Abstract
Norovirus (NoV) genogroup I (GI) and GII are responsible for most human infections with NoV. Because of the high genetic variability of NoV, natural infection does not induce sufficient protective immunity to different genotypes or to variants of the same genotype and there is little or no cross-protection against different genogroups. NoV-derived virus-like particles (VLPs) are promising vaccine candidates that induce high levels of NoV-specific humoral and cellular immune responses. It is believed that a bivalent NoV vaccine consisting of a representative VLP from GI and GII is a minimum requirement for an effective vaccine. Here, we compared the abilities of monovalent immunizations with NoV GI.1-2001, GI.3-2002, GII.4-1999, and GII.4-2010 New Orleans VLPs to induce NoV type-specific and cross-reactive immune responses and protective blocking antibody responses in BALB/c mice. All of the VLPs induced comparable levels of type-specific serum IgG antibodies, as well as blocking antibodies to the VLPs used for immunization. However, the abilities of different VLP genotypes to induce cross-reactive IgG and cross-blocking antibodies varied remarkably. Our results confirm previous findings of a lack of cross-protective immune responses between GI and GII NoVs. These data support the rationale for including NoV GI.3 and GII.4-1999 VLPs in the bivalent vaccine formulation, which could be sufficient to induce protective immune responses across NoV genotypes in the two common genogroups in humans.
INTRODUCTION
Noroviruses (NoVs) are the leading cause of sporadic and epidemic nonbacterial gastroenteritis worldwide (1, 2). NoV disease is characterized by a short duration of symptoms (3), which can be severe, especially for people in high-risk groups, such as young children, the elderly, or immunocompromised patients. There is currently no vaccine available to prevent NoV infection. Cell culture models to support the propagation of human NoVs have previously failed, hampering the use of live or attenuated NoV vaccines. However, Jones et al. recently described a cell culture model to productively infect human B cells with NoV (4) that might be a step closer to successful NoV propagation. NoV capsid VP1 protein spontaneously forms virus-like particles (VLPs) morphologically and antigenically similar to NoV virions (5, 6). NoV VLPs can be efficiently produced in insect cells with baculovirus expression systems and a variety of other protein expression systems (5, 7). VLPs are promising candidates for use in a vaccine against NoV (8–10), as well as several other viruses, including influenza virus (11), parvovirus (12), and HIV-1 (13). VLP-based vaccines against hepatitis B virus (14, 15) and human papillomavirus (16) are currently licensed and used worldwide. As VLPs are highly immunogenic particulate structures, it is believed that addition of external adjuvants is not needed (17). This is very important, particularly when designing NoV vaccines for a pediatric population (9). However, clinical trials of NoV VLP vaccine conducted with adults have used adjuvants (18, 19) and proven that adjuvanted NoV VLP vaccine is safe and immunogenic.
NoVs are single-stranded, positive-sense RNA viruses in the Caliciviridae family and are genetically very heterogeneous, with six genogroups (GI to GVI) recognized so far (20, 21). GI and GII NoVs are responsible for most human NoV infections, comprising more than 30 genotypes that evolve rapidly to novel immune escape variants (22). GII viruses are responsible for approximately 90% of the human NoV infections that occur each year, most of which are caused by variants of a single GII.4 genotype (2, 23). New emerging strains develop approximately every 2 to 3 years, and they have been related to changes in blocking antibody epitopes in the hypervariable P2 domain of VP1 (24, 25).
Diverse putative receptors/attachment factors for NoVs, histo-blood group antigen (HBGA) carbohydrates, are found on mucosal epithelial cells and as free antigens in body secretions (22, 26). HBGA expression is associated with susceptibility or resistance to certain NoV strains (26, 27). GII.4 strain NoVs have an exceptionally broad HBGA binding repertoire and high transmissibility (2), explaining the predominance of GII.4 NoV infections worldwide (28). The quantity of genotype-specific antibodies that can block the binding of NoV VLP to the HBGA has been shown to increase remarkably after NoV infection or NoV VLP immunization in humans (18, 19, 29, 30). Prechallenge levels of blocking antibodies in human serum have been shown to positively correlate with the protection of both NoV infection and illness, and it is generally accepted that especially blocking antibodies in serum play a substantive role in protection from NoV infection (18, 31).
Natural immunity to NoV has been believed to have a short duration (32, 33); however, a more recent estimate suggests that protection could last up to 8 years (34). However, induction of long-term protective immunity is extremely challenging because of the rapid evolution of NoV strains that result in high genetic variability and insufficient cross-protective immunity, especially between GI and GII NoVs (30, 35, 36). It is believed that a representative of each genogroup is a minimum requirement for cross-protective NoV vaccine (10, 37, 38). Indeed, research groups working on NoV vaccine development have used VLP combinations to constitute their vaccine candidates (8, 19, 35, 38).
We have tested NoV GI.1, GI.3, GII.4-1999, and GII.4-2010 New Orleans (NO) VLPs representing GI and GII NoVs as vaccine candidates in BALB/c mice. GI.1 (Norwalk virus) represents a historic prototype NoV (39), and GI.3 is a commonly found GI virus that also efficiently infects children (40, 41). As NoV GII.4 has dominated worldwide for close to 2 decades (24), we have chosen an ancestor GII.4 strain (1999) and a more recent GII.4 NO (2010) strain as representatives of GII NoVs.
MATERIALS AND METHODS
VLP generation.
Six NoV VLPs, GI.1-2001 (GenBank accession no. AY502016), GI.3-2002 (GenBank accession no. AF414403), GII.4-1999 (GenBank accession no. AF080551), GII.4-2010 NO (GenBank accession no. GU445325), GII.4-2012 Sydney (GenBank accession no. AFV08795.1), and GII.12-1998 (GenBank accession no. AJ277618), were expressed with a baculovirus expression system and purified from insect cells by sucrose ultracentrifugation as described previously (42, 43). VLP preparations were analyzed as described elsewhere (38, 42, 43). In brief, the total protein concentration was determined with the Pierce bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL). The purity and integrity of the VLP preparations were analyzed by SDS-PAGE and densitometric analysis. Endotoxins (<0.1 endotoxin unit/10 μg of protein) were quantified by Limulus amebocyte lysate assay (Lonza, Walkersville, MD). VLP morphology was confirmed by transmission electron microscopy, with all VLPs displaying particles of approximately 30 to 40 nm (38). NoV GI.1-, GI.3-, GII.4-1999-, and GII.4-2010 NO-derived VLPs were used to immunize mice as described below. In addition, GII.4-2012 Sydney and GII.12 VLPs were used in analytical methods.
Mouse immunization and sample collection.
Female 7- to 8-week-old BALB/c mice obtained from Harlan Laboratories (Netherlands) were used for immunization. Two doses of 10 μg of monovalent (10 mice/group) NoV VLPs (GI.1, GI.3, GII.4-1999, and GII.4-2010 NO) in phosphate-buffered saline (PBS) were administered intramuscularly. In another set of experiments, mice were immunized with a bivalent combination of GI.3 and GII.4-1999 VLPs (five mice/group). Control mice were immunized with PBS only. Mice were immunized at weeks 0 and 3 and euthanized 2 weeks after the second immunization. Animals were anesthetized before immunization and euthanasia with a mixture of medetomidine (Dorbenevet, 1 mg/ml; Laboratorios SYVA S.A., Leon, Spain) and ketamine (Ketaminol vet, 50 mg/ml; Intervet International B.V., Boxmeer, Netherlands). Blood and spleens were collected at the time of termination for the analysis of serological and cell-mediated immune responses as previously described (44). Serum samples were stored at −20°C, and spleen cell suspensions were stored in liquid nitrogen. Experiments were performed in accordance with the guidelines of the Finnish National Animal Experiment Board.
Serum IgG, IgG1, and IgG2a ELISA.
Serum of immunized mice was analyzed by enzyme-linked immunosorbent assay (ELISA) (42) to determine type-specific and cross-reactive IgG, IgG1, and IgG2a titers. Briefly, 0.4 to 1.0 μg/ml of GI.1, GI.3, GII.4-1999, GII.4-2010 NO, GII.4-2012 Sydney, or GII.12 VLPs in PBS was used to coat 96-well half-area polystyrene plates (Corning Inc., Corning, NY). Duplicates of 2-fold serial dilutions of serum samples (starting at 1:200) were incubated for 2 h at room temperature, and antibodies were detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Sigma-Aldrich, Saint Louis, MO), IgG1 (Invitrogen, Carlsbad, CA), or IgG2a (Invitrogen), followed by reaction with the SIGMAFAST o-phenylenediamine dihydrochloride (OPD) substrate (Sigma-Aldrich). Optical density (OD) at 490 nm was measured with a Victor2 1420 Multilabel Counter (Wallac, PerkinElmer) plate reader. The background signal in wells without serum was subtracted from all of the OD readings of a plate. The wells of control mouse serum were used to count the cutoff value as the mean OD plus three standard deviations. Samples with a net OD value above the set cutoff and an OD of at least 0.100 were considered positive (45). The endpoint antibody titer was defined as the highest dilution of serum giving an OD above the set cutoff value.
Blocking of HBGA binding.
ELISAs measuring antibodies able to block NoV VLP binding to HBGAs were performed by previously published methods based on the use of synthetic or human saliva HBGAs (28, 42, 44). Groupwise pooled serum was diluted 2-fold starting at 1:100 for homologous blocking and 1:10 for heterologous blocking. For a blocking assay using synthetic HBGAs, serum was preincubated for 1 h at 37°C with 0.4 μg/ml of NoV VLPs before plating on biotin-conjugated synthetic HBGA (GlycoTech Corporation, Rockville, MD)-coated NeutrAvidin plates (Pierce, Rockford, IL). Synthetic H type 1 HBGA was used for binding of all VLPs, except for GI.3 VLPs and GII.12 VLPs, which were tested on Lewisa (Lea) and blood type B trimer (Btri) HBGAs. For a saliva blocking assay, secretor-positive human type A or O saliva (for GI.1 VLPs only) was used at a dilution of 1:3,000 in PBS to coat half-area 96-microtiter plates before adding the preincubated mixture of serum and 0.1 μg/ml of NoV VLP. Maximum VLP binding was determined in wells containing VLPs without serum. Bound VLPs were detected with human NoV antiserum (29) and an anti-human IgG-HRP secondary antibody, followed by OPD substrate as described above. OD readings were determined spectrophotometrically at 490 nm with a microplate reader. The blocking index (percent) was calculated as follows: 100% − [(OD wells with VLP and serum/OD wells without serum, maximum binding) × 100%].
IFN-γ ELISPOT assay.
A gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay was used to enumerate GII.4 NoV-specific IFN-γ-producing T cells of immunized mice as previously described (38). Mouse splenocytes from either individual or groupwise pooled mice were plated on MultiScreenHTS-IP filter plates (Millipore, Billerica, MA) coated with an anti-mouse IFN-γ monoclonal antibody (Mabtech AB, Nacka Strand, Sweden) at 5 μg/ml and blocked with 10% fetal bovine serum (FBS; Sigma-Aldrich). A total of 1 × 105 splenocytes were stimulated with synthetic peptides (5 μg/ml) for 20 h in cell culture medium (CM; RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-mercaptoethanol, and 2 mM l-glutamine; Sigma-Aldrich). The following NoV capsid-derived synthetic 15-mer peptides (ProImmune Ltd., Oxford, United Kingdom) were used: NP-4, a previously published T-cell epitope of an ancestor GII.4 virus (CLLPQEWVQHFYQEA, amino acids 461 to 475) (46); NP-4 NO, a corresponding GII.4 NO-specific peptide (CLLPQEWVQYFYQEA); and NP-12, a GII.12-specific peptide (CLLPQEWIQHLYQES). Stimulation with concanavalin A (ConA; Sigma-Aldrich) at 10 μg/ml was used as a positive control. CM alone and irrelevant rotavirus 15-mer peptide R6-3 (IFPYSASFTLNRSQP) were added as negative controls to each assay. IFN-γ was detected with a biotinylated anti-mouse IFN-γ monoclonal antibody (Mabtech; 0.5 μg/ml in PBS–0.5% FBS), followed by 1:1,000-diluted streptavidin-alkaline phosphatase (Mabtech), and spots were developed with 5-bromo-4-chloro-3-indolylphosphate (BCIP)-Nitro Blue Tetrazolium substrate (Mabtech). Plate reading and analysis were done by an ImmunoSpot automatic cytotoxic T-lymphocyte analyzer (CTL-Europe GmbH, Bonn, Germany), and the results are expressed as the mean number of spot-forming cells (SFC)/106 splenocytes in duplicate wells.
Statistics.
A nonparametric Mann-Whitney U test was performed to assess the statistical significance of differences. Data were analyzed with IBM SPSS Statistics version 20.0 (SPSS Inc., Chicago, IL). A statistically significant difference was defined as a P value of ≤0.05.
RESULTS
Genotype-specific serum IgG, IgG1, and IgG2a titers.
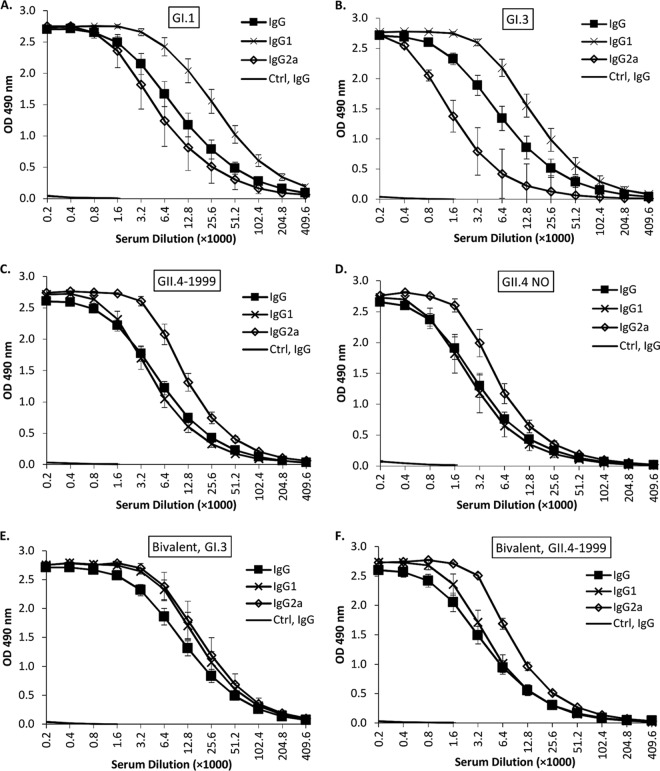
Mice were immunized with monovalent NoV VLPs or a bivalent combination of GI.3 and GII.4-1999 VLPs. The total serum IgG antibodies in mouse serum were analyzed 2 weeks after the second immunization. Robust NoV genotype-specific IgG responses against each VLP were detected (Fig. 1A to F) with endpoint titers of >51,200. In addition, IgG antibody subclass IgG2a (representing a Th1-type response) and IgG1 (representing a Th2-type response) titers in serum were measured. All VLP immunizations induced both IgG1 and IgG2a antibodies (Fig. 1A to F). Mice immunized with the bivalent combination of GI.3 and GII.4-1999 VLPs developed IgG, IgG1, and IgG2a antibody responses comparable to those of mice immunized with each of the single VLPs (all P > 0.6) (Fig. 1E and F). Control mouse serum was completely negative for all VLP antigens (Fig. 1A to F).
FIG 1.
NoV genotype-specific serum IgG, IgG1, and IgG2a antibody titers. Termination sera of mice immunized with monovalent NoV GI.1 (A), GI.3 (B), GII.4-1999 (C), or GII.4 NO (D) VLPs were titrated against homotypic NoV VLP antigen in an ELISA. Bivalent GI.3 and GII.4-1999 VLP-immunized mouse serum was analyzed for GI.3 (E)- and GII.4-1999 (F)-specific antibodies. Serum of mice immunized with only the carrier (PBS) served as a control (Ctrl). Shown are the mean ODs of the groups with error bars representing the standard errors of the means.
Cross-reactive IgG antibodies.
In addition to type-specific antibody responses, cross-reactive serum IgG against GI.1, GI.3, GII.4-1999, GII.4 NO, GII.4 Sydney, and GII.12 NoV genotypes were analyzed. Strong intragenogroup-specific IgG responses were observed in each experimental group, but only limited, significantly lower (P < 0.05) intergenogroup-specific antibody responses were detected (Fig. 2). Immunization with the bivalent GI.3 and GII.4-1999 VLPs induced a response similar to that induced by immunization with each VLP alone; thus, it generated the broadest serum IgG response. All of the ODs of the PBS-immunized control mice were <0.05.
FIG 2.
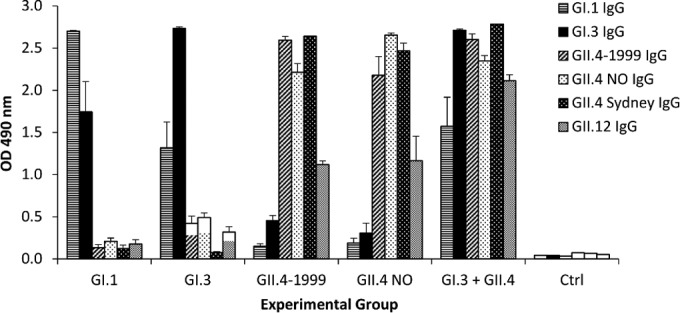
NoV VLP genotype-specific and cross-reactive serum IgG responses against five heterologous NoV genotypes. Serum was analyzed by ELISA at a dilution of 1:200 after monovalent GI.1, GI.3, GII.4-1999, or GII.4 NO or bivalent GI.3 and GII.4-1999 VLP immunization. Serum of PBS-immunized mice was used as a negative control (Ctrl). Shown are the mean ODs of the experimental groups with error bars representing the standard errors of the means.
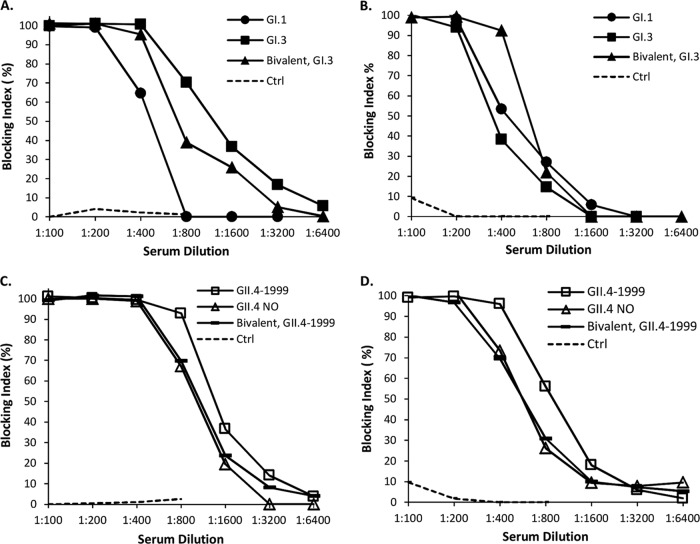
Blocking activity of immune mouse sera.
The capability of mouse immune sera to block NoV VLP binding was determined by incubating serially diluted sera with NoV VLPs before plating on microtiter plates coated with synthetic HBGAs or human type A or O saliva. On the basis of the data recently reported by our group (28), synthetic H type 1 HBGA was chosen to be used for GI.1, GII.4-1999, GII.4 NO, and GII.4 Sydney blocking assays, while Lea was used for GI.3 VLP blocking and Btri was used for GII.12 VLP blocking. Homologous blocking activity in sera from each experimental group measured by a saliva assay is shown in Fig. 3A and C, and that measured by a synthetic HBGA assay is shown in Fig. 3B and D. All immunizations with monovalent VLPs, as well as the bivalent VLP, generated strong blocking activity in serum. The serum antibody titers needed to block ≥90% of the VLPs from binding either to saliva or to the synthetic HBGAs ranged from 1:200 to 1:400. Control mouse sera blocked ≤10% of the VLP binding.
FIG 3.
NoV genotype-specific blocking activities of monovalent and bivalent VLP-immunized mouse sera. Serum samples were diluted 2-fold and assayed for the blocking of homologous NoV VLP binding to human saliva (A and C) and synthetic (B and D) HBGA-coated plates. Type A saliva was used, except for GI.1 VLP binding, where type O saliva was used. H type 1 HBGA was used in GI.1, GII.4-1999, and GII.4 NO synthetic blocking assays, while Lea HBGA was used for GI.3 VLP blocking. GI-specific blocking is shown in the upper panels, and GII-specific blocking is shown in the lower panels. Nonspecific blocking by control (Ctrl) mouse serum is shown as dashed lines. The blocking index (percent) was calculated as follows: 100% − [(OD wells with serum/OD wells without serum, maximum binding) × 100%].
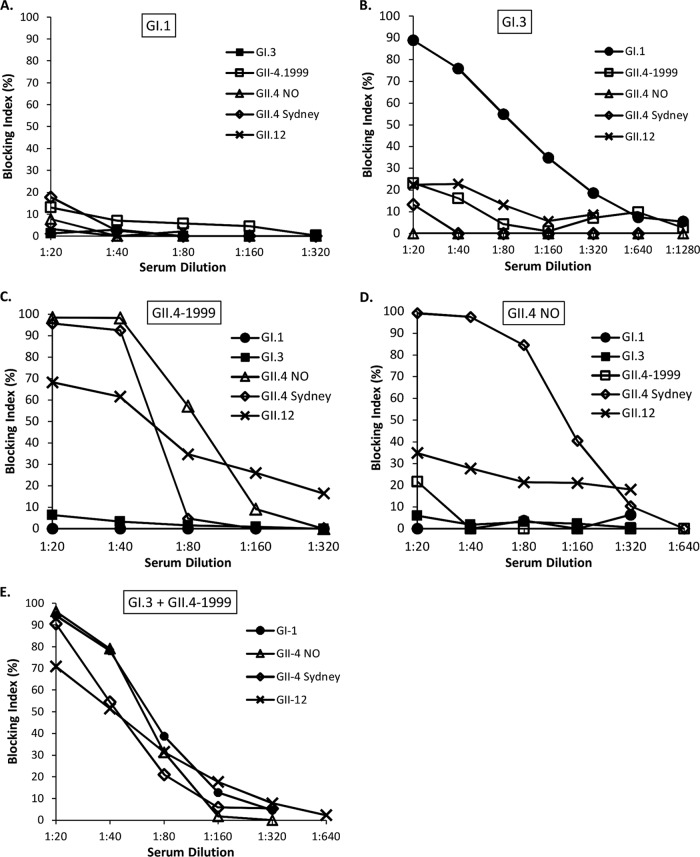
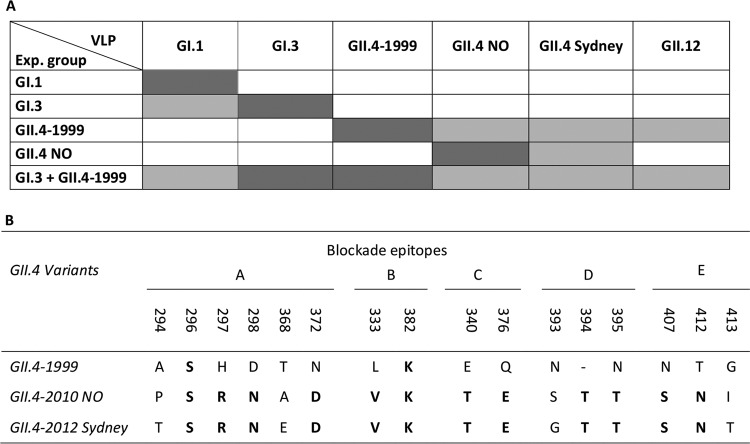
Serum blocking activity against heterologous VLPs not used for immunization was also investigated. Remarkable differences between the experimental groups were observed (Fig. 4A to E and 5A). GI.1 VLP-immunized mice did not generate any cross-blocking serum activity (Fig. 4A and 5A), while GI.3 VLP-immunized mouse serum blocked heterologous GI.1 VLP binding but no GII VLP binding (Fig. 4B and 5A). Serum of GII.4-1999 VLP-immunized mice blocked the binding of all of the GII VLPs tested, i.e., GII.4 NO, GII.4 Sydney, and GII.12 (Fig. 4C and 5A). On the contrary, GII.4 NO VLP-immunized mouse serum was able to cross-block only GII.4 Sydney VLP binding (Fig. 4D and 5A). Mice immunized with the bivalent VLP combination blocked the binding of all of the heterologous VLPs tested (Fig. 4E and 5A). The control mouse serum blocking index against each VLP at a dilution of 1:20 was <20% in every assay (data not shown).
FIG 4.
Serum blocking of heterologous VLP binding. The serum of mice immunized with GI.1 (A), GI.3 (B), GII.4-1999 (C), GII.4 NO (D), or bivalent GI.3 and GII.4 (E) VLPs was serially 2-fold diluted starting at a 1:20 dilution and analyzed in a blocking assay against heterologous GI and GII VLPs.
FIG 5.
Serum blocking activity and blockade epitopes. (A) Schematic representation of homologous and heterologous blocking activities of immune mouse serum. Dark gray shading indicates homologous blocking, and light gray shading indicates heterologous blocking. Absence of shading indicates absence of blocking activity. (B) Variation in the amino acid sequences of blockade epitopes A to E (22, 51) in subdomain P2 of the three GII.4 variants used in this study. Identical amino acids are in boldface.
IFN-γ ELISPOT assay.
We further tested the T cell responses of mice immunized with GII.4-1999 and GII.4 NO VLPs to 15-mer synthetic peptides specific for GII.4 (NP-4 and NP-4 NO, respectively) and GII.12 (NP-12) capsid proteins in an IFN-γ ELISPOT assay (44). Immunization with GII.4-1999 VLPs induced robust self NP-4 peptide-specific (304 SFC/106 cells) and cross-reactive NP-4 NO-specific (206 SFC/106 cells) and NP-12-specific (269 SFC/106 cells) IFN-γ responses (Fig. 6). On the contrary, GII.4 NO VLP-immunized mice did not respond to any of the peptides (Fig. 6). Negative-control 15-mer peptide R6-3 did not induce IFN-γ responses in any of the mice (<50 SFC/106 cells). ConA stimulation indicated good cell viability (all >5,000 SFC/106 cells, data not shown). Control mice did not respond to any peptide antigen stimulation (<50 SFC/106 splenocytes).
FIG 6.
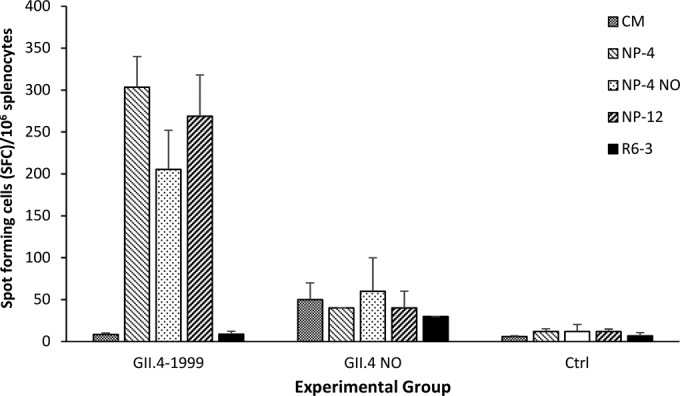
NoV-specific IFN-γ responses. Splenocytes of mice immunized with the monovalent GII.4-1999 and GII.4 NO VLPs or control (Ctrl) mice immunized with the carrier (PBS) only were stimulated in vitro with 15-mer NoV capsid-derived synthetic peptides specific for GII.4-1999 (NP-4), GII.4 NO (NP-4 NO), and GII.12 (NP-12). An irrelevant peptide (R6-3) was used as a negative control, and CM served as a background control. The results shown are the mean numbers of SFC/106 cells and the standard errors of the means.
DISCUSSION
NoV GI.1, GI.3, GII.4-1999, and GII.4-2010 NO VLPs representing GI and GII NoVs were used as monovalent vaccine candidates to immunize BALB/c mice. NoV VLP immunization of mice induced humoral and cell-mediated immune responses, which is in concordance with previously published results (38, 42, 44). Immunizations with each monovalent VLP resulted in high genotype-specific and cross-reactive intragenogroup-specific serum IgG titers. Low levels of cross-reactive intergenogroup-specific IgG antibodies detected were probably directed to the conserved N-terminal region and S domain of NoV capsid VP1 (47). Importantly, strong blocking of NoV VLP binding by homologous immune sera was observed in each experimental group.
Comparison of monovalent immunizations with several NoV VLPs revealed different potentials of the proteins to induce cross-reactive blocking antibodies. As GI NoVs are very well conserved (48), we expected to see similar results induced by the GI.1 and GI.3 VLPs. However, GI.3 VLPs induced serum antibodies able to block GI.1 VLP binding to the HBGAs but not vice versa. Also, although NoV VLPs GII.4-1999 and GII.4-2010 NO belong to the same genotype, having approximately 95% homology in the VP1 capsid protein amino acid sequences (21, 49), there is remarkable difference in the putative protective immune responses they induce. We demonstrated in this study that GII.4-1999 ancestor VLPs induced cross-blocking antibodies against distant and rare but recently reemerging GII NoV genotype GII.12 (23, 50), as well as to GII.4 NO and to the most recent GII.4-2012 Sydney VLPs. On the contrary, GII.4 NO VLP immunization induced cross-blocking antibodies only to closely related GII.4 Sydney. Figure 5B shows the variation in the blockade epitopes (22, 51) of GII.4-1999, GII.4-2010 NO, and GII.4-2012 Sydney variants. Although 14/16 amino acid residues differ between the 1999 and 2012 variants and only 4/16 amino acid residues differ between the 2010 and 2012 variants, it is remarkable that GII.4-1999 VLP immunization induced maximum blocking against GII.4 Sydney at a level similar to that of more closely related GII.4 NO VLPs. An explanation for the different cross-protective responses seen in this study might be that GII.4-1999 and GI.3 VLPs induce serum antibodies with much stronger affinity that better tolerate the variations within the target epitopes (8, 22, 35). In addition, there might exist as-yet-undetermined cross-reactive blockade epitopes across the NoV capsid protein. Furthermore, Zhu et al. recently showed that two genetically highly related intracluster murine NoV strains displayed very different abilities to induce protective immune responses (52). We also showed that GII.4-1999 VLPs had a T cell immune response-inducing ability superior to that of GII.4-2010 NO VLPs, which is congruent with the cross-blocking antibody responses detected. It is possible that the stronger the T cell responses generated are, the better the cross-reactive blocking antibodies induced are.
Altogether, the results of this study indicate that measurement of the NoV strain-specific IgG level or the cross-reactive binding antibodies induced by immunization with different NoV VLPs alone is not adequate to determine the quality of immune responses (31, 51). This is in line with our results obtained with human serum (30), where genogroup-specific cross-reactive IgG titers were raised after natural NoV infection but no equivalent blocking antibody titer increase was induced.
The challenge for the development of a NoV vaccine is related to multiple circulating NoV strains that readily escape the protective immunity induced by previous infections (53, 54). To the contrary, there are indications that immunization of mice with a cocktail of different NoV VLP genotypes may induce heterotypic antibodies that can bind novel variants not included in the cocktail (35). Therefore, it is important to generate as broad a response as possible by vaccination, particularly when immunizing young children with no or a less extensive NoV exposure history. It has been suggested that although the NoV genome accumulates mutations over time, they tend to revert to the amino acid composition of the older strains (40, 55). This would mean that updating the vaccine composition on a regular basis according to the prevalent circulating strain might not be necessary, but instead, cross-reactive immunity should be induced at a sufficient level.
Our results indicate that a bivalent NoV vaccine containing GI.3 and GII.4-1999 VLPs might be sufficient to induce protective immune responses across NoV genotypes and genogroups. The bivalent VLP combination showed an additive effect of the VLPs without mutual inhibition. Moreover, as no cross-protective immunity between the two NoV genogroups was induced, our results confirm the previous findings (38) and support the idea that a NoV vaccine should consist of at least two NoV VLPs belonging to GI and GII (10, 37, 38). However, the genotypes chosen for vaccine design should be carefully considered, as even variants of a single genotype, as shown here, have different abilities to induce cross-protective immune responses. Eventually, only field trials with humans with complex pre-existing NoV immunity will give a definitive picture of the protection induced by VLP-based vaccines.
ACKNOWLEDGMENTS
We thank the laboratory personnel of the Vaccine Research Center of the School of Medicine at the University of Tampere for expert technical assistance during this study.
No external funding was used for this study.
No conflicts of interests exist in publishing this work.
REFERENCES
- 1.Hall AJ, Lopman BA, Payne DC, Patel MM, Gastanaduy PA, Vinje J, Parashar UD. 2013. Norovirus disease in the United States. Emerg Infect Dis 19:1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siebenga JJ, Vennema H, Zheng DP, Vinje J, Lee BE, Pang XL, Ho EC, Lim W, Choudekar A, Broor S, Halperin T, Rasool NB, Hewitt J, Greening GE, Jin M, Duan ZJ, Lucero Y, O'Ryan M, Hoehne M, Schreier E, Ratcliff RM, White PA, Iritani N, Reuter G, Koopmans M. 2009. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001-2007. J Infect Dis 200:802–812. doi: 10.1086/605127. [DOI] [PubMed] [Google Scholar]
- 3.Devasia T, Lopman B, Leon J, Handel A. 8 December 2014. Association of host, agent and environment characteristics and the duration of incubation and symptomatic periods of norovirus gastroenteritis. Epidemiol Infect doi: 10.1017/S0950268814003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinje J, Tibbetts SA, Wallet SM, Karst SM. 2014. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang X, Wang M, Graham DY, Estes MK. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol 66:6527–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst-Kralovetz M, Mason HS, Chen Q. 2010. Norwalk virus-like particles as vaccines. Expert Rev Vaccines 9:299–307. doi: 10.1586/erv.09.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington PR, Yount B, Johnston RE, Davis N, Moe C, Baric RS. 2002. Systemic, mucosal, and heterotypic immune induction in mice inoculated with Venezuelan equine encephalitis replicons expressing Norwalk virus-like particles. J Virol 76:730–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parra GI, Bok K, Taylor R, Haynes JR, Sosnovtsev SV, Richardson C, Green KY. 2012. Immunogenicity and specificity of norovirus consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine 30:3580–3586. doi: 10.1016/j.vaccine.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesikari T, Blazevic V. 2015. Norovirus vaccine: one step closer. J Infect Dis 211:853–855. doi: 10.1093/infdis/jiu498. [DOI] [PubMed] [Google Scholar]
- 10.Atmar RL, Estes MK. 2012. Norovirus vaccine development: next steps. Expert Rev Vaccines 11:1023–1025. doi: 10.1586/erv.12.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haynes JR. 2009. Influenza virus-like particle vaccines. Expert Rev Vaccines 8:435–445. doi: 10.1586/erv.09.8. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein DI, El Sahly HM, Keitel WA, Wolff M, Simone G, Segawa C, Wong S, Shelly D, Young NS, Dempsey W. 2011. Safety and immunogenicity of a candidate parvovirus B19 vaccine. Vaccine 29:7357–7363. doi: 10.1016/j.vaccine.2011.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young KR, McBurney SP, Karkhanis LU, Ross TM. 2006. Virus-like particles: designing an effective AIDS vaccine. Methods 40:98–117. doi: 10.1016/j.ymeth.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Vesikari T, Martin JC, Liss CL, Liska V, Schodel FP, Bhuyan PK. 2011. Safety and immunogenicity of a modified process hepatitis B vaccine in healthy infants. Pediatr Infect Dis J 30:e109-13. doi: 10.1097/INF.0b013e31821ed1a4. [DOI] [PubMed] [Google Scholar]
- 15.Roldão A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. 2010. Virus-like particles in vaccine development. Expert Rev Vaccines 9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 16.Pomfret TC, Gagnon JM Jr, Gilchrist AT. 2011. Quadrivalent human papillomavirus (HPV) vaccine: a review of safety, efficacy, and pharmacoeconomics. J Clin Pharm Ther 36:1–9. doi: 10.1111/j.1365-2710.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- 17.Bachmann MF, Jennings GT. 2010. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 18.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, Mendelman PM. 2011. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med 365:2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treanor JJ, Atmar RL, Frey SE, Gormley R, Chen WH, Ferreira J, Goodwin R, Borkowski A, Clemens R, Mendelman PM. 2014. A novel intramuscular bivalent norovirus virus-like particle vaccine candidate—reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J Infect Dis 210:1763–1771. doi: 10.1093/infdis/jiu337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesquita JR, Costantini VP, Cannon JL, Lin SC, Nascimento MS, Vinje J. 2013. Presence of antibodies against genogroup VI norovirus in humans. Virol J 10:176. doi: 10.1186/1743-422X-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroneman A, Vega E, Vennema H, Vinje J, White PA, Hansman G, Green K, Martella V, Katayama K, Koopmans M. 2013. Proposal for a unified norovirus nomenclature and genotyping. Arch Virol 158:2059–2068. doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debbink K, Lindesmith LC, Donaldson EF, Baric RS. 2012. Norovirus immunity and the great escape. PLoS Pathog 8:e1002921. doi: 10.1371/journal.ppat.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vega E, Barclay L, Gregoricus N, Shirley SH, Lee D, Vinje J. 2014. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009 to 2013. J Clin Microbiol 52:147–155. doi: 10.1128/JCM.02680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eden JS, Tanaka MM, Boni MF, Rawlinson WD, White PA. 2013. Recombination within the pandemic norovirus GII.4 lineage. J Virol 87:6270–6282. doi: 10.1128/JVI.03464-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindesmith LC, Costantini V, Swanstrom J, Debbink K, Donaldson EF, Vinje J, Baric RS. 2013. Emergence of a norovirus GII.4 strain correlates with changes in evolving blockade epitopes. J Virol 87:2803–2813. doi: 10.1128/JVI.03106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutson AM, Atmar RL, Graham DY, Estes MK. 2002. Norwalk virus infection and disease is [sic] associated with ABO histo-blood group type. J Infect Dis 185:1335–1337. doi: 10.1086/339883. [DOI] [PubMed] [Google Scholar]
- 27.Rockx BH, Vennema H, Hoebe CJ, Duizer E, Koopmans MP. 2005. Association of histo-blood group antigens and susceptibility to norovirus infections. J Infect Dis 191:749–754. doi: 10.1086/427779. [DOI] [PubMed] [Google Scholar]
- 28.Uusi-Kerttula H, Tamminen K, Malm M, Vesikari T, Blazevic V. 2014. Comparison of human saliva and synthetic histo-blood group antigens usage as ligands in norovirus-like particle binding and blocking assays. Microbes Infect 16:472–480. doi: 10.1016/j.micinf.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Nurminen K, Blazevic V, Huhti L, Rasanen S, Koho T, Hytonen VP, Vesikari T. 2011. Prevalence of norovirus GII-4 antibodies in Finnish children. J Med Virol 83:525–531. doi: 10.1002/jmv.21990. [DOI] [PubMed] [Google Scholar]
- 30.Malm M, Uusi-Kerttula H, Vesikari T, Blazevic V. 2014. High serum levels of norovirus genotype-specific blocking antibodies correlate with protection from infection in children. J Infect Dis 210:1755–1762. doi: 10.1093/infdis/jiu361. [DOI] [PubMed] [Google Scholar]
- 31.Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, Atmar RL. 2010. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis 202:1212–1218. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parrino TA, Schreiber DS, Trier JS, Kapikian AZ, Blacklow NR. 1977. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N Engl J Med 297:86–89. doi: 10.1056/NEJM197707142970204. [DOI] [PubMed] [Google Scholar]
- 33.Johnson PC, Mathewson JJ, DuPont HL, Greenberg HB. 1990. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis 161:18–21. [DOI] [PubMed] [Google Scholar]
- 34.Simmons K, Gambhir M, Leon J, Lopman B. 2013. Duration of immunity to norovirus gastroenteritis. Emerg Infect Dis 19:1260–1267. doi: 10.3201/eid1908.130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LoBue AD, Lindesmith L, Yount B, Harrington PR, Thompson JM, Johnston RE, Moe CL, Baric RS. 2006. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine 24:5220–5234. doi: 10.1016/j.vaccine.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 36.Farkas T, Thornton SA, Wilton N, Zhong W, Altaye M, Jiang X. 2003. Homologous versus heterologous immune responses to Norwalk-like viruses among crew members after acute gastroenteritis outbreaks on 2 US Navy vessels. J Infect Dis 187:187–193. doi: 10.1086/367809. [DOI] [PubMed] [Google Scholar]
- 37.Debbink K, Lindesmith LC, Baric RS. 2014. The state of norovirus vaccines. Clin Infect Dis 58:1746–1752. doi: 10.1093/cid/ciu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamminen K, Lappalainen S, Huhti L, Vesikari T, Blazevic V. 2013. Trivalent combination vaccine induces broad heterologous immune responses to norovirus and rotavirus in mice. PLoS One 8:e70409. doi: 10.1371/journal.pone.0070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapikian AZ, Wyatt RG, Dolin R, Thornhill TS, Kalica AR, Chanock RM. 1972. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol 10:1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rackoff LA, Bok K, Green KY, Kapikian AZ. 2013. Epidemiology and evolution of rotaviruses and noroviruses from an archival WHO global study in children (1976-79) with implications for vaccine design. PLoS One 8:e59394. doi: 10.1371/journal.pone.0059394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puustinen L, Blazevic V, Huhti L, Szakal ED, Halkosalo A, Salminen M, Vesikari T. 2012. Norovirus genotypes in endemic acute gastroenteritis of infants and children in Finland between 1994 and 2007. Epidemiol Infect 140:268–275. doi: 10.1017/S0950268811000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blazevic V, Lappalainen S, Nurminen K, Huhti L, Vesikari T. 2011. Norovirus VLPs and rotavirus VP6 protein as combined vaccine for childhood gastroenteritis. Vaccine 29:8126–8133. doi: 10.1016/j.vaccine.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 43.Huhti L, Tamminen K, Vesikari T, Blazevic V. 2013. Characterization and immunogenicity of norovirus capsid-derived virus-like particles purified by anion exchange chromatography. Arch Virol 158:933–942. doi: 10.1007/s00705-012-1565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamminen K, Huhti L, Koho T, Lappalainen S, Hytonen VP, Vesikari T, Blazevic V. 2012. A comparison of immunogenicity of norovirus GII-4 virus-like particles and P-particles. Immunology 135:89–99. doi: 10.1111/j.1365-2567.2011.03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridge SE, Vizard AL. 1993. Determination of the optimal cutoff value for a serological assay: an example using the Johne's Absorbed EIA. J Clin Microbiol 31:1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LoBue AD, Lindesmith LC, Baric RS. 2010. Identification of cross-reactive norovirus CD4+ T cell epitopes. J Virol 84:8530–8538. doi: 10.1128/JVI.00727-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansman GS, Natori K, Shirato-Horikoshi H, Ogawa S, Oka T, Katayama K, Tanaka T, Miyoshi T, Sakae K, Kobayashi S, Shinohara M, Uchida K, Sakurai N, Shinozaki K, Okada M, Seto Y, Kamata K, Nagata N, Tanaka K, Miyamura T, Takeda N. 2006. Genetic and antigenic diversity among noroviruses. J Gen Virol 87:909–919. doi: 10.1099/vir.0.81532-0. [DOI] [PubMed] [Google Scholar]
- 48.Lindesmith LC, Donaldson E, Leon J, Moe CL, Frelinger JA, Johnston RE, Weber DJ, Baric RS. 2010. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol 84:1800–1815. doi: 10.1128/JVI.02179-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng DP, Widdowson MA, Glass RI, Vinje J. 2010. Molecular epidemiology of genogroup II-genotype 4 noroviruses in the United States between 1994 and 2006. J Clin Microbiol 48:168–177. doi: 10.1128/JCM.01622-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puustinen L, Blazevic V, Salminen M, Hamalainen M, Rasanen S, Vesikari T. 2011. Noroviruses as a major cause of acute gastroenteritis in children in Finland, 2009-2010. Scand J Infect Dis 43:804–808. doi: 10.3109/00365548.2011.588610. [DOI] [PubMed] [Google Scholar]
- 51.Lindesmith LC, Beltramello M, Donaldson EF, Corti D, Swanstrom J, Debbink K, Lanzavecchia A, Baric RS. 2012. Immunogenetic mechanisms driving norovirus GII.4 antigenic variation. PLoS Pathog 8:e1002705. doi: 10.1371/journal.ppat.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu S, Regev D, Watanabe M, Hickman D, Moussatche N, Jesus DM, Kahan SM, Napthine S, Brierley I, Hunter RN III, Devabhaktuni D, Jones MK, Karst SM. 2013. Identification of immune and viral correlates of norovirus protective immunity through comparative study of intra-cluster norovirus strains. PLoS Pathog 9:e1003592. doi: 10.1371/journal.ppat.1003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saito M, Goel-Apaza S, Espetia S, Velasquez D, Cabrera L, Loli S, Crabtree JE, Black RE, Kosek M, Checkley W, Zimic M, Bern C, Cama V, Gilman RH, Norovirus Working Group in Peru . 2014. Multiple norovirus infections in a birth cohort in a Peruvian periurban community. Clin Infect Dis 58:483–491. doi: 10.1093/cid/cit763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parra GI, Green KY. 2014. Sequential gastroenteritis episodes caused by 2 norovirus genotypes. Emerg Infect Dis 20:1016–1018. doi: 10.3201/eid2006.131627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boon D, Mahar JE, Abente EJ, Kirkwood CD, Purcell RH, Kapikian AZ, Green KY, Bok K. 2011. Comparative evolution of GII.3 and GII.4 norovirus over a 31-year period. J Virol 85:8656–8666. doi: 10.1128/JVI.00472-11. [DOI] [PMC free article] [PubMed] [Google Scholar]