Abstract
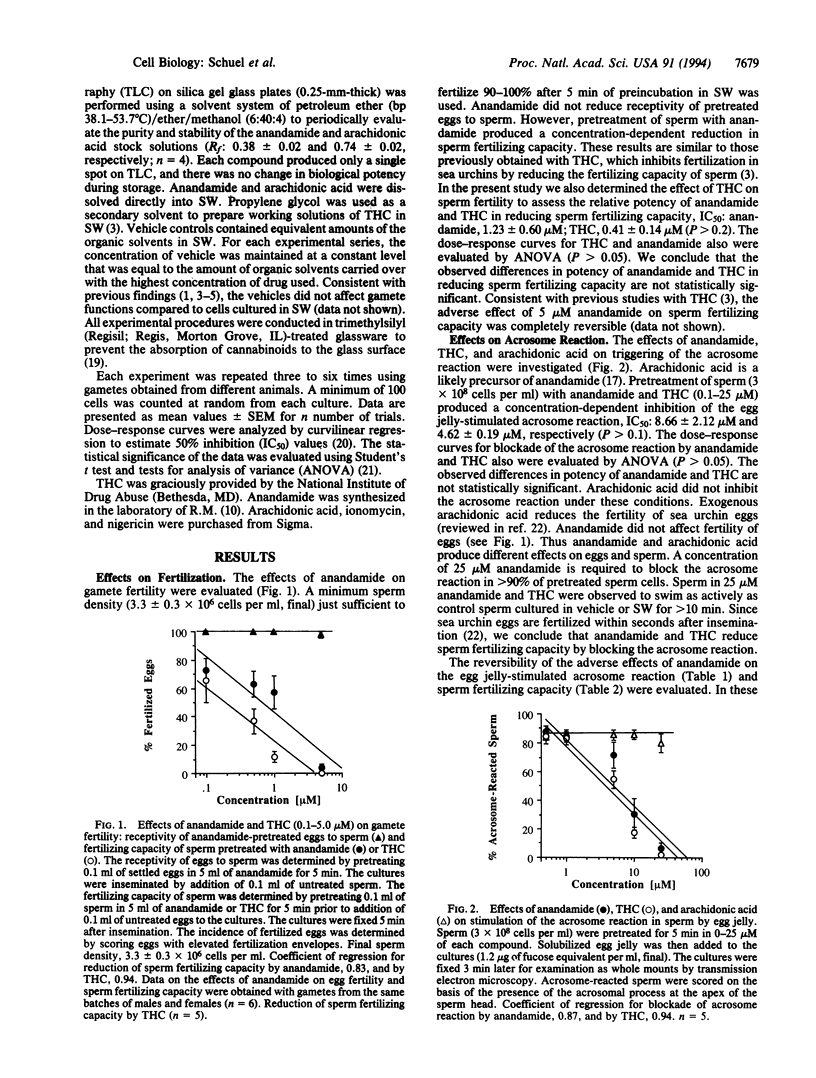
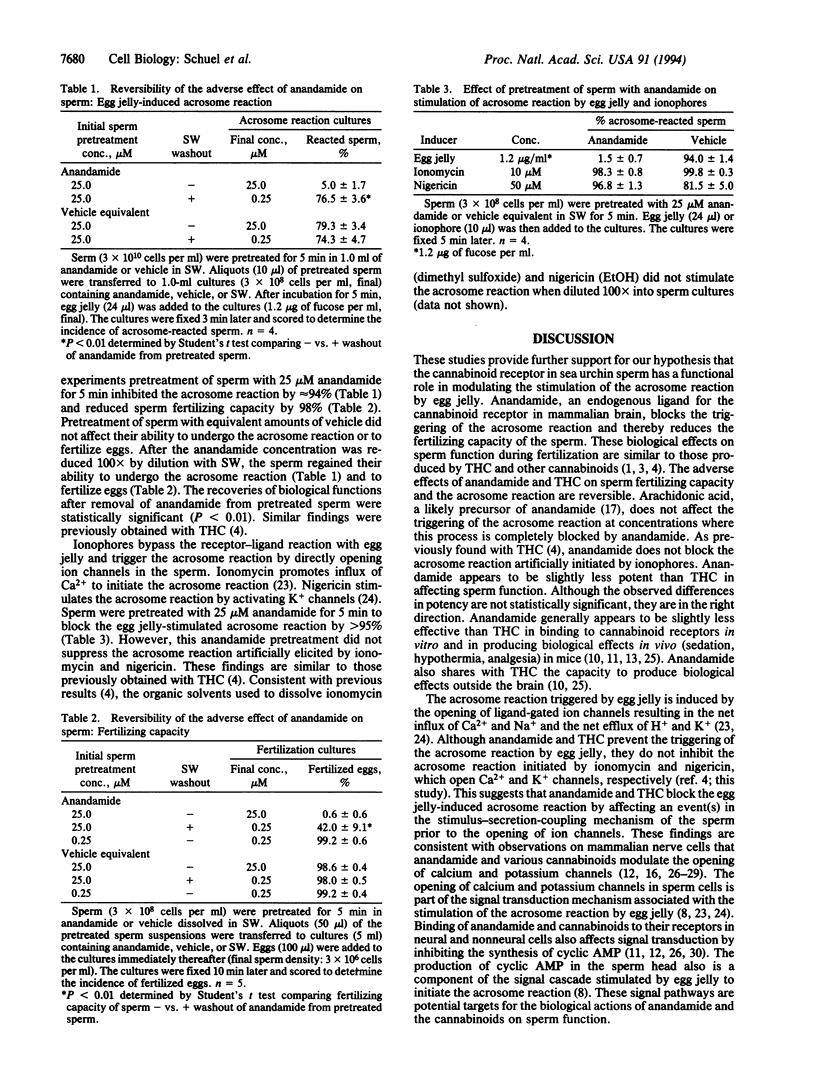
Anandamide (arachidonylethanolamide) is an endogenous cannabinoid receptor agonist in mammalian brain. Sea urchin sperm contain a high-affinity cannabinoid receptor similar to the cannabinoid receptor in mammalian brain. (-)-delta 9-Tetrahydrocannabinol (THC), the primary psychoactive cannabinoid in marihuana, reduces the fertilizing capacity of sea urchin sperm by blocking the acrosome reaction that normally is stimulated by a specific ligand in the egg's jelly coat. We now report that anandamide produces effects similar to those previously obtained with THC in Strongylocentrotus purpuratus in reducing sperm fertilizing capacity and inhibiting the egg jelly-stimulated acrosome reaction. Arachidonic acid does not inhibit the acrosome reaction under similar conditions. The adverse effects of anandamide on sperm fertilizing capacity and the acrosome reaction are reversible. The receptivity of unfertilized eggs to sperm and sperm motility are not impaired by anandamide. Under conditions where anandamide completely blocks the egg jelly-stimulated acrosome reaction, it does not inhibit the acrosome reaction artificially initiated by ionomycin, which promotes Ca2+ influx, and nigericin, which activates K+ channels in sperm. These findings provide additional evidence that the cannabinoid receptor in sperm plays a role in blocking the acrosome reaction, indicate that anandamide or a related molecule may be the natural ligand for the cannabinoid receptor in sea urchin sperm, and suggest that binding of anandamide to the cannabinoid receptor modulates stimulus-secretion-coupling in sperm by affecting an event prior to ion channel opening.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACHUR N. R., MASEK K., MELMON K. L., UDENFRIEND S. FATTY ACID AMIDES OF ETHANOLAMINE IN MAMMALIAN TISSUES. J Biol Chem. 1965 Mar;240:1019–1024. [PubMed] [Google Scholar]
- Blackmore P. F., Neulen J., Lattanzio F., Beebe S. J. Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. J Biol Chem. 1991 Oct 5;266(28):18655–18659. [PubMed] [Google Scholar]
- Bouaboula M., Rinaldi M., Carayon P., Carillon C., Delpech B., Shire D., Le Fur G., Casellas P. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993 May 15;214(1):173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- Chang M. C., Berkery D., Laychock S. G., Schuel H. Reduction of the fertilizing capacity of sea urchin sperm by cannabinoids derived from marihuana. III. Activation of phospholipase A2 in sperm homogenate by delta 9-tetrahydrocannabinol. Biochem Pharmacol. 1991 Jul 25;42(4):899–904. doi: 10.1016/0006-2952(91)90051-6. [DOI] [PubMed] [Google Scholar]
- Chang M. C., Berkery D., Schuel R., Laychock S. G., Zimmerman A. M., Zimmerman S., Schuel H. Evidence for a cannabinoid receptor in sea urchin sperm and its role in blockade of the acrosome reaction. Mol Reprod Dev. 1993 Dec;36(4):507–516. doi: 10.1002/mrd.1080360416. [DOI] [PubMed] [Google Scholar]
- Chang M. C., Schuel H. Reduction of the fertilizing capacity of sea urchin sperm by cannabinoids derived from marihuana. II. Ultrastructural changes associated with inhibition of the acrosome reaction. Mol Reprod Dev. 1991 May;29(1):60–71. doi: 10.1002/mrd.1080290110. [DOI] [PubMed] [Google Scholar]
- Cornett L. E., Meizel S. Stimulation of in vitro activation and the acrosome reaction of hamster spermatozoa by catecholamines. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4954–4958. doi: 10.1073/pnas.75.10.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAN J., OHORI Y., KUSHIDA H. STUDIES ON THE ACROSOME. VII. FORMATION OF THE ACROSOMAL PROCESS IN SEA URCHIN SPERMATOZOA. J Ultrastruct Res. 1964 Dec;11:508–524. doi: 10.1016/s0022-5320(64)80079-4. [DOI] [PubMed] [Google Scholar]
- Deadwyler S. A., Hampson R. E., Bennett B. A., Edwards T. A., Mu J., Pacheco M. A., Ward S. J., Childers S. R. Cannabinoids modulate potassium current in cultured hippocampal neurons. Receptors Channels. 1993;1(2):121–134. [PubMed] [Google Scholar]
- Deutsch D. G., Chin S. A. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993 Sep 1;46(5):791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992 Dec 18;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Briley E. M., Axelrod J., Simpson J. T., Mackie K., Devane W. A. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C., Veluz J. S., Williams H. L., Briley E. M., Matsuda L. A. Cannabinoid agonists stimulate both receptor- and non-receptor-mediated signal transduction pathways in cells transfected with and expressing cannabinoid receptor clones. Mol Pharmacol. 1992 Nov;42(5):838–845. [PubMed] [Google Scholar]
- Fride E., Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur J Pharmacol. 1993 Feb 9;231(2):313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- Garbers D. L. Molecular basis of fertilization. Annu Rev Biochem. 1989;58:719–742. doi: 10.1146/annurev.bi.58.070189.003443. [DOI] [PubMed] [Google Scholar]
- Garrett E. R., Hunt C. A. Physiochemical properties, solubility, and protein binding of delta9-tetrahydrocannabinol. J Pharm Sci. 1974 Jul;63(7):1056–1064. doi: 10.1002/jps.2600630705. [DOI] [PubMed] [Google Scholar]
- Guerrero A., Darszon A. Evidence for the activation of two different Ca2+ channels during the egg jelly-induced acrosome reaction of sea urchin sperm. J Biol Chem. 1989 Nov 25;264(33):19593–19599. [PubMed] [Google Scholar]
- Gérard C. M., Mollereau C., Vassart G., Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991 Oct 1;279(Pt 1):129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L., Gopher A., Almog S., Mechoulam R. Two new unsaturated fatty acid ethanolamides in brain that bind to the cannabinoid receptor. J Med Chem. 1993 Oct 1;36(20):3032–3034. doi: 10.1021/jm00072a026. [DOI] [PubMed] [Google Scholar]
- Hawkins D. J., Brash A. R. Eggs of the sea urchin, Strongylocentrotus purpuratus, contain a prominent (11R) and (12R) lipoxygenase activity. J Biol Chem. 1987 Jun 5;262(16):7629–7634. [PubMed] [Google Scholar]
- Heindel J. J., Keith W. B. Specific inhibition of FSH-stimulated cAMP accumulation by delta 9-tetrahydrocannabinol in cultures of rat Sertoli cells. Toxicol Appl Pharmacol. 1989 Oct;101(1):124–134. doi: 10.1016/0041-008x(89)90218-4. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Heald S. L., Dally R. D., Janis R. A. Isolation, identification and synthesis of an endogenous arachidonic amide that inhibits calcium channel antagonist 1,4-dihydropyridine binding. Prostaglandins Leukot Essent Fatty Acids. 1993 Jun;48(6):429–437. doi: 10.1016/0952-3278(93)90048-2. [DOI] [PubMed] [Google Scholar]
- Keller S. H., Vacquier V. D. The isolation of acrosome-reaction-inducing glycoproteins from sea urchin egg jelly. Dev Biol. 1994 Mar;162(1):304–312. doi: 10.1006/dbio.1994.1087. [DOI] [PubMed] [Google Scholar]
- Mackie K., Devane W. A., Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol Pharmacol. 1993 Sep;44(3):498–503. [PubMed] [Google Scholar]
- Mackie K., Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990 Aug 9;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McClean D. K., Zimmerman A. M. Action of delta 9-tetrahydrocannabinol on cell division and macromolecular synthesis in division-synchronized protozoa. Pharmacology. 1976;14(4):307–321. doi: 10.1159/000136610. [DOI] [PubMed] [Google Scholar]
- Munro S., Thomas K. L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993 Sep 2;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murphy L. L., Newton S. C., Dhali J., Chávez D. Evidence for a direct anterior pituitary site of delta-9-tetrahydrocannabinol action. Pharmacol Biochem Behav. 1991 Nov;40(3):603–607. doi: 10.1016/0091-3057(91)90370-h. [DOI] [PubMed] [Google Scholar]
- Nadler V., Mechoulam R., Sokolovsky M. Blockade of 45Ca2+ influx through the N-methyl-D-aspartate receptor ion channel by the non-psychoactive cannabinoid HU-211. Brain Res. 1993 Sep 17;622(1-2):79–85. doi: 10.1016/0006-8993(93)90804-v. [DOI] [PubMed] [Google Scholar]
- Nelson L., Young M. J., Gardner M. E. Sperm motility and calcium transport: a neurochemically controlled process. Life Sci. 1980 May 26;26(21):1739–1749. doi: 10.1016/0024-3205(80)90573-1. [DOI] [PubMed] [Google Scholar]
- Parmentier M., Libert F., Schurmans S., Schiffmann S., Lefort A., Eggerickx D., Ledent C., Mollereau C., Gérard C., Perret J. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature. 1992 Jan 30;355(6359):453–455. doi: 10.1038/355453a0. [DOI] [PubMed] [Google Scholar]
- Perry G., Epel D. Fertilization stimulates lipid peroxidation in the sea urchin egg. Dev Biol. 1985 Jan;107(1):58–65. doi: 10.1016/0012-1606(85)90375-6. [DOI] [PubMed] [Google Scholar]
- Ralt D., Goldenberg M., Fetterolf P., Thompson D., Dor J., Mashiach S., Garbers D. L., Eisenbach M. Sperm attraction to a follicular factor(s) correlates with human egg fertilizability. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2840–2844. doi: 10.1073/pnas.88.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S. H. Stereospecific presynaptic inhibitory effect of delta9-tetrahydrocannabinol on cholinergic transmission in the myenteric plexus of the guinea pig. Can J Physiol Pharmacol. 1978 Dec;56(6):968–975. doi: 10.1139/y78-154. [DOI] [PubMed] [Google Scholar]
- Schuel H., Berkery D., Schuel R., Chang M. C., Zimmerman A. M., Zimmerman S. Reduction of the fertilizing capacity of sea urchin sperm by cannabinoids derived from marihuana. I. Inhibition of the acrosome reaction induced by egg jelly. Mol Reprod Dev. 1991 May;29(1):51–59. doi: 10.1002/mrd.1080290109. [DOI] [PubMed] [Google Scholar]
- Schuel H., Schuel R., Zimmerman A. M., Zimmerman S. Cannabinoids reduce fertility of sea urchin sperm. Biochem Cell Biol. 1987 Feb;65(2):130–136. doi: 10.1139/o87-018. [DOI] [PubMed] [Google Scholar]
- SeGall G. K., Lennarz W. J. Chemical characterization of the component of the jelly coat from sea urchin eggs responsible for induction of the acrosome reaction. Dev Biol. 1979 Jul;71(1):33–48. doi: 10.1016/0012-1606(79)90080-0. [DOI] [PubMed] [Google Scholar]
- Stanley-Samuelson D. W. Comparative eicosanoid physiology in invertebrate animals. Am J Physiol. 1991 May;260(5 Pt 2):R849–R853. doi: 10.1152/ajpregu.1991.260.5.R849. [DOI] [PubMed] [Google Scholar]
- Storey B. T., Hourani C. L., Kim J. B. A transient rise in intracellular Ca2+ is a precursor reaction to the zona pellucida-induced acrosome reaction in mouse sperm and is blocked by the induced acrosome reaction inhibitor 3-quinuclidinyl benzilate. Mol Reprod Dev. 1992 May;32(1):41–50. doi: 10.1002/mrd.1080320108. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Barg J., Levy R., Saya D., Heldman E., Mechoulam R. Anandamide, a brain endogenous compound, interacts specifically with cannabinoid receptors and inhibits adenylate cyclase. J Neurochem. 1993 Jul;61(1):352–355. doi: 10.1111/j.1471-4159.1993.tb03576.x. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. The biology and chemistry of fertilization. Science. 1987 Jan 30;235(4788):553–560. doi: 10.1126/science.3027891. [DOI] [PubMed] [Google Scholar]
- Yazigi R. A., Odem R. R., Polakoski K. L. Demonstration of specific binding of cocaine to human spermatozoa. JAMA. 1991 Oct 9;266(14):1956–1959. [PubMed] [Google Scholar]
- Yebra M., Klein T. W., Friedman H. Delta 9-tetrahydrocannabinol suppresses concanavalin A induced increase in cytoplasmic free calcium in mouse thymocytes. Life Sci. 1992;51(2):151–160. doi: 10.1016/0024-3205(92)90009-e. [DOI] [PubMed] [Google Scholar]
- Young R. J., Laing J. C. The binding characteristics of cholinergic sites in rabbit spermatozoa. Mol Reprod Dev. 1991 Jan;28(1):55–61. doi: 10.1002/mrd.1080280109. [DOI] [PubMed] [Google Scholar]
- Zimmerman S., Zimmerman A. M., Laurence H. Effect of delta 9-tetrahydrocannabinol on cyclic nucleotides in synchronously dividing Tetrahymena. Can J Biochem. 1981 Jul;59(7):489–493. doi: 10.1139/o81-068. [DOI] [PubMed] [Google Scholar]



