Abstract
The cheetah population in Namibia is the largest free-ranging population in the world and a key population for research regarding the health status of this species. We used serological methods and quantitative real-time PCR to test free-ranging and captive Namibian cheetahs for the presence of feline leukemia virus (FeLV), a gammaretrovirus that can be highly aggressive in populations with low genetic diversity, such as cheetahs. We also assessed the presence of antibodies to other gammaretroviruses and the responses to a FeLV vaccine developed for domestic cats. Up to 19% of the free-ranging cheetahs, 27% of the captive nonvaccinated cheetahs, and 86% of the captive vaccinated cheetahs tested positive for FeLV antibodies. FeLV-antibody-positive free-ranging cheetahs also tested positive for Rauscher murine leukemia virus antibodies. Nevertheless, FeLV was not detectable by quantitative real-time PCR and no reverse transcriptase activity was detectable by product-enhanced reverse transcriptase assay in the plasma of cheetahs or the supernatants from cultures of peripheral blood mononuclear cells. The presence of antibodies to gammaretroviruses in clinically healthy specimens may be caused either by infection with a low-pathogenic retrovirus or by the expression of endogenous retroviral sequences. The strong humoral immune responses to FeLV vaccination demonstrate that cheetahs can respond to the vaccine and that vaccination against FeLV infection may be beneficial should FeLV infection ever become a threat, as was seen in Iberian lynx and Florida panthers.
INTRODUCTION
The cheetah population in Namibia is the largest free-ranging population of this vulnerable species (1). For more than 2 decades, cheetahs have been considered highly susceptible to infectious diseases because of low genetic variability, which is assumed to impair immune responses to viral challenges (2–5). Evidence for fatal viral infections in cheetahs comes from an outbreak of feline infectious peritonitis (FIP), a consequence of feline coronavirus (FCoV) infections, in a captive population in the United States that was kept at nonethologically high density (6–8) and from a single case of very rapid feline leukemia virus (FeLV) disease progression in a captive Namibian cheetah in 1995 (9). No disease outbreaks have been reported in any free-ranging cheetah population, but several studies have identified antibodies against viruses such as feline herpesvirus (FHV), feline calicivirus (FCV), feline parvovirus (FPV), FCoV, canine distemper virus (CDV), feline immunodeficiency virus (FIV), and rabies virus (10–13). In Namibia, free-ranging cheetahs are generally in good health; no clinical signs of viral infections were detected during sampling, and none of the histopathological examinations conducted after necropsies showed lesions related to viral infections (12–15). A recent study on major histocompatibility complex (MHC) class I and class II confirmed the relatively low genetic variability in cheetahs (2). Despite the small number of MHC class I alleles (10 alleles), Namibian cheetahs can still mount effective immune responses against some viral challenges, although their immunocompetence might be limited when they are confronted with new pathogens (2, 16). Thus, it is important to continuously monitor the free-ranging cheetah population in Namibia, particularly for viruses for which no antibodies have been reported so far, such as FeLV, an oncogenic gammaretrovirus (10–13).
FeLV is of particular interest because, in the 1995 case, a cheetah experienced rapid deterioration and died from an infection transmitted from a captive cheetah that tested positive for FeLV antigens. Circumstantial evidence indicated that the origins of the infection were nonvaccinated feral and domestic cats viremic with FeLV (9). Such a method of transmission and a course of disease were also observed in Florida panthers (Puma concolor coryi) (17) and Iberian lynx (Lynx pardinus), the most endangered free-ranging felid species (18). In Namibia, cheetahs roam mainly on commercially used farmland, where they might come into contact with nonvaccinated feral and domestic cats and dogs, as well as free-ranging leopards (Panthera pardus) and smaller carnivores (19, 20). Previous studies documented that cheetahs in north-central Namibia have higher seroprevalences of FHV, FCV, FCoV, and CDV than do cheetahs in east-central Namibia (12, 13). This is probably a consequence of greater contact frequency, due to a likely higher density of mostly nonvaccinated feral and domestic cats and dogs in regions with greater human populations (13, 21, 22).
The goals of the present report were to study and to discuss the nature of antibodies to FeLV or a closely related retrovirus that were present in a portion of serum samples from free-ranging and captive Namibian cheetahs. We also examined the efficacy of vaccination against FeLV to induce antibodies to FeLV in captive cheetahs.
MATERIALS AND METHODS
Study animals and samples.
In this retrospective study, blood samples that had been collected between June 2002 and July 2009 from 88 free-ranging cheetahs (63 males and 25 females) on commercial farmland in east-central Namibia were analyzed. To study samples from animals living close to human settlements, with potential contact with domestic cats, we also sampled 56 cheetahs (37 males and 19 females) held in enclosures within their natural habitat. Thirty of the captive cheetahs were housed at the AfriCat Foundation, a nonprofit conservation facility for carnivores in central Namibia, and 26 on privately owned farms. We collected heparinized plasma samples from the free-ranging cheetahs, and we collected heparinized plasma and/or serum samples and EDTA-treated whole-blood samples from the cheetahs housed at AfriCat and the 26 captive cheetahs housed on private farms. Because samples collected over time may provide evidence of seroconversion, some animals were repeatedly used for sample collection. Of the free-ranging cheetahs, five were sampled twice, one was sampled three times, and one was sampled four times. Among the captive cheetahs, seven were sampled twice, two were sampled three times, and one was sampled four times. One animal was sampled as a free-ranging cheetah and later as a captive cheetah and was counted in both categories. The intervals between repeated sampling times ranged between 1 month and 28 months. In this study, we used 72 serum and 26 heparinized plasma samples from free-ranging cheetahs and 29 serum and 45 heparinized plasma samples from captive cheetahs. In addition, we used 26 EDTA-treated whole-blood samples from free-ranging cheetahs and 15 from captive cheetahs. Depending on the question examined, different sample sets were used (see “Statistical analysis,” below).
Free-ranging and captive animals were captured, anesthetized, sampled, clinically examined (body size determination, weight and temperature measurements, body palpation, and visual check for signs of infection), and released again, as described previously (13, 23). Captive animals were examined as part of the annual health check-up, which included vaccinations developed for domestic cats against FHV, FCV, FPV, and FeLV (Tricat [Nobivac], containing attenuated FHV, FCV, and FPV and the nonglycosylated envelope gene product expressed in Escherichia coli, or Fel-o-Vax Lv-K, containing killed FeLV) and rabies (Rabisin [Merial, Midrand, South Africa], containing inactivated rabies virus). Thirty of the plasma samples originated from cheetahs that had been vaccinated for several years. Five plasma samples originated from nonvaccinated cheetahs that were vaccinated for the first time on the day of examination and were tested again between 23 months and 28 months later.
Samples were collected from the vena saphena or vena cephalica into heparin, serum, and EDTA blood collection tubes (BD Vacutainer Systems, Plymouth, United Kingdom). The samples were kept at 4°C during transport to the field station or the Central Veterinary Laboratory in Windhoek, Namibia. All plasma, serum, and whole-blood samples were stored in liquid nitrogen. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples from 35 captive and 22 free-ranging cheetahs through a Ficoll gradient using Histopaque (Sigma-Aldrich, Buchs, Switzerland) and Hanks' balanced salt solution (HBSS) (Invitrogen, Basel, Switzerland), as described previously (24), and were stored in liquid nitrogen. Plasma, serum, and whole-blood samples and PBMCs were transported to Switzerland at −80°C on dry ice or at −196°C in a dry shipper (Voyageur 12; Air Liquide, Paris, France), in full compliance with the Convention on International Trade in Endangered Species (CITES).
Enzyme-linked immunoabsorbent assays.
Serum and plasma samples were tested for FeLV p27 antigen using a sandwich enzyme-linked immunoabsorbent assay (ELISA), as described previously (25, 26). Samples that reached an optical density (OD) of >20% of the positive-control value were considered true-positive samples, samples that reached ≥4% to 20% of the positive-control value were considered questionable, and samples with values below 4% were considered true-negative samples (25, 27). We also determined the presence of antibodies against FeLV p45 (the nonglycosylated form of the gp70 surface unit of the envelope glycoprotein) and whole FeLV, purified by ultracentrifugation (FL-74), by ELISA as described (28, 29), at a dilution of 1:200. Antibody levels were assessed by comparison with predefined positive-control sera, which consisted of pooled sera collected from FeLV regressor cats (29). To determine FL-74 ELISA cutoff values, we measured the mean plasma OD values for specific-pathogen-free (SPF) cats (n = 15, assayed in duplicate), as a percentage of the positive-control value (assigned to be 100%). The cutoff value was set at the mean value plus 2.58 times the standard deviation (99% confidence interval for all negative results). Determination of the cutoff value for the p45 ELISA was performed in the same way, with 5 SPF cats. A compilation of the numbers of animals and samples from free-ranging, captive nonvaccinated, and captive vaccinated cheetahs used for each test is presented in Table 1.
TABLE 1.
Serological results of ELISAs for the presence of FeLV p27 antigens and antibodies against FeLV p45 and FeLV whole virus (FL-74) in free-ranging, captive nonvaccinated, and captive vaccinated cheetahs
| ELISAa | Free-ranging |
Captive nonvaccinated |
Captive vaccinated |
|||
|---|---|---|---|---|---|---|
| No. (%) of animals | No. (%) of samples | No. (%) of animals | No. (%) of samples | No. (%) of animals | No. (%) of samples | |
| P27 | ||||||
| >20% (positive) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ≥4% to ≤20% | 6 (7) | 6 (6) | 3 (11) | 3 (8) | 2 (6) | 2 (6) |
| <4% (negative) | 82 (93) | 92 (94) | 25 (89) | 36 (92) | 33 (94) | 33 (94) |
| Total tested | 88 | 98 | 26 | 39 | 35 | 35 |
| Sum | 88 (100) | 98 (100) | 28 (100)b,c | 39 (100) | 35 (100) | 35 (100) |
| P45 | ||||||
| >20% (positive) | 1 (1) | 1 (1) | 3 (11) | 6 (20) | 25 (71) | 25 (71) |
| ≤20% (negative) | 69 (99) | 74 (99) | 24 (89) | 24 (80) | 10 (29) | 10 (29) |
| Total tested | 70 | 75 | 26 | 30 | 35 | 35 |
| Sum | 70 (100) | 75 (100) | 27 (100)b,c | 30 (100) | 35 (100) | 35 (100) |
| FL-74 | ||||||
| >22% (positive) | 17 (19) | 21 (21) | 7 (25) | 9 (23) | 30 (86) | 30 (86) |
| ≤22% (negative) | 72 (81) | 77 (79) | 21 (75) | 30 (77) | 5 (14) | 5 (14) |
| Total tested | 88 | 98 | 26 | 39 | 35 | 35 |
| Sum | 89 (100)b | 98 (100) | 28 (100)b | 39 (100) | 35 (100) | 35 (100) |
Samples with OD values of ≥20% (P27 and P45) or ≥22% (FL-74) were considered positive; samples with OD values of ≥4% and ≤20% were considered questionable and samples with OD values of <4% were considered negative for p27 antigens.
The sum of the animals is greater than the total number of animals because animals captured and tested repeatedly were classified in different OD levels (see the text).
The sum of the animals is greater than the total number of animals because animals tested once with plasma and once with serum were classified in different OD levels (see the text).
Western blot analysis.
Western blotting (WB) was used to determine the presence of antibodies against FeLV gp70, p58, p27, and the two fragments of p15(E), using 1:100 dilutions of the samples (30). We used 24 serum and 26 plasma samples from 45 free-ranging cheetahs and 11 serum and 45 plasma samples from 45 captive cheetahs; depending on the question examined, different sample sets were used (see “Statistical analysis,” below). Samples that showed antibodies to FeLV p27 plus one or two p15(E) fragments or to both p15(E) fragments only were considered positive (29, 31).
Antibody reactivity against other retroviruses was tested with serum samples from six randomly chosen FeLV-WB-positive and six FeLV-WB-negative free-ranging cheetahs. Samples were tested for reactivity against the following retroviruses (a generous gift to H.L. from the U.S. National Cancer Institute): baboon endogenous retrovirus, feline RD114 endogenous retrovirus, Rauscher murine leukemia virus (RMuLV), and AKR murine leukemia virus (AKR-MuLV). Antibodies were visually assessed by WB using 0.5 μg of antigen per strip for each virus preparation, with 1:50 dilution of the samples.
Total nucleic acid extraction.
Total nucleic acids (TNA) were extracted from 100 μl EDTA-treated blood (n = 41) and 100 μl heparinized plasma (n = 71) after the addition of 100 μl of MgCl2- and CaCl2-free phosphate-buffered saline (PBS) (Invitrogen) to each volume. For this, the MagNA Pure LC TNA isolation kit (Roche Diagnostics, Rotkreuz, Switzerland) was used, following the manufacturer's instructions. TNA were eluted with 100 μl buffer (Roche) and stored at −20°C until further analysis.
Real-time PCR.
The presence of amplifiable DNA in each of the 41 whole-blood TNA samples and the extraction controls was tested by a quantitative real-time PCR assay for feline glyceraldehyde-3-phosphate dehydrogenase (fGAPDH), as described previously (32). The presence of amplifiable exogenous FeLV viral RNA or DNA was determined by real-time reverse transcriptase (RT)-PCR or real-time PCR, respectively, in whole-blood and plasma TNA. Whole-blood TNA were tested for amplifiable endogenous FeLV sequences as described previously (33–35).
Product-enhanced reverse transcriptase assay.
PBMCs were cultured in 5 ml medium consisting of RPMI 1640 medium (Sigma), 10% inactivated fetal calf serum (Invitrogen), 1% l-glutamine (Invitrogen), and 1% antibiotic/antimycotic (Invitrogen), in a 25-cm2 cell culture flask (TPP, Trasadingen, Switzerland), and 10 μl of concanavalin A (5 mg/ml; Sigma) was added. After 1 day, 40 U/ml of interleukin 2 (IL-2) (Sandoz Pharmaceuticals AG, Cham, Switzerland) was added to the culture. Five milliliters of medium and IL-2 at 8 U/ml were added to the culture at day 3, and then 5 ml of medium was replaced and IL-2 at 8 U/ml was added to the culture every 3 to 4 days. Once a week, 5 ml of supernatant was collected, centrifuged for 10 min at 1,500 × g, and stored in 1-ml aliquots at −80°C. After 4 weeks, cells were frozen again and stored in liquid nitrogen. RT activity was assessed in supernatants collected in the fourth week of the PBMC cultures, at the Swiss National Center for Retroviruses, University of Zurich (Zurich, Switzerland), using the product-enhanced reverse transcriptase (PERT) assay (36). RT activity was also assessed in 12 heparinized plasma samples from WB- and ELISA-negative animals. Potential inhibition by heparin was excluded by testing a positive RT sample together with aliquots with and without heparin. As no inhibition was observed, no correction of the measured RT values was necessary.
Statistical analysis.
All tests were performed using Systat 13.0 (Systat Software Inc., Richmond, VA). To assess whether vaccinated animals differed from nonvaccinated animals in the serological assays, we used the three categories of free-ranging, captive nonvaccinated, and captive vaccinated cheetahs. For nonvaccinated animals sampled repeatedly, we randomly selected one test result for the analyses, so that each animal was represented only once in the analyses. For the five captive animals tested both as nonvaccinated and as vaccinated, we used only the nonvaccinated results, because all other vaccinated animals had been vaccinated for several years (see Results). As serological data were not normally distributed (Lilliefors test, P < 0.05), comparisons were performed using nonparametric Kruskal-Wallis tests. WB results were assessed using a chi-square test. Results for male and female animals of each group and for each test were compared with Fisher's exact test.
RESULTS
Study animals.
None of the study animals showed any clinical signs of an infectious disease, such as fever, enlarged lymph nodes, pale mucus membranes, anemia, or prolonged capillary refill time, or was lethargic or dehydrated.
Serological assays. (i) P27 ELISA.
None of the free-ranging, captive nonvaccinated, or captive vaccinated cheetahs tested positive for p27 (Table 1). All seven repeatedly tested free-ranging cheetahs and five of the seven repeatedly tested captive nonvaccinated cheetahs (71%) were negative in all tests. None of the five captive cheetahs that were sampled as nonvaccinated animals and 2 years later as vaccinated against FeLV turned positive. Three of the 30 AfriCat cheetahs (10%) kept at the facility of the AfriCat Foundation, all of which had been vaccinated for several years, tested questionable; all others tested negative.
(ii) P45 ELISA.
One free-ranging (1%), three captive nonvaccinated (11%), and 25 captive vaccinated (71%) cheetahs tested positive (Table 1). All three repeatedly tested free-ranging cheetahs and two of the three repeatedly tested captive nonvaccinated cheetahs revealed the same results for the repeated tests. Two of the five captive cheetahs that were sampled as nonvaccinated animals and 2 years later as vaccinated animals tested positive for both samples, and two tested negative for both samples; only one of the animals turned from negative to positive. Twenty-two of the AfriCat cheetahs (73%) tested positive. The OD values differed for the three categories (Kruskal-Wallis test statistic, 58.46; P < 0.0001; free-ranging, n = 70; nonvaccinated, n = 25; vaccinated, n = 30), with captive vaccinated cheetahs having higher values than free-ranging cheetahs (Conover-Inman test for pairwise comparisons, P < 0.0001) or captive nonvaccinated cheetahs (P < 0.0001). Free-ranging cheetahs had OD values similar to those for captive nonvaccinated cheetahs (P = 0.11) (Fig. 1).
FIG 1.
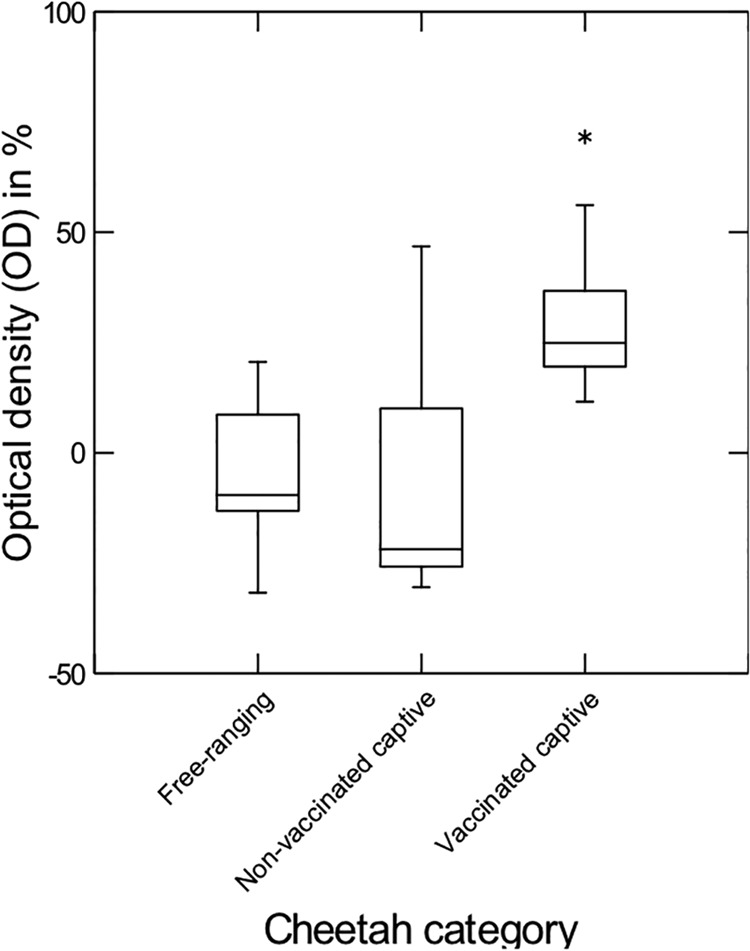
Optical densities obtained for samples from free-ranging, captive nonvaccinated, and captive vaccinated cheetahs in antibody tests for FeLV p45. Captive vaccinated cheetahs had higher OD levels than free-ranging (P < 0.0001) and captive nonvaccinated (P < 0.0001) cheetahs, whereas the latter two had similar OD levels (P = 0.11). Box and whisker plot: lower line, 25th percentile; middle line, 50th percentile; upper line, 75th percentile; whiskers, 5th and 95th percentiles, respectively. *, outlier.
(iii) FL-74 whole-virus ELISA.
Seventeen free-ranging (19%), seven captive nonvaccinated (27%), and 30 captive vaccinated cheetahs (86%) tested positive (Table 1). Six of the seven repeatedly tested free-ranging cheetahs (86%) and five of the seven repeatedly tested captive nonvaccinated cheetahs (29%) revealed the same results for the repeated tests. Two of the five captive cheetahs that were sampled as nonvaccinated animals and 2 years later as vaccinated animals tested positive for both samples, and two tested negative for both samples; only one of the animals turned from negative to positive. Twenty-seven of the AfriCat cheetahs (90%) tested positive. The OD values differed for the three categories (Kruskal-Wallis test statistic, 46.38; P < 0.0001; free-ranging, n = 88; nonvaccinated, n = 25; vaccinated, n = 30), with captive vaccinated cheetahs having higher values than free-ranging cheetahs (Conover-Inman test for pairwise comparisons, P < 0.0001) or captive nonvaccinated cheetahs (P < 0.0001). Antibodies to FL-74 virus increased with the time of presence in the AfriCat facility (R = 0.37, P = 0.044). Free-ranging cheetahs had OD values similar to those for captive nonvaccinated cheetahs (P = 0.20) (Fig. 2).
FIG 2.
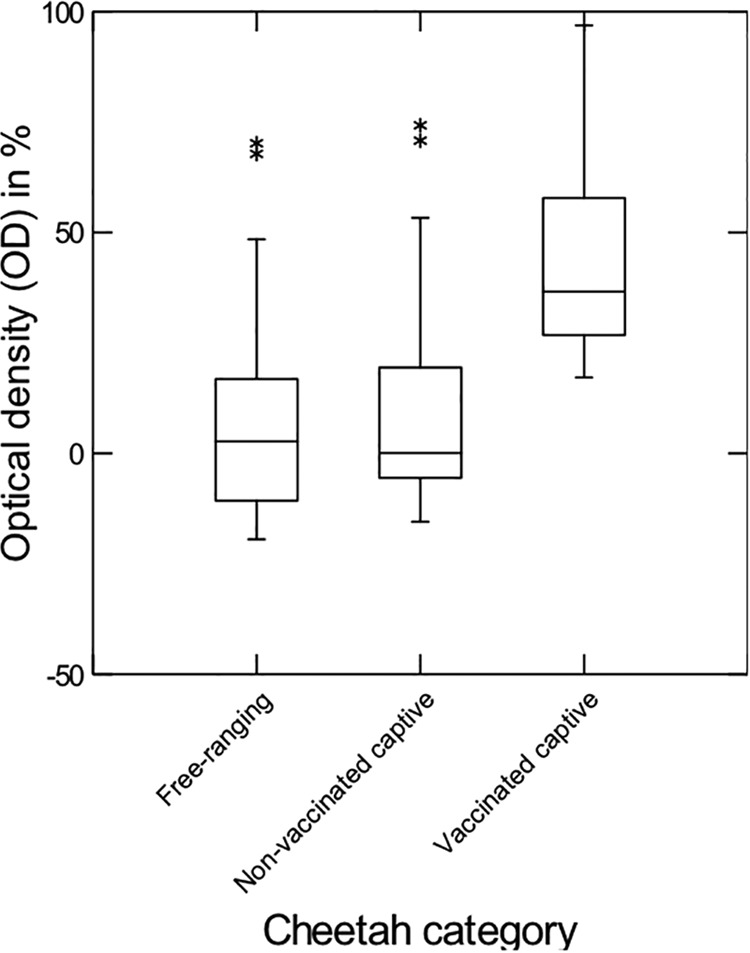
Optical densities obtained for samples from free-ranging, captive nonvaccinated, and captive vaccinated cheetahs in antibody tests for FeLV FL-74. Captive vaccinated cheetahs had higher OD levels than free-ranging (P < 0.0001) and captive nonvaccinated (P < 0.0001) cheetahs, whereas the latter two had similar OD levels (P = 0.20). Box and whisker plot: lower line, 25th percentile; middle line, 50th percentile; upper line, 75th percentile; whiskers, 5th and 95th percentiles, respectively. *, outliers.
Western blotting.
Twenty-seven free-ranging (60%), 10 captive nonvaccinated (60%), and 24 captive vaccinated cheetahs (69%) were positive (Table 2). All three repeatedly tested free-ranging cheetahs revealed the same results for the repeated tests. Two of five captive cheetahs that were sampled as nonvaccinated animals and 2 years later as vaccinated animals tested positive for both samples, and two tested negative for both samples; one animal turned, contrary to expectation, from positive to negative. The vaccinated AfriCat cheetahs did not differ from captive nonvaccinated and free-ranging cheetahs in their proportions of positive and negative samples (χ2 = 1.43; free-ranging positive, n = 27; free-ranging negative, n = 18; nonvaccinated positive, n = 10; nonvaccinated negative, n = 5; vaccinated positive, n = 22; vaccinated negative, n = 8; P = 0.49).
TABLE 2.
Western blot results for the presence of antibodies against FeLV p27 and FeLV p15(E) in free-ranging, captive nonvaccinated, and captive vaccinated cheetahs
| Western blot | Free-ranging |
Captive nonvaccinated |
Captive vaccinated |
|||
|---|---|---|---|---|---|---|
| No. (%) of animals | No. (%) of samples | No. (%) of animals | No. (%) of samples | No. (%) of animals | No. (%) of samples | |
| FeLVa | ||||||
| Positive for both p15(E) fragments | 13 (29) | 16 (32) | 6 (37) | 10 (47) | 23 (66) | 23 (66) |
| Positive for p27 + p15(E) | 14 (31) | 15 (30) | 4 (26) | 5 (24) | 1 (3) | 1 (3) |
| Negative | 18 (40) | 19 (38) | 6 (37) | 6 (29) | 11 (31) | 11 (31) |
| Total tested | 45 | 50 | 15 | 21 | 35 | 35 |
| Sum | 45 (100) | 50 (100) | 16 (100)b | 21 (100) | 35 (100) | 35 (100) |
| Positive for p15(E) ofc | ||||||
| BeRV | 0 (0) | 0 (0) | ND | ND | ND | ND |
| FeRD114eRV | 0 (0) | 0 (0) | ND | ND | ND | ND |
| RMuLV | 6 (50)d | 6 (50)d | ND | ND | ND | ND |
| AKR-MLV | 0 (0) | 0 (0) | ND | ND | ND | ND |
| Total tested | 12 | 12 | ||||
Samples were considered positive when antibodies were detected against FeLV p27 plus one or two p15(E) fragments or against both p15(E) fragments only.
The sum of the animals is greater than the total number of animals because animals captured and tested repeatedly were positive for different fragments (see the text).
Six FeLV-p15(E)-positive and six FeLV-p15(E)-negative samples from 12 free-ranging cheetahs were used to test antibody reactivity against four additional retroviruses. BeRV, baboon endogenous retrovirus; FeRD114eRV, feline RD114 endogenous retrovirus; RMuLV, Rauscher murine leukemia virus; AKR-MLV, AKR murine leukemia virus; ND, not done.
All six RMuLV-p15(E)-positive samples were also FeLV-p15(E)-positive samples.
None of the cheetahs tested positive for all four tests performed. Of the 34 free-ranging, 13 captive nonvaccinated, and 34 captive vaccinated cheetahs that tested positive for at least one of the tests, nine (27%), five (39%), and nine (27%), respectively, were positive for a second test and four (12%), one (8%), and 18 (53%), respectively, were positive for three tests. There were no significant differences in the proportions of seropositive or seronegative males and females for p45 ELISA, FL-74 ELISA, and WB.
Of the six FeLV-WB-positive and six FeLV-WB-negative free-ranging animals that were selected for further WB evaluation for four other retroviruses, the six WB-positive animals also demonstrated antibodies against the two p15(E) fragments of RMuLV. The six WB-negative animals tested negative for all four retroviruses.
Real-time PCR.
Forty of 41 whole-blood TNA samples showed acceptable amounts of genomic DNA (>10,000 copies/reaction [33]) and were selected for further analysis. PCR assays for endogenous and exogenous FeLV were negative for all animals.
PERT assay.
We tested PBMC cultures from seven WB-positive samples and one WB-negative sample from free-ranging animals. The PERT assays were negative. The RT activity of cell culture supernatants was 0 to 16 nU/ml, and that of plasma from 12 WB- and ELISA-negative animals was 0 to 1 nU/ml. Positive-control samples had an activity range of 10,000 to 20,000 nU/ml.
DISCUSSION
FeLV infecting free-ranging felids can threaten entire populations (18, 37). To date, the free-ranging Namibian felid population has been spared retroviral infections (12, 13, 38, 39); the Kalahari Desert may act as a natural barrier, keeping the Namibian populations naive to pathogens present in adjacent countries (10, 11, 40, 41). In our study, we detected antibodies against FeLV and RMuLV but no FeLV viral DNA or reverse transcriptase activity in PBMCs or plasma samples. If the Kalahari Desert is a natural barrier to pathogens, then the undetected retrovirus triggering measurable antibodies against FeLV and RMuLV in Namibian cheetahs might be an endemic one. Previous studies testing carnivores for FeLV used only antigen tests (12, 13), whereas we also used antibody tests in this study. Using antibody tests also in countries across the Kalahari Desert might provide new insights into the distribution of retroviruses in southern Africa.
Because antibodies that are able to recognize FeLV can cross-react with RMuLV (29, 42) but not with the more distantly related baboon or feline RD114 endogenous retrovirus, we speculate that Namibian cheetahs are either infected with a retrovirus or harbor a differentially expressed endogenous retrovirus that belongs to the gammaretrovirus mammalian group subfamily. No antibody reactivity to p15(E) of AKR-MuLV was detected, although AKR-MuLV p15(E) differs from FeLV and RMuLV p15(E) at only one amino acid position each. Thus, the observation that cheetah antibodies do not recognize AKR-MuLV p15(E) could be explained by the fact that AKR-MuLV p15(E) is different in a conformational epitope not present in FeLV and RMuLV p15(E).
Alternatively, the presence of reactive antibodies against RMuLV in all six FeLV-WB-positive samples might be due to the activation of endogenous gammaretroviruses not directly related to an infectious disease. A similar observation has been reported recently for humans diagnosed with recent-onset psychosis (43). Those subjects developed antibodies cross-reactive to MuLV and the Mason-Pfizer monkey virus (a simian D-type betaretrovirus), whereas patients with long-term schizophrenia or control subjects did not exhibit such cross-reactive antibodies (43). Although this underlying cause is unlikely to be important for cheetahs, aberrant endogenous retrovirus expression might also be triggered by other factors and was reported to be involved in defective mice spermatogenesis (44). Defective spermatogenesis can result in teratospermia, i.e., the production of >60% morphologically abnormal sperm per ejaculate, which is a well-known phenomenon in felids, particularly cheetahs (45–47). However, the capacities of ejaculates from proven and unproven cheetah male breeders to inseminate oozytes in vitro do not differ (46), suggesting that teratospermia in cheetahs is not of major concern for captive or free-ranging populations, particularly not for the world's largest population in Namibia. Still, aberrant endogenous retrovirus expression might be involved in the production of teratospermic ejaculates in cheetahs, eventually becoming problematic for particular individuals if teratospermia reaches levels that lead to infertility.
In domestic cats viremic with FeLV, antibody-positive samples may show low levels of replicating virus, whereas viremic cats with samples with low or undetectable antibody levels may have productive infections with high levels of replicating virus (48). Therefore, samples from FeLV-WB-positive and WB-negative animals were used in the PERT assay. The negative PERT results may be due to complete repression of the virus in the antibody-positive animals or the absence of infection in the antibody-negative animals. Animals with productive infections might exist in the wild only rarely or infections might have only a very short phase of viremia, with such animals having a rather low probability of being captured and sampled. Additional analyses using, for example, targeted high-throughput sequencing of genomic DNA would be required to identify the unknown retroviral strain.
The fact that all of the animals were healthy also suggests that this virus may not be very pathogenic and/or has a short course of infection. This hypothesis is supported by the recent demonstration of nearly asymptomatic experimental infection of Mus pahari (a mice strain lacking endogenous murine leukemia viruses and gammaretrovirus restriction factor fv1 and seen as a transspecies transmission model for gammaretroviruses) with xenotropic murine leukemia virus-related gammaretrovirus (XMRV) (49, 50); while viral RNA was detected in the plasma of only 1/10 infected mice, 10/12 infected mice developed persistent neutralizing antibodies directed against Gag and Env proteins.
Therefore, in analogy to the situation in Mus pahari, we speculate that cheetahs, in the context of hunting (22), become exposed to a gammaretrovirus present in wild mice. This virus might be able to transiently infect cheetahs, which develop an effective immune response, seroconvert, and are able to overcome this retroviral infection. Alternatively, cheetahs may react to low-level exposure to FeLV like domestic cats. In domestic cats exposed experimentally to low levels of FeLV, seroconversion to FeLV was observed but FeLV proviral DNA was found to be present only in very small amounts in some internal organs (31, 51).
The good health status of the seropositive cheetahs further suggests that, despite the relatively low MHC class I variability, the cheetah population can mount effective immune responses against the gammaretrovirus investigated in this study. Low MHC variability, however, might limit the immunocompetence of individuals when confronted with new pathogens (2, 16).
Because Namibian cheetahs are potentially susceptible to FeLV (9), captive animals were preventively vaccinated with a vaccine widely used for domestic cats. We attempted to assess the magnitude of the immune responses after cheetahs have been vaccinated according to a protocol used for domestic cats (28). The cheetahs housed at AfriCat that had been vaccinated for several years had higher OD values for p45 and FL-74 than did free-ranging and captive nonvaccinated cheetahs but similar OD values for p27 and similar patterns of antigen recognition in WB. The increased levels of antibodies to FL-74 virus in cheetahs with long residence at AfriCat can be explained by repeated FeLV vaccinations. Thus, tests for antibodies to p45 and FL-74 virus seem to be useful to assess the effects of FeLV vaccination on antibody induction also in cheetahs. From the 71% and 86% positive responses to p45 and FL-74 antigens, respectively, in the vaccinated cheetahs at AfriCat, it can be concluded that cheetahs readily respond to FeLV vaccination. However, of the five captive animals that were vaccinated once and retested 2 years later, only one animal (20%) demonstrated higher OD values for p45 and FL-74 in the second test. This supports the concept that boosting may induce increased levels of antibodies. However, increased levels of antibodies are not necessarily identical to protection. In view of the presence of domestic cats infected with FeLV, it might be useful to continue with the vaccination program in captive animals.
In conclusion, we detected the presence of antibodies reacting with a FeLV-related gammaretrovirus in Namibian cheetahs. As the animals were in good health, we conclude that this infection of unknown origin may be endemic in Namibian cheetahs and that the affected animals can mount efficient immune responses and cope with the virus despite low MHC variability. Because no replicating virus was detected, further studies are required to identify its origin. As most of the FeLV-vaccinated cheetahs could mount immune responses to the vaccine, it appears to be meaningful to continue with the vaccination program against FeLV as a preventive measure in captive animals.
ACKNOWLEDGMENTS
We thank the Ministry of Environment and Tourism in Namibia for permission to conduct the study in the Seeis, Hochfeld, and Khomas Conservancies, and we thank the AfriCat Foundation and the private facilities housing captive cheetahs for cooperation. We also thank B. Förster and H. Förster, whose preparatory work provided the basis for the cooperation with the conservancies, and J. Lonzer, for his valuable work in the field. Laboratory work was performed using the facilities of the Central Veterinary Laboratory in Windhoek and the logistic capabilities of the Center for Clinical Studies at the Vetsuisse Faculty of the University of Zurich (Zurich, Switzerland). RMuLV, AKR-MuLV, baboon endogenous virus, and RD114 virus were donated by the U.S. National Cancer Institute to H. Lutz in 1985. We thank G. Wolf-Jäckel, C. P. Geret, V. Rüegger, C. Robert, T. Meili Prodan, E. Gönczi, and B. Weibel in Zurich, Switzerland, and D. Thierer and K. Wilhelm in Berlin, Germany, for expert laboratory assistance and technical support.
This project was funded by the Messerli Foundation in Switzerland and a grant to A. Krengel from the German Academic Exchange Service.
REFERENCES
- 1.Durant S, Marker L, Purchase N, Belbachir F, Hunter L, Packer C, Breitenmoser-Wursten C, Sogbohossou E, Bauer H. 2008. Acinonyx jubatus, on The IUCN Red List of Threatened Species. http://www.iucnredlist.org/details/219/0 Accessed 24 July 2011.
- 2.Castro-Prieto A, Wachter B, Sommer S. 2011. Cheetah paradigm revisited: MHC diversity in the world's largest free-ranging population. Mol Biol Evol 28:1455–1468. doi: 10.1093/molbev/msq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Brien SJ, Roelke ME, Marker L, Newman A, Winkler CA, Meltzer D, Colly L, Evermann JF, Bush M, Wildt DE. 1985. Genetic basis for species vulnerability in the cheetah. Science 227:1428–1434. doi: 10.1126/science.2983425. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien SJ, Wildt DE, Bush M. 1986. The cheetah in genetic peril. Sci Am 254:84–92. doi: 10.1038/scientificamerican0586-84.3704622 [DOI] [Google Scholar]
- 5.O'Brien SJ, Evermann JF. 1988. Interactive influence of infectious disease and genetic diversity in natural populations. Trends Ecol Evol 3:254–259. doi: 10.1016/0169-5347(88)90058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evermann JF, Roelke ME, Briggs MB. 1986. Feline coronavirus infection of cheetahs. Feline Pract 16:21–30. [Google Scholar]
- 7.Evermann JF, Heeney JL, Roelke ME, McKeirnan AJ, O'Brien SJ. 1988. Biological and pathological consequences of feline infectious peritonitis virus infection in the cheetah. Arch Virol 102:155–171. doi: 10.1007/BF01310822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marker L, O'Brien SJ. 1989. Captive breeding of the cheetahs (Acinonyx jubatus) in North American zoos (1871–1986). Zoo Biol 8:3–16. doi: 10.1002/zoo.1430080103. [DOI] [Google Scholar]
- 9.Marker L, Munson L, Basson PA, Quackenbush S. 2003. Multicentric T-cell lymphoma associated with feline leukemia virus infection in a captive Namibian cheetah (Acinonyx jubatus). J Wildl Dis 39:690–695. doi: 10.7589/0090-3558-39.3.690. [DOI] [PubMed] [Google Scholar]
- 10.Olmsted RA, Langley R, Roelke ME, Goeken RM, Adger-Johnson D, Goff JP, Albert JP, Packer C, Laurenson MK, Caro TM, Scheepers L, Wildt DE, Bush M, Martenson JS, O'Brien SJ. 1992. Worldwide prevalence of lentivirus infection in wild feline species: epidemiologic and phylogenetic aspects. J Virol 66:6008–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown EW, Miththapala S, O'Brien SJ. 1993. Prevalence of exposure to feline immunodeficiency virus in exotic felids. J Zoo Wildl Med 24:357–364. [Google Scholar]
- 12.Munson L, Marker L, Dubovi E, Spencer JA, Evermann JF, O'Brien SJ. 2004. Serosurvey of viral infections in free-ranging Namibian cheetahs (Acinonyx jubatus). J Wildl Dis 40:23–31. doi: 10.7589/0090-3558-40.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Thalwitzer S, Wachter B, Robert N, Wibbelt G, Muller T, Lonzer J, Meli ML, Bay G, Hofer H, Lutz H. 2010. Seroprevalences to viral pathogens in free-ranging and captive cheetahs (Acinonyx jubatus) on Namibian farmland. Clin Vaccine Immunol 17:232–238. doi: 10.1128/CVI.00345-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thalwitzer S. 2007. Reproductive activity in cheetah females, cub survival and health of male and female cheetahs on Namibian farmland. Ph.D. dissertation Freie Universität, Berlin, Germany. [Google Scholar]
- 15.Munson L, Terio KA, Worley M, Jago M, Bagot-Smith A, Marker L. 2005. Extrinsic factors significantly affect patterns of disease in free-ranging and captive cheetah (Acinonyx jubatus) populations. J Wildl Dis 41:542–548. doi: 10.7589/0090-3558-41.3.542. [DOI] [PubMed] [Google Scholar]
- 16.Radwan J, Biedrzycka A, Babik W. 2010. Does reduced MHC diversity decrease viability of vertebrate populations? Biol Conserv 143:537–544. doi: 10.1016/j.biocon.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown MA, Cunningham MW, Roca AL, Troyer JL, Johnson WE, O'Brien SJ. 2008. Genetic characterization of feline leukemia virus from Florida panthers. Emerg Infect Dis 14:252–259. doi: 10.3201/eid1402.070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meli ML, Cattori V, Martinez F, Lopez G, Vargas A, Simon MA, Zorrilla I, Munoz A, Palomares F, Lopez-Bao JV, Pastor J, Tandon R, Willi B, Hofmann-Lehmann R, Lutz H. 2009. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus). PLoS One 4:e4744. doi: 10.1371/journal.pone.0004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider HP. 1991. Animal health and veterinary medicine in Namibia. Agrivet, Windhoek, Namibia. [Google Scholar]
- 20.Marker-Kraus L, Kraus D, Barnett D, Hurlbut S. 1996. Cheetah survival on Namibian farmlands. Cheetah Conservation Fund, Otjiwarongo, Namibia. [Google Scholar]
- 21.Mendelsohn J, Jarvis A, Roberts C, Robertson T. 2002. Atlas of Namibia: a portrait of the land and its people. David Philip Publisher, Cape Town, South Africa. [Google Scholar]
- 22.Malan JS. 1995. Peoples of Namibia. Rhino Publisher, Pretoria, South Africa. [Google Scholar]
- 23.Wachter B, Thalwitzer S, Hofer H, Lonzer J, Hildebrandt TB, Hermes R. 2011. Reproductive history and absence of predators are important determinants of reproductive fitness: the cheetah controversy revisited. Conserv Lett 4:47–54. doi: 10.1111/j.1755-263X.2010.00142.x. [DOI] [Google Scholar]
- 24.Pepin AC, Tandon R, Cattori V, Niederer E, Riond B, Willi B, Lutz H, Hofmann-Lehmann R. 2007. Cellular segregation of feline leukemia provirus and viral RNA in leukocyte subsets of long-term experimentally infected cats. Virus Res 127:9–16. doi: 10.1016/j.virusres.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Lutz H, Pedersen NC, Theilen GH. 1983. Course of feline leukemia virus infection and its detection by enzyme-linked immunosorbent assay and monoclonal antibodies. Am J Vet Res 44:2054–2059. [PubMed] [Google Scholar]
- 26.Hofmann-Lehmann R, Huder JB, Gruber S, Boretti F, Sigrist B, Lutz H. 2001. Feline leukaemia provirus load during the course of experimental infection and in naturally infected cats. J Gen Virol 82:1589–1596. [DOI] [PubMed] [Google Scholar]
- 27.Lutz H, Pedersen NC, Durbin R, Theilen GH. 1983. Monoclonal antibodies to three epitopic regions of feline leukemia virus p27 and their use in enzyme-linked immunosorbent assay of p27. J Immunol Methods 56:209–220. doi: 10.1016/0022-1759(83)90413-1. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann R, Franchini M, Aubert A, Wolfensberger C, Cronier J, Lutz H. 1991. Vaccination of cats experimentally infected with feline immunodeficiency virus, using a recombinant feline leukemia virus vaccine. J Am Vet Med Assoc 199:1446–1452. [PubMed] [Google Scholar]
- 29.Lutz H, Pedersen N, Higgins J, Hubscher U, Troy FA, Theilen GH. 1980. Humoral immune reactivity to feline leukemia virus and associated antigens in cats naturally infected with feline leukemia virus. Cancer Res 40:3642–3651. [PubMed] [Google Scholar]
- 30.Lutz H, Arnold P, Hubscher U, Egberink H, Pedersen N, Horzinek MC. 1988. Specificity assessment of feline T-lymphotropic lentivirus serology. Zentralbl Veterinarmed B 35:773–778. [DOI] [PubMed] [Google Scholar]
- 31.Gomes-Keller MA, Gönczi E, Grenacher B, Tandon R, Hofman-Lehmann R, Lutz H. 2009. Fecal shedding of infectious feline leukemia virus and its nucleic acids: a transmission potential. Vet Microbiol 134:208–217. doi: 10.1016/j.vetmic.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Leutenegger CM, Mislin CN, Sigrist B, Ehrengruber MU, Hofmann-Lehmann R, Lutz H. 1999. Quantitative real-time PCR for the measurement of feline cytokine mRNA. Vet Immunol Immunopathol 71:291–305. doi: 10.1016/S0165-2427(99)00100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tandon R, Cattori V, Willi B, Meli ML, Gomes-Keller MA, Lutz H, Hofmann-Lehmann R. 2007. Copy number polymorphism of endogenous feline leukemia virus-like sequences. Mol Cell Probes 21:257–266. doi: 10.1016/j.mcp.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Tandon R, Cattori V, Willi B, Lutz H, Hofmann-Lehmann R. 2008. Quantification of endogenous and exogenous feline leukemia virus sequences by real-time PCR assays. Vet Immunol Immunopathol 123:129–133. doi: 10.1016/j.vetimm.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Tandon R, Cattori V, Gomes-Keller MA, Meli ML, Golder MC, Lutz H, Hofmann-Lehmann R. 2005. Quantitation of feline leukaemia virus viral and proviral loads by TaqMan® real-time polymerase chain reaction. J Virol Methods 130:124–132. doi: 10.1016/j.jviromet.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Pyra H, Böni J, Schüpbach J. 1994. Ultrasensitive retrovirus detection by a reverse transcriptase assay based on product enhancement. Proc Natl Acad Sci U S A 91:1544–1548. doi: 10.1073/pnas.91.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunningham MW, Brown MA, Shindle DB, Terrell SP, Hayes KA, Ferree BC, McBride RT, Blankenship EL, Jansen D, Citino SB, Roelke ME, Kiltie RA, Troyer JL, O'Brien SJ. 2008. Epizootiology and management of feline leukemia virus in the Florida puma. J Wildl Dis 44:537–552. doi: 10.7589/0090-3558-44.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spencer JA, Morkel P. 1993. Serological survey of sera from lions in Etosha National Park. S Afr J Wildl Res 23:60–61. [Google Scholar]
- 39.Letcher JD, O'Conner TP. 1991. Incidence of antibodies reacting to feline immunodeficiency virus in a population of Asian lions. J Zoo Wildl Med 22:324–329. [Google Scholar]
- 40.Roelke ME, Brown MA, Troyer JL, Winterbach H, Winterbach C, Hemson G, Smith D, Johnson RC, Pecon-Slattery J, Roca AL, Alexander KA, Klein L, Martelli P, Krishnasamy K, O'Brien SJ. 2009. Pathological manifestations of feline immunodeficiency virus (FIV) infection in wild African lions. Virology 390:1–12. doi: 10.1016/j.virol.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osofsky SA, Hirsch KJ, Zuckermann EE, Hardy WD. 1996. Feline lentivirus and feline oncovirus status of free-ranging lions (Panthera leo), leopards (Panthera pardus), and cheetahs (Acinonyx jubatus) in Botswana: a regional perspective. J Zoo Wildl Med 27:453–467. [Google Scholar]
- 42.Parks WP, Scolnick EM. 1972. Radioimmunoassay of mammalian type-C viral proteins: interspecies antigenic reactivities of the major internal polypeptide. Proc Natl Acad Sci U S A 69:1766–1770. doi: 10.1073/pnas.69.7.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickerson F, Lillehoj E, Stallings C, Wiley M, Origoni A, Vaughan C, Khushalani S, Sabunciyan S, Yolken R. 2012. Antibodies to retroviruses in recent onset psychosis and multi-episode schizophrenia. Schizophr Res 138:198–205. doi: 10.1016/j.schres.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 44.Ollinger R, Childs AJ, Burgess HM, Speed RM, Lundegaard PR, Reynolds N, Gray NK, Cooke HJ, Adams IR. 2008. Deletion of the pluripotency-associated Tex19.1 gene causes activation of endogenous retroviruses and defective spermatogenesis in mice. PLoS Genet 4:e1000199. doi: 10.1371/journal.pgen.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pukazhenthi BS, Wildt DE, Howard JG. 2001. The phenomenon and significance of teratospermia in felids. J Reprod Fertil Suppl 57:423–433. [PubMed] [Google Scholar]
- 46.Pukazhenthi BS, Neubauer K, Jewgenow K, Howard J, Wildt DE. 2006. The impact and potential etiology of teratospermia in the domestic cat and its wild relatives. Theriogenology 66:112–121. doi: 10.1016/j.theriogenology.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 47.Wildt DE, Bush M, Howard JG, O'Brien SJ, Meltzer D, Van Dyk A, Ebedes H, Brand DJ. 1983. Unique seminal quality in the South African cheetah and a comparative evaluation in the domestic cat. Biol Reprod 29:1019–1025. doi: 10.1095/biolreprod29.4.1019. [DOI] [PubMed] [Google Scholar]
- 48.Hofmann-Lehmann R, Cattori V, Tandon R, Boretti FS, Meli ML, Riond B, Lutz H. 2008. How molecular methods change our views of FeLV infection and vaccination. Vet Immunol Immunopathol 123:119–123. doi: 10.1016/j.vetimm.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Sakuma T, Tonne JM, Squillace KA, Ohmine S, Thatava T, Peng KW, Barry MA, Ikeda Y. 2011. Early events in retrovirus XMRV infection of the wild-derived mouse Mus pahari. J Virol 85:1205–1213. doi: 10.1128/JVI.00886-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakuma T, Tonne JM, Malcolm JA, Thatava T, Ohmine S, Peng KW, Ikeda Y. 2012. Long-term infection and vertical transmission of a gammaretrovirus in a foreign host species. PLoS One 7:e29682. doi: 10.1371/journal.pone.0029682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Major A, Cattori V, Boenzli E, Riond B, Ossent P, Meli ML, Hofmann-Lehmann R, Lutz H. 2010. Exposure of cats to low doses of FeLV: seroconversion as the sole parameter of infection. Vet Res 41:17. doi: 10.1051/vetres/2009065. [DOI] [PMC free article] [PubMed] [Google Scholar]


