Abstract
Activated monocytes/macrophages and T lymphocytes that produce a cytokine storm are assumed to play a pivotal role in the pathogenesis of dengue. Interleukin-18 (IL-18) is a proinflammatory cytokine that is increased during dengue and known to induce gamma interferon (IFN-γ), which is crucial for dengue immune response. No data are available regarding the balance between IL-18 and its natural inhibitor IL-18 binding protein (IL-18BP) and how they interact within the inflammatory reaction of patients with dengue virus infections. Circulating levels of IL-18; IL-18BP; free, biologically active IL-18; the IL-18-dependent proinflammatory cytokine IFN-γ; monocyte-derived cytokines; and ferritin were assessed in adult Indonesian dengue patients (n = 95). Healthy individuals (n = 22) and leptospirosis (n = 19) and enteric fever (n = 6) patients served as controls. Total IL-18 levels were increased during dengue, leptospirosis, and enteric fever compared to healthy controls. However, due to a concurrent increase in IL-18BP levels, biologically active IL-18 levels remained similar in the different phases of dengue and in patients with leptospirosis. Biologically active IL-18 levels were also similar in patients with severe and nonsevere dengue. In conclusion, high total IL-18 and IL-18BP levels concur in dengue virus infections, leptospirosis, and enteric fever. This resulted in unchanged levels of free, biologically active IL-18 in dengue and leptospirosis, which underlines the importance of measuring both IL-18 and IL-18BP when studying the role of IL-18 in diseases.
INTRODUCTION
Dengue has become one of the most important arboviral infections in the world (1). Dengue virus infection usually manifests as a self-limiting febrile illness. A subset of patients, however, develops life-threatening complications, which include plasma leakage, bleeding, and/or severe organ failure (2, 3). These complications usually occur during or shortly after defervescence in the so-called critical phase. The precise pathogenesis of these complications is not yet fully understood, but a massive secretion of cytokines by activated monocytes/macrophages and T lymphocytes is assumed to play a pivotal role (3–6).
Interleukin-18 (IL-18) is a member of the IL-1 family of cytokines. It is synthesized as an inactive precursor requiring processing by caspase-1 into an active cytokine. The precursor is constitutively expressed by nearly all cells in humans. Dengue virus has been shown to activate the inflammasome and induce the production of IL-18 by human macrophages (7). IL-18, together with IL-12, plays a major role in the production of gamma interferon (IFN-γ). This was also shown in a murine dengue model, in which IL-18 acted in synergism with IL-12 to induce IFN-γ and other T-helper 1 cytokines, which are considered central mediators in dengue host defense (8, 9).
Previous studies in patients with acute dengue have reported high serum IL-18 levels, which correlated with disease severity (10–12). However, the activity of IL-18 is balanced by a naturally occurring IL-18 binding protein (IL-18BP). IFN-γ stimulates the production of IL-18BP (13), prompting a classical feedback loop whereby IL-18BP neutralizes excessive IL-18 and attenuates the IFN-γ response. Hyperferritinemia has recently been reported to be a common feature of dengue (14). Hyperferritinemia is also commonly observed under conditions with macrophage activation such as adult-onset Still's disease, and IL-18 has been implicated in the pathogenesis of these conditions (15). To gain a better insight into the biological activity of IL-18 during dengue, we determined plasma levels of IL-18, together with levels of IL-18BP, IFN-γ, and other proinflammatory cytokines, in a cohort of Indonesian dengue patients. We also determined the association with ferritin levels and included a group of healthy individuals and patients suffering from leptospirosis and enteric fever as controls.
MATERIALS AND METHODS
Study design.
This study was part of a prospective study performed between March 2011 and March 2012 in Rumah Sakit Hasan Sadikin, an academic referral hospital in Bandung, Indonesia. Patients clinically suspected of having dengue, leptospirosis, or Salmonella enterica serovar Typhi/Salmonella enterica serovar Paratyphi infection were eligible to be included in the study. Blood was drawn at admission for routine laboratory investigations and diagnosis. Samples of clinically suspected dengue cases were tested for the presence of viral RNA by reverse transcriptase PCR (RT-PCR) and for dengue virus-specific IgM and IgG (Panbio, Windsor, Australia). A sample with a positive RT-PCR, a minimum 4-fold IgM and/or IgG titer increase, and/or an IgM and/or IgG conversion was considered a proven dengue virus infection. Samples that were IgM positive in at least one sample and/or with IgG levels comparable to a hemagglutination inhibition (HI) titer of at least 1:2,560 (the IgG cutoff point set by the manufacturer to detect secondary infection) were considered highly suggestive dengue cases (2). Patients with a proven or highly suggestive dengue virus infection were included and retrospectively classified as nonsevere or severe dengue according to WHO 2009 criteria (2). Dengue patients were systematically evaluated by daily history, physical examination, laboratory investigation, and ultrasonography to detect plasma leakage. Additional blood sampling was scheduled once in each clinical phase of dengue virus infection: the febrile phase (temperature of 37.5°C or more on that day), the critical phase (period within 48 h after defervescence and before platelet counts increased again), early recovery phase (increasing platelet counts and clinical improvement), and convalescence phase (>2 weeks after discharge).
Patients with a clinical leptospirosis diagnosis in combination with both a positive IgM rapid test (16) and a positive enzyme-linked immunosorbent assay (ELISA) IgM result (Panbio, Windsor, Australia) were included, as well as patients with a clinical diagnosis of enteric fever who had a positive blood culture (Bactec blood culture system; BD Diagnostics, Sparks, MD, USA) for S. Typhi or S. Paratyphi. Furthermore, a group of healthy volunteers, recruited among hospital staff, served as healthy controls.
The study was approved by the local Medical Ethical Committee, and written informed consent was obtained before enrollment from all patients and healthy controls.
Cytokines, IL-18 binding protein, and ferritin measurements.
Plasma was obtained from citrate-anticoagulated whole blood, which was immediately centrifuged at 1,700 × g for 15 min and stored at −80°C until further analysis. IL-18, IFN-α, IFN-γ, tumor necrosis factor alpha (TNF-α), IL-6, IL-1 receptor agonist (IL-1Ra), and ferritin levels were determined using commercially available Magpix Milliplex kits (Merck Millipore). A minimum of 50 beads was acquired with a Luminex Magpix instrument (Luminex Corporation, Austin, TX, USA). The minimum detectable concentrations were 9.8 pg/ml for IL-18; 2.4 pg/ml for IFN-α, IFN-γ, TNF-α, IL-6, and IL-1Ra; and 0.03 ng/ml for ferritin, respectively. Plasma IL-1α (GenProbe Dioclone) and IL-18BP (IL-18BP-α; R&D Systems) were determined by commercially available ELISA kits according to the manufacturer's instructions and had minimum detection limits of 0.03 ng/ml and 0.1 ng/ml, respectively. The level of free, bioactive IL-18 was calculated based on the mass-action law, using a dissociation constant of 400 pM and a stoichiometric ratio of 1:1 (17).
Statistical analysis.
Data are expressed as medians with interquartile ranges (IQRs) or numbers with percentages. Differences in noncontinuous data of 2 groups were analyzed by Pearson's chi-square test or by Fisher's exact test in case of expected counts of <5. Continuous variables between 2 groups were analyzed by unpaired t test in cases of normally distributed data and by Mann-Whitney U test in cases of nonparametric data. Relationships between continuous data were examined by Spearman's correlation for nonparametric data. A P value of <0.05 was considered statistically significant.
RESULTS
Clinical characteristics.
A total number of 95 patients with acute dengue, 19 patients with acute leptospirosis, 6 patients with enteric fever, and 22 healthy controls were enrolled. Of the dengue patients, 80 (84%) were retrospectively classified as having nonsevere dengue and 15 (16%) were classified as having severe dengue (2). All patients with severe dengue had plasma leakage in combination with shock; two patients with severe dengue also had respiratory distress accompanied by pleural effusion. None of the severe dengue cases had severe bleeding or organ failure. Characteristics and baseline data of the enrolled subjects are presented in Table 1.
TABLE 1.
Patient and healthy control characteristics and baseline dataa
| Characteristic | Nonsevere dengue (n = 80 [84%]) | Severe dengue (n = 15 [16%]) | Leptospirosis (n = 19) | Enteric fever (n = 6) | Healthy control (n = 22) |
|---|---|---|---|---|---|
| Age, yr | 24 (19–34) | 23 (16–30) | 47 (39–55)*** | 25 (17–27) | 25 (23–31) |
| Male sex, n (%) | 46 (58)+* | 6 (40) | 14 (74)** | 3 (50) | 7 (32) |
| Duration of illness at enrollment, days | 6 (6–7) | 6 (5–7) | 7 (5–9) | 12 (9–17) | NA |
| Blood pressure, mm Hg | |||||
| Systolic | 110 (100–120)++ | 100 (90–110) | 100 (90–110) | 100 (100–110) | ND |
| Diastolic | 70 (60–80) | 70 (60–80) | 70 (60–70) | 62 (60–70) | ND |
| Pulse pressure, mm Hg | 40 (30–40)++ | 30 (20–40) | 40 (30–50) | 40 (34–43) | ND |
| Pulse rate, per min | 80 (72–92) | 84 (80–108) | 88 (80–92) | 90 (79–96) | ND |
| Respiratory rate, per min | 22 (20–24) | 20 (20–24) | 24 (20–26) | 24 (22–25) | ND |
| Temp, °C | 36.4 (35.8–37.3) | 36.3 (35.8–37.6) | 36.6 (36.3–37.2) | 37.7 (36.7–38.0) | ND |
| Hemoglobin, g/dl | 14.3 (13.0–15.8) | 13.9 (11.1–15.5) | 11.5 (10.0–11.9)*** | 11.6 (7.0–13.3) | 13.9 (12.7–15.4) |
| Hematocrit, % | 42 (39–45) | 43 (33–46) | 32 (28–35)*** | 33 (22–39)* | 42 (39–46) |
| Cell count, ×109/liter | |||||
| Platelets | 46 (30–70)+++*** | 20 (14–26)*** | 151 (62–293)** | 107 (62–159)*** | 294 (240–318) |
| Leukocytes | 3.8 (2.9–5.0)*** | 4.1 (3.3–4.8)*** | 13.6 (7.9–14.9)** | 4.0 (3.4–4.3)** | 7.0 (6.8–8.1) |
| Albumin, g/dl | 3.7 (3.5–4.0) | 3.3 (3.1–3.7) | ND | ND | ND |
| ALAT, U/dl | 44 (25–79) | 44 (35–58) | 29 (17–58) | 46 (28–223) | ND |
| Ferritin, ng/ml | 3,260 (918–8,305)*** | 3,760 (980–8,150)*** | 509 (209–1,069)*** | 3,165 (533–9,113)** | 42 (28–90) |
Data are represented as median (interquartile range) for continuous data or number with percentage for noncontinuous data. Mann-Whitney U tests or chi-square tests were performed for comparison of each group with healthy controls (*) or for comparison between nonsevere and severe dengue (+). A P value of <0.05 was considered significant and is indicated as *** or +++ (P < 0.001), ** or ++ (P < 0.01), or * or + (P < 0.05). Abbreviations: ALAT, alanine aminotransferase; NA, not applicable; ND, not determined.
The RT-PCR for dengue was positive in 33 (35%) patients, with dengue virus type 2 (DENV-2) being the most common serotype (n = 16), followed by DENV-3 (n = 8), DENV-1 (n = 5), and DENV-4 (n = 4). In the remainder of the dengue patients, the diagnosis was based on results of the serological tests. Most dengue patients were admitted in the critical phase (n = 76; 80%), and the others were admitted in the febrile phase (n = 15; 16%) and the early recovery phase (n = 4; 4%). Plasma leakage in the form of ascites, pleural effusion, and/or significant hematocrit changes occurred in 51 (54%) of the patients. Ferritin levels were most increased in dengue and enteric fever patients and to a lesser extent increased in leptospirosis patients, compared to healthy controls.
IL-18BP neutralizes increased IL-18 levels in early dengue and in leptospirosis.
Patients with acute dengue, leptospirosis, and enteric fever all had significantly higher total median IL-18 plasma levels than did healthy controls (Fig. 1A). However, plasma IL-18BP levels were also significantly increased in all 3 patient groups (Fig. 1B). This resulted in a calculated free, biologically active IL-18 level that was unchanged in dengue and leptospirosis patients compared with healthy controls, while patients with enteric fever had increased free IL-18 levels despite a simultaneous increase in IL-18BP (Fig. 1A to C). The critical phase of dengue occurs around defervescence and is the phase of dengue in which most severe complications take place. This phase was defined as the period within 48 h after defervescence and before platelet counts increased again. Total IL-18, IL-18BP, and biologically active IL-18 plasma levels did not differ between nonsevere and severe dengue (Fig. 2A to C) or between patients with and without plasma leakage and/or bleeding in the critical phase of dengue (data not shown). In the critical phase of dengue, median total IL-18 levels were 218.5 and 163.0 pg/ml (interquartile ranges, 104.5 to 354.0 and 101.5 to 258.5 pg/ml), median IL-18BP levels were 14.6 and 16.7 ng/ml (interquartile ranges, 9.4 to 22.1 and 12.9 to 22.8 ng/ml), and median biologically active IL-18 levels were 77.4 and 58.1 pg/ml (interquartile ranges, 44.9 to 158.1 and 37.2 to 77.3 pg/ml) for severe and nonsevere dengue patients, respectively. There was a moderate positive correlation between total IL-18 as well as IL-18BP levels and ferritin levels in dengue patients (Rs = 0.36, P = 0.001; Rs = 0.29, P < 0.05, respectively).
FIG 1.
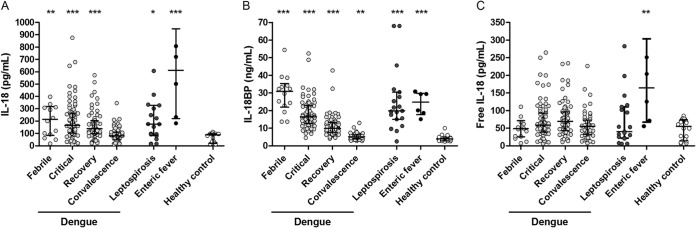
IL-18, IL-18BP, and free, biologically active IL-18 levels in dengue, leptospirosis, and enteric fever patients and healthy controls. Plasma levels of total interleukin-18 (IL-18) (A); IL-18 binding protein (IL-18BP) (B); and free, biologically active IL-18 (C) in Indonesian adults with dengue fever (n = 95) during the febrile (n = 14), critical (n = 77), early recovery (n = 72), and convalescence (n = 54) phases; with leptospirosis (n = 19); and with enteric fever (n = 6) and in a control group of healthy adult Indonesians (n = 22). The minimum detectable concentration was 9.8 pg/ml for total IL-18 and 0.1 ng/ml for IL-18BP. The level of free, bioactive IL-18 was calculated based on the mass-action law, using a dissociation constant of 400 pM and a stoichiometric ratio of 1:1. The dots represent the individual measurements, the horizontal lines represent median values, and the whiskers represent interquartile ranges. Outliers were left out of the figure as follows: (A) 1 febrile dengue, 1 leptospirosis, and 1 enteric fever patient (IL-18 = 2,077, 2,207, and 1,367 pg/ml, respectively) and (B) 1 leptospirosis patient (IL-18BP = 441 ng/ml). P values were determined by Mann-Whitney U test for comparison of dengue, leptospirosis, and enteric fever patients with the healthy control group. A P value of <0.05 was considered significant. P values are indicated as follows: *, <0.05; **, <0.01; ***, <0.001.
FIG 2.
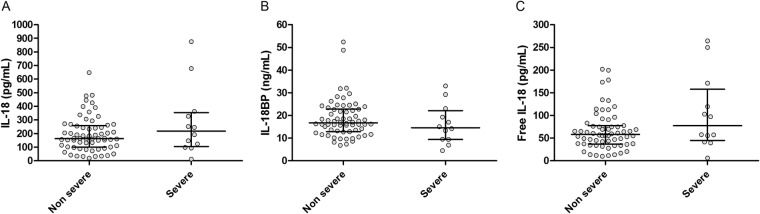
IL-18; IL-18BP; and free, biologically active IL-18 levels in nonsevere versus severe dengue cases. Plasma levels of total interleukin-18 (IL-18) (A); IL-18 binding protein (IL-18BP) (B); and free, biologically active IL-18 (C) in Indonesian adults with nonsevere dengue fever (n = 65; n = 4 missing) and severe dengue fever (n = 12; n = 3 missing) during the critical phase. The minimum detectable concentration was 9.8 pg/ml for total IL-18 and 0.1 ng/ml for IL-18BP. The level of free, bioactive IL-18 was calculated based on the mass-action law, using a dissociation constant of 400 pM and a stoichiometric ratio of 1:1. The dots represent the individual measurements, the horizontal lines represent median values, and the whiskers represent interquartile ranges. P values were determined by Mann-Whitney U test for comparison of nonsevere dengue and severe dengue. A P value of <0.05 was considered significant.
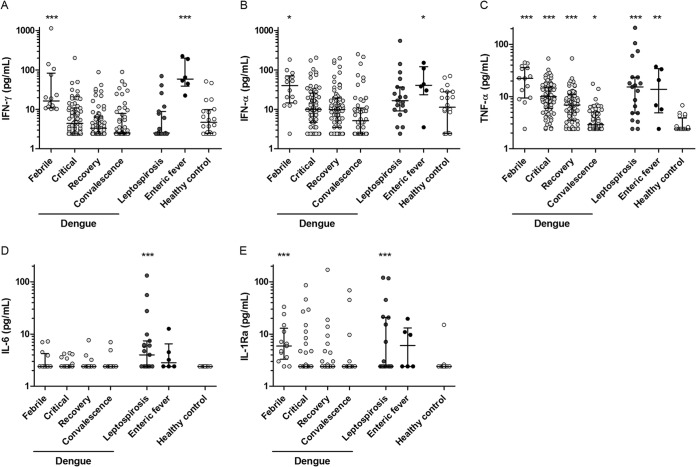
IFN-γ, IFN-α, and TNF-α increased, while IL-6, IL-1Ra, and IL-1α levels remained low during dengue.
Plasma levels of IFN-γ, IFN-α, and TNF-α were all increased during early dengue virus infection (Fig. 3A to C). Remarkably, high TNF-α levels were found in dengue patients, while IL-6 remained below the detection limit of 2.4 pg/ml, except for 7/13 (54%) patients in the febrile phase (Fig. 3D). The same trend was observed for IL-1Ra (Fig. 3E) and IL-1α (data not shown; 156 patients and control samples were below the detection limit, and therefore, no further analysis of the remaining samples was performed). Compared to patients with nonsevere dengue, patients with severe dengue had similar IFN-γ levels (medians, 6.3 and 4.4 pg/ml; interquartile ranges, 2.4 to 19.1 and 2.4 to 10.5 pg/ml, respectively) but decreased IFN-α levels (medians, 4.6 and 11.1 pg/ml; interquartile ranges, 2.4 to 10.9 and 6.4 to 27.9 pg/ml, respectively; P < 0.05) and increased TNF-α levels (medians, 14.6 and 9.6 pg/ml; interquartile ranges, 8.9 to 19.9 and 5.0 to 14.3 pg/ml, respectively; P < 0.05) in the critical phase. Total IL-18 was moderately associated with TNF-α (Rs = 0.28, P < 0.01), and IL-18BP was associated with IFN-α (Rs = 0.29, P < 0.01) but not with IFN-γ, whereas biologically active IL-18 was not associated with any of the determined cytokines.
FIG 3.
Cytokine levels in dengue, leptospirosis, and enteric fever patients and healthy controls. Plasma levels of gamma interferon (IFN-γ) (A), IFN-α (B), tumor necrosis factor alpha (TNF-α) (C), IL-6 (D), and IL-1 receptor antagonist (IL-1Ra) (E) in Indonesian adults with dengue fever (n = 95) during the febrile (n = 14), critical (n = 77), early recovery (n = 72), and convalescence (n = 54) phases; with leptospirosis (n = 19); and with enteric fever (n = 6) and in a control group of healthy adult Indonesians (n = 22). The minimum detectable concentration was 2.4 pg/ml for all cytokines. The dots represent the individual measurements, the horizontal lines represent median values, and the whiskers represent interquartile ranges. P values were determined by Mann-Whitney U test for comparison of dengue, leptospirosis, and enteric fever patients with the healthy control group. A P value of <0.05 was considered significant. P values are indicated as follows: *, <0.05; **, <0.01; ***, <0.001.
Enteric fever patients also had increased IFN-γ, IFN-α, and TNF-α plasma levels (Fig. 3A to C), while leptospirosis was associated with increased IL-6 (Fig. 3D) and IL-1Ra (Fig. 3E). In leptospirosis, IL-18BP positively correlated with TNF-α levels (Rs = 0.68, P = 0.001) (data not shown).
DISCUSSION
Three clinical studies documented high IL-18 levels in dengue virus-infected patients (10–12). However, the interpretation of these data is limited by the fact that no information on its natural antagonist IL-18BP is given as well. The same applies to the in vitro and animal studies that also suggested an important role for IL-18 in the pathogenesis of dengue virus (7–9). In the present study, we document high plasma IL-18 levels concurring with increased IL-18BP levels in dengue, especially during the febrile and critical phases of dengue and to a lesser extent in the recovery phase of dengue. As a result, the calculated levels of circulating biologically active IL-18 levels did not increase during any of the phases of dengue. Furthermore, IL-18BP and biologically active IL-18 levels were not related to dengue severity during any of the phases of dengue virus infection. Our findings therefore suggest that the importance of high IL-18 levels in dengue should be put into perspective.
The concurrent elevation of IL-18 and IL-18BP was not specific for dengue virus infection but also occurred in our cohort of leptospirosis and enteric fever patients. Only enteric fever patients ultimately had increased biologically active IL-18 levels, although this group was too small to draw any firm conclusions.
IFN-γ is a central cytokine in the host defense against dengue. IFN-γ production can be induced, as shown in a murine dengue model, by the combined action of IL-18 and IL-12, while blocking IL-18 activity by administration of recombinant IL-18BP results in lower IFN-γ levels, higher TNF-α and IL-6 serum levels, and more severe disease (8). In contrast, the present study showed that IFN-γ was increased during the febrile phase of dengue, while IL-18 levels were unchanged. Our findings that circulating levels of biologically active IL-18 were not increased in dengue do not preclude a role for IL-18 in IFN-γ production, because IL-18-secreting macrophages are especially located in the liver and spleen and levels in the peripheral blood may not reflect local IL-18 production. Furthermore, we cannot exclude the possibility that IL-18-independent IFN-γ production by the adaptive part of the immune system, especially specific Th1 memory cells, also plays a role.
Apart from dengue, IL-18BP levels were also measured previously in patients with acute Chikungunya virus infection. IL-18BP levels were higher in acute-phase sera than in convalescent-phase sera, but levels in that study were significantly lower than our data (18). In patients with sepsis, total IL-18 and IL-18BP and calculated biologically active IL-18 were all increased (19), while the clearly elevated IL-18BP levels again did not reach levels similar to what we found. These studies, together with the current study, indicate that biologically active IL-18 should be determined when analyzing the role of IL-18 in the pathogenesis of diseases.
A limitation of this study is that patients were admitted relatively late in the course of dengue, enteric fever, and leptospirosis, probably due to late help-seeking behavior of the patient and the study setting in an academic referral hospital. We cannot rule out the possibility that IL-18BP was induced significantly later than IL-18 and that higher biologically active IL-18 levels may have been present earlier in the course of dengue.
In conclusion, in this study we report that increased levels of IL-18 concur with increased levels of its antagonist IL-18BP, resulting in unchanged biologically active IL-18 levels in dengue virus infections. This demonstrates the need for both total IL-18 and IL-18BP measurements when studying the role of IL-18 in diseases. Increased IL-18 and IL-18BP levels seem not to be specific for dengue virus infection, as our data in leptospirosis and enteric fever patients indicate.
ACKNOWLEDGMENTS
We thank the patients and healthy volunteers for participating in this study. We thank D. Novick for sharing her expertise on calculating free IL-18; Suharyani Soedarmo, Fitria Utami, and Tenny Putri Wikayani for their assistance in blood sampling and processing; and Helga Toenhake-Dijkstra, Heidi Lemmers, and Trees Jansen for assisting in the IL-18 and IL-18 binding protein measurements and validation.
M.M. was funded by a Junior Research Grant provided by the Radboud University Medical Center, Nijmegen; M.G.N. was supported by a Vici grant of the Netherlands Organization for Scientific Research. C.A.D. was supported by National Institutes of Health grants AI-15614, CA-04-6934, and AR-45584. None of the funders had a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2009. Dengue: guidelines for diagnosis, treatment, prevention and control. WHO/HTM/NTD/DEN/2009.1. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 3.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. 2010. Dengue: a continuing global threat. Nat Rev Microbiol 8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturvedi UC, Agarwal R, Elbishbishi EA, Mustafa AS. 2000. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol Med Microbiol 28:183–188. doi: 10.1111/j.1574-695X.2000.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 5.Rothman AL, Ennis FA. 1999. Immunopathogenesis of dengue hemorrhagic fever. Virology 257:1–6. doi: 10.1006/viro.1999.9656. [DOI] [PubMed] [Google Scholar]
- 6.Costa VV, Fagundes CT, da Gloria de Souza D, Teixeira MM. 2013. Inflammatory and innate immune responses in dengue infection: protection versus disease induction. Am J Pathol doi: 10.1016/j.ajpath.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 7.Wu MF, Chen ST, Yang AH, Lin WW, Lin YL, Chen NJ, Tsai IS, Li L, Hsieh SL. 2013. CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood 121:95–106. doi: 10.1182/blood-2012-05-430090. [DOI] [PubMed] [Google Scholar]
- 8.Fagundes CT, Costa VV, Cisalpino D, Amaral FA, Souza PR, Souza RS, Ryffel B, Vieira LQ, Silva TA, Atrasheuskaya A, Ignatyev G, Sousa LP, Souza DG, Teixeira MM. 2011. IFN-gamma production depends on IL-12 and IL-18 combined action and mediates host resistance to dengue virus infection in a nitric oxide-dependent manner. PLoS Negl Trop Dis 5:e1449. doi: 10.1371/journal.pntd.0001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa VV, Fagundes CT, Valadao DF, Cisalpino D, Dias AC, Silveira KD, Kangussu LM, Avila TV, Bonfim MR, Bonaventura D, Silva TA, Sousa LP, Rachid MA, Vieira LQ, Menezes GB, de Paula AM, Atrasheuskaya A, Ignatyev G, Teixeira MM, Souza DG. 2012. A model of DENV-3 infection that recapitulates severe disease and highlights the importance of IFN-gamma in host resistance to infection. PLoS Negl Trop Dis 6:e1663. doi: 10.1371/journal.pntd.0001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathakrishnan A, Wang SM, Hu Y, Khan AM, Ponnampalavanar S, Lum LC, Manikam R, Sekaran SD. 2012. Cytokine expression profile of dengue patients at different phases of illness. PLoS One 7:e52215. doi: 10.1371/journal.pone.0052215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mustafa AS, Elbishbishi EA, Agarwal R, Chaturvedi UC. 2001. Elevated levels of interleukin-13 and IL-18 in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol 30:229–233. doi: 10.1111/j.1574-695X.2001.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 12.van de Weg CA, Huits RM, Pannuti CS, Brouns RM, van den Berg RW, van den Ham HJ, Martina BE, Osterhaus AD, Netea MG, Meijers JC, van Gorp EC, Kallas EG. 2014. Hyperferritinaemia in dengue virus infected patients is associated with immune activation and coagulation disturbances. PLoS Negl Trop Dis 8:e3214. doi: 10.1371/journal.pntd.0003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muhl H, Kampfer H, Bosmann M, Frank S, Radeke H, Pfeilschifter J. 2000. Interferon-gamma mediates gene expression of IL-18 binding protein in nonleukocytic cells. Biochem Biophys Res Commun 267:960–963. doi: 10.1006/bbrc.1999.2064. [DOI] [PubMed] [Google Scholar]
- 14.Soundravally R, Agieshkumar B, Daisy M, Sherin J, Cleetus CC. 2015. Ferritin levels predict severe dengue. Infection 43:13–19. doi: 10.1007/s15010-014-0683-4. [DOI] [PubMed] [Google Scholar]
- 15.Kadavath S, Efthimiou P. 2015. Adult-onset Still's disease—pathogenesis, clinical manifestations, and new treatment options. Ann Med 47:6–14. doi: 10.3109/07853890.2014.971052. [DOI] [PubMed] [Google Scholar]
- 16.Smits HL, Eapen CK, Sugathan S, Kuriakose M, Gasem MH, Yersin C, Sasaki D, Pujianto B, Vestering M, Abdoel TH, Gussenhoven GC. 2001. Lateral-flow assay for rapid serodiagnosis of human leptospirosis. Clin Diagn Lab Immunol 8:166–169. doi: 10.1128/CDLI.8.1.166-169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. 1999. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity 10:127–136. doi: 10.1016/S1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 18.Chirathaworn C, Rianthavorn P, Wuttirattanakowit N, Poovorawan Y. 2010. Serum IL-18 and IL-18BP levels in patients with Chikungunya virus infection. Viral Immunol 23:113–117. doi: 10.1089/vim.2009.0077. [DOI] [PubMed] [Google Scholar]
- 19.Novick D, Schwartsburd B, Pinkus R, Suissa D, Belzer I, Sthoeger Z, Keane WF, Chvatchko Y, Kim SH, Fantuzzi G, Dinarello CA, Rubinstein M. 2001. A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine 14:334–342. doi: 10.1006/cyto.2001.0914. [DOI] [PubMed] [Google Scholar]