Abstract
Conventional formalin-ether concentration method is a gold standard for the diagnosis of parasite infection. However, it may be time-consuming and laborious. We aimed to reveal the clinical usefulness of a modified formalin-ether concentration method using the Para Tube (KS Corporation, Korea) compared with the conventional method. A total of 117 fresh, unpreserved fecal samples composed to 90 negative controls and 27 positive controls with ova of Diphyllobothrium latum/D. nihonkaiense, ova of Clonorchis sinensis and cysts of Giardia lamblia were used in this study. Both methods showed comparable correct identification rate (87.2% for the Para Tube vs. 86.3% for the conventional method).When five samples were examined at once, the Para Tube method reduced the procedure time compared with the conventional method (19 min 58 sec vs. 23 min 18 sec, P=0.0286). We concluded that the modified formalin-ether concentration method using the Para Tube is a rapid, simple, and reliable fecal concentration method for clinical use.
Keywords: Para Tube, Formalin-ether concentration, Parasite, Diagnosis
Although the incidence of intestinal parasite infection has recently declined in Korea, it is still a major health problem worldwide [1, 2]. Microscopic examination of feces is essential to detect intestinal parasites for diagnosis. Direct fecal microscopy is useful for detection of motile protozoan trophozoites, but is inadequate for routine examination of feces with suspected parasitic infection [3]. To improve the sensitivity of fecal examination, concentration of fecal sample is recommended to increase the chance of detecting parasitic ova, cysts, and larvae [3]. The conventional formalin-ether concentration (C-FEC) method is an unpleasant and time-consuming technique involving high-risk due to gauze-filtration of fecal material. Recently, the Para Tube (KS Corporation, Sungnam, Korea) was developed as a commercial kit for fecal concentration. The Para Tube method involves the use of two disposable, modular tubes, with a safer procedure and comparable efficiency. The aims of our study were to compare the performance of the Para Tube with the C-FEC method, and to compare the procedure time required for both methods.
A total of 117 fresh, unpreserved fecal samples submitted for fecal examination at Chonnam National University Hospital between October 2014 and December 2014 were used in this prospective study. The collection of fecal samples for this study was conducted in accordance with the guidelines and approval of the Institutional Review Board of Chonnam National University Hospital, Korea. Samples were concentrated and examined by the C-FEC method and the modified formalin-ether concentration with the Para Tube (Para-FEC). To set up positive controls, ova of Diphyllobothrium latum/D. nihonkaiense and Clonorchis sinensis were added to formalinized samples collected from the patients for laboratory quality control. Additionally, cysts of Giardia lamblia, stored for educational purposes, were used. A total of 27 positive controls were used, with three samples each, for D. latum/D. nihonkaiense, C. sinensis, and G. lamblia at respective concentrations of 20 ova or cysts/mL (n=9), 40 ova or cysts/mL (n=9), and 80 ova or cysts/mL (n=9). D. latum/D. nihonkaiense and C. sinensis ova were diluted in 600 µL distilled water to obtain a concentration of 10 ova/10 µL, and G. lamblia cysts were diluted in 200 µL distilled water to obtain a concentration of 80 cysts/10 µL. Each concentration was confirmed from 10 slide observations showing the average concentration as 10 ova/10 µL or 80 cysts/10 µL. According to the different proportions of the ova and cysts, 20, 40, and 80 µL of ovum samples and 2.5, 5, and 10 µL of cyst samples were added to parasite-free fecal samples and emulsified with 1 mL distilled water.
Briefly, the C-FEC procedure involved emulsification of fresh or formalinized feces (1 g) in 10 mL distilled water and filtration through gauze into a 15-mL centrifuge tube [4]. The tube was centrifuged at 500g for 5 min. After decanting the supernatant, the tube was filled with 10 mL formalin (10%) and 3 mL ethyl ether. The tube was sealed and vigorously mixed in a vortex mixer to allow diethyl ether to be exposed to all remaining fecal material. After a second centrifugation at 500g for 5 min, the supernatant fluid was discarded, the top plug of debris was rimmed with an applicator stick, and the remaining sediment was examined under a microscope. The Para-FEC procedure involved the use of two clear, modular plastic tubes: a 12-mL, flat-bottomed tube for filtration and another 15-mL, calibrated, cone-bottomed tube (See Supplemental Data Figure S1). Fecal samples were processed as per manufacturer instructions. Briefly, 1 g fresh or formalinized feces were suspended in 10 mL formalin (10%) in the Para Tube followed by centrifugation at 500 g for 1 min. After centrifugation, the inner tube with filtered debris was discarded. Then, 3 mL ethyl acetate was added to the cone-bottomed tube. The tube was centrifuged at 500 g for 10 min, supernatant was decanted, and the top plug of debris was rimmed with an applicator stick. For both methods, the remaining sediment was diluted in a few drops of 10% formalin and 20 µL was placed onto a slide to be examined for parasites.
We compared the performance of two methods for 117 fecal samples using the result by the single observation at first. In addition, we evaluated the recovery of positivity using 27 positive control samples, by observation of single slide and triplicate slides per one sample, to know whether multiple observations could be helpful for detection or not. The detection rates of the two methods were compared in a single examination, while three slides were examined for positive controls. All slide examinations were performed in a blinded manner, using only serial accession codes. The procedure time for the two methods was compared for both single-sample and five samples performed simultaneously. Chi-squared or Fisher's exact test was used to compare the detection rates, while Student's t-test was used to compare the time for each procedure. Statistical analysis was performed by using PASW version 18.0 (SPSS Inc., Chicago, IL, USA), and significance was defined as P<0.05.
From 117 fecal samples, including 27 positive controls and 90 negative controls, the overall correct identification rates of Para-FEC and C-FEC were 87.2% (102/117) and 86.3% (101/117), respectively (Table 1). Para-FEC showed 44.4% (95% confidence interval [CI], 25.50% to 64.66%) sensitivity, 100% (95% CI, 95.94% to 100.00%) specificity, with 100% (95% CI, 73.35% to 100.00%) positive and 85.7% (95% CI, 77.53% to 91.77%) negative predictive values, which were comparable to the overall performance of C-FEC. The positive controls had relatively low concentration of ova, from 20 to 80 ova/mL feces in this study, which might have contributed to the low sensitivity and high false-negative rate in both concentration methods. Para-FEC also showed improved performance in terms of recovery of ova or cysts in the positive controls, compared with C-FEC (average, 23.7 ova or cysts from Para-FEC vs. 17.0 ova or cysts from C-FEC) (Table 1). Although the pore-size used was similar to the 600-µm pore gauze filter, the first short centrifuge step in Para-FEC might be helpful to filter out more sediment. However, it was apparent that more debris was obtained from samples prepared by Para-FEC than by C-FEC method. The amount of debris, distribution, and clarity of fecal material could be important variables that influence parasite detection [5]. The performance of Para-FEC was superior to other commercial fecal concentration kits, in the detection of cysts or helminth ova (average number observed per slide, for Para Tube vs. Fecal Parasite Concentrator [Evergreen Scientific, Los Angeles, CA, USA]; 1.0 vs. 0.5 for G. lamblia cysts; 0.2 vs.0.3 for helminth ova) (See Supplemental Data Table S1) [5].
Table 1. Comparison of fecal examination results using Para Tube (Para-FEC) and conventional tube (C-FEC) with positive samples (ova of Diphyllobothrium latum/D. nihonkaiense and Clonorchis sinensis, cysts of Giardia lamblia) and negative samples obtained from healthy controls.
| Characteristics | N of samples | Correct identification N (%) | Total N (Average N) of eggs observed in a cover slip | ||
|---|---|---|---|---|---|
| Para-FEC | C-FEC | Para-FEC | C-FEC | ||
| Negative for any parasites | 90 | 90 (100.0) | 90 (100.0) | ||
| Positive for any parasites | 27 | 12 (44.4) | 11 (40.7) | ||
| Ova of D. latum/D. nihonkaiense | 1 (0.1) | 1 (0.1) | |||
| 20 eggs/1 mL feces | 3 | 0 | 0 | 0 | 0 |
| 40 eggs/1 mL feces | 3 | 0 | 1 | 0 | 1 |
| 80 eggs/1 mL feces | 3 | 1 | 0 | 1 | 0 |
| Ova of Clonorchis sinensis | 15 (1.7) | 10 (1.1) | |||
| 20 eggs/1 mL feces | 3 | 1 | 2 | 1 | 3 |
| 40 eggs/1 mL feces | 3 | 2 | 1 | 5 | 1 |
| 80 eggs/1 mL feces | 3 | 3 | 3 | 9 | 6 |
| Cysts of Giardia lamblia | 55 (6.1) | 40 (4.4) | |||
| 20 cysts/1 mL feces | 3 | 0 | 0 | 0 | 0 |
| 40 cysts/1 mL feces | 3 | 2 | 1 | 3 | 1 |
| 80 cysts/1 mL feces | 3 | 3 | 3 | 52 | 39 |
| Total | 117 | 102 (87.2) | 101 (86.3) | 71 (23.7) | 51 (17.0) |
According to the number of slides examined, the detection rates using Para-FEC were 44.4% (12/27) for the single slide and 59.3% (16/27) for triplicate slides, while those using C-FEC were 40.7% (11/27) and 51.9% (14/27), respectively (Table 2). Although there was no statistical difference between the two methods, the detection rate of multiple examinations was higher for multiple observations in Para-FEC (single slide vs. triplicate slides, P=0.035 for Para-FEC and P=0.113 for C-FEC). Low sensitivity might be inevitable in case of clinical samples with low parasite concentration, considering that only 20 µL sediment was observed per sample. However, considering that the 10-20 µL sample was adequate for microscopic observation, multiple observations using at least three slides per sample could increase the detection rate for samples with high index of suspicion.
Table 2. Recovery of positivity according to the number of tested slides per sample by the modified formalin-ether concentration using Para Tube (Para-FEC) and the conventional formalin-ether concentration (C-FEC).
| Tested slides per one sample | Positive number/total positive controls (%) | |
|---|---|---|
| Para-FEC | C-FEC | |
| Single slide | 12/27 (44.4) | 11/27 (40.7) |
| Three slides | 16/27 (59.3)* | 14/27 (51.9) |
*P value <0.05, significant statistical difference was found between the single observation and triplicate observations using Para-FEC (P=0.035), but not between the observations using C-FEC (P=0.113).
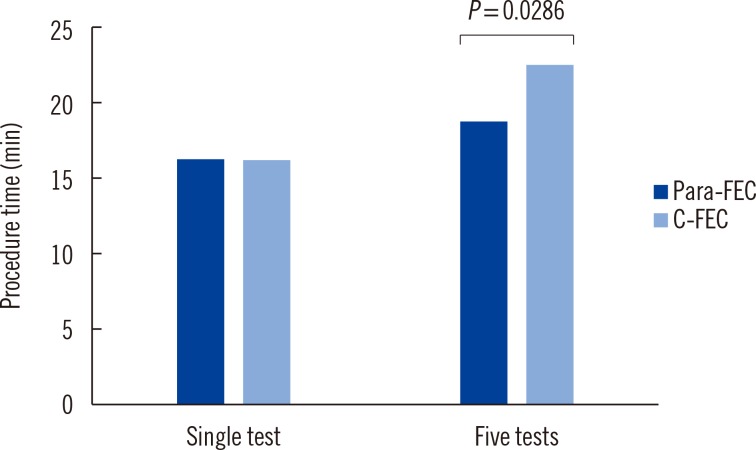
It is noteworthy that use of the Para Tube shortened the total procedure time required by the C-FEC method. The mean time for a single test using Para-FEC was comparable to that using C-FEC method (16 min and 22 sec for Para-FEC vs. 16 min and 14 sec for C-FEC, P=0.3527). However, when five samples were tested together, the procedure time for Para-FEC method was reduced (19 min and 58 sec for Para-FEC vs. 23 min and 18 sec for C-FEC, P=0.0286) (Fig. 1). This might be an advantage in the clinical laboratory when multiple samples are examined at once.
Fig. 1. Comparison of procedure time by the modified formalin-ether concentration using the Para Tube (Para-FEC) and the conventional formalin-ether concentration (C-FEC) methods, according to the increasing number of tests simultaneously. While the mean time for a single test was comparable in the two methods, the procedure time using the Para Tube was reduced compared with that required by the conventional method, when five samples were tested simultaneously.
In summary, Para-FEC showed clinical performance comparable to C-FEC and a reduction in procedure time when multiple samples were tested together. A limitation of this study was that only three types of parasites were used as positive control. However, the results from this study suggest that Para-FEC may be suitable to detect other ova and cysts as well, as it is based on the C-FEC method. Although the Para Tube could be more expensive than the conventional conical tube ($0.8 for the Para Tube vs. $0.4 for the conventional tube), it is still affordable and within the range of the test fee. As a single-use, closed-system device, the Para Tube is safer and more user friendly in the laboratory setting. We believe that the modified formalin-ether concentration method using the Para Tube is useful as a rapid, simple, and reliable fecal concentration technique for clinical use.
Acknowledgments
Thanks to Se-Jin Jeon, M.T. in the department of the Parasitology Laboratory at Chonnam National University Hospital for technical assistance. This work was partially supported by the Fund of Annals of Clinical Microbiology.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
Supplementary Materials
Picture of the Para Tube (KS Corporation, Sungnam, Korea). (A) Para Tube is composed of two-pieces of clear plastic tubes; (B) One piece was a calibrated 15-mL-capacity conical tube and the other one was a flat-bottom filtering tube with 12-mL capacity.
Supplemental Data Table S1. Comparison of the commercial kits for the fecal concentration in the detection of helminthes ova and the protozoan cysts.
| Specimens | Average N of ova or cysts observed in a cover slip* | ||||||
|---|---|---|---|---|---|---|---|
| Direct Ex† | FPC† | PMC† | TFC† | FCK† | C-FEC | Para-FEC | |
| Cysts of Giardia lamblia | 0.1 | 0.5 | 0.0 | 0.1 | 0.1 | 0.3 | 1.0 |
| Ova of Necator americanus | 1.4 | 0.3 | 0.1 | 0.4 | 0.2 | - | - |
| Ova of Diphyllobothrium latum/D. nihonkaiense | - | - | - | - | - | 0.2 | 0.2 |
*Average numbers of ova or cysts observed in a cover slip are calculated according to the adjustment with the concentration of 40 ova or cysts per 1 mL of fecal samples; †indicates the results of Perry et al. comparing the parasite detection efficiencies of five fecal concentration systems [5].
Abbreviations: Direct Ex, Direct examination; FPC, Fecal Parasite Concentrator (Evergreen Scientific, Los Angeles, CA, USA); PMC, Para-Pak Macro-Con (Meridian Diagnostics, Inc., Cincinnati, OH, USA); TFC, Trend FeKal CON-Trate (Trend Scientific, Inc., St. Paul, MN, USA); FCK, Fecal Concentrator Kit (Remel, Lenexa, Kansas); C-FEC, conventional formalin-ether concentration; Para-FEC, modified formalin-ether concentration using Para Tube.
References
- 1.Stepek G, Buttle DJ, Duce IR, Behnke JM. Human gastrointestinal nematode infections: are new control methods required? Int J Exp Pathol. 2006;87:325–341. doi: 10.1111/j.1365-2613.2006.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim TS, Cho SH, Huh S, Kong Y, Sohn WM, Hwang SS, et al. A nationwide survey on the prevalence of intestinal parasitic infections in the Republic of Korea, 2004. Korean J Parasitol. 2009;47:37–47. doi: 10.3347/kjp.2009.47.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barda BD, Rinaldi L, Ianniello D, Zepherine H, Salvo F, Sadutshang T, et al. Mini-FLOTAC, an innovative direct diagnostic technique for intestinal parasitic infections: experience from the field. PLoS Negl Trop Dis. 2013;7:e2344. doi: 10.1371/journal.pntd.0002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JW, Bartlett MS. Diagnostic parasitology: introduction and methods. In: Lenette EH, Balows A, Hausler WJ Jr, Shadomy HJ, editors. Manual of clinical microbiology. 4th ed. Washington, D.C.: American Society for Microbiology; 1985. pp. 595–611. [Google Scholar]
- 5.Perry JL, Matthews JS, Miller GR. Parasite detection efficiencies of five stool concentration systems. J Clin Microbiol. 1990;28:1094–1097. doi: 10.1128/jcm.28.6.1094-1097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Picture of the Para Tube (KS Corporation, Sungnam, Korea). (A) Para Tube is composed of two-pieces of clear plastic tubes; (B) One piece was a calibrated 15-mL-capacity conical tube and the other one was a flat-bottom filtering tube with 12-mL capacity.
Supplemental Data Table S1. Comparison of the commercial kits for the fecal concentration in the detection of helminthes ova and the protozoan cysts.
| Specimens | Average N of ova or cysts observed in a cover slip* | ||||||
|---|---|---|---|---|---|---|---|
| Direct Ex† | FPC† | PMC† | TFC† | FCK† | C-FEC | Para-FEC | |
| Cysts of Giardia lamblia | 0.1 | 0.5 | 0.0 | 0.1 | 0.1 | 0.3 | 1.0 |
| Ova of Necator americanus | 1.4 | 0.3 | 0.1 | 0.4 | 0.2 | - | - |
| Ova of Diphyllobothrium latum/D. nihonkaiense | - | - | - | - | - | 0.2 | 0.2 |
*Average numbers of ova or cysts observed in a cover slip are calculated according to the adjustment with the concentration of 40 ova or cysts per 1 mL of fecal samples; †indicates the results of Perry et al. comparing the parasite detection efficiencies of five fecal concentration systems [5].
Abbreviations: Direct Ex, Direct examination; FPC, Fecal Parasite Concentrator (Evergreen Scientific, Los Angeles, CA, USA); PMC, Para-Pak Macro-Con (Meridian Diagnostics, Inc., Cincinnati, OH, USA); TFC, Trend FeKal CON-Trate (Trend Scientific, Inc., St. Paul, MN, USA); FCK, Fecal Concentrator Kit (Remel, Lenexa, Kansas); C-FEC, conventional formalin-ether concentration; Para-FEC, modified formalin-ether concentration using Para Tube.