Abstract
Because of the high incidence of morbidity and mortality associated with invasive fungal infections, antifungal prophylaxis is often used in solid organ transplant recipients. However, this prophylaxis is not universally effective and may contribute to the selection of emerging, resistant pathogens. Here we present a rare case of invasive infection caused by Microascus trigonosporus species complex in a human, which developed during voriconazole prophylaxis in a lung transplant recipient. Nebulized liposomal amphotericin B was used in addition to systemic therapy in order to optimize antifungal drug exposure; this regimen appeared to reduce the patient's fungal burden. Despite this apparent improvement, the patient's pulmonary status progressively declined in the setting of multiple comorbidities, ultimately leading to respiratory failure and death.
1. Introduction
Invasive fungal infections, most commonly with Candida spp. and Aspergillus spp., are associated with a high morbidity and mortality in immunocompromised hosts [1]. For this reason, antifungal prophylaxis active against these pathogens is often employed. However, antifungal prophylaxis is not universally effective and may contribute to the selection of emerging, resistant pathogens [2]. Here we present a rare case of invasive infection caused by Microascus trigonosporus species complex in a human, which developed during voriconazole prophylaxis.
2. Case Report
A 64-year-old male underwent bilateral lung transplantation at an outside hospital in 2011 for idiopathic pulmonary fibrosis with pulmonary hypertension. His comorbidities at the time of transplant were notable for New York Heart Association Class III Heart Failure with implantable cardioverter defibrillator placement. His postoperative course was complicated by severe primary graft dysfunction (PGD 3), acute rejection, renal failure requiring hemodialysis, tracheostomy placement, Clostridium difficile colitis and ileitis, cytomegaloviral viremia, bilateral upper extremity deep venous thrombi, pleurocutaneous fistula of the right chest necessitating pleurodesis, atrial fibrillation, and a gastrointestinal bleed. His postoperative course spanned six weeks in the intensive care unit. At hospital discharge, his forced expiratory volume in one second (FEV1) was 1.31 L/s or 43% of predicted.
Three months after discharge, he presented to our facility in follow-up after moving to our region. His immunosuppression consisted of tacrolimus (trough = 6.4 ng/mL), mycophenolate mofetil 250 mg twice daily, prednisone 10 mg daily, and inhaled fluticasone/salmeterol 250/50 mcg twice daily. He had been receiving voriconazole 200 mg twice daily for fungal prophylaxis since day 1 postoperatively in addition to sulfamethoxazole-trimethoprim and valganciclovir. At presentation he was noted to have worsening dyspnea, dry cough, increasing malaise, and low-grade fevers. Physical exam findings were consistent with distal airway narrowing by auscultation. FEV1 was 0.92 L/s (29% predicted). Chest radiography (CXR) showed diffuse patchy infiltrates and small bilateral pleural effusions (Figure 1). Bronchoscopy revealed multiple endobronchial strictures but no endobronchial plaques, and intravenous (IV) high-dose steroids (methylprednisolone 10 mg/kg once daily × 3 days) were given for probable acute rejection (AR). Transbronchial biopsies did not demonstrate AR (A0), but the bronchoalveolar lavage (BAL) fluid grew moderate mold 5 days later. Posaconazole 400 mg twice daily was started for empiric treatment of a breakthrough fungal infection, and the patient's esomeprazole was held to facilitate absorption. Speciation of the mold occurred at day 11, revealing moderate Scopulariopsis sp. on culture, with morphology most consistent with S. brumptii by microscopy (Figure 2). Later, DNA sequencing of the internal transcribed spacer (ITS) region would identify the mold as Microascus trigonosporus species complex. Confirmatory DNA sequencing of the organism was attempted at a second laboratory and was unsuccessful.
Figure 1.
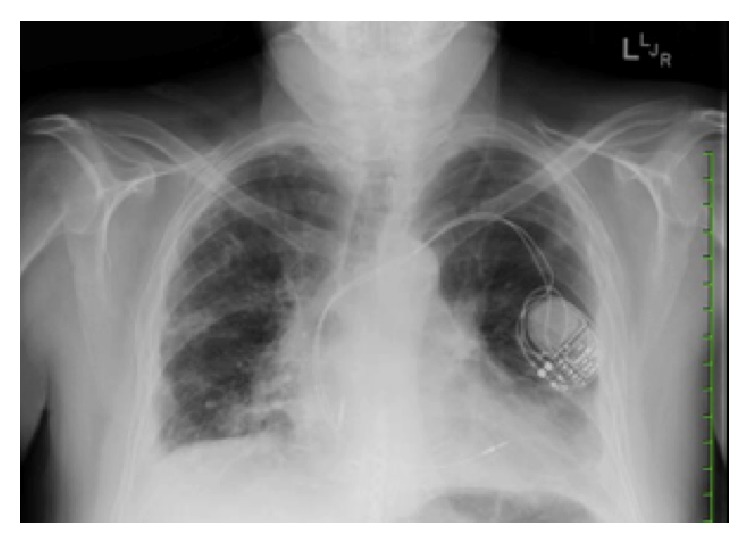
Chest radiography (CXR) showed diffuse patchy infiltrates and small bilateral pleural effusions.
Figure 2.
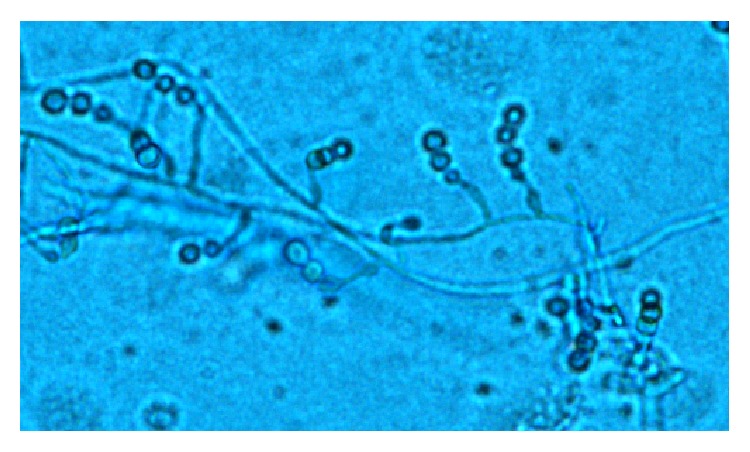
Microscopy. Lactophenol cotton blue stain. Note many overlapping conidiophores and conidia of Scopulariopsis sp. grown from patient's BAL sample fluid (10x).
Following two weeks of posaconazole therapy, repeat CXR denoted increased right-sided pleural effusion and new infiltrates in the right lower lobe (Figure 3). FEV1 was 1.08 L/s (33% predicted). A chest computed tomography scan was notable for a thick-walled cavitary lesion with mural nodularity involving the subpleural posterior right lower lobe and tree-in-bud centrilobular nodules within the right lower lobe (Figure 4). Repeat bronchoscopy demonstrated endobronchial, adherent tan-colored plaques throughout, tan secretions, and diffusely abnormal appearing mucosa (Figure 5). Pathology results from a transbronchial biopsy of the cavitary lesion demonstrated septated fungal hyphae, consistent with possible Aspergillus spp. Endobronchial biopsies of the plaques were notable for the finding of a small, detached fragment of matted fungal hyphae adjacent to but not penetrating the endobronchial mucosa (Figure 6). The BAL fluid became positive at day 4 after biopsy for moderate mold, again identified as Scopulariopsis sp. The patient was deemed not to be a surgical candidate for resection of the mycetoma based on his comorbidities. He was admitted for treatment of his invasive fungal infection with IV liposomal amphotericin B.
Figure 3.
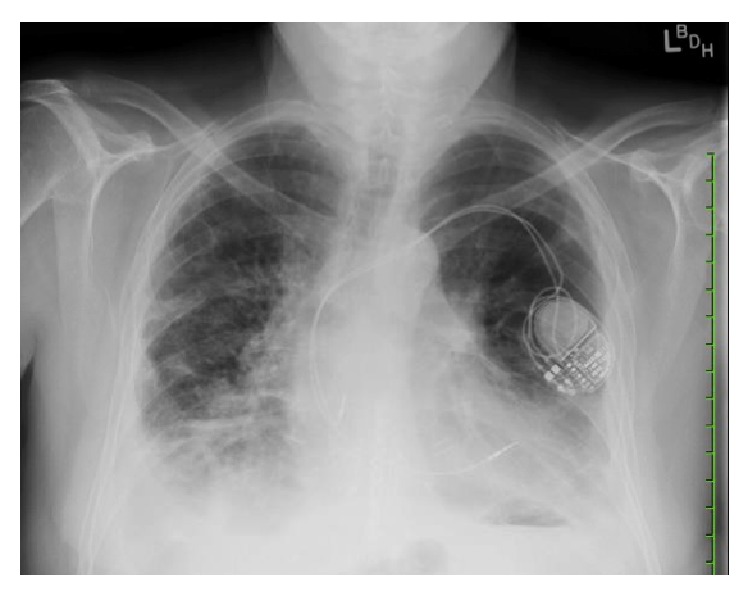
Chest radiography after two weeks of antifungal therapy showing right lower lobe opacity.
Figure 4.
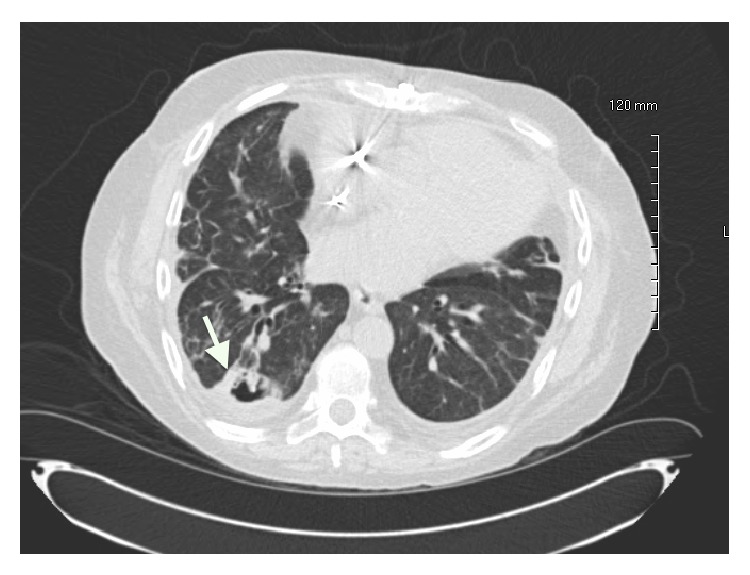
Axial chest computed tomography scan after two weeks of antifungal therapy showing thick-walled, subpleural cavitation and nodules (arrow).
Figure 5.
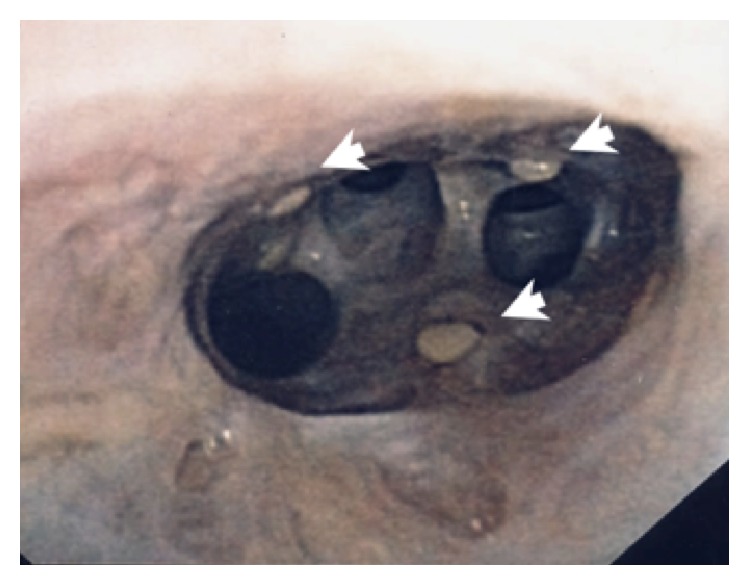
Bronchoscopy images after two weeks of antifungal therapy with diffusely abnormal appearing mucosa and tan adherent plaques (arrows).
Figure 6.
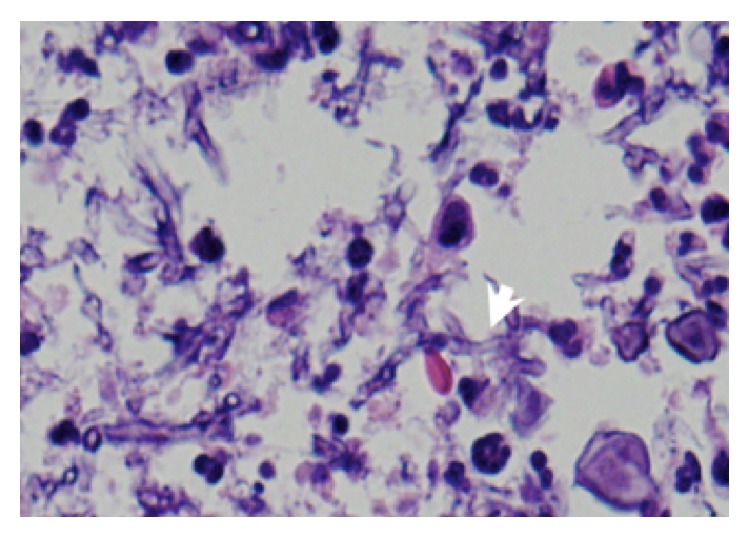
BAL cytology. H&E stain (high power, zoom) showing fungus hyphae which were hyaline and septated with 45-degree branching (arrow).
Treatment with liposomal amphotericin B IV 4 mg/kg intravenously every 24 hours was initiated. In order to maximize antifungal drug concentrations at the site of the infection, liposomal amphotericin B 25 mg via nebulizer was given every 24 hours for five days and then reduced to three times a week. Posaconazole was also continued. Inhaled fluticasone/salmeterol and mycophenolate mofetil were held given the patient's active fungal infection. The patient was discharged on hospital day 8 in stable condition.
Three weeks after therapy was started, the patient presented to the hospital with hypoxemic respiratory failure with increased oxygen requirement. CXR was notable for worsened parenchymal opacities reflecting edema or pneumonia. The patient was admitted and treated with IV furosemide and an increased dose of liposomal amphotericin B IV (5 mg/kg daily). The patient developed respiratory failure requiring intubation and developed difficult to control atrial flutter with a rapid ventricular rate. Repeat bronchoscopy demonstrated a markedly lower volume of tan secretions, and only a single endobronchial plaque in the left mainstem bronchus was identified. However, the patient's pulmonary status continued to decline secondary to chronic rejection, cardiac dysrhythmia with pulmonary edema, and pulmonary infection. On the 11th day of hospitalization, the patient's advance directive to withdraw care resulted in death. His medical power of attorney refused autopsy on his behalf.
Further workup of the Microascus trigonosporus species complex later revealed the following minimum inhibitory concentrations (MICs, mcg/mL) obtained by the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method: amphotericin B ≥ 8, itraconazole ≥ 16, posaconazole = 2, and voriconazole = 2. Minimum effective concentration (MEC, mcg/mL) for the echinocandins was also obtained: micafungin = 2, anidulafungin = 2, and caspofungin = 2.
3. Discussion
Here we report a rare case of invasive infection with Microascus trigonosporus species complex in a lung transplant recipient. DNA sequencing of the cultured organism proved necessary to distinguish clearer identification of the pathogen having a clinical appearance similar to more common pathogens in the lung transplant patient such as Aspergillus and a morphologic appearance similar to other species of Scopulariopsis (teleomorph Microascus).
Fungi of the genus Scopulariopsis are asexual and filamentous; their teleomorphs, Microascus, are also included in the genus. The fungi grow worldwide and are known to inhabit soil, plant material, insects, and other material. Scopulariopsis and Microascus spp. are more frequently the cause of superficial infections, including onychomycosis and keratitis [3]. Rarely, invasive disease caused by this genus of fungi has been described, including endocarditis, brain abscess, cutaneous infections, and localized and disseminated pulmonary infections [3–6]. Four Scopulariopsis species (S. acremonium, S. brevicaulis, S. brumptii, and S. candida) and two Microascus species (M. cirrosus and M. cinereus) have been definitively identified and reported as causing invasive human infection [3]. One reported case of Microascus trigonosporus pneumonia exists in the literature; however, this case did not describe evidence of the fungus on tissue biopsy but rather identified the organism based on morphology from a positive culture from the BAL fluid (nonsterile fluid) [7]. Confirmation test via DNA sequencing was not performed.
Invasive disease caused by Microascus and Scopulariopsis spp. primarily affects immunocompromised hosts and is associated with a high mortality in this population [3]. A paucity of data exists describing the susceptibility profile of Microascus and Scopulariopsis spp. In vitro studies have indicated that MICs of fluconazole, itraconazole, and flucytosine against Scopulariopsis spp. are high, and those of amphotericin B, voriconazole, and ketoconazole are variable [8–13]. Case reports also describe high-level resistance of S. brevicaulis and S. acremonium to amphotericin B, which correlated with poor clinical outcomes [14–16].
Because we knew little about the susceptibility pattern of the organism we were treating prior to obtaining the MICs postmortem other than its growth despite voriconazole prophylaxis, we used a combination of inhaled and systemic liposomal amphotericin B. In a study of inhaled liposomal amphotericin B for Aspergillus prophylaxis in lung transplant recipients, drug concentrations measured between the segmental bronchus and the parenchyma at 2, 7, and 14 days after nebulization were shown to exceed the MICs for the large majority of Aspergillus isolates [17, 18]. No evidence of lipid pneumonitis was found [17]. The addition of nebulized liposomal amphotericin B in this patient offered direct airway delivery of the antifungal, which allowed for an increase in total amphotericin B exposure without a high risk of additive systemic toxicity. This regimen appeared to reduce the endobronchial fungal burden despite high amphotericin MICs. Furthermore, the use of nebulized liposomal amphotericin B allowed for a convenient transition of care to the outpatient setting, as the pharmacokinetic properties of liposomal amphotericin B allow for three times weekly administration [17].
Identification of rare pathogenic molds infecting human hosts is imperative both for the optimization of treatment in the affected patient and for advancing our understanding in order to improve treatment strategies for future patients. Identification of molds based on phenotypic criteria alone can be flawed, and molecular methods of mold identification are the gold standard for species identification [19, 20]. DNA sequencing of the organism in this case was performed at two distinct clinical laboratories, and, despite this, definitive identification of the species was not possible. Therefore, this case is unique not only because of the rarity of the organism involved and the innovative treatment strategy that was utilized but also because it demonstrates the inherent difficulties in identification of rare molds that remain despite the recent advancements of molecular methods. The nucleotide sequence of the case isolate was deposited in the GenBank database under accession number HQ676488.
4. Conclusion
Here we report a rare case of invasive infection with Microascus trigonosporus species complex in a human. It developed in a lung transplant recipient despite antifungal prophylaxis with voriconazole. Nebulized liposomal amphotericin B was used in addition to systemic therapy in order to optimize drug exposure; this regimen appeared to reduce the patient's fungal burden. Despite this, this patient's Microascus trigonosporus species complex infection likely contributed to his worsening pulmonary status, which ultimately led to his death.
Acknowledgment
The authors would like to acknowledge Sudha Chaturvedi, Ph.D., Wadsworth Center, New York State Department of Health, for her assistance with DNA sequencing.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Neofytos D., Fishman J. A., Horn D., et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transplant Infectious Disease. 2010;12(3):220–229. doi: 10.1111/j.1399-3062.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 2.Singh N. Trends in the epidemiology of opportunistic fungal infections: predisposing factors and the impact of antimicrobial use practices. Clinical Infectious Diseases. 2001;33(10):1692–1696. doi: 10.1086/323895. [DOI] [PubMed] [Google Scholar]
- 3.Iwen P. C., Schutte S. D., Florescu D. F., Noel-Hurst R. K., Sigler L. Invasive Scopulariopsis brevicaulis infection in an immunocompromised patient and review of prior cases caused by Scopulariopsis and Microascus species. Medical Mycology. 2012;50(6):561–569. doi: 10.3109/13693786.2012.675629. [DOI] [PubMed] [Google Scholar]
- 4.Célard M., Dannaoui E., Piens M. A., et al. Early Microascus cinereus endocarditis of a prosthetic valve implanted after Staphylococcus aureus endocarditis of the native valve. Clinical Infectious Diseases. 1999;29(3):691–692. doi: 10.1086/598662. [DOI] [PubMed] [Google Scholar]
- 5.Baddley J. W., Moser S. A., Sutton D. A., Pappas P. G. Microascus cinereus (anamorph Scopulariopsis) brain abscess in a bone marrow transplant recipient. Journal of Clinical Microbiology. 2000;38(1):395–397. doi: 10.1128/jcm.38.1.395-397.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wuyts W. A., Molzahn H., Maertens J., et al. Fatal Scopulariopsis infection in a lung transplant recipient: a case report. Journal of Heart and Lung Transplantation. 2005;24(12):2301–2304. doi: 10.1016/j.healun.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Mohammedi I., Piens M. A., Audigier-Valette C., et al. Fatal Microascus trigonosporus (anamorph Scopulariopsis) pneumonia in a bone marrow transplant recipient. European Journal of Clinical Microbiology and Infectious Diseases. 2004;23(3):215–217. doi: 10.1007/s10096-003-1096-y. [DOI] [PubMed] [Google Scholar]
- 8.Aguilar C., Pujol I., Guarro J. In vitro antifungal susceptibilities of Scopulariopsis isolates. Antimicrobial Agents and Chemotherapy. 1999;43(6):1520–1522. doi: 10.1128/aac.43.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson E. M., Szekely A., Warnock D. W. In vitro activity of Syn-2869, a novel tri-azole agent, against emerging and less common mold pathogens. Antimicrobial Agents and Chemotherapy. 1999;43(5):1260–1263. doi: 10.1128/aac.43.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGinnis M. R., Pasarell L. In vitro testing of susceptibilities of filamentous ascomycetes to voriconazole, itraconazole, and amphotericin B, with consideration of phylogenetic implications. Journal of Clinical Microbiology. 1998;36(8):2353–2355. doi: 10.1128/jcm.36.8.2353-2355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radford S. A., Johnson E. M., Warnock D. W. In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogens. Antimicrobial Agents and Chemotherapy. 1997;41(4):841–843. doi: 10.1128/aac.41.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szekely A., Johnson E. M., Warnock D. W. Comparison of E-test and broth microdilution methods for antifungal drug susceptibility testing of molds. Journal of Clinical Microbiology. 1999;37(5):1480–1483. doi: 10.1128/jcm.37.5.1480-1483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuenca-Estrella M., Gomez-Lopez A., Mellado E., Buitrago M. J., Monzón A., Rodriguez-Tudela J. L. Scopulariopsis brevicaulis, a fungal pathogen resistant to broad-spectrum antifungal agents. Antimicrobial Agents and Chemotherapy. 2003;47(7):2339–2341. doi: 10.1128/aac.47.7.2339-2341.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmon A., Debourgogne A., Vasbien M., et al. Disseminated Scopulariopsis brevicaulis infection in an allogeneic stem cell recipient: case report and review of the literature. Clinical Microbiology and Infection. 2010;16(5):508–512. doi: 10.1111/j.1469-0691.2009.02878.x. [DOI] [PubMed] [Google Scholar]
- 15.Beltrame A., Sarmati L., Cudillo L., et al. A fatal case of invasive fungal sinusitis by Scopulariopsis acremonium in a bone marrow transplant recipient. International Journal of Infectious Diseases. 2009;13(6):e488–e492. doi: 10.1016/j.ijid.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Shaver C. M., Castilho J. L., Cohen D. N., et al. Fatal scuplariopsis infection in a lung transplant recipient: lessons of organ procurement. American Journal of Transplantation. 2014;14:2893–2897. doi: 10.1111/ajt.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monforte V., Ussetti P., López R., et al. Nebulized liposomal amphotericin B prophylaxis for Aspergillus infection in lung transplantation: pharmacokinetics and safety. Journal of Heart and Lung Transplantation. 2009;28(2):170–175. doi: 10.1016/j.healun.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Baddley J. W., Marr K. A., Andes D. R., et al. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the transplant-associated infections surveillance network. Journal of Clinical Microbiology. 2009;47:3271–3275. doi: 10.1128/JCM.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summerbell R. C., Lévesque C. A., Seifert K. A., et al. Microcoding: the second step in DNA barcoding. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360(1462):1897–1903. doi: 10.1098/rstb.2005.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balajee S. A., Borman A. M., Brandt M. E., et al. Sequence-based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: where are we and where should we go from here? Journal of Clinical Microbiology. 2009;47(4):877–884. doi: 10.1128/jcm.01685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


