Abstract
Several serological tests designed to detect antibodies to immunodominant Mycobacterium bovis antigens have recently emerged as ancillary tests for the detection of bovine tuberculosis in cattle, particularly when used after the injection of purified protein derivative (PPD) for skin testing, which significantly boosts M. bovis-specific antibody responses. The present findings demonstrate the onset and duration of boosted antibody responses after the injection of M. bovis PPD for the caudal fold test (CFT) and Mycobacterium avium and M. bovis PPDs for the comparative cervical test (CCT), administered in series in cattle experimentally infected with M. bovis. While skin tests boosted the responses to certain antigens (i.e., MPB83 and MPB70), they did not affect the responses to other antigens (e.g., ESAT-6, CFP10, MPB59, and MPB64). Administration of the CCT 105 days after the CFT resulted in an even greater secondary boost in antibody responses to MPB83 and MPB70 and to a proteinase K-digested whole-cell sonicate (WCS-PK) of M. bovis. Both IgM and IgG contributed to the initial boost in the MPB83/MPB70-specific antibody response after the CFT. The secondary boost after the CCT was primarily due to increased IgG levels. Also, the avidity of antibodies to MPB83 and MPB70 increased after the CCT in M. bovis-infected cattle. The avidity of antibodies to the WCS-PK antigens increased in the interval between the CFT and the CCT but did not increase further after the CCT. Together, these findings demonstrate that the administration of PPDs for skin tests results in additive enhancement (i.e., when the CFT and CCT are performed in series), both qualitative and quantitative, of MPB83/MPB70-specific antibody responses.
INTRODUCTION
Bovine tuberculosis of cattle results primarily from infection with Mycobacterium bovis, a member of the Mycobacterium tuberculosis complex. Despite intensive control efforts, bovine tuberculosis persists as a costly disease, with adverse impacts on animal health and welfare, the trade of animals and animal products, and the livelihoods of producers. In addition, the persistence of this disease necessitates the maintenance of costly regional and federal networks for control/eradication campaigns. The mainstays of bovine tuberculosis control are (i) abattoir surveillance with epidemiological investigations after detection of M. bovis-infected animals, to identify bovine tuberculosis-affected herds (1); (ii) application of antemortem testing for routine surveillance, movement of animals, and identification and removal of infected animals from tuberculosis-affected herds (2); and (iii) management of the disease in wildlife reservoirs, such as white-tailed deer in Michigan (3), brushtail possums (Trichosurus vulpecula) in New Zealand, Eurasian wild boar (Sus scrofa) and red deer (Cervus elaphus) in Spain (4), and Eurasian badgers (Meles meles) in the United Kingdom/Ireland (5). Tuberculin-based cellular immune assays, including measurements of in vitro interferon gamma release and measurements of delayed-type hypersensitivity (DTH) reactions via skin test procedures, are the principal diagnostic tests used for the control of bovine tuberculosis in cattle in most countries (6, 7). In the United States, the caudal fold test (CFT) (intradermal injection of M. bovis purified protein derivative [PPD] in the caudal skin fold) is used as a primary test and the comparative cervical test (CCT) (intradermal injection of Mycobacterium avium and M. bovis PPDs at separate sites in the neck) and the Bovigam assay (Prionics Ag, Schlieren, Switzerland) (an interferon gamma release assay) are used as secondary or confirmatory tests (8).
Several serological tests designed to detect antibodies (Abs) to immunodominant M. bovis antigens (e.g., MPB83, MPB70, ESAT-6, and CFP10) have emerged for potential application in cattle (9–13). A commercial enzyme-linked immunosorbent assay (ELISA) for MPB83 and MPB70 (M. bovis Ab test; IDEXX Laboratories, Westbrook, ME) (9) has been approved by the Office International des Epizooties and the U.S. Department of Agriculture (USDA) for use in cattle in bovine tuberculosis control programs, although applications of this test are primarily limited to ancillary purposes such as confirmation of infections and potentially detection of M. bovis-infected cattle anergic in the skin test. A commercial immunochromatographic test (dual-path platform [DPP] VetTB assay; Chembio Diagnostic Systems, Medford, NY) (14) also has been approved for official use as a primary test for the detection of tuberculosis in deer in the United States. With serological tests for bovine tuberculosis, injection of purified protein derivatives (PPDs) for skin testing boosts antibody responses to complex (e.g., PPD and whole-cell sonicates of M. bovis) and individual protein antigens in M. bovis-infected cattle, including animals without detectable antibody responses prior to skin testing (11, 15–18). Currently, it is generally recommended that serological tests for bovine tuberculosis in cattle be performed after skin tests. Recently, Casal et al. demonstrated that the use of serological testing performed after skin testing (specifically, the IDEXX M. bovis Ab test and Enferplex TB immunoassay [Enfer Scientific, Co., Kildare, Republic of Ireland]), in combination with traditional skin test procedures, increased the number of tuberculous animals detected within tuberculosis-affected cattle herds, compared to skin tests alone (15). However, the effects of serial injections of PPDs for skin tests on serological responses, as well as the duration and quality of the antibody boosts, have not been fully evaluated. In this study, we utilized serological assays for complex antigens (i.e., proteinase K-digested whole-cell sonicate [WCS-PK] of M. bovis) and specific antigens (i.e., MPB83 and MPB70 in the IDEXX M. bovis Ab test and an MPB83-MPB70 fusion protein in the DPP format) to determine the effects of tuberculin administration for the CFT and CCT, performed in series, on the quantity and quality (i.e., avidity, isotypes, and antigen recognition patterns) of boosted antibodies produced in response to M. bovis infections in cattle.
MATERIALS AND METHODS
Aerosol challenge procedures, mycobacterial culture, and assessment of lesions for experimental infection of cattle with Mycobacterium bovis.
For experimental infection, Holstein steers (n = 8) received ∼104 CFU of M. bovis strain 10-7428 by aerosol. Strain 10-7428 is a virulent (19) field isolate from a dairy cow in Colorado (20). In a separate study, treatment groups included noninfected Holstein steers (n = 7), M. bovis strain 10-7428-infected (∼104 CFU by aerosol) Holstein steers (n = 8), or M. bovis strain 95-1315-infected (∼104 CFU by aerosol) Holstein steers (n = 8). For the challenge inoculum, low-passage-number cultures (≤3 passages) of M. bovis were prepared, using standard techniques (21), in Middlebrook 7H9 liquid medium (Becton Dickinson, Franklin Lakes, NJ) supplemented with 10% oleic acid-albumin-dextrose complex (OADC) plus 0.05% Tween 80. Holstein steers were obtained from tuberculosis-free and paratuberculosis-monitored herds in Iowa and were housed in a biosafety level 3 (BSL-3) facility at the National Animal Disease Center (Ames, IA), according to institutional biosafety (permit IBC-0004RA) and animal care and use committee guidelines (with ethical approval via animal care and use protocol ACUP-3859). For aerosol infection, an M. bovis challenge inoculum was delivered to restrained calves (∼9 months of age) by nebulization of the inoculum into a mask (Trudell Medical International, London, ON, Canada) covering the nostrils and mouth. The inoculum was inhaled through a one-way valve into the mask, for inhalation into the respiratory tract via the nostrils. The process continued until the inoculum, a 1-ml phosphate-buffered saline (PBS) wash of the inoculum tube, and an additional 2 ml of PBS were delivered, a process that required ∼10 min. Enumeration of M. bovis challenge inocula, necropsy procedures (7 months after the experimental challenge), and gross and microscopic assessments of lesions were each performed as described previously (22). Qualitative assessment of mycobacterial colonization was performed using standard mycobacterial culture techniques (19–23), using Middlebrook 7H11 selective agar plates (Becton Dickinson) incubated for 8 weeks at 37°C, as well as IS6110 real-time PCR for confirmation of colonies, as described by Thacker et al. (24). Strict biosafety protocols were followed throughout the study to protect personnel from exposure to M. bovis, including BSL-3 containment upon initiation of M. bovis challenges in animal rooms and standard BSL-3 laboratory practices for handling M. bovis cultures and samples from M. bovis-infected animals.
Tuberculin skin test procedures.
For the caudal fold test (CFT), all calves received 0.1 ml (100 μg) of M. bovis purified protein derivative (PPD) intradermally in the right caudal skin fold adjacent to the tailhead (administered 89 days after the experimental challenge), according to USDA guidelines (8). For the comparative cervical test (CCT), calves received 0.1 ml (100 μg) of M. bovis PPD and 0.1 ml (40 μg) of M. avium PPD intradermally at separate clipped sites in the midcervical region (administered 194 days after the experimental challenge), according to USDA guidelines (8). Balanced PPDs for skin tests were obtained from the Brucella and Mycobacterial Reagents section of the National Center for Animal Health (Ames, IA). For the CFT, injection sites were observed and animals with palpable reactions were considered responders, according to USDA guidelines. For the CCT, data are presented as skin thicknesses before and after administration of PPDs, as well as the change in skin thickness. A scattergram for interpretation of CCT results was used to characterize responses as negative, suspect, or reactor, as prescribed by the Animal and Plant Health Inspection Service (APHIS), USDA (8). For comparison to antibody results, responses to M. avium PPD (i.e., changes in skin thickness) were subtracted from responses to M. bovis PPD.
Proteinase K-digested whole-cell sonicate of M. bovis ELISA.
Mycobacterial antigen was prepared from M. bovis strain 95-1315 as described previously (25) and following a procedure originally described by Jark et al. for detection of antibody responses to Mycobacterium avium subsp. paratuberculosis infection of cattle (26). Briefly, M. bovis bacilli were harvested from 4-week-old cultures, sonicated in PBS, further disrupted with 0.1- to 0.15-mm glass beads (Biospec Products, Bartlesville, OK) in a bead beater (Biospec Products), centrifuged, filtered (0.22 μm), digested in a 1-mg/ml proteinase K (Roche Molecular Biochemicals, Indianapolis, IN) solution (50 mM Tris, 1 mM CaCl2 buffer [pH 8.0]) for 1 h at 50°C, and stored at −20°C. Immulon II 96-well microtiter plates (Thermo Scientific, Hudson, NJ) were coated with 100 μl/well (10 μg) antigen diluted in carbonate/bicarbonate coating buffer (pH 9.7). Antigen-coated plates, including control wells containing coating buffer alone, were incubated for 20 h at 4°C. Plates were washed three times with 200 μl/well PBS containing 0.05% Tween 20 (PBST) (Sigma, St. Louis, MO) and were blocked for 1 h at 22°C with 200 μl/well of a commercial milk diluent/blocking solution (Kirkegaard and Perry Laboratories, Gaithersburg, MD). Wells were washed three times with 200 μl/well PBST and incubated for 1 h at 39°C with 1:100 test serum diluted in milk diluent/blocking solution, in duplicate. Plates were washed six times with 200 μl/well PBST and incubated for 1 h at 39°C with 100 μl/well of peroxidase-conjugated protein G (Sigma) diluted 1:2,000 in milk diluent/blocking solution. Wells were washed six times with 200 μl/well PBST and incubated at 22°C with 100 μl/well of SureBlue microwell peroxidase substrate (Kirkegaard & Perry Laboratories) for kinetic ELISA readings (at 650 nm) at 1-min intervals for 15 min. Kinetic analysis of samples (determination of Vmax) was performed using a FlexStation 3 benchtop multimode microplate reader (Molecular Devices, Sunnyvale, CA). Acquired data were analyzed using Softmax Pro software (version 5.2; Molecular Devices). Data are presented as ΔVmax, calculated by subtracting the mean Vmax (in milliunits per minute) of the wells with no antigen from the mean Vmax of antigen-coated wells.
MPB83/MPB70 ELISA (IDEXX M. bovis Ab test).
The IDEXX M. bovis antibody ELISA (IDEXX Laboratories) was performed as described previously (11) and according to the manufacturer's instructions, with the exception of the addition of NH4SCN for avidity analysis and the use of a kinetic instead of endpoint ELISA. Briefly, diluted serum samples (100 μl/well, 1:50 dilution with kit diluent, in duplicate) plus kit controls (positive and negative) were added to Immulon I 96-well microtiter plates (Thermo Scientific, Hudson, NJ) coated with MPB83 and MPB70, and the plates were incubated at 22°C for 60 min. After four washes, 100 μl of a monoclonal anti-bovine IgG-horseradish peroxidase conjugate was added to each well, and the plates were incubated at 22°C for 30 min. After four washes, 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was added to each well, and the plates were incubated at 22°C for kinetic ELISA readings (450 nm) at 1-min intervals for 15 min. Kinetic analysis of samples (determination of Vmax) was performed using a FlexStation 3 benchtop multimode microplate reader (Molecular Devices). Acquired data were analyzed using Softmax Pro software (version 5.2; Molecular Devices). For kinetic assays, data are presented as sample/positive (S/P) ratios, which were derived by subtracting the mean kit negative-control optical density (OD) from each sample value and dividing this value by the corrected positive-control value (mean positive-control OD minus mean negative-control OD). Samples with S/P ratios of ≥0.30 were considered positive for M. bovis antibodies, as determined in prior studies (11) and recommended in the package insert for the test kit. For avidity assays, data are presented as ΔVmax, calculated by subtracting the mean Vmax (in milliunits per minute) of the wells with no antigen from the mean Vmax of antigen-coated wells.
Avidity ELISAs.
Both ELISAs were modified to enable determination of the avidity of antibodies reacting to complex (M. bovis proteinase K-digested whole-cell sonicate [WCS-PK]) and specific (MPB83 and MPB70) antigens. Antigen-coated plates were incubated with 100 μl test serum for 1 h and washed three times with PBST, 100 μl dissociating buffer (NH4SCN in PBST) was added to each well, the contents were mixed via pipetting, and the plates were incubated for 15 min at 22°C. Treatments included NH4SCN concentrations of 0, 0.3125, 0.625, 1.25, 2.5, 5.0, 6.0, and 7.0 M. An additional experiment was performed to determine the effects of washing the antigen-coated plates prior to the addition of NH4SCN. Therefore, after incubation of antigen-coated plates with test serum, wells were either (i) washed three times with PBST prior to the addition of NH4SCN, to remove any unbound antibodies, (ii) not washed with PBST (i.e., NH4SCN was added directly to the antigen-coated plates), or (iii) washed three times with PBST after which 50 μl test serum was added back to the wells in addition to NH4SCN (i.e., to add back unbound antibodies). Data are presented as the ΔVmax for each concentration of NH4SCN and as avidity indices. Avidity indices were calculated by dividing the ΔVmax for samples that received NH4SCN (i.e., 0.3125 to 7 M) by the ΔVmax for samples that received diluent buffer alone (i.e., 0 M NH4SCN) and multiplying that value by 100. For the avidity indices, data are presented as mean ± standard error of the mean (SEM) for each NH4SCN concentration at individual time points relative to the skin tests.
Multiantigen print immunoassay.
The multiantigen print immunoassay (MAPIA) (10) was performed as described for use with samples from cattle (18). Briefly, 12 M. tuberculosis complex antigens, i.e., M. bovis culture filtrate (MBCF), M. bovis PPD, ESAT-6 (Rv3875), CFP-10 (Rv3874), MPB59 (Rv1886c), MPB64 (Rv1980c), MPB70 (Rv2875), MPB83 (Rv2873), Acr1 (Rv3391), 38-kDa protein (Rv0934), ESAT-6-CFP10 (polyepitope fusion of ESAT-6 and CFP10), and F10 (polyepitope fusion of Mtb8 [Rv0379], CFP10, and 38-kDa protein), were immobilized on nitrocellulose membrane strips, blocked for 1 h with 1% nonfat skim milk in PBS with 0.05% Tween 20, and then incubated for 1 h with serum samples diluted 1:40 in blocking solution. After washing, strips were incubated for 1 h with peroxidase-conjugated protein G (Sigma, St. Louis, MO) diluted 1:1,000, washed, and developed with 3,3′,5,5′-tetramethylbenzidine (TMB) (Kirkegaard & Perry Laboratories).
Dual-path platform testing.
Chembio DPP technology was used to detect bovine IgG and IgM responses to the polyepitope fusion protein MPB83-MPB70 (14, 19). As a detection system, goat anti-bovine IgG and anti-bovine IgM antibodies (Kirkegaard & Perry Laboratories) were conjugated to colloidal gold nanoparticles using Chembio standard procedures. Serum samples were tested at a dilution of 1:40 in assay running buffer, and results were recorded at 20 min using an optical reader to measure test band reflectance in relative light units (RLU), as described previously (14).
Statistical analysis.
Data were analyzed by analysis of variance (ANOVA) followed by Tukey's multiple-comparison test, Student's t test, or Pearson's correlation, using commercially available software (Prism 6.0c, GraphPAD Software, La Jolla, CA). P values of <0.05 were considered statistically significant.
RESULTS
Skin test results, lesions, and colonization after experimental infection.
All animals developed DTH responses after experimental infection, as determined by the CFT and CCT (performed ∼3 and 6.5 months after the M. bovis challenge, respectively) (Table 1). Upon necropsy (∼7 months after the challenge), each animal had tuberculous lesions primarily in the lungs and lung-associated (i.e., tracheobronchial and mediastinal) lymph nodes, typical of a low-dose aerosol M. bovis challenge (19, 22). Infection was confirmed in each animal by isolation of M. bovis from the lesions.
TABLE 1.
Delayed-type hypersensitivity responses
| Animal no. | Skin thickness (mm) |
Status | |||||
|---|---|---|---|---|---|---|---|
| CFT, M. bovis PPD |
CCT |
||||||
|
M. avium PPD |
M. bovis PPD |
||||||
| Pre-PPD/post-PPD | Change | Pre-PPD/post-PPD | Change | Pre-PPD/post-PPD | Change | ||
| 394 | 6/21 | 15 | 8/11.5 | 3.5 | 6/34.5 | 28.5 | Reactor |
| 397 | 7/19 | 12 | 11.5/15 | 3.5 | 9.5/21 | 11.5 | Reactor |
| 671 | 6/24 | 18 | 9.5/15 | 5.5 | 8.5/41 | 32.5 | Reactor |
| 673 | 7/21.5 | 14.5 | 10.5/18 | 7.5 | 8.5/50 | 41.5 | Reactor |
| 1207 | 6/22 | 14.5 | 9/11 | 2 | 6.5/26 | 19.5 | Reactor |
| 1208 | 7/29 | 22 | 11/16.5 | 5.5 | 9/27.5 | 18.5 | Reactor |
| 1209 | 7/24 | 17 | 10.5/16 | 5.5 | 8/30 | 22.0 | Reactor |
| 1210 | 7/22 | 15 | 12.5/17.5 | 5 | 10/22.5 | 12.5 | Reactor |
| Mean | 6.6/22.8 | 16.2 | 10.3/15.1 | 4.8 | 8.3/31.6 | 23.3 | |
| SEM | 0.2/1.1 | 1.0 | 0.5/0.9 | 0.6 | 0.5/3.5 | 3.6 | |
Effects of skin tests on levels of serum antibody responses and antigen recognition profiles.
Experimental infection of cattle resulted in serum antibody responses to M. bovis WCS-PK (Fig. 1A) and MPB83 and MPB70 antigens (Fig. 1B). Antibody responses to the WCS-PK antigen exceeded (P < 0.05) prechallenge values beginning 61 days after the M. bovis challenge (Fig. 1A). Responses elicited early after experimental infection and prior to administration of PPDs for skin tests were minimal, as demonstrated previously (11, 15, 27). Injection of PPDs for the CFT and CCT boosted (P < 0.05) antibody responses to the WCS-PK antigen (Fig. 1A) and MPB83 and MPB70 (Fig. 1B) as early as 7 days after the CFT and CCT. Responses after the CCT exceeded all prior responses (P < 0.05). Seven days after the CFT, 3/8 animals had responses considered positive (i.e., S/P ratio of >0.3) with the IDEXX MPB83/MPB70 ELISA. From 10 to 62 days after the CFT, S/P ratios were >0.3 for all 8 animals. At 70, 77, and 105 days after the CFT, S/P ratios were >0.3 for 7/8, 6/8, and 4/8 animals, respectively. As early as 7 days after the CCT (and 112 days after the CFT), responses were dramatically boosted in all 8 animals, with S/P ratios ranging from 4.4 to 13.4. Overall, antibody responses to MPB83/MPB70 and WCS-PK antigens were correlated (Pearson's r = 0.7, P < 0.0001). As with prior studies (11), which also included the use of the CFT and CCT in series (27), antibody responses for either the WCS-PK (ΔVmax of <1) or MPB83/MPB70 (S/P ratio of <0.1) antigens were not detected in noninfected cattle (n = 7) before or after administration of PPDs for skin tests (see Fig. S1 in the supplemental material).
FIG 1.
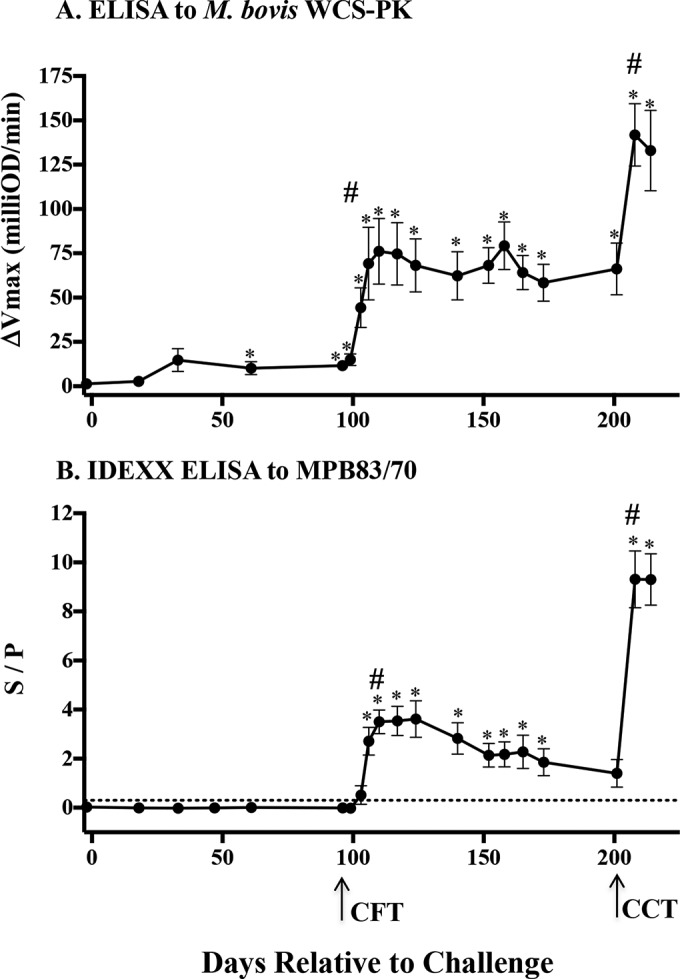
Kinetics of serum antibody responses to complex (WCS-PK) and specific (MPB83/MPB70) antigens and effects of skin tests, as measured by ELISA. (A) ELISA results for proteinase K-digested whole-cell sonicate (WCS-PK) of M. bovis over time after an M. bovis challenge (n = 8). For the WCS-PK ELISA, data are presented as the ΔVmax (mean ± SEM), which was calculated by subtracting the mean Vmax (milliunits per minute) for duplicate wells with no antigen from the mean Vmax for antigen-coated wells. (B) ELISA results for MPB83 and MPB70 antigens over time after an M. bovis challenge (n = 8). For the IDEXX ELISA for MPB83/MPB70 antigens, data are presented as S/P ratios (mean ± SEM), which were derived by subtracting the mean kit negative-control optical density (OD) from each sample value and dividing that value by the corrected positive-control value (mean positive-control OD minus mean negative-control OD). Arrows, timing of the caudal fold test (CFT) and the comparative cervical test (CCT) at 96 and 201 days, respectively, after an M. bovis challenge. Dotted line, level of response (S/P ratio of 0.3) considered positive in the IDEXX test kit package insert. Data were analyzed by ANOVA followed by Tukey's multiple-comparison test. #, boosted (P < 0.05) responses to skin tests. *, responses differing (P < 0.05) from preinfection responses.
Antigen recognition profiles were determined by the MAPIA (Fig. 2 and Table 2). Injection of PPDs for the CFT and CCT resulted in increased frequencies of responders to certain antigens, including both complex (i.e., M. bovis PPD and MBCF) and specific (i.e., MPB70, MPB83, and Acr1/MPB83) antigens. In contrast, responses to other antigens, including ESAT-6, CFP10, MPB59, MPB64, Acr1, 38 kDa, TBF10, and Mtb8, were not affected or were only minimally affected by PPD administration. Injection of PPD for the CFT boosted (P < 0.05) both IgM and IgG responses to MPB83/MPB70 antigens, as measured by the DPP assay (Table 3). Injection of PPDs for the CCT 105 days after the CFT provided an additional boost to IgG responses but not IgM responses. As expected, the IgG DPP (i.e., to MPB83-MPB70) and IDEXX ELISA responses were highly correlated (r = 0.92, P < 0.0001). The MPB83/MPB70-specific IgG/IgM ratio (as measured by the DPP assay) at the peak of the boosted antibody response after the CFT was negatively correlated (r = −0.81, P = 0.01) with the level of the subsequent DTH response (i.e., CCT response) measured ∼3 months later (Fig. 3). Thus, animals with high IgG/IgM ratios had lower M. bovis-specific skin test responses.
FIG 2.
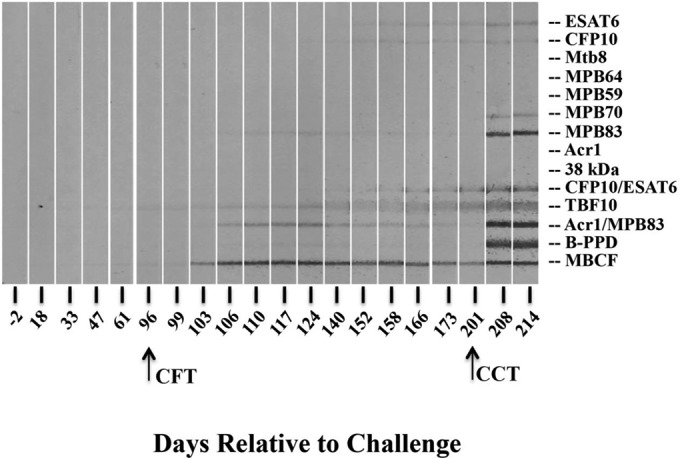
Antigen recognition patterns for antibodies from Mycobacterium bovis-infected cattle, measured by the multiantigen print immunoassay (MAPIA). Serological reactivity to M. tuberculosis complex antigens was determined by the MAPIA for sequential serum samples. Representative results from a single animal (calf 1208) are provided, and the antigens (described in Materials and Methods) are indicated at the right. Arrows, timing of the caudal fold test (CFT) and the comparative cervical test (CCT) at 96 and 201 days, respectively, after an M. bovis challenge.
TABLE 2.
Serum IgG reactivity to defined antigens measured by multiantigen immunoprint assay
| Antigen | No. of respondersa |
||
|---|---|---|---|
| Pre-CFTb | Post-CFTc | Post-CCTd | |
| ESAT-6 | 1 | 1 | 1 |
| CFP10 | 0 | 2 | 2 |
| Mtb8 | 0 | 0 | 0 |
| MPB64 | 0 | 1 | 1 |
| MPB59 | 0 | 1 | 1 |
| MPB70 | 2 | 4 | 7 |
| MPB83 | 0 | 6 | 8 |
| Acr1 | 0 | 0 | 0 |
| 38 kDa | 0 | 0 | 0 |
| ESAT-6/CFP10 | 0 | 2 | 2 |
| TBF10 | 1 | 2 | 2 |
| Acr1/MPB83 | 2 | 6 | 8 |
| M. bovis PPD | 0 | 2 | 7 |
| MBCF | 1 | 8 | 8 |
Number of responders to each antigen used in the MAPIA among the 8 M. bovis-infected cattle.
From 0 to 3 months postinfection, prior to the CFT.
From 3 to 6.5 months postinfection, after the CFT and before the CCT.
From 6.5 to 7 months postinfection, after the CCT.
TABLE 3.
Serum IgM and IgG responses to MPB83-MPB70 measured by DPP assay
| Animal no. | Reflectance (RLU)a |
|||||
|---|---|---|---|---|---|---|
| IgM |
IgG |
|||||
| Pre-CFTb | Post-CFTc | Post-CCTd | Pre-CFTb | Post-CFTc | Post-CCTd | |
| 394 | 313 | 238 | 87 | 89 | 379 | 530 |
| 397 | 0 | 98 | 384 | 97 | 542 | 1281 |
| 671 | 38 | 285 | 402 | 0 | 428 | 1295 |
| 673 | 53 | 605 | 668 | 138 | 1014 | 1276 |
| 1207 | 0 | 169 | 184 | 204 | 966 | 1301 |
| 1208 | 0 | 312 | 389 | 171 | 695 | 1300 |
| 1209 | 0 | 107 | 362 | 79 | 297 | 1172 |
| 1210 | 0 | 102 | 106 | 106 | 587 | 1303 |
| Mean ± SEM | 51 ± 38e | 240 ± 60f | 322 ± 68f | 111 ± 22e | 614 ± 93f | 1182 ± 94g |
Optical reader data obtained at the peak of antibody responses during each period of infection.
From 0 to 3 months postinfection, prior to the CFT.
From 3 to 6.5 months postinfection, after the CFT and before the CCT.
From 6.5 to 7 months postinfection, after the CCT.
Different from post-CFT and post-CCT means (IgM and IgG) (P < 0.05).
Different from pre-CFT mean (IgM) or from pre-CFT and post-CCT means (IgG) (P < 0.05).
Different from pre-CFT and post-CFT means (IgG) (P < 0.05).
FIG 3.
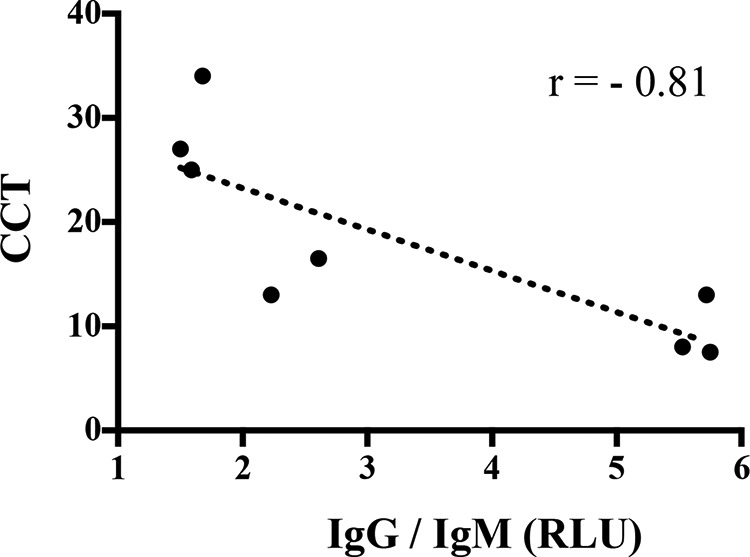
Correlation of the MPB83/MPB70-specific IgG/IgM ratio after the initial skin test (CFT) with subsequent delayed-type hypersensitivity responses (i.e., CCT results) ∼3 months later. Antibody responses to MPB83 and MPB70 were measured by a DPP reader in relative light units (RLU) and are presented as IgG/IgM ratios. Values represent the peak antibody responses for individual animals (n = 8) after injection of M. bovis PPD for the initial skin test (i.e., CFT). Skin test responses for the CCT are presented as the change in skin thickness in response to M. bovis PPD minus the change in skin thickness in response to M. avium PPD (in millimeters). Responses were correlated (Pearson's r = −0.81).
Effects of skin tests on avidity of antibodies to complex (WCS-PK) and specific (MPB83/MPB70) antigens.
Ammonium thiocyanate (NH4SCN, a chaotropic agent that interferes with antigen-antibody binding) is commonly used to estimate the overall strength of binding of multiple antigenic determinants to multivalent antibodies (i.e., avidity). The addition of NH4SCN reduced the level of antibody binding (ΔVmax) to both complex (WCS-PK) and specific (MPB83/MPB70) antigens (data not shown). Avidity indices for the WCS-PK antigen were higher (P < 0.05) with pre- and post-CCT samples (i.e., 166 and 208 days after the challenge, respectively) than with post-CFT samples (i.e., 103 days after the challenge) (Fig. 4A). Avidity indices for the WCS-PK antigen did not differ between pre- and post-CCT samples at 0.3 to 1.25 M NH4SCN. In contrast, avidity indices for the MPB83/MPB70 antigens were higher (P < 0.05) with post-CCT samples than with pre-CCT and post-CFT samples (Fig. 4B). As antibody responses to MPB83/MPB70 were not detectable with the IDEXX ELISA prior to injection of M. bovis PPD for the CFT (Fig. 1B), it was not possible to determine whether antibody avidity increased in response to the CFT. For the MPB83/MPB70 ELISA, antibody binding to antigen was completely abrogated with NH4SCN concentrations of ≥5.0 M. In contrast, responses to the WCS-PK antigen, albeit low, were detectable in post-CCT samples at exceedingly high NH4SCN concentrations (i.e., 5 to 7 M), possibly due to detection of antibody binding to degradation products.
FIG 4.
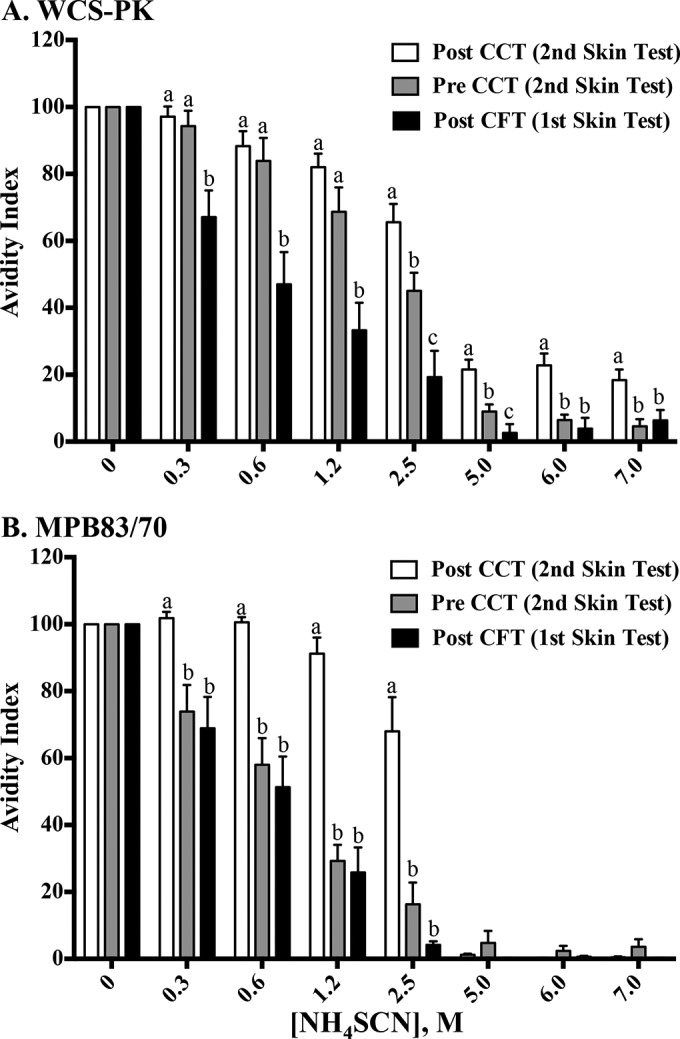
Effects of PPD administration for skin tests on avidity indices for proteinase K-digested whole-cell sonicate of M. bovis (WCS-PK) antigens (A) or MPB83/MPB70 antigens (B) measured by ELISA. Sample collection time points were as follows: post CFT, 14 days after the CFT and 103 days after the M. bovis challenge; pre CCT, 28 days prior to the CCT and 173 days after the M. bovis challenge; post CCT, 14 days after the CCT and 215 days after the challenge. Avidity indices were calculated by dividing the ΔVmax for samples that received NH4SCN (i.e., 0.3125 to 7.0 M) by the ΔVmax for samples that received diluent buffer alone (i.e., 0 M NH4SCN) and multiplying that value by 100. Data were analyzed by ANOVA followed by Tukey's multiple-comparison test. a to c, different letters indicate that responses (mean ± SEM, n = 8) differ (P < 0.05) within the NH4SCN concentration.
In initial studies using several serum sets, including the sera used in the present study, it was noted that avidity indices (particularly with samples containing high antibody levels) with lower concentrations of NH4SCN (i.e., 0.3 to 2.5 M) exceeded 100 (i.e., the avidity index for samples with 0 M NH4SCN) for both the WCS-PK and MPB83/MPB70 antigens. Also, the addition of exceedingly high concentrations of NH4SCN (i.e., 5 to 7.5 M) did not completely eliminate detection of antibody binding to antigens. Thus, we designed a study to evaluate the effects on the avidity assay of a washing step prior to the addition of NH4SCN and after the addition of sera. Minimal effects of washing were detected with the samples containing low antibody levels (Fig. 5A); however, significant differences were detected with samples containing high levels of MPB83/MPB70-specific antibodies, particularly at the higher concentrations of NH4SCN (Fig. 5B), resulting in concurrent differences in avidity indices (data not shown). Interestingly, the addition of 50 μl of sera back into wells that had been washed with PBST prior to the addition of NH4SCN resulted in a partial increase in the responses, intermediate between those with no washes and with three washes with PBST. These findings suggest that unbound specific antibodies may bind to epitopes unmasked by the addition of NH4SCN, potentially of higher affinity than the original antibody binding site.
FIG 5.
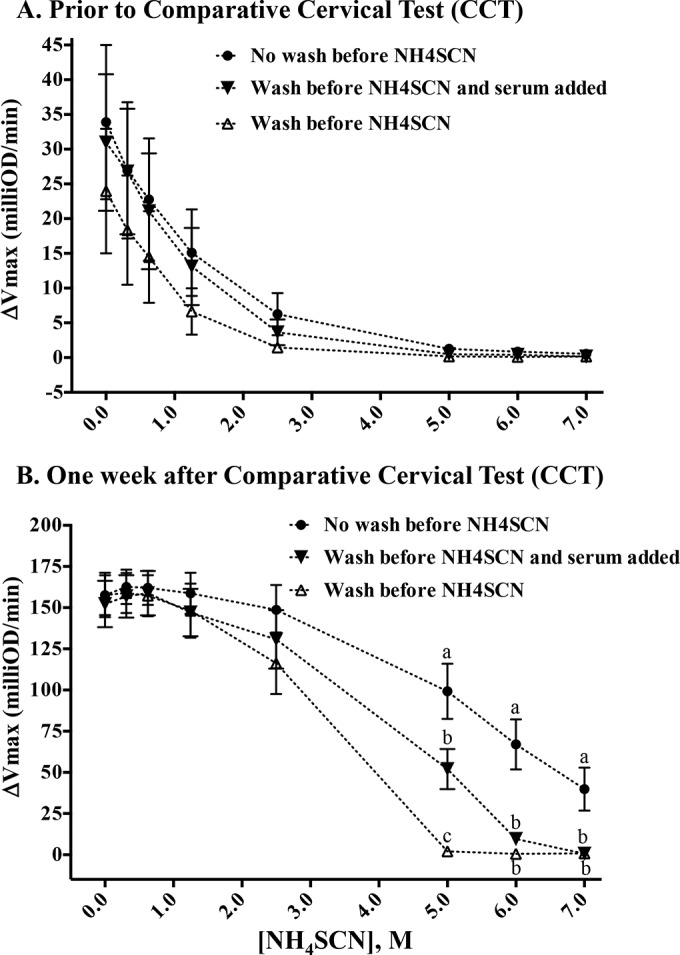
Effects of a washing step to remove residual nonbound antibodies prior to the addition of NH4SCN for the avidity assay for MPB83 and MPB70 measured by ELISA. The levels of antibody binding (ΔVmax) to MPB83 and MPB70 after incubation with a range of NH4SCN concentrations were determined at 28 days prior to the comparative cervical skin test (CCT) (A) and 14 days after the CCT (B). After incubation of antigen-coated plates with test serum, wells were either not washed with PBST (i.e., NH4SCN was added directly to antigen-coated plates) (●), washed three times with PBST after which 50 μl test serum was added back to the wells in addition to NH4SCN (▼), or washed three times with PBST prior to the addition of NH4SCN, to remove any unbound antibody (△). Data were analyzed by ANOVA followed by Tukey's multiple-comparison test. a to c, different letters indicate that responses (mean ± SEM, n = 8) differ (P < 0.05) within the NH4SCN concentration.
DISCUSSION
The present findings demonstrate that (i) serum antibody responses increase after injection of M. bovis PPD for the CFT, (ii) boosted responses to a complex antigen (WCS-PK) peak ∼2 weeks after the CFT and remain steady for at least another 3 months after the CFT, (iii) responses to MPB83 and MPB70 peak ∼2 weeks after the CFT and wane over the next 2 to 3 months, and (iv) injection of M. avium and M. bovis PPDs for the CCT in series with the CFT (i.e., 105 days after the CFT) results in a remarkable boost to preexisting serum antibody responses to both WCS-PK and MPB83/MPB70 antigens. In addition, injection of PPDs for skin tests boosts the responses to certain antigens (i.e., MPB83 and MPB70) but not other antigens (e.g., ESAT-6, CFP10, MPB59, and MPB64). MPB83 and MPB70 are present at relatively high concentrations within M. bovis PPD (16, 28, 29) and thus are the likely causes of this anamnestic response. Both IgM and IgG contribute to the initial boost in MPB83/MPB70-specific antibody responses after the CFT. However, the secondary boost, upon performance of the CCT, is mainly due to increased IgG. Also, the IgG/IgM ratio at the peak of the antibody response to MPB83 and MPB70 elicited by injection of M. bovis PPD for the CFT negatively correlated with the ensuing DTH response (i.e., CCT results), perhaps providing hints to mechanisms of DTH anergy (30) and the possible role for antituberculin antibodies in this enigmatic phenomenon (31).
A practical aspect of the current study was the determination of the duration of the antibody boost with a single intradermal skin test (i.e., the CFT) as used in the United States, Mexico, New Zealand, and several other countries. With the IDEXX M. bovis Ab ELISA, positive responses (i.e., S/P ratios of >0.3) were detected with samples from 3/8 and 8/8 animals at 7 and 10 days after the CFT, respectively. The responses waned between 62 and 70 days after the CFT, with 7/8 and 6/8 animals testing positive at 70 and 77 days after the CFT, respectively. The present findings are in general support of the current USDA, APHIS, guidance document (http://www.aphis.usda.gov/animal_health/animal_diseases/tuberculosis/downloads/idexx_guidance_document.pdf), stating that blood samples are to be collected 7 to 60 days after the CFT for use with the IDEXX M. bovis Ab ELISA, although minor amendments may be warranted.
Antibody avidity assays are routinely used for the diagnosis and/or discrimination of clinically relevant stages of infection (e.g., to predict primary infection versus reinfection, responses to therapy, or reactivation) for a variety of infectious agents for humans, including rubella virus (32), cytomegalovirus (33), Toxoplasma gondii (34), and herpesvirus (35) infections, as well as to estimate the protective potential of responses to vaccination (e.g., with Haemophilus influenzae type b vaccine [36]). Antibody avidity assays are less commonly used for diagnostic purposes with samples from veterinary species (e.g., Neospora caninum infection of cattle [37]). Early after antimycobacterial therapy for M. tuberculosis infections in humans, there is a decrease in antibody avidity accompanied by an initial increase in serum antibody levels, likely due to the bactericidal activity of antituberculous drugs leading to low-affinity antibodies to released antigens (38). Also, humans with active tuberculosis produce low-avidity antibodies with low IgG/IgM ratios for surface antigens of M. tuberculosis but higher-avidity antibodies with higher IgG/IgM ratios for cellular fractions of antigens, potentially indicating immune evasion strategies of this highly successful pathogen, given the association with protection for highly avid antibodies to surface components of other bacteria (39). With experimental M. bovis infection, the disease is generally considered slowly progressive (40, 41), thus most closely resembling the active form of the disease in humans. The present findings demonstrate increased avidity of antibodies to MPB83 and MPB70 after injection of PPD for the CCT (i.e., the second skin test) in M. bovis-infected cattle. Avidity for the complex antigen (i.e., WCS-PK) increased in the interval between the CFT and the CCT but did not increase further after the CCT (Fig. 5). Estimates of antibody avidity are dependent on the homogeneity of antibodies present in the sample (42). Given the diversity of antigens within the WCS-PK, it is likely that the estimate of antibody avidity for these complex antigens is less accurate than that for MPB83/MPB70 antigens, as responses to the various antigens in the WCS-PK may vary over the course of infection and in response to PPD injection. Thus, responses to certain individual antigens within the WCS-PK may be masked, and it is impossible to optimize the procedure (i.e., reagent dilutions) for each of the numerous antigens within the mixture. With M. bovis infections, however, the host is called upon to respond to a large diversity of antigens; thus, use of complex antigens may be more representative of the host-pathogen interface and more reflective of the collective antibody responses to M. bovis infections. Indeed, studies with human tuberculosis showed that patients with a long duration of symptoms had higher antibody avidity for a Triton X-100 extract/whole-cell sonicate antigen preparation than patients with recent onset of infection (38). The current study is the first evaluation of antibody avidity for complex and specific antigens during M. bovis infections in cattle.
Several technical aspects related to the use of NH4SCN for antibody avidity assessments during M. bovis infections in cattle were evaluated. Initial studies that did not include a wash step prior to the addition of NH4SCN to antigen-coated plates containing test serum resulted in avidity indices exceeding those for wells that did not receive NH4SCN (i.e., avidity indices of >100 in wells that received 0.3 to 2.5 M NH4SCN but only with samples containing high levels of high-avidity antibodies). Additionally, high concentrations of NH4SCN (i.e., 5 to 7.5 M) did not completely eliminate detection of antibody binding to antigens for samples containing high levels of antibodies. The addition of a washing step eliminated the unusual responses (almost complete dissolution of antigen-antibody binding with 5 to 7.5 M NH4SCN and decreasing avidity indices to ≤100 with increasing NH4SCN concentrations), and the addition of 50 μl of test serum partially restored the responses. Thus, addition of NH4SCN directly to antigen-coated plates containing test serum in solution might have led to (i) interference of low-affinity antibodies that were replaced with high-affinity antibodies, (ii) reduced binding by nonspecific antibodies and other serum proteins that were replaced with specific antibodies, and/or (iii) displacement of antigen-specific IgM with higher-affinity antigen-specific IgG, with avidity indices exceeding 100 in each of these possible scenarios.
In summary, administration of PPDs for skin tests results in additive boosts to serum antibody responses when used in series. The boosted responses are targeted to specific antigens (e.g., MPB83 and MPB70) and the quality of the responses (i.e., avidity) is also increased. Boosted antibody responses of high avidity provide positive diagnostic benefit for bovine tuberculosis tests, such as the DPP assay and the M. bovis Ab ELISA.
ACKNOWLEDGMENT
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00119-15.
REFERENCES
- 1.Kaneene JB, Miller R, Meyer RM. 2006. Abattoir surveillance: the U.S. experience. Vet Microbiol 112:273–282. doi: 10.1016/j.vetmic.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 2.de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, Clifton-Hadley RS. 2006. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques. Res Vet Sci 81:190–210. doi: 10.1016/j.rvsc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Fitzgerald SD, Kaneene JB. 2013. Wildlife reservoirs of bovine tuberculosis worldwide: hosts, pathology, surveillance, and control. Vet Pathol 50:488–499. doi: 10.1177/0300985812467472. [DOI] [PubMed] [Google Scholar]
- 4.Gortazar C, Cowan P. 2013. Introduction to this issue: dealing with TB in wildlife. Epidemiol Infect 141:1339–1341. doi: 10.1017/S0950268813000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corner LA, Murphy D, Gormley E. 2011. Mycobacterium bovis infection in the Eurasian badger (Meles meles): the disease, pathogenesis, epidemiology and control. J Comp Pathol 144:1–24. doi: 10.1016/j.jcpa.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Bezos J, Casal C, Romero B, Schroeder B, Hardegger R, Raeber AJ, López L, Rueda P, Domínguez L. 2014. Current ante-mortem techniques for diagnosis of bovine tuberculosis. Res Vet Sci 97(Suppl):S44–S52. doi: 10.1016/j.rvsc.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, Orloski KA, Buddle BM, Thacker TC, Lyashchenko KP, Waters WR. 2010. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound Emerg Dis 57:205–220. doi: 10.1111/j.1865-1682.2010.01148.x. [DOI] [PubMed] [Google Scholar]
- 8.USDA, Animal and Plant Health Inspection Service. 2007. Bovine tuberculosis eradication: uniform methods and rules. APHIS 91-45-011. U.S. Government Printing Office, Washington, DC. [Google Scholar]
- 9.Green LR, Jones CC, Sherwood AL, Garkavi IV, Cangelosi GA, Thacker TC, Palmer MV, Waters WR, Rathe CV. 2009. Single-antigen serological testing for bovine tuberculosis. Clin Vaccine Immunol 16:1309–1313. doi: 10.1128/CVI.00028-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyashchenko KP, Singh M, Colangeli R, Gennaro ML. 2000. A multi-antigen print immunoassay for the development of serological diagnosis of infectious diseases. J Immunol Methods 242:91–100. doi: 10.1016/S0022-1759(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 11.Waters WR, Buddle BM, Vordermeier HM, Gormley E, Palmer MV, Thacker TC, Bannantine JP, Stabel JR, Linscott R, Martel E, Milian F, Foshaug W, Lawrence JC. 2011. Development and evaluation of an enzyme-linked immunosorbent assay for use in the detection of bovine tuberculosis in cattle. Clin Vaccine Immunol 18:1882–1888. doi: 10.1128/CVI.05343-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whelan C, Shuralev E, O'Keeffe G, Hyland P, Kwok HF, Snoddy P, O'Brien A, Connolly M, Quinn P, Groll M, Watterson T, Call S, Kenny K, Duignan A, Hamilton MJ, Buddle BM, Johnston JA, Davis WC, Olwill SA, Clarke J. 2008. Multiplex immunoassay for serological diagnosis of Mycobacterium bovis infection in cattle. Clin Vaccine Immunol 15:1834–1838. doi: 10.1128/CVI.00238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whelan C, Whelan AO, Shuralev E, Kwok HF, Hewinson G, Clarke J, Vordermeier HM. 2010. Performance of the Enferplex TB assay with cattle in Great Britain and assessment of its suitability as a test to distinguish infected and vaccinated animals. Clin Vaccine Immunol 17:813–817. doi: 10.1128/CVI.00489-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyashchenko KP, Greenwald R, Esfandiari J, O'Brien DJ, Schmitt SM, Palmer MV, Waters WR. 2013. Rapid detection of serum antibody by dual-path platform VetTB assay in white-tailed deer infected with Mycobacterium bovis. Clin Vaccine Immunol 20:907–911. doi: 10.1128/CVI.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casal C, Díez-Guerrier A, Álvarez J, Rodriguez-Campos S, Mateos A, Linscott R, Martel E, Lawrence JC, Whelan C, Clarke J, O'Brien A, Domínguez L, Aranaz A. 2014. Strategic use of serology for the diagnosis of bovine tuberculosis after intradermal skin testing. Vet Microbiol 170:342–351. doi: 10.1016/j.vetmic.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 16.Lightbody KA, Skuce RA, Neill SD, Pollock JM. 1998. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet Rec 142:295–300. doi: 10.1136/vr.142.12.295. [DOI] [PubMed] [Google Scholar]
- 17.Lightbody KA, McNair J, Neill SD, Pollock JM. 2000. IgG isotype antibody responses to epitopes of the Mycobacterium bovis protein MPB70 in immunised and in tuberculin skin test-reactor cattle. Vet Microbiol 75:177–188. doi: 10.1016/S0378-1135(00)00215-7. [DOI] [PubMed] [Google Scholar]
- 18.Waters WR, Palmer MV, Thacker TC, Bannantine JP, Vordermeier HM, Hewinson RG, Greenwald R, Esfandiari J, McNair J, Pollock JM, Andersen P, Lyashchenko KP. 2006. Early antibody responses to experimental Mycobacterium bovis infection of cattle. Clin Vaccine Immunol 13:648–654. doi: 10.1128/CVI.00061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters WR, Thacker TC, Nelson JT, DiCarlo DM, Maggioli MF, Greenwald R, Esfandiari J, Lyashchenko KP, Palmer MV. 2014. Virulence of two strains of Mycobacterium bovis in cattle following aerosol infection. J Comp Pathol 151:410–419. doi: 10.1016/j.jcpa.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Francisco TI, Orloski KA, Roberts NJ. 2014. Investigation of a Mycobacterium bovis outbreak in cattle at a Colorado dairy in 2010. J Am Vet Med Assoc 244:805–812. doi: 10.2460/javma.244.7.805. [DOI] [PubMed] [Google Scholar]
- 21.Larsen MH, Biermann K, Jacobs WR. 2007. Laboratory maintenance of Mycobacterium tuberculosis. Curr Protoc Microbiol 10:Unit 10A.1. doi: 10.1002/9780471729259.mc10a01s6. [DOI] [PubMed] [Google Scholar]
- 22.Palmer MV, Waters WR, Whipple DL. 2003. Aerosol delivery of virulent Mycobacterium bovis to cattle. Tuberculosis (Edinb) 82:275–282. doi: 10.1054/tube.2002.0341. [DOI] [PubMed] [Google Scholar]
- 23.Waters WR, Whelan AO, Lyashchenko KP, Greenwald R, Palmer MV, Harris BN, Hewinson RG, Vordermeier HM. 2010. Immune responses in cattle inoculated with Mycobacterium bovis, Mycobacterium tuberculosis, or Mycobacterium kansasii. Clin Vaccine Immunol 17:247–252. doi: 10.1128/CVI.00442-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thacker TC, Harris B, Palmer MV, Waters WR. 2011. Improved specificity for detection of Mycobacterium bovis in fresh tissues using IS6110 real-time PCR. BMC Vet Res 7:50. doi: 10.1186/1746-6148-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waters WR, Palmer MV, Whipple DL. 2002. Mycobacterium bovis-infected white-tailed deer (Odocoileus virginianus): detection of immunoglobulin specific to crude mycobacterial antigens by ELISA. J Vet Diagn Invest 14:470–475. doi: 10.1177/104063870201400604. [DOI] [PubMed] [Google Scholar]
- 26.Jark U, Ringena I, Franz B, Gerlach GF, Beyerbach M, Franz B. 1997. Development of an ELISA technique for serodiagnosis of bovine paratuberculosis. Vet Microbiol 57:189–198. doi: 10.1016/S0378-1135(97)00125-9. [DOI] [PubMed] [Google Scholar]
- 27.Palmer MV, Waters WR, Thacker TC, Greenwald R, Esfandiari J, Lyashchenko KP. 2006. Effects of different tuberculin skin-testing regimens on gamma interferon and antibody responses in cattle experimentally infected with Mycobacterium bovis. Clin Vaccine Immunol 13:387–394. doi: 10.1128/CVI.13.3.387-394.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harboe M, Wiker HG, Duncan JR, Garcia MM, Dukes TW, Brooks BW, Turcotte C, Nagai S. 1990. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J Clin Microbiol 28:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiker HG. 2009. MPB70 and MPB83: major antigens of Mycobacterium bovis. Scand J Immunol 69:492–499. doi: 10.1111/j.1365-3083.2009.02256.x. [DOI] [PubMed] [Google Scholar]
- 30.Lepper AW, Pearson CW, Corner LA. 1977. Anergy to tuberculin in beef cattle. Aust Vet J 53:214–216. doi: 10.1111/j.1751-0813.1977.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 31.Encinales L, Zuñiga J, Granados-Montiel J, Yunis M, Granados J, Almeciga I, Clavijo O, Awad C, Collazos V, Vargas-Rojas MI, Bañales-Mendez JL, Vazquez-Castañeda L, Stern JN, Romero V, Fridkis-Hareli M, Terreros D, Fernandez-Viña M, Yunis EJ. 2010. Humoral immunity in tuberculin skin test anergy and its role in high-risk persons exposed to active tuberculosis. Mol Immunol 47:1066–1073. doi: 10.1016/j.molimm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nedeljkovic J, Jovanovic T, Oker-Blom C. 2001. Maturation of IgG avidity to individual rubella virus structural proteins. J Clin Virol 22:47–54. doi: 10.1016/S1386-6532(01)00161-5. [DOI] [PubMed] [Google Scholar]
- 33.Baccard-Longere M, Freymuth F, Cointe D, Seigneurin JM, Grangeot-Keros L. 2001. Multicenter evaluation of a rapid and convenient method for determination of cytomegalovirus immunoglobulin G avidity. Clin Diagn Lab Immunol 8:429–431. doi: 10.1128/CDLI.8.2.429-431.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi C. 1998. A simple, rapid enzyme-linked immunosorbent assay for evaluating immunoglobulin G antibody avidity in toxoplasmosis. Diagn Microbiol Infect Dis 30:25–30. doi: 10.1016/S0732-8893(97)00194-6. [DOI] [PubMed] [Google Scholar]
- 35.Ward KN, Turner DJ, Couto Parada X, Thiruchelvam AD. 2001. Use of immunoglobulin G antibody avidity for differentiation of primary human herpesvirus 6 and 7 infections. J Clin Microbiol 39:959–963. doi: 10.1128/JCM.39.3.959-963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denoël PA, Goldblatt D, de Vleeschauwer I, Jacquet JM, Pichichero ME, Poolman JT. 2007. Quality of the Haemophilus influenzae type b (Hib) antibody response induced by diphtheria-tetanus-acellular pertussis/Hib combination vaccines. Clin Vaccine Immunol 14:1362–1369. doi: 10.1128/CVI.00154-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguado-Martínez A, Alvarez-García G, Arnaiz-Seco I, Innes E, Ortega-Mora LM. 2005. Use of avidity enzyme-linked immunosorbent assay and avidity Western blot to discriminate between acute and chronic Neospora caninum infection in cattle. J Vet Diagn Invest 17:442–450. doi: 10.1177/104063870501700506. [DOI] [PubMed] [Google Scholar]
- 38.Arias-Bouda LM, Kuijper S, Van der Werf A, Nguyen LN, Jansen HM, Kolk AH. 2003. Changes in avidity and level of immunoglobulin G antibodies to Mycobacterium tuberculosis in sera of patients undergoing treatment for pulmonary tuberculosis. Clin Diagn Lab Immunol 10:702–709. doi: 10.1128/CDLI.10.4.702-709.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perley CC, Frahm M, Click EM, Dobos KM, Ferrari G, Stout JE, Frothingham R. 2014. The human antibody response to the surface of Mycobacterium tuberculosis. PLoS One 9:e98938. doi: 10.1371/journal.pone.0098938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer MV, Waters WR. 2006. Advances in bovine tuberculosis diagnosis and pathogenesis: what policy makers need to know. Vet Microbiol 112:181–190. doi: 10.1016/j.vetmic.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 41.Palmer MV, Waters WR, Thacker TC. 2007. Lesion development and immunohistochemical changes in granulomas from cattle experimentally infected with Mycobacterium bovis. Vet Pathol 44:863–874. doi: 10.1354/vp.44-6-863. [DOI] [PubMed] [Google Scholar]
- 42.Romero-Steiner S, Holder PF, Gomez de Leon P, Spear W, Hennessy TW, Carlone GM. 2005. Avidity determinations for Haemophilus influenzae type b anti-polyribosylribitol phosphate antibodies. Clin Diagn Lab Immunol 12:1029–1035. doi: 10.1128/CDLI.12.9.1029-1035.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


