survive from an acute MI, most of them suffer from heart failure (HF) [1].
It is likely that multiple mechanisms are at play in the development of HF post MI. In this regard, a surge of studies that have shown involvement of increased oxidative stress [2,3], and left ventricular (LV) myocardial remodeling, which are collectively characterized by chamber dilatation and impaired ventricular functioning [3,4] in both humans and animals. Resveratrol (RES), a polyphenol phytoalexin is found in grapes and red wine. RES has received attention for preventing or slowing down cardiovascular disorders [6]. RES has been reported to have a preconditioning effect against ischemic injury [8] and reduces ischemia-reperfusion injury and infarction [9]. Regarding mechanisms, RES has been shown to reduce cardiomyocyte apoptosis, attenuate ventricular arrhythmias, improve post-ischemic LV functioning, and improve long-term survival in animal models of MI due to its antioxidant and anti-inflammatory activities [6,10,11]. However, in all of these studies, no complete cure of MI was achieved by RES alone.
CoQ10 (ubiquinone, CoQ10) is ubiquitous and has a central role against the depletion of ATP as an electron carrier in the mitochondrial respiratory chain and in oxidative phosphorylation [12]. Extra mitochondrial CoQ10 is also an efficient lipid soluble antioxidant, protecting against lipid peroxidation [12]. Endogenous synthesis of CoQ10 in the body declines with age, therefore, it is provided as supplement for the elderly [14]. Upto 75% of ischemic heart disease patients exhibited low levels of CoQ10 in the plasma and the heart as the heart disease progressed [14]. Low levels of cardiac CoQ10 is observed in patients with cardiomyopathy [13]. Interestingly, non-surviving heart failure patients had lower levels of CoQ10 in the plasma than surviving patients [15]. These cardioprotective effects of CoQ10 are most likely explained by its antioxidant effect and its ability to generate ATP, which requires continuous reduction of ubiquinone and regeneration to the active ubiquinol form [16].
In this study, we hypothesized that, when combined, RES and CoQ10 will effectively attenuate the infarct size and improve acute cardiac consequences after experimental MI in rats. Infract size, LV hemodynamics, oxidative stress and inflammation were investigated to shed light on the mechanism underlying the combined effects of RES and CoQ10.
Materials and methods
Drugs and chemicals
Resveratrol (C14H12O3, Cat No. R5010), CoQ10 (C59H90O4, Cat No. C9538), Evans blue dye (Cat No. 206334) and 2, 3, 5-triphenyltetrazolium chloride (TTC, Cat T8877) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Animals and experimental design
Eight-week-old male adult Wistar rats (weighing 230 ± 10 g) were supplied by the animal house facility at King Khalid University, Abha, Kingdom of Saudi Arabia. The Animal Care and Use Committee of the King Khalid University approved the experimental protocols. They were housed four per cage in a controlled environment (23 ± 1°C; 45%-50% relative humidity; fixed 12/12 h light/dark cycle, lights on at 08:00 h) with food and water ad libitum. Rats were randomized into six groups (n = 12 each) as follows: (1) Control un-infarcted group: rats were not exposed to any surgical procedure; (2) Sham operated group: rats underwent surgical procedure used to ligate the left anterior descending (LAD) coronary artery as shown later except that the silk suture was placed around the left coronary artery without being tied; (3) MI group: rats underwent LAD ligation; (4) RES treated group (RES then MI): rats were pre-treated with resveratrol (RES, 20 mg/kg/body wt, orally) for seven consecutive days and then underwent LAD ligation. RES was prepared by dissolving in a saline solution (0.9% NaCl) of 20% hydroxypropyl cyclodextrin (American Maize-Products Co., Hammond, IN, USA) to the desired final volume used in the experimental procedure; (5) CoQ10 treated group (CoQ10 then MI): rats were pre-treated with CoQ10 (20 mg/kg/bwt, intramuscular) for seven consecutive days and then underwent LAD ligation. CoQ10 was prepared in 1% aqueous solution of Tween 80; (6) RES and CoQ10 treated group (CoQ10 and RES then MI): rats pre-treated with concomitant doses of RES and CoQ10 as in groups 4 and 5, then underwent LAD ligation. Dose selection was based on previous studies [17,18].
Surgical MI procedure
Myocardial infarction was produced according to Xing et al. [19]. Sixty rats were anaesthetised by intraperitoneal (i.p.) injection of a 1% solution of sodium pentobarbital (50 mg/kg). The trachea was intubated via the mouth and mechanically ventilated with a small-rodent ventilator (Model 863 rodent ventilator, Harvard Apparatus, Holliston, MA) to perform a left thoracotomy to expose the heart. A heat pad maintained the temperature of the animals. The electrocardiogram (ECG, lead II), heart rate, and respiratory rate were continuously monitored. MI was induced by permanent ligation of the LAD coronary artery at the location between the pulmonary cone and the left atrial appendage, using an 8-0 polypropylene suture. Myocardial blanching and ECG ST-segment elevation confirmed MI. During surgery, saline and gel were dripped on the conjunctiva and cornea to prevent blindness caused by corneal drying in rats. Afterwards, the thorax was closed using 5-0 sutures. To reduce post-operative infection and pain, the rats were given intramuscular penicillin and subcutaneous buprenorphine (0.1 mg/kg). After restoring breathing, endotracheal intubation was removed, and rats were placed on an electric blanket, waiting for their revival, and returned to their cages.
Cardiac hemodynamic measurements
Cardiac hemodynamic measurements were assessed at 24 h post-MI for the 12 rats in each groups. In brief, rats were anaesthetized with 1% solution of sodium pentobarbital (50 mg/kg; i.p.) and placed on a heating pad to maintain body temperature. After performing tracheal intubation and ventilation, an open chest surgery was performed. SPR-320 pressure catheter was inserted directly into the LV to measure LV systolic pressure (LVSP), LV end diastolic pressure (LVEDP), maximal rate of rise in LV pressure (+dP/dt), and maximal rate of decline in LV pressure (-dP/dt). All data were recorded and analyzed with a PowerLab data acquisition system (ML780 PowerLab/8channels, AD Instruments Ltd., Australia).
Preparation of plasma and LV homogenates
During sacrifice, 2 ml blood was withdrawn from the aorta into EDTA-treated tubes, centrifuged at 4,000 rpm for 10 min to obtain the plasma. Parts of the LV obtained from six rats per group were homogenized in cold phosphate buffer, containing EDTA. The supernatant obtained was stored at -70°C for biochemical assays. Other parts of these LVs were frozen at -80°C and used for RNA extraction.
ELISA of inflammatory markers
Plasma brain natriuretic peptide (BNP) levels were measured using BNP ELISA Kit (Cat. No. KT-8580, Kamiya Biomedical Company, WA, USA) as per manufacturer’s instructions. This utilized competitive inhibition enzyme immunoassay technique in which the competitive inhibition reaction is launched between biotin labeled rat BNP and unlabeled rat BNP (Calibrators or samples) with the pre-coated antibody specific for rat BNP. The intensity of color developed was measured at 450 nm and was inversely proportional to the concentration of BNP in the sample. BNP levels were expressed as pg/ml.
Levels of TNF-α and IL-6 in LV homogenates were also determined by ELISA (Cat no. ab46070, Abcam, Cambridge, MA, USA and Cat No. ELR-IL6-001, RayBio, MO, USA, respectively) as per the manufacturer’s instruction. In brief, 100 μl of homogenate supernatant was used in the reaction and the intensity of the developed color at 450nm was directly proportional to the concentration of TNF-α and IL-6 contained in the samples . LV levels of TNF-α and IL-6 levels were expressed as pg/mg protein.
Oxidative stress assays
Lipid peroxidation in the LV homogenates were measured by the Thiobarbituric Acid (TBA) reaction using commercial kits (Cat No. NWK-MDA01, NWLSS, which city, province? USA) as per manufacturer’s instructions. TBA reacts with Malondialdehyde (MDA) forming an MDA-TBA2 adduct at 532 nm. In brief, tissue supernatant (50 μL) was added to test tubes containing 2 μL of butylated hydroxytoluene (BHT) in methanol to prevent oxidation of sample. Next, 50 μL of acid (1 M phosphoric acid) and 50 μL of TBA solution were added. The tubes were mixed, incubated for 60 min at 60°C and centrifuged. The supernatant was aliquoted and absorbance measured at 532 nm. TBARS levels were expressed as nmol/mg protein.
Superoxide dismutase (SOD) activity in LV homogenates was measured using a commercial kit (Cat. No. 706002, Cayman Chemical, Ann Arbor, MI, USA) as per manufacturer’s instructions. Briefly, the kit uses hypoxanthine and xanthine oxidase to generate superoxide radical that is detected through its reaction with a tetrazolium salt to form a formazan dye that absorbs at 440 nm. SOD dismutates superoxide to hydrogen peroxide, resulting in a decrease in the amount of formazan dye and absorbance at 440 nm. The calculated SOD activity was expressed as U/mg protein.
Glutathione peroxidase (GPx) activity in LV homogenates was measured using a commercial kit (Cat. No. 703102, Cayman Chemical), as per manufacturer’s instructions. The kit measures GPx activity using glutathione reductase (GR). Oxidized Glutathione (GSSG) is produced upon reduction of hydroperoxide by GPX and is recycled to its reduced state by GR and NADPH. The oxidation of NADPH to NADP+ is accompanied by a decrease in NADPH absorbance at 340 nm. Under conditions in which the GPX activity is limiting, the rate of decrease in the A340 is directly proportional to the GPX activity in the sample. The enzyme activity was depicted as nmol/min/ml.
Determination of myocardialiInfarct area and histological assessment
This procedure was done on six rats from each experimental group. After blood collection, a catheter (24G) was inserted in the clamped aorta. Evans blue dye (2% solution) was injected via this catheter into the heart to delineate the ischemic zone. The heart was then rapidly excised, horizontally sectioned into five 1-mm-thick sections that were then incubated in 1.0% 2, 3, 5-triphenyltetrazolium chloride (TTC, pH = 37) for 5 min at 37°C to demarcate the viable and nonviable myocardium within the risk zone. Normal uninfarcted myocardium typically stains blue when dyed with Evans blue and TTC blue, while ischemic myocardium stains red and infarcted region remains pale. Post-dye digital analysis of myocardial sections was performed. The area of LV at risk and the area of infarcted tissue in the risk zone were quantified by planimetry (using Adobe Photoshop software in a blinded fashion). The extent of ischemic myocardium (area at risk) was expressed as the percentage of the total left ventricle. The extent of the myocardial infarct was expressed as percentage of infarcted size over ischemic size [20]. For each heart, four sections were analyzed for determinations of infarct size. Values were then averaged and labeled as a percentage of single infarct per LV. The mean percentage and SD of all infracted areas in a single group were then calculated.
The fifth section of each heart from each group was rapidly fixed in 10% formaldehyde, dehydrated and embedded in paraffin, then cut into 4 μm slices that were stained with hematoxylin and eosin for histological assessment.
Semi quantitative RT-PCR
The procedure was optimized for semi quantitative detection using the primer pairs and conditions described in Table 1. Published sequences of PCR primers used for the detection of Bcl-2 associated X protein (BAX) and p53 were used according to the procedure established already in our labs, where β-actin was used to control for loading [21]. Total RNA was extracted from the frozen parts of LV (30 mg) using an RNeasy Mini Kit (Qiagen Pty. Ltd., Victoria, Australia) according to manufacturer’s instructions. RNA purity was estimated by the 260/280 nm absorbance ratio. Single-strand cDNA synthesis was performed as follows: 30 μl of reverse transcription mixture contained 1 μg of DNase I pre-treated total RNA, 0.75 μg of oligo d(T) primer, 6 μl of 5x RT buffer, 10 mM dithiothreitol, 0.5 mM deoxynucleotides, 50 U of RNase inhibitor, and 240 U of reverse transcriptase (Invitrogen, which city, province, country?). The reverse transcription (RT) reaction was carried out at 40°C for 70 min, followed by heat inactivation at 95°C for 3 min. The tested genes and the internal control (β-actin) were amplified by PCR using 2 μl RT reaction products from each sample in a 20 μl reaction containing Taq polymerase (0.01 U/ml), dNTPs (100 mM), MgCl2 (1.5 mM), and buffer (50 mM Tris-HCl). PCR reactions consisted of a first denaturing cycle at 97°C for 5 min, followed by amplification cycles, consisting of denaturation at 96°C for 30 sec, annealing for 30 sec, and extension at 72°C for 1 min. A final extension cycle of 72°C for 15 min was included. Annealing temperature was adjusted as: 60°C for p53 and 55°C for BAX and β-actin. A control reaction without reverse transcriptase was included for every sample of RNA isolated to verify the absence of contamination. PCR products (10 μl) were electrophoresed on 2% agarose gels containing 100 ng/ml ethidium bromide and photographed with a Polaroid camera under ultraviolet illumination.
Table 1.
Primers and conditions used in PCR reactions
| Target | Primer sequence (5’ to 3’) | AT (C) | Size (bp) | |
|---|---|---|---|---|
| p53 | Sense | 5-CTACTAAGGTCGTGAGACGCTGCC-3c | 60 | 106 |
| Antisense | 5-TCAGCATACAGGTTTCCTTCCACC-3d | |||
| Bax | Sense | 5-5_-GGTTGCCCTCTTCTACTTT-3c | 55 | 143 |
| Antisense | 5-AGCCACCCTGGTCTTG-3d | |||
| β-actin | Sense | 5-CGTTGACATCCGTAAAGAC-3c | 55 | 110 |
| Antisense | 5-TAGGAGCCAGGGCAGTA-3d |
Statistical analysis
Statistical analysis was performed using Graphpad Prism statistical software package (version 6). Data was presented as means with standard deviation (mean ± SD). Normality and homogeneity of the data were confirmed before ANOVA. Differences among the experimental groups were assessed by one-way ANOVA, followed by Tukey’s test.
Results
ST elevation and infarct size 24 hours Post-MI
As shown in Figure 1, normal ECG waves with normal ST segment height were seen in the control and sham groups of rats (A and B, respectively). The success of LAD ligation in MI model group was confirmed by ST segment elevation (Figure 1C). Normal ECG waves without any significant ST elevation were seen in control, sham, CoQ10 (alone), and CoQ10-RES (combined) groups. Evans blue and TTC staining were used to detect the size of infarct area as a percentage of area at risk (white area) and total LV (red area) in the hearts from all groups. As shown in Figure 2, deep blue colors of LVs were obtained from hearts isolated from the control and sham groups, indicating normal myocardium. However, compared with the sham group, the MI model group showed significant increases in the percentage of area at risk (64.83% ± 3.71%) and infract areas (47.33% ± 4.08%). In the MI-induced group of rats pre-treated with RES, there was no improvement in the area at risk and infract area. However, the mean area at risk and infarct size were significantly reduced in the CoQ10 pre-treated (20% ± 1.67% and 19.17% ± 2.97%) and both CoQ10 and RES treated groups (16.33% ± 3.52% and 13.5% ± 2.66%, respectively) when compared to the MI group.
Figure 1.
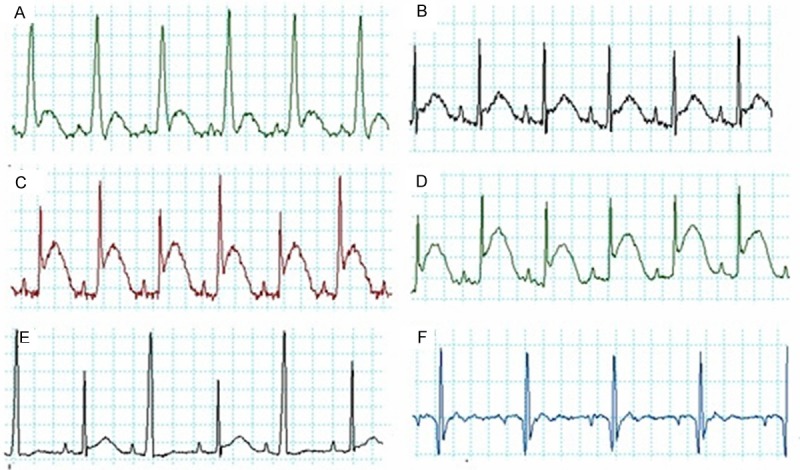
Examples of ECG trace recordings obtained from all experimental groups using PowerLab showing a clear ST segment elevation in groups C and D. A: Control, B: Sham operated, C: MI, D: RES then MI, E: CoQ10 then MI and F: RES + CoQ10 then MI. RES: Resveratrol, CoQ10: Coenzyme Q10 and MI: Myocardial Infarction.
Figure 2.
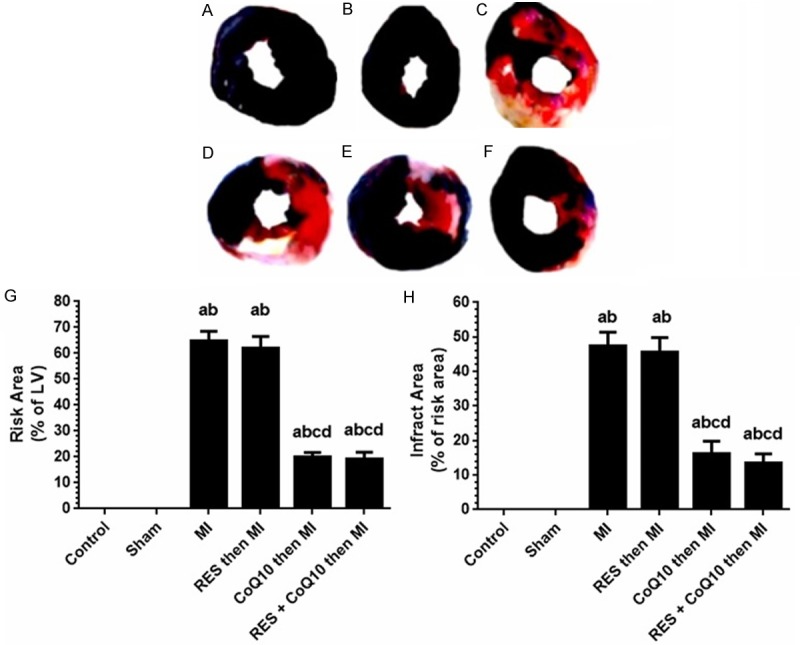
Assessment of myocardial infarct size induced by left anterior descending coronary artery (LAD) ligation in all groups of rats. A-F: Representative Evan’s blue dye and TTC staining of control and all experimental groups. A: Control, B: Sham operated, C: MI model, D: RES then MI, E: CoQ10 then MI and F: RES + CoQ10 then MI. G: Percent Area at Risk (red color in the upper panel) as expressed of percent of total size the left ventricle. H: Percentages of infracted area (necrotic white areas in the upper panel). RES: Resveratrol, CoQ10: Coenzyme Q10 and MI: Myocardial infarction. Values are expressed as Mean ± SD for 6 rats in each group. Values were considered significantly different at P < 0.05. aSignificantly different to control group. bSignificantly different to sham group. cSignificantly different to MI group. dSignificantly different to RES (alone). RES: Resveratrol, CoQ10: Coenzyme Q10, MI: Myocardial Infarction.
LV histology
Histological changes in the left ventricle in rats from all groups were assessed (Figure 3). Both control (Figure 3A) and sham (Figure 3B) groups showed normal myocardial tissue with orderly striated heart muscle fibers, intercalated discs, and a clear nuclear and muscle bands staining (Figure 3A and 3B). However, sections obtained from MI (Figure 3C) and RES (alone) group (Figure 3D) lacked transverse band structure, and demonstrated shrinkage, fragmentation, or disappearance of nucleus. Many nuclei were pyknotic (shrunken and dark) and underwent karyorrhexis (fragmentation) and karyolysis (dissolution). Inflammatory cell infiltration was seen in both groups with MI and RES alone. Sections obtained from CoQ10 (alone; Figure 3E) or in combination with RES (Figure 3G) showed normal architecture of cardiac cells, normal fiber striation, and clear nuclear and fiber staining and minimal inflammatory cell invasion.
Figure 3.
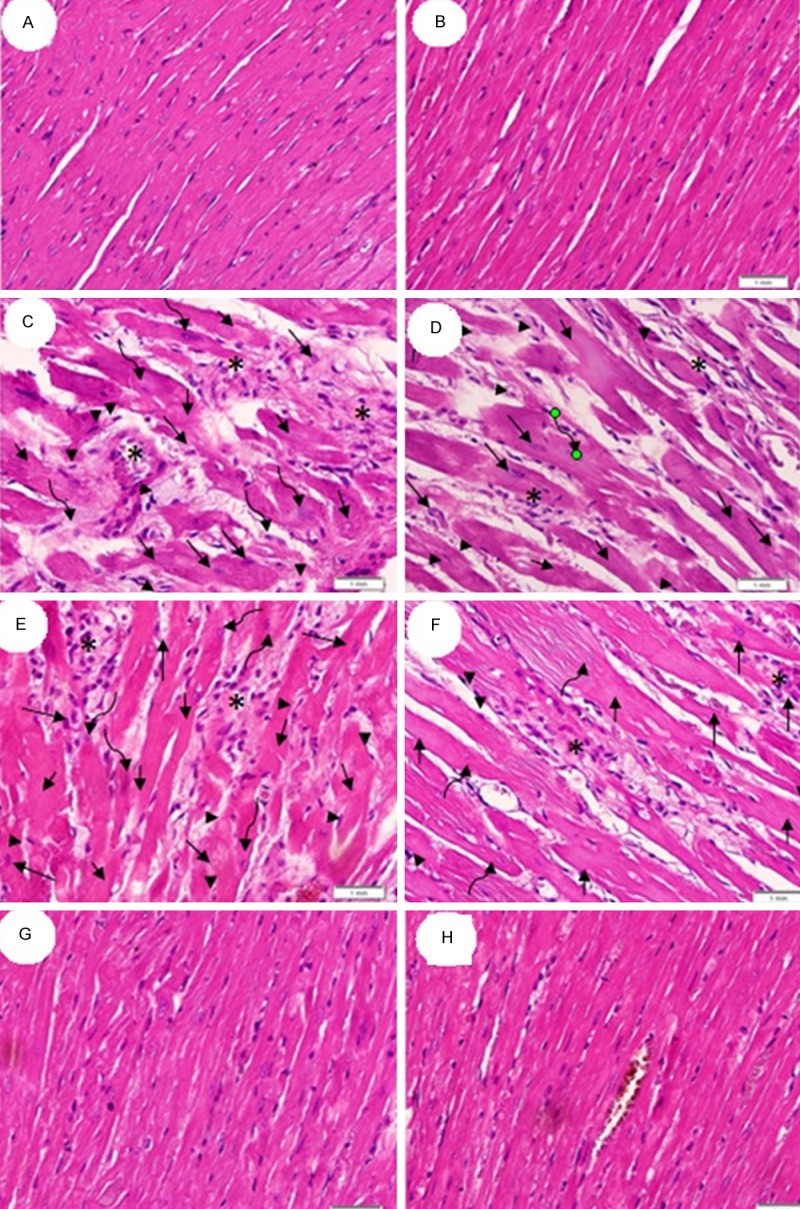
Histology of left ventricle. (A and B) are from control and sham, respectively, The myocardium appears orderly with striated fibers, intercalated discs, and clear nuclear and muscle staining. (C and D) are from MI group while sections (E and F) are from RES pre-treated MI group. Sections (C-F) lack transverse band, exhibit shrinkage (arrow head) and fragmentation (long arrow) or disappearance of nucleus (short arrow). Many nuclei are pyknotic (shrunken and dark, arrow head) and have undergone karorrhexis (fragmentation, long arrow) and karyolysis (dissolution, curved arrow). Inflammatory cell infiltration was also noted (star). Sections (G and H) are from CoQ10 (alone) and CoQ10-RES groups, respectively, and exhibit normal architectures of cardiac cells, normal fiber striation, clear nuclear and fiber staining and minimal inflammatory cell invasion. All images are at 400X magnification and captured using light microscopy.
Cardiac hemodynamic measurements 24 hours post-MI
As shown in Table 2, LV systolic pressure (LVSP), LV end diastolic pressure (LVEDP), maximal rate of rise in LV pressure (+dP/dt), and maximal rate of decline in LV pressure (-dP/dt) were not significantly different between the control and sham operated rats. However, compared with the sham group, the rats of the MI group showed a significant increase in the LVEDP as well as significant decreases in LVSP, LV +dP/dt and LV -dp/dt. RES pretreatment did not improve any of the MI-induced deterioration of hemodynamic parameters. In contrast, CoQ10 pre-administration alone or in combination with RES, restored LVEDP by 100% and 98.7%, respectively, and significantly increased LVSP by 95.4% and 91.4%, LV +dP/dt by 102% and 89.2%, and LV -dp/dt by 73.1% and 84.6%, respectively. However, there was no significant difference between hemodynamic values obtained between groups given CoQ10 alone or in combination with RES.
Table 2.
Cardiac hemodynamic parameters 24 hours post MI
| Group | HR (Beats/min) | LVESP (mmHg) | LVEDP (mmHg) | +dp/dt (mmHg/s) | -dp/dt (mmHg/s) |
|---|---|---|---|---|---|
| Control | 281 ± 10.1 | 124.7 ± 5.2 | 15.5 ± 2.2 | 5191 ± 227 | 3190 ± 287 |
| Sham | 277 ± 9.2 | 123.8 ± 5.4 | 15.1 ± 2.3 | 5290 ± 247 | 3292 ± 60 |
| MI | 279 ± 9.8 | 101.8 ± 3.5a,b | 27.3 ± 1.8a,b | 4016 ± 397a,b | 2528 ± 185a,b |
| Res then MI | 276 ± 7.3 | 102.8 ± 3.4a,b | 26.7 ± 2.1a,b | 4024 ± 379a,b | 2439 ± 111a,b |
| CoQ10 then MI | 273 ± 7.5 | 122.8 ± 6.1c,d | 14.4 ± 1.9c,d | 5293 ± 80c,d | 3089 ± 87c,d |
| RES + CoQ10 then MI | 280 ± 7.1 | 122.7 ± 5.4c,d | 15.8 ± 1.6c,d | 5161 ± 193c,d | 3176 ± 224c,d |
Values are expressed as Mean ± SD for 12 rats in each group and considered significantly different at P < 0.05.
Significantly different when compared to control group.
Significantly different when compared to sham group.
Significantly different when compared to MI group.
Significantly different when compared to RES (alone).
RES: Resveratrol, CoQ10: Coenzyme Q10, MI: Myocardial Infarction, HR: Heart Rate, LVESP: Left Ventricle End Systolic Pressure, LEEDP: Left Ventricle End Diastolic Pressure, +dp/dt: Maximal Rate of Rise in LV Pressure -dp/dt: Maximal Rate of Decline in LV Pressure.
Plasma BNP and LV inflammatory markers
As shown in Figure 4, no significant change in the levels of plasma BNP was seen in the sham operated group compared to unoperated controls, which also demonstrated very low TNF-α and IL-6 in the LV homogenate. However, BNP, TNF-α, and IL-6 levels were significantly elevated in the MI (alone) group by 78%, 337%, and 314%, respectively. The administration of RES (alone) to the MI group did not significantly alter BNP, TNF-α, and IL-6 levels. In contrast, a significant decrease in the plasma BNP and LV levels of TNF-α and IL-6 were noted in the MI-groups pre-treated with either CoQ10 alone or in combination with RES. The percentage decrease in plasma BNP in these groups of rats were 70% and 78.9%, respectively, whereas the percentage decrease in LV TNF-α were 83.2% and 89.1%, respectively, whereas IL-6 was decreased by 83.2% and 80% respectively. There was no difference between the effects of CoQ10 alone or in combination with RES.
Figure 4.
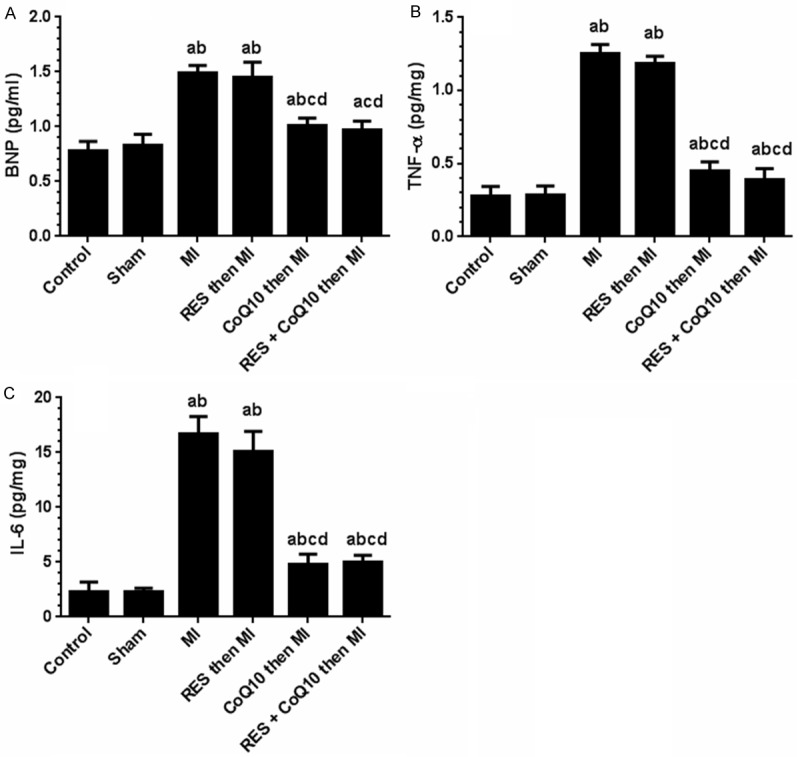
Levels of Brain Natriuretic Peptide (BNP), Tumor Necrosis Factor-α (TNF-α) and Interleukin 6 (IL-6) in the left ventricle homogenate from all experimental groups. Values are expressed as Means ± SD for 12 rats in each group for BNP and for 6 rats for TNF-α and IL-6. Values were considered significantly different at P < 0.05. aSignificantly different when compared to control group. bSignificantly different when compared to sham group. bSignificantly different when compared to sham group. cSignificantly different when compared to MI group. dSignificantly different when compared to RES (alone). RES: Resveratrol, CoQ10: Coenzyme Q10, MI: Myocardial Infarction.
LV oxidative stress markers
No significant difference was detected between the control and the sham groups with regard to TBARS, SOD, and GPx activities (Figure 5). However, in the MI group and the RES (alone) groups, significant increases were noted with TBARS (304% and 287%) while SOD activity (72.3% and 70.3%), and GPx activity (56.3% and 59%) decreased significantly. In contrast, CoQ10 alone or in combination with RES, normalized these parameters to sham levels. TBARS decreased by 355% and 339%, respectively, SOD activity increased by 266% and 250%, respectively and GPx activity was increased by 139% and 135%, respectively with CoQ10 alone or in combination.
Figure 5.
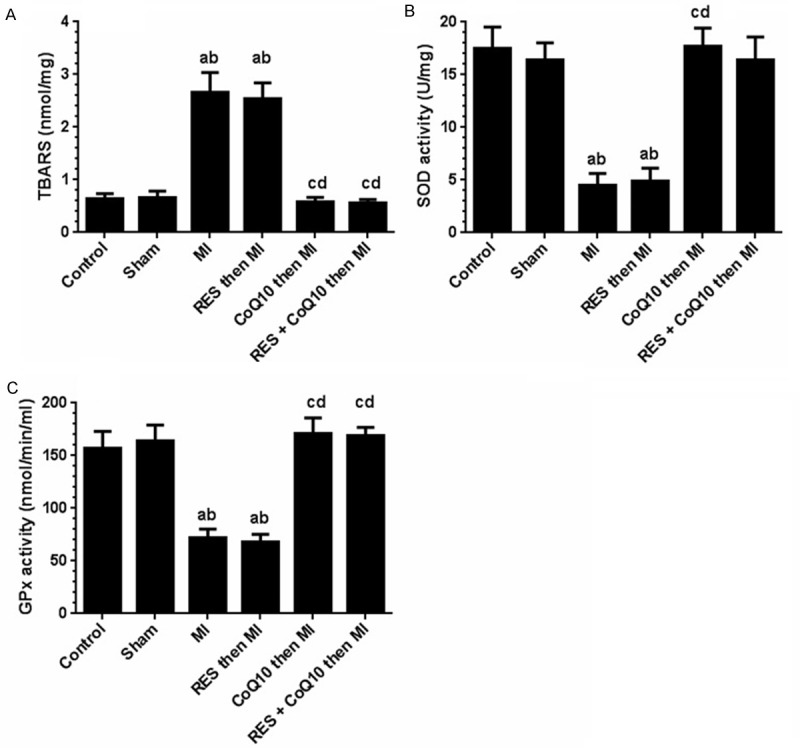
Levels of Thiobarbituric Acid reactive substances (TBARS) and activities of superoxide dismutase (SOD) and glutathione peroxidise (GPx) in the left ventricle homogenates of all experimental group of rats. Values are expressed as Means ± SD for 6 rats in each group and considered significantly different at P < 0.05. aSignificantly different when compared to control group. bSignificantly different when compared to sham group. cSignificantly different when compared to MI group. dSignificantly different when compared to RES (alone). RES: Resveratrol, CoQ10: Coenzyme Q10, MI: Myocardial Infarction.
BAX and p53 gene expression in LV
Figure 6 shows the transcriptional changes of p53 and BAX mRNAs in the LVs obtained from all groups of rats. All tested transcripts were detected and resulted in fragments similar in size to those expected (Table 1). The levels of the β-actin transcript remained relatively constant in all groups. In the control or sham operated groups, p53 and BAX mRNA were barely detectable. However, mRNA levels of both BAX and p53 increased several fold following MI. Their levels remained elevated in the RES (alone) group. Both p53 and BAX mRNA were significantly reduced upon CoQ10 administration (i.e., alone or in combination with RES). Importantly, while CoQ10 completely normalized BAX, p53 mRNA remained slightly elevated compared to sham levels.
Figure 6.
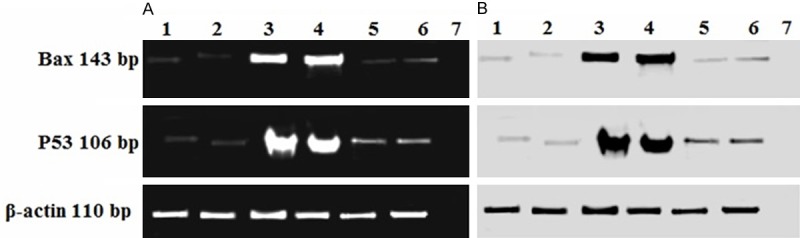
Expression of Bax and p53 mRNA in the left ventricle. The RT-PCR products obtained from all groups were separated by 2% agarose gel electrophoresis with 100 ng/ml ethidium bromide. 1: Control, 2: Sham operated, 3: MI model group, 4: MI then RES, 5: CoQ10 then MI, 6: CoQ10 and RES then MI and 7: Negative control. (B) Negative exposure of (A).
Discussion
In the present study we investigated the protective effects two antioxidants, resveratrol and coenzyme Q10 on acute consequences of experimental MI. The major results of this study indicate CoQ10 is a better prophylactic agent than resveratrol against acute sequelae of MI in rats. The mechanism of the cardioprotective action of CoQ10 involved blocking exaggerated oxidative stress and inflammation post MI, resulting in normalized/optimized hemodynamics and reduced infarct area of the left ventricle. CoQ10 alone or in combination with RES conferred cardioprotection characterized by a reduction in myocardial infarct size, improved cardiac muscle structure, and normalized hemodynamics as well as improved contractility of LV. The fact that RES alone did not alter MI-induced changes in LV but did so when co-administered with CoQ10, demonstrates that the cardioprotective effect in such combination therapy is primarily due to CoQ10.
In the current study, coinciding LV functional impairment, there was a significant increase in the levels of lipid peroxides (TBARS) and inflammatory markers TNF-α and IL-6 with a concomitant decrease of the endogenous antioxidant enzymes (SOD and GPx), in the cardiac homogenates of the infracted areas taken from the MI group. Considerable evidence indicates that the reactive oxygen species (ROS), such as superoxide anion (O2-), hydrogen radicals, and hydrogen peroxide, play an important role in mediating myocardial ischemic injury and cardiac cell apoptosis [22]. It has been reported that high levels of ROS disrupt the inner and outer mitochondrial membranes, induce relocation of BAX, and apoptosis. ROS induced DNA damage also stimulates p53 expression [23]. In fact, excessive O2- formation was detected in patients with coronary artery disease, acute MI, and unstable angina pectoris [24,25].
There is evidence that cytokines may also be involved in the pathogenesis of myocardial dysfunction and cardiomyocyte death in MI and HF [26,27]. Indeed, increased plasma levels as well as local myocardial production of several cytokines such as TNF-α, IL-1β, IL-6, and transforming growth factor b1 (TGF-β1) have been observed in patients with early post-MI [28]. In our current study, a significant increase in TNF-α and IL-6 was detected in the cardiac homogenates of the MI group 24 hours after LAD. Upregulation and production of these cytokines represent an intrinsic or an innate stress response against myocardial injury [29]. Indeed, in rodent models of MI, within the first few hours to one day, there is robust upregulation of intramyocardial cytokines, including TNF-α and IL-6 mRNA expression in the infarct area (up to 50-fold), as well as in the uninfarcted myocardium (up to 15-fold) [30]. As cytokines can regulate cardiac myocyte growth, contractile protein synthesis, and extracellular matrix gene expression [31], they may be important modulators in the post-MI process and reducing their levels may attenuate post-MI HF. Interestingly, cytokines are capable of decreasing left ventricle performance and myocyte contractility directly and indirectly. TNF-α and IL-6 can attenuate myocyte contractility directly through the immediate reduction of systolic cytosolic [Ca2+] via alterations in sarcoplasmic reticulum function, which is found to be reversible by the removal of the cytokine exposure [32]. However, TNF-α is also capable of decreasing myocyte contractility indirectly through nitric oxide-dependent attenuation of myofilament Ca2+ sensitivity [33].
It has been previously reported that when there is an irreversible and severe DNA damage, p53 induces the expression of Bcl-2 proapoptotic family members: BAX, Puma, and noxa [34]. In the current study, control and sham groups had low gene expression of BAX and p53 (as characterized by very thin bands) in the cardiac cells of LV. Following MI, an upregulation of cardiac BAX and p53 gene expression were noted in the MI group. Similar to our findings, CoQ10 normalize the increased levels of both P53 and BAX in the hearts of nutritional induced aged rats model [35]. In the same line, in other ischemic retinal or neural injuries, CoQ10 significantly decreased Bax protein expression [36,37]. However, caution must be exercised while interpreting these results, as protein levels for these DNA damage response factors were not measured in any group.
CoQ10 encompasses a group of homologous molecules that are present in almost all tissues. CoQ10 plays a key role in mitochondrial oxidative phosphorylation and ATP production and is found in the membranes of many organelles in humans [38]. It is therefore essential for all energy-dependent processes in the heart, including heart-muscle contraction. Since its primary function in cells is in generating energy, the highest concentration is found on the inner membrane of the mitochondrion [39]. Exogenous CoQ10 is taken up by CoQ10-deficient cells and is incorporated into the mitochondria for maintenance of optimal cellular and mitochondrial function [38]. CoQ10 can also reduce ROS by the suppression of NADPH oxidase expression [40], scavenging lipid peroxides [41] and preventing nitrative stress by the inhibition of excess NO production [42]. Furthermore, CoQ10 is a membrane stabilizer and preserves myocardial sodium-potassium ATPase activity and stabilizes myocardial calcium-dependent ion channels, thus providing energy and enhancing contractile function in the failing heart [39]. This could be one reason behind the improved LV function seen in the MI-induced treated rats treated with CoQ10.
In summary, these results demonstrate CoQ10 to be an effective prophylactic agent again experimental MI induced acute cardiac changes. The mechanisms probed included attenuation of oxidative stress, induction of antioxidants like SOD and GPx as well as lowering of cardiac inflammatory markers like TNF-a and IL-6. DNA damage to the heart following MI was likely also reduced with CoQ10, as supported by reduced Bax and p53 gene expression in the LV. We propose that CoQ10 is an effective antioxidant/anti-inflammatory agent to combat against acute cardiac changes induced by MI and can be an attractive therapeutic option in patients undergoing cardiac changes post MI.
Acknowledgements
The authors wish to thank Prof. Mohamad Samir Zaki from the Department of Anatomy at King Khalid University, Abha, Saudi Arabia for evaluating the histopathological changes and wish to thank Mr. Riyad Alessa from the Department of Biochemistry at King Khalid University, Abha, Saudi Arabia for his contribution in the biochemical assays used in the current study. S.G. and MA want to thank supporting grants from the Dairy Farmers of Canada. S. G. is a Michael Smith Foundations for Heath Research and Canadian Diabetes Association Scholar.
References
- 1.Fox CS, Coady S, Sorlie PD, D’Agostino RB, Pencina MJ, Vasan RS, Meigs JB, Levy D, Peter JS. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–50. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 2.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbate A, Bonanno E, Mauriello A, Bussani R, Biondi-Zoccai GG, Liuzzo G, Silvestri F, Dobrina A, Baldi F, Pandolfi F, Biasucci LM, Baldi A, Spagnoli LG, Crea F. Widespread myocardial inflammation and infarct-related artery patency. Circulation. 2004;110:46–50. doi: 10.1161/01.CIR.0000133316.92316.81. [DOI] [PubMed] [Google Scholar]
- 4.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 5.Center for Drug Evaluation and Research (CDER), U.S. Department of Health and Human Services Food and Drug Administration, June 2013. Guidance for Industry Codevelopment of Two or More New Investigational Drugs for Use in Combination. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM236669.pdf.
- 6.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 7.Di Castelnuovo A, Rotondo S, Iacoviello L, Donati MB, de Gaetano G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation. 2002;105:2836–2844. doi: 10.1161/01.cir.0000018653.19696.01. [DOI] [PubMed] [Google Scholar]
- 8.Das S, Alagappan VK, Bagchi D, Sharma HS, Maulik N, Das DK. Coordinated induction of iNOS-VEGF-KDR-eNOS after Resveratrol consumption: a potential mechanism for Resveratrol preconditioning of the heart. Vascul Pharmacol. 2005;42:281–289. doi: 10.1016/j.vph.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Mokni M, Limam F, Elkahoui S, Amri M, Aouani E. Strong cardioprotective effect of resveratrol, a red wine polyphenol, on isolated rat hearts after ischemia/reperfusion injury. Arch Biochem Biophys. 2007;457:1–6. doi: 10.1016/j.abb.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Chen YR, Yi FF, Li XY, Wang CY, Chen L, Yang XC, Su PX, Cai JR. Resveratrol attenuates ventricular arrhythmias and improves the long-term survival in rats with myocardial infarction. Cardiovasc Drugs Ther. 2008;22:479–485. doi: 10.1007/s10557-008-6141-8. [DOI] [PubMed] [Google Scholar]
- 11.Lin JF, Lin SM, Chih CL, Nien MW, Su HH, Hu BR, Huang SS, Tsai SK. Resveratrol reduces infarct size and improves ventricular function after myocardial ischemia in rats. Life Sci. 2008;83:313–317. doi: 10.1016/j.lfs.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Liehn EA, Postea O, Curaj A, Marx N. Repair after myocardial infarction, between fantasy and reality: the role of chemokines. J Am Coll Cardiol. 2011;58:2357–2362. doi: 10.1016/j.jacc.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Senes M, Erbay AR, Yilmaz FM, Topkaya BC, Zengi O, Doğan M, Yücel D. Coenzyme Q10 and high-sensitivity C-reactive protein in ischemic and idiopathic dilated cardiomyopathy. Clin Chem Lab Med. 2008;46:382–386. doi: 10.1515/CCLM.2008.061. [DOI] [PubMed] [Google Scholar]
- 14.Kalén A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24:579–584. doi: 10.1007/BF02535072. [DOI] [PubMed] [Google Scholar]
- 15.Molyneux SL, Florkowski CM, George PM, Pilbrow AP, Frampton CM, Lever M, Richards AM. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol. 2008;52:1435–1441. doi: 10.1016/j.jacc.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 16.Witte KK, Nikitin NP, Parker AC, von Haehling S, Volk HD, Anker SD, Clark AL, Cleland JG. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26:2238–2244. doi: 10.1093/eurheartj/ehi442. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama H, Lingle DM, Crestanello JA, Kamelgard J, Kott BR, Momeni R, Millili J, Mortensen SA, Whitman GJ. Coenzyme Q10 protects coronary endothelial function from ischemia reperfusion injury via an antioxidant effect. Surgery. 1996;120:189–196. doi: 10.1016/s0039-6060(96)80287-x. [DOI] [PubMed] [Google Scholar]
- 18.Penumathsa SV, Thirunavukkarasu M, Koneru S, Juhasz B, Zhan L, Pant R, Menon VP, Otani H, Maulik N. Statin and resveratrolin combination induces cardioprotection against myocardial infarction in hypercholesterolemic Rat. J Mol Cell Cardiol. 2007;42:508–516. doi: 10.1016/j.yjmcc.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing Y, Gao Y, Chen J, Zhu H, Wu A, Yang Q, Teng F, Zhang DM, Xing Y, Gao K, He Q, Zhang Z, Wang J, Shang H. Wenxin-Keli regulates the calcium/calmodulin-dependent protein kinase II signal transduction pathway and inhibits cardiac arrhythmia in rats with myocardial infarction. Evid Based Complement Alternat Med. 2013;2013:464508. doi: 10.1155/2013/464508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang LP, Yang CY, Wang YP, Cui F, Zhang Y. Protective effect of polydatin against ischemia/reperfusion injury in rat heart. Acta Physiologica Sinica. 2008;60:161–168. [PubMed] [Google Scholar]
- 21.Elewa SM, Alkhateeb MA, Alhasem FA, Bin-Jaliah I, Sakr HF, Elreaey HM, Elkarib AO, Alessa RM, Haidara MA, Shatoor AS, Khalil MA. Resveratrol reverses cadmium chloride-induced testicular damage and subfertility by downregulating p53 and Bax and upregulating gonadotropins and Bcl-2 gene expression. J Reprod Dev. 2014;60:115–127. doi: 10.1262/jrd.2013-097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh RB, Kartikey K, Charu AS, Niaz MA, Schaffer S. Effect of taurine and coenzyme Q10 in patients with acute myocardial infarction. Adv Exp Med Biol. 2003;526:41–48. doi: 10.1007/978-1-4615-0077-3_6. [DOI] [PubMed] [Google Scholar]
- 23.Jia Y, Castellanos J, Wang C, Sinha-Hikim I, Lue Y, Swerdloff RS, Sinha-Hikim AP. Mitogen-activated protein kinase signaling in male germ cell apoptosis in the rat. Biol Reprod. 2009;80:771–780. doi: 10.1095/biolreprod.108.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois-Rande JL, Artigou JY, Darmon JY, Habbal R, Manuel C, Tayarani I, Castaigne A, Grosgogeat Y. Oxidative stress in patients with unstable angina. Eur Heart J. 1994;15:179–83. doi: 10.1093/oxfordjournals.eurheartj.a060473. [DOI] [PubMed] [Google Scholar]
- 25.McMurray J, Mclay J, Chopra M, Bridges A, Belch JJF. Evidence for enhanced free radical activity in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1990;65:1261–1262. doi: 10.1016/0002-9149(90)90985-a. [DOI] [PubMed] [Google Scholar]
- 26.Blum A, Miller H. Role of cytokines in heart failure. Am Heart J. 1998;135:181–186. doi: 10.1016/s0002-8703(98)70080-8. [DOI] [PubMed] [Google Scholar]
- 27.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda U, Ohkawa F, Seino Y, Yamamoto K, Hidaka Y, Kasahara T, Kawai T, Shimada K. Serum interleukin-6 levels become elevated in acute myocardial infarction. J Mol Cell Cardiol. 1992;24:579–584. doi: 10.1016/0022-2828(92)91042-4. [DOI] [PubMed] [Google Scholar]
- 29.Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Ann Rev Physiol. 2003;65:81–101. doi: 10.1146/annurev.physiol.65.092101.142249. [DOI] [PubMed] [Google Scholar]
- 30.Deten A, Volz HC, Briest W, Zimmer HG. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc Res. 2002;55:329–340. doi: 10.1016/s0008-6363(02)00413-3. [DOI] [PubMed] [Google Scholar]
- 31.Sasayama S, Matsumori A, Kihara Y. New insights into the pathophysiological role for cytokines in heart failure. Cardiovasc Res. 1999;42:557–564. doi: 10.1016/s0008-6363(99)00050-4. [DOI] [PubMed] [Google Scholar]
- 32.Goldhaber JI. Free radicals enhance Na+/Ca+2 exchange in ventricular myocytes. Am J Physiol. 1996;271:H823–833. doi: 10.1152/ajpheart.1996.271.3.H823. [DOI] [PubMed] [Google Scholar]
- 33.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 34.Thornborrow EC, Patel S, Mastropietro AE, Schwartzfarb EM, Manfredi JJ. A conserved intronic response element mediates direct p53-dependent transcriptional activation of both the human and murine bax genes. Oncogene. 2002;21:990–999. doi: 10.1038/sj.onc.1205069. [DOI] [PubMed] [Google Scholar]
- 35.Denise S, Twinn F, McConnell J, Hargreaves J, Giussan D, Ozanne S. Adkins J, Blackmore H and Martin-Gronert M. Coenzyme Q10 prevents acceleratedcardiacaging in aratmodelofpoormaternalnutritionand accelerated postnatalgrowth. Molecular Metabolism. 2013;2:480–490. doi: 10.1016/j.molmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee D, Kim K, Shim M, Kim S, Ellisman M, Weinreb R. Coenzyme Q10 ameliorates oxidative stress and prevents mitochondrial alteration in ischemic retinal injury. Apoptosis. 2014;19:603–614. doi: 10.1007/s10495-013-0956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zamani M, Majid Katebi, Mehdizadeh M, Mohamadzadeh F, Soleiman M. Coenzyme Q10 Protects Hippocampal Neurons against Ischemia/ Reperfusion Injury via Modulation of BAX/Bcl-2 Expression. Basic and Clinical Science. 2012;3:5–9. [Google Scholar]
- 38.Nakamura T, Sanma H, Himeno M, Kato K. Transfer of exogenous coenzyme Q10 to the inner membrane of heart mitochondria in rats. In: Folkers K, Yamamura Y, editors. Biomedical and clinical aspects of coenzyme Q. Vol 2. Elsevier/North-Holland Press; 1980. pp. 3–14. [Google Scholar]
- 39.Kumar A, Kaur B, Devi P, Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol Ther. 2009;124:259–268. doi: 10.1016/j.pharmthera.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Sohet FM, Neyrinck AM, Pachikian BD, de Backer FC, Bindels LB, Niklowitz P, Menke T, Cani PD, Delzenne NM. Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem Pharmacol. 2009;78:1391–1400. doi: 10.1016/j.bcp.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Tsuneki H, Sekizaki N, Suzuki T, Kobayashi S, Wada T, Okamoto T, Kimura I, Sasaoka T. Coenzyme Q10 prevents high glucose-induced oxidative stress in human umbilical vein endothelial cells. Eur J Pharmacol. 2007;566:1–10. doi: 10.1016/j.ejphar.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Jung HJ, Park EH, Lim CJ. Evaluation of anti-angiogenic, anti-inflammatory and antinociceptive activity of coenzyme Q(10) in experimental animals. J Pharm Pharmacol. 2009;61:1391–1395. doi: 10.1211/jpp/61.10.0017. [DOI] [PubMed] [Google Scholar]


