Abstract
Amorphous silica nanoparticles (SiNP) are being investigated for their potential use in various industrial and medical fields. Therefore, the assessment of their possible pathophysiological effect on circulating cells such as platelets is essential. We recently showed that intraperitoneal administration of SiNP causes proinflammatory and prothrombotic responses in vivo. However, little is known about the interaction of amorphous SiNP with platelets in vitro. Presently, we investigated the in vitro effects of SiNP (1, 5 and 25 μg/ml) on platelet aggregation, oxidative stress and intracellular calcium in mouse platelets. Incubation of platelets with SiNP caused a significant and dose-dependent platelet aggregation. Similarly, the activity of lactate dehydrogenase (as a marker of cell membrane integrity) was significantly increased by SiNP. Total antioxidant activity and lipid platelets vulnerability to in vitro peroxidation (measured by malondialdehyde production) were significantly increased after SiNP exposure. Additionally, SiNP exposure significantly increased the cytosolic calcium concentration. In conclusion, our in vitro data show that incubation of platelets with SiNP caused platelet aggregation, oxidative stress and increased intracellular calcium. This finding provides evidence on the toxicity of SiNP on platelet physiology.
Keywords: Amorphous silica nanoparticles, platelets, Oxidative stress, mice
Introduction
Amorphous silica nanoparticles (SiNP) are now being used in various fields such as industrial manufacturing, packaging, high-molecule composite materials and ceramics synthesis, drug delivery, cancer therapy and imaging [1].
Human exposure to SiNP may occur during production, storage, transportation, and consumer use [1]. Due to their very small density, SiNP can be readily evaporated into air, and can be inhaled. Following inhalation, nanoparticles have been reported to rapidly cross the alveolar capillary barrier and penetrate into to the systemic circulation and reach various organs [2-5]. Furthermore, with their medical usage, after injection or skin application, nanoparticles can be distributed into the blood and affect the circulatory cells including platelets [1]. Consequently, studying the effects of engineered nanoparticles on platelet physiology is relevant and much needed.
It has been reported that intravenous administration of SiNP induces consumptive coagulopathy in mice [6]. More recently, we have demonstrated that intraperitoneal administration of amorphous SiNP cause prothrombotic events, endothelial dysfunction and systemic inflammation in mice [7]. However, little is known about the direct in vitro effect of amorphous SiNP on platelets. Only a single study showed that exposure of washed human platelets in vitro to amorphous SiNP induced a low [NO]/[ONOO-] ratio leading to platelet aggregation [8]. We recently found that the in vitro incubation of SiNP in whole blood caused platelet aggregation in a dose-dependent fashion [7]. However, the direct influence of SiNP on washed mouse platelets and the mechanisms related to their effects have not, as far as we are aware, been reported. Consequently, the specific aims of this in vitro study were four-fold: (1) to assess the proaggregatory effects of three graded doses of SiNP on mouse platelets; (2) to evaluate the effect of SiNP on lactate dehydrogenase release by platelets; (3) to assess platelet lipid peroxidation and oxidant/antioxidant status following exposure to SiNP, including malondialdehyde (MDA) concentration and total antioxidant activity; and (4) to assess the effects of SiNP on platelet cytosolic calcium concentration.
Materials and methods
Amorphous silica nanoparticles
Amorphous silica nanoparticles (50 nm) were purchased from Polysciences (Warrington, PA, USA). Data regarding their structure, shape, size and charge were recently published [8,9].
For electron microscopy, droplets (10 μL) of a suspension of 0.1 mg of SiNP in 500 μL were placed on matured formvar/carbon film for 30 seconds. The samples were then drained and inverted onto droplets of ultrapure water for 1 hour before being drained, dried, and examined in a Philips CM10 Transmission Electron Microscope.
Blood collection
This project was reviewed and approved by our Institutional Review Board, and experiments were performed in accordance with protocols approved by the Institutional Animal Care and Research Advisory Committee.
mice (30-35 g, HsdOla:TO, Harlan, UK) were housed in light (12-h light:12-h dark cycle) and temperature-controlled (22 ± 1°C) rooms. They had free access to commercial laboratory chow and were provided tap water ad libitum. For blood collection, the animals were anesthetized intraperitoneally with sodium pentobarbital (45 mg/kg), and then blood was drawn from the inferior vena cava into syringes containing one-tenth volume Acid Citrate Dextrose (130 mM citric acid, 124 mM sodium citrate, 110 mM dextrose) anticoagulant [10].
Isolation and exposure of platelets and assessment of platelet aggregation
Washed platelets were obtained as described previously [11]. The blood was centrifuged at 200g for 20 min at 25°C. The platelet rich plasma was separated, added with Tyrode buffer (137 mM NaCl, 2.8 mM KCl, 12 mM NaHCO3, 5 mM glucose, 0.4 mM Na2HPO4, 10 mM HEPES, 0.1% BSA), pH 6.5 in 1:7 volumetric ratio and centrifuged at 900 g for 10 min. The platelet pellet was resuspended in 1 ml of Tyrode buffer (pH 7.0).
The platelets were exposed in Tyrode buffer (pH 7.4). Each reaction included 1 million of platelets in a total volume of 1 ml. Where indicated, control consisting of normal saline (NaCl 0.9%) containing Tween 80 (0.01%) or SiNP were incubated at 37°C at the indicated concentrations (1, 5, 25 μg/ml) for 3 min, and then stirred for another 3 min. At the end of this period, 25-μl samples were removed and fixed on ice in 225 ml cell Fix (Becton Dickinson, Franklin Lakes, NJ). After fixation, single platelets were counted in a VET ABX Micros with mouse card (ABX, Montpellier, France). The occurrence of of platelet aggregation was quantified by measuring the fall in single platelets counted due to aggregation induced by the treatment with SiNP or saline compared with untreated platelets [12,13]. Therefore, the degree of platelet aggregation following SiNP or saline (control) exposure was expressed as ℅ of that obtained in untreated platelets [12,13].
LDH leakage
One hundred μl of the platelet suspension (1 x 106 cells/ml) was incubated with SiNP at final concentrations of 1, 5 and 25 μg/ml, in a 24-well plate. The platelets treated with normal saline (NaCl 0.9%) containing Tween 80 (0.01%) were taken as control. The plates were incubated for 30 min at 37°C. At the end of the incubation period, the cell suspension was centrifuged, and aliquots of the resulting supernatant were subjected to LDH analysis by UV assay using a commercially available kit produced by Roche (Basel, Switzerland) in blood chemistry analyzer (Roche COBAS, Switzerland).
Measurement of markers of oxidative stress
Measurement of markers of oxidative stress was carried out as described previously with slight modifications [14,15]. One hundred μl of the platelet suspension (1 x 106 cells/ml) was incubated with SiNP at final concentrations of 1, 5 and 25 μg/ml, in a 24-well plate. The platelets treated with normal saline (NaCl 0.9%) containing Tween 80 (0.01%) were taken as control. The plates were incubated for 30 min at 37°C. At the end of the incubation period, cell suspensions were centrifuged for 2 min in an Eppendorf microcentrifuge. Platelet pellets were re-suspended in previous volume of Tyrode’s buffer, pH 7.4. After that the platelets were lysed and then centrifuged at 15,000 xg for 10 min. The concentrations of MDA and total antioxidant were quantified colorimetrically with commercially available kits (Cayman Chemicals, Ann Arbor, MI, USA) according to methods described by the manufacturer.
Measurement of intracellular Ca2+
Intracellular Ca2+ was measured after incubation with either vehicle or various concentrations of SiNP, as described above, according to previously described techniques [11,14,16]. Briefly, platelets were washed in Tyrode buffer and then loaded with the fluorescent indicator dye fura 2AM (Calbiochem; La Jolla, CA) in Tyrode buffer containing 2 mM CaCl2 and 2 μM fura 2 [11]. The cells were incubated at 37°C for 10 min and washed once in Tyrode buffer containing 2 mM CaCl2. The fura 2-loaded platelets were resuspended in Tyrode buffer. Then, Ca2+-dependent fluorescence intensity was then monitored with a fluorimeter (model SFM 25, Kontron; Zurich, Switzerland) set at 340-nm excitation and 510-nm emission [14].
Statistics
All statistical analyses were performed with GraphPad Prism Software version 5 (San Diego, CA, USA). Comparisons between groups were performed by one way analysis of variance (ANOVA), followed by Dunnett’s multiple range tests. P-values < 0.05 were considered as significant. All the data in the figures were reported as mean ± SEM.
Results
Particle characterization
Transmission electron microscopy analysis revealed the presence of nanosized amorphous SiNP (Figure 1). In our analysis, we found SiNP of around 50 nm in size. We have also seen small aggregates of probably two SiNPs with an approximate size of 100 nm. The same particles obtained from the same vendor were recently analysed by Corbalan et al. [9]. They reported the presence of significant deviations from nominal specifications which is usual in commercially supplied samples [9], and confirmed a size of around 50 nm [9].
Figure 1.
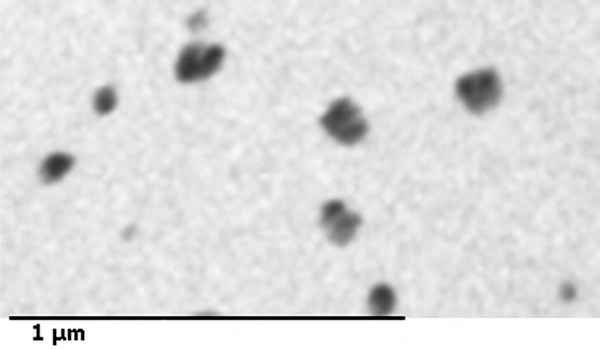
Transmission electron micrographs of the amorphous silica nanoparticle (SiNP) suspension showing the presence of of nanosized particles.
Effect of SiNP on platelet aggregation
Compared with control group, incubation of platelets with SiNP caused significant and dose dependent proaggregatory effects (Figure 2). The level of significance was achieved at 5 μg/ml SiNP (P < 0.0001) and 25 μg/ml SiNP (P < 0.0001).
Figure 2.
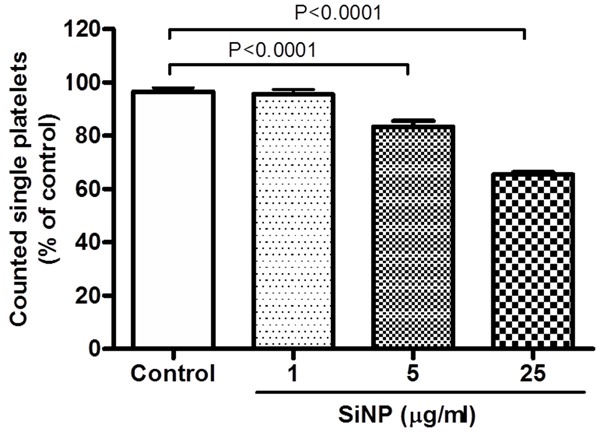
Effect of silica nanoparticle (SiNP) on mouse platelet aggregation in vitro. Platelets were incubated at 37°C with SiNP for 3 min, stirred for another 3 min, and then single platelets were counted. Platelet aggregation was quantified by measuring the fall in single platelets counted due to aggregation induced by SiNP or saline. The degree of platelet aggregation following SiNP or saline (control) exposure was expressed as ℅ of that obtained in untreated platelets. Data are mean ± SEM (n = 5). Statistical analysis by one-way ANOVA followed by Dunnett’s multiple range tests.
Effect of SiNP on LDH activity
Figure 3 shows the effect of SiNP on the activity of LDH in mouse platelets. LDH was used as a marker of cell membrane integrity. Compared with control group, incubation of platelets with 25 μg/ml SiNP caused a significant increase in LDH activity (P < 0.05).
Figure 3.
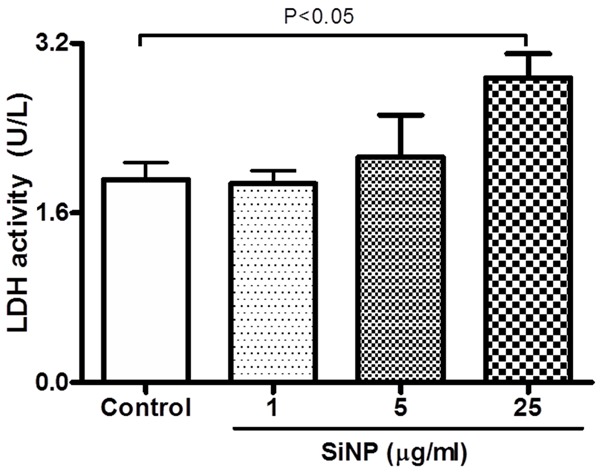
Effect of silica nanoparticles (SiNP) on platelet membrane integrity. Lactate dehydrogenase (LDH) activity was measured in experimental medium after incubation of platelets with various concentrations of SiNP. Data are mean ± SEM. Statistical analysis by one-way ANOVA followed by Dunnett’s multiple range tests.
Effect of SiNP on concentrations of MDA
Figure 4 illustrates the effect of SiNP on the concentrations of MDA in mouse platelets. MDA was used to assess the susceptibility of platelet lipid to in vitro peroxidation. The incubation of platelets with SiNP caused a significant increase in MDA concentrations (Figure 4). The effect was statistically significant at 5 μg/ml (P < 0.0001) and 25 μg/ml (P < 0.0001).
Figure 4.
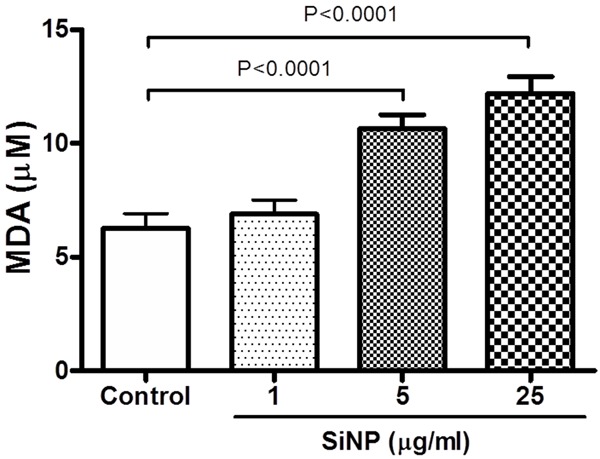
Effect of of silica nanoparticles (SiNP) on the concentrations of malondialdehyde (MDA) in mouse platelets. Data are mean ± SEM (n = 8 in each group). Statistical analysis by one-way ANOVA followed by Dunnett’s multiple range tests.
Effect of SiNP on concentrations of total antioxidant activity
Figure 5 shows the effect of SiNP on total antioxidant activity. Compared with the control group, the antioxidant activity in platelets was significantly increased following the incubation of platelets with SiNP 25 μg/ml (P < 0.0001).
Figure 5.
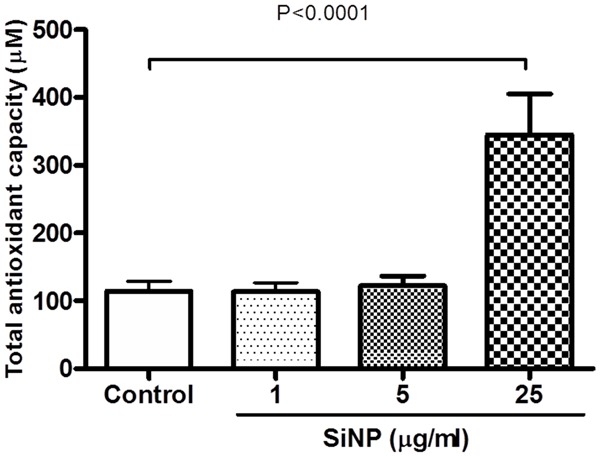
Effect of of silica nanoparticles (SiNP) on the total antioxidant activity in mouse platelets. Data are mean ± SEM (n = 8 in each group). Statistical analysis by one-way ANOVA followed by Dunnett’s multiple range tests.
Effect of SiNP on intracellular calcium
Figure 6 represents the effect of SiNP on platelet cytosolic calcium concentration. Compared with the control group, the incubation of platelets with SiNP induced a significant increase in platelet cytosolic calcium concentration. The level of significance was achieved at 25 μg/ml (P < 0.05).
Figure 6.
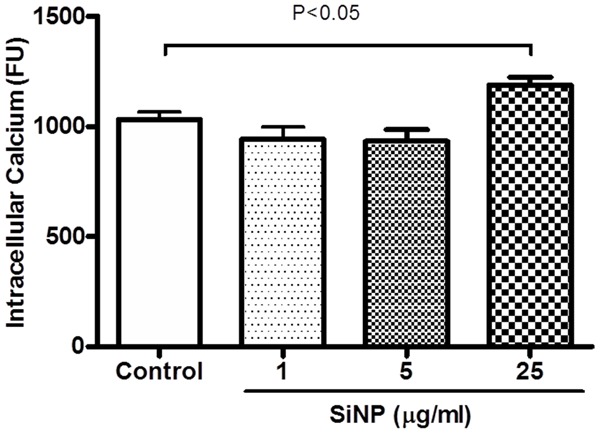
Effect of silica nanoparticles (SiNP) on cytosolic calcium concentration in mouse platelets. Data are mean ± SEM (n = 4-6 in each group). Statistical analysis by one-way ANOVA followed by Dunnett’s multiple range tests.
Discussion
In this work, we showed that in vitro incubation of platelets with SiNP caused platelet aggregation, oxidative stress and increased intracellular calcium.
The distinctive characteristics of nanoparticles including their very small size, large surface area and cellular penetration capacity are responsible for their specific biological responses that differ from larger particle of similar chemical composition [1,17]. In this context, nanoparticles have been demonstrated to cause greater biological responses and particle-mediated toxicity than larger particles per given mass [17]. Exposure to silica nanoparticles, both colloidal and crystalline silica, induced more lung inflammation and injury compared with larger particles [1]. Nanoparticles can reach the systemic circulation via various routes including injection, skin penetration, ingestion or inhalation and interact with circulatory cells including platelets [1,17]. Therefore, studies on the interaction of nanoparticles with platelets are pertinent and can possibly have significant health implications.
In the present study, we assessed the effect of three graded concentrations of SiNP on platelets. These concentrations are comparable with those reported in recent studies which assessed the in vitro effects of SiNP on human platelet aggregation (10-100 μg/ml) and mouse erythrocytes (1-125 μg/ml). Also, they are very small compared with intravenously injected SiNP in mice which consisted of 29.5-177.5 mg/kg [18] and 10-30 mg/kg [19]. Additional studies are required assess whether the concentrations of SiNP used in the current study can be reached in the blood or tissue of humans.
We have recently demonstrated that intraperitoneal administration of amorphous SiNP caused prothrombotic effects in cerebral microvessel of mice, altered endothelial function and induced systemic inflammation [7]. Moreover, we found that the in vitro incubation of SiNP in whole blood caused platelet aggregation in a dose-dependent fashion [7]. The last finding has prompted us to conduct the present study to specifically assess the direct effects of SiNP on washed platelets. Our data show that SiNP induced significant and dose-dependent aggregation of mouse platelets. Our results are in agreement with those of Corbalan et al. [8] who showed that exposure of human platelets in vitro to amorphous SiNP induced a low [NO]/[ONOO-] ratio leading to platelet aggregation [8]. The last effect was greater when nanoparticle size decreased and particle concentration increased [8].
Our data show that incubation of mouse platelets with SiNP caused a significant increase of LDH activity, suggesting that SiNP can adversely affect platelet membrane integrity and induce cytolysis. Such an effect was not reported in the context of interaction of SiNP with platelets. It has been reported that incubation of endothelial cell line with SiNP cause a cytotoxic damage assessed by LDH release and a decrease in cell survival in a dose-related manner [20].
Oxidative stress results from an imbalance between radical-generating and radical-scavenging systems leading to cell membrane impairment or DNA damage [21]. In view of the observed proaggregatory and cytotoxic effects of SiNP, we wanted to assess the mechanisms underlying these effects by verifying whether, and to what extent, would SiNP affect markers of oxidative stress, comprising MDA and total antioxidants. Our data show that SiNP cause significant and dose-dependent increase of platelet MDA which is a reflection of lipid peroxidation [21]. This finding is in line with the alteration of platelet membrane integrity assessed by LDH increase. Furthermore, we found that SiNP cause a significant increase in total antixidants. It is possible that the increase in oxidative stress induced by SiNP may have been accompanied by increased antioxidant enzymes, indicating that SiNP could trigger adaptive responses that counterbalance the potentially damaging activity of oxygen radicals. An increase of total antioxidants in bronchoalveolar lavage has been observed following the pulmonary exposure to particulate air pollution in mice [22]. It has been reported that amorphous SiNP penetrate the platelet plasma membrane and stimulate a rapid and prolonged NO release leading to eNOS uncoupling, ONOO- over-production and eventually, a low [NO]/[ONOO-] ratio and high nitroxidative/oxidative stress [8].
Ca2+ is an essential second messenger in practically all cells, controlling a large range of cellular processes [23]. It is well known that platelets that have not been activated maintain a low cytosolic Ca2+ concentration and a steep plasma membrane Ca2+ gradient [23]. However, when activated by agonists, a significant increase in cytosolic Ca2+ is observed. The elevation in intracellular Ca2+ concentration contributes to various steps of cellular activation, such as reorganization of the actin cytoskeleton necessary for shape change, degranulation or inside-out activation of integrin αIIbß3, essential for platelet aggregation [23]. Our data show a significant increase in cytosolic Ca2+ after the incubation of platelets with 25 μg/ml SiNP. This finding is in line the proaggregatory effects induced by SiNP. This action is novel and as far as we are aware not previously reported. In human aortic endothelial cells, the possibility that nanoparticles can affect Ca2+ channels as well as the endothelial nitric oxide synthase that is associated with caveolae has been raised [24]. It was also speculated that SiNP are likely to stimulate a Ca2+-dependent NO release in human platelets [8].
In conclusion, our data show that incubation of platelets with SiNP caused platelet aggregation, oxidative stress and increased intracellular Ca2+ in vitro. This finding provides new insights into the toxicity of SiNP on platelet physiology. Further work is warranted to uncover the mechanisms underlying our observed effects and to study the interaction of SiNP with platelet by electron microscopy.
Acknowledgements
The authors would like to thank Mr Saeed Tariq for his technical help with EM picture. This work was supported by the funds of the College of Medicine and Health Sciences, United Arab Emirates University (UAEU), and UAEU-Sultan Qaboos University grant.
Disclosure of conflict of interest
No conflicts of interest to disclose.
References
- 1.Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH. The nanosilica hazard: another variable entity. Part Fibre Toxicol. 2010;7:39. doi: 10.1186/1743-8977-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 3.Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med. 2001;164:1665–1668. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- 4.Pery AR, Brochot C, Hoet PH, Nemmar A, Bois FY. Development of a physiologically based kinetic model for 99m-Technetium-labelled carbon nanoparticles inhaled by humans. Inhal Toxicol. 2009;21:1099–1107. doi: 10.3109/08958370902748542. [DOI] [PubMed] [Google Scholar]
- 5.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particle following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- 6.Nabeshi H, Yoshikawa T, Matsuyama K, Nakazato Y, Arimori A, Isobe M, Tochigi S, Kondoh S, Hirai T, Akase T, Yamashita T, Yamashita K, Yoshida T, Nagano K, Abe Y, Yoshioka Y, Kamada H, Imazawa T, Itoh N, Kondoh M, Yagi K, Mayumi T, Tsunoda S, Tsutsumi Y. Amorphous nanosilicas induce consumptive coagulopathy after systemic exposure. Nanotechnology. 2012;23:045101. doi: 10.1088/0957-4484/23/4/045101. [DOI] [PubMed] [Google Scholar]
- 7.Nemmar A, Albarwani S, Beegam S, Yuvaraju P, Yasin J, Attoub S, Ali BH. Amorphous silica nanoparticles impair vascular homeostasis and induce systemic inflammation . Int J Nanomedicine. 2014;9:2779–89. doi: 10.2147/IJN.S52818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbalan JJ, Medina C, Jacoby A, Malinski T, Radomski MW. Amorphous silica nanoparticles aggregate human platelets: potential implications for vascular homeostasis. Int J Nanomedicine. 2012;7:631–639. doi: 10.2147/IJN.S28293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbalan JJ, Medina C, Jacoby A, Malinski T, Radomski MW. Amorphous silica nanoparticles trigger nitric oxide/peroxynitrite imbalance in human endothelial cells: inflammatory and cytotoxic effects. Int J Nanomedicine. 2011;6:2821–2835. doi: 10.2147/IJN.S25071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulaftali Y, Adam F, Venisse L, Ollivier V, Richard B, Taieb S, Monard D, Favier R, Alessi MC, Bryckaert M, Arocas V, Jandrot-Perrus M, Bouton MC. Anticoagulant and antithrombotic properties of platelet protease nexin-1. Blood. 2010;115:97–106. doi: 10.1182/blood-2009-04-217240. [DOI] [PubMed] [Google Scholar]
- 11.Towhid ST, Tolios A, Munzer P, Schmidt EM, Borst O, Gawaz M, Stegmann E, Lang F. Stimulation of platelet apoptosis by balhimycin. Biochem Biophys Res Commun. 2013;435:323–326. doi: 10.1016/j.bbrc.2013.01.120. [DOI] [PubMed] [Google Scholar]
- 12.Nemmar A, Al-Salam S, Zia S, Marzouqi F, Al-Dhaheri A, Subramaniyan D, Yasin J, Ali BH, Kazzam EE. Contrasting actions of diesel exhaust particles on the pulmonary and cardiovascular systems and the effects of thymoquinone. Br J Pharmacol. 2011;164:1871–1882. doi: 10.1111/j.1476-5381.2011.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemmar A, Yuvaraju P, Beegam S, John A, Raza H, Ali BH. Cardiovascular effects of nose-only water-pipe smoking exposure in mice. Am J Physiol Heart Circ Physiol. 2013;305:H740–H746. doi: 10.1152/ajpheart.00200.2013. [DOI] [PubMed] [Google Scholar]
- 14.Pignatelli P, Lenti L, Sanguigni V, Frati G, Simeoni I, Gazzaniga PP, Pulcinelli FM, Violi F. Carnitine inhibits arachidonic acid turnover, platelet function, and oxidative stress. Am J Physiol Heart Circ Physiol. 2003;284:H41–H48. doi: 10.1152/ajpheart.00249.2002. [DOI] [PubMed] [Google Scholar]
- 15.Olas B, Kedzierska M, Wachowicz B, Stochmal A, Oleszek W. Effects of polyphenol-rich extract from berries of Aronia melanocarpa on the markers of oxidative stress and blood platelet activation. Platelets. 2010;21:274–281. doi: 10.3109/09537101003612821. [DOI] [PubMed] [Google Scholar]
- 16.Foller M, Mahmud H, Gu S, Wang K, Floride E, Kucherenko Y, Luik S, Laufer S, Lang F. Participation of leukotriene C(4) in the regulation of suicidal erythrocyte death. J Physiol Pharmacol. 2009;60:135–143. [PubMed] [Google Scholar]
- 17.Nemmar A, Holme JA, Rosas I, Schwarze PE, Alfaro-Moreno E. Recent advances in particulate matter and nanoparticle toxicology: a review of the in vivo and in vitro studies. Biomed Res Int. 2013;2013:279371. doi: 10.1155/2013/279371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y, Li Y, Wang W, Jin M, Du Z, Li Y, Duan J, Yu Y, Sun Z. Acute toxicity of amorphous silica nanoparticles in intravenously exposed ICR mice. PLoS One. 2013;8:e61346. doi: 10.1371/journal.pone.0061346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimori H, Kondoh M, Isoda K, Tsunoda S, Tsutsumi Y, Yagi K. Histological analysis of 70-nm silica particles-induced chronic toxicity in mice. Eur J Pharm Biopharm. 2009;72:626–629. doi: 10.1016/j.ejpb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Napierska D, Thomassen LC, Rabolli V, Lison D, Gonzalez L, Kirsch-Volders M, Martens JA, Hoet PH. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small. 2009;5:846–853. doi: 10.1002/smll.200800461. [DOI] [PubMed] [Google Scholar]
- 21.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemmar A, Al Salam S, Dhanasekaran S, Sudhadevi M, Ali BH. Pulmonary exposure to diesel exhaust particles promotes cerebral microvessel thrombosis: protective effect of a cysteine prodrug l-2-oxothiazolidine-4-carboxylic acid. Toxicology. 2009;263:84–92. doi: 10.1016/j.tox.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7:1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa T, Iwakiri N, Kaneko Y, Taguchi A, Fukushima K, Mori H, Morone N, Kadokawa J. Nitric oxide release in human aortic endothelial cells mediated by delivery of amphiphilic polysiloxane nanoparticles to caveolae. Biomacromolecules. 2009;10:2074–2085. doi: 10.1021/bm900128x. [DOI] [PubMed] [Google Scholar]


