Abstract
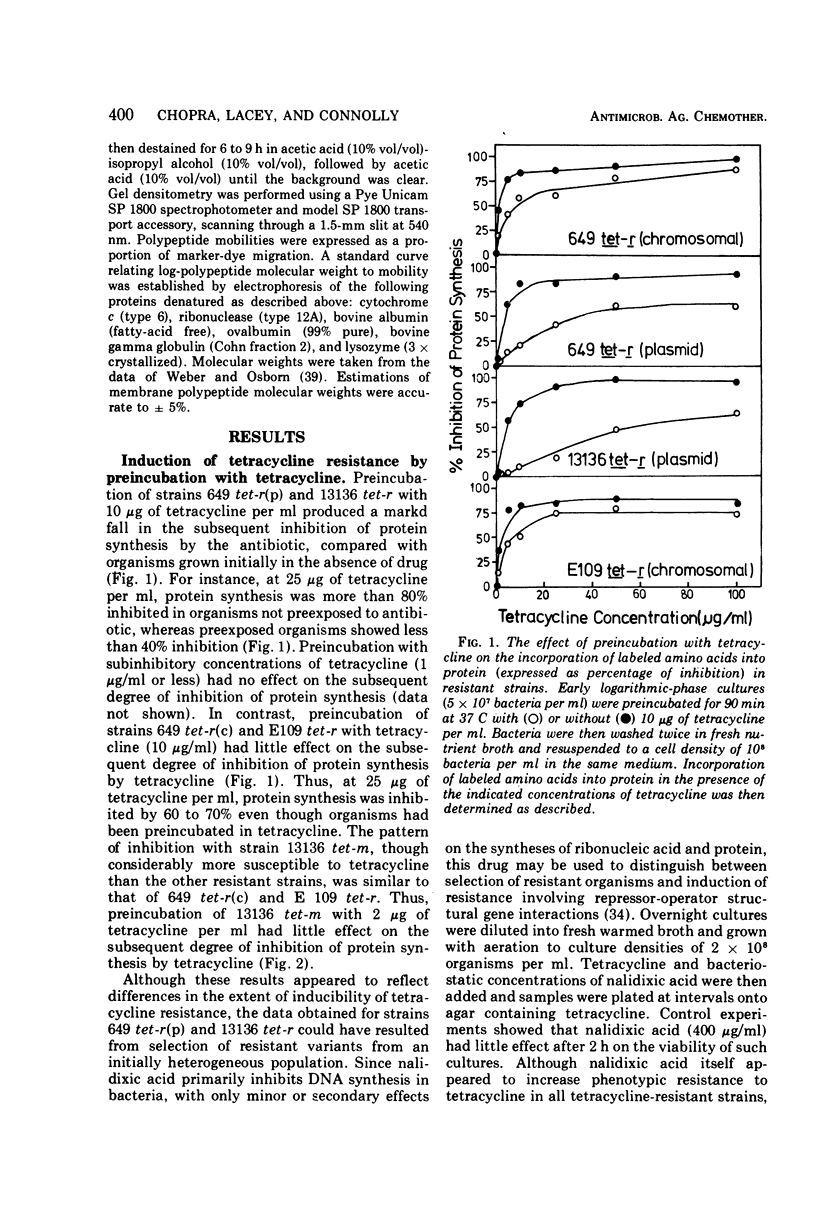
Both the genetic and biochemical basis of tetracycline resistance in a number of staphylococcal strains was investigated. The strains examined could be classified into three groups: (i) those possessing a high basal level of resistance and in which resistance could be induced to higher levels (macro-inducible); (ii) those which had a high uninduced level of resistance, but which were virtually uninducible (macro-constitutive); (iii) one derivative which had a low basal level of resistance and was also uninducible (micro-constitutive). Resistance in macro-constitutive strains was plasmid mediated and typical of organisms possessing wild-type plasmids. The macro-constitutive pattern of resistance appeared to be correlated with a chromosomal location for the resistance genes, whereas the micro-constitutive pattern was correlated with loss of a region from the wild-type plasmid. Analysis of membrane proteins by sodium dodecyl sulfate polyacrylamide gel electrophoresis suggested that a number of membrane polypeptides became unstable in staphylococci possessing high-level tetracycline resistance. In particular, the absence of a polypeptide of 22,000 daltons was always associated with high-level resistance. There was no evidence that multiple gene copies are required for expression of tetracycline resistance in Staphylococcus aureus.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Asheshov E. H. Loss of antibiotic resistance in Staphylococcus aureus resulting from growth at high temperature. J Gen Microbiol. 1966 Mar;42(3):403–410. doi: 10.1099/00221287-42-3-403. [DOI] [PubMed] [Google Scholar]
- Avtalion R. R., Ziegler-Schlomowitz R., Pearl M., Wojdani A., Sompolinsky D. Depressed resistance to tetracycline in Staphylococcus aureus. Microbios. 1971 Mar;3(10):165–180. [PubMed] [Google Scholar]
- Chopra I., Bennett P. M., Lacey R. W. A variety of Staphylococcal plasmids present as multiple copies. J Gen Microbiol. 1973 Dec;79(2):343–345. doi: 10.1099/00221287-79-2-343. [DOI] [PubMed] [Google Scholar]
- De Zeeuw J. R. Accumulation of tetracyclines by Escherichia coli. J Bacteriol. 1968 Feb;95(2):498–506. doi: 10.1128/jb.95.2.498-506.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN T. J., GODFREY A. RESISTANCE OF ESCHERICHIA COLI TO TETRACYCLINES. Biochem J. 1965 Jan;94:54–60. doi: 10.1042/bj0940054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Franklin T. J., Cook J. M. R factor with a mutation in the tetracycline resistance marker. Nature. 1971 Jan 22;229(5282):273–274. doi: 10.1038/229273a0. [DOI] [PubMed] [Google Scholar]
- Franklin T. J., Foster S. J. Effect of osmotic shock on tetracycline resistance in Escherichia coli bearing an R-factor. Biochem J. 1971 Jan;121(2):287–292. doi: 10.1042/bj1210287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J., Higginson B. Active accumulation of tetracycline by Escherichia coli. Biochem J. 1970 Jan;116(2):287–297. doi: 10.1042/bj1160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J. Resistance of Escherichia coli to tetracyclines. Changes in permeability to tetracyclines in Escherichia coli bearing transferable resistance factors. Biochem J. 1967 Oct;105(1):371–378. doi: 10.1042/bj1050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Lacey R. W. Genetic variation of streptomycin resistance in clinical strains of Staphylococcus aureus. J Med Microbiol. 1973 Aug;6(3):351–361. doi: 10.1099/00222615-6-3-351. [DOI] [PubMed] [Google Scholar]
- Hutchings B. L. Tetracycline transport in Staphylococcus aureus H. Biochim Biophys Acta. 1969 Feb 18;174(2):734–748. doi: 10.1016/0005-2787(69)90302-5. [DOI] [PubMed] [Google Scholar]
- Izaki K., Kiuchi K., Arima K. Specificity and mechanism of tetracycline resistance in a multiple drug resistant strain of Escherichia coli. J Bacteriol. 1966 Feb;91(2):628–633. doi: 10.1128/jb.91.2.628-633.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser F. H., Wüst J., Corrodi P. Transduction and elimination of resistance determinants in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1972 Sep;2(3):217–223. doi: 10.1128/aac.2.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W., Chopra I. Evidence for mutation to streptomycin resistance in clinical strains of Staphylococcus aureus. J Gen Microbiol. 1972 Nov;73(1):175–180. doi: 10.1099/00221287-73-1-175. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Chopra I. Genetic studies of a multi-resistant strain of Staphylococcus aureus. J Med Microbiol. 1974 May;7(2):285–297. doi: 10.1099/00222615-7-2-285. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Genetic control in methicillin-resistant strains of Staphylococcus aureus. J Med Microbiol. 1972 Nov;5(4):497–508. doi: 10.1099/00222615-5-4-497. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Grinsted J. Genetic analysis of methicillin-resistant strains of Staphylococcus aureus; evidence for their evolution from a single clone. J Med Microbiol. 1973 Nov;6(4):511–526. doi: 10.1099/00222615-6-4-511. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Grinsted J. Linkage of fusidic acid resistance to the penicillinase plasmid in Staphylococcus aureus. J Gen Microbiol. 1972 Dec;73(3):501–508. doi: 10.1099/00221287-73-3-501. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Lewis E., Rosdahl V. T. Evolution of plasmids in vivo in a strain of Staphylococcus aureus. J Med Microbiol. 1974 Feb;7(1):117–125. doi: 10.1099/00222615-7-1-117. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Rosdahl V. T. An unusual "penicillinase plasmid" in staphylococcus aureus; evidence for its transfer under natural conditions. J Med Microbiol. 1974 Feb;7(1):1–9. doi: 10.1099/00222615-7-1-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- Nordström K., Ingram L. C., Lundbäck A. Mutations in R factors of Escherichia coli causing an increased number of R-factor copies per chromosome. J Bacteriol. 1972 May;110(2):562–569. doi: 10.1128/jb.110.2.562-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Penicillinase plasmids of Staphylococcus aureus. Fed Proc. 1967 Jan-Feb;26(1):29–38. [PubMed] [Google Scholar]
- Reynard A. M., Nellis L. F., Beck M. E. Uptake of 3H-Tetracycline by resistant and sensitive Escherichia coli. Appl Microbiol. 1971 Jan;21(1):71–75. doi: 10.1128/am.21.1.71-75.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Kasamatsu H., Mickel S. The molecular nature and replication of drug resistance factors of the Enterobacteriaceae. Ann N Y Acad Sci. 1971 Jun 11;182:188–206. doi: 10.1111/j.1749-6632.1971.tb30656.x. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr, Strominger J. L. C 55 -isoprenoid alcohol phosphokinase: an extremely hydrophobic protein from the bacterial membrane. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2441–2443. doi: 10.1073/pnas.68.10.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Krawitz T., Zaidenzaig Y., Abramova N. Inducible resistance to tetracycline in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):341–349. doi: 10.1099/00221287-62-3-341. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Zaidenzaig Y., Ziegler-Schlomowitz R., Abramova N. Mechanism of tetracycline resistance in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):351–362. doi: 10.1099/00221287-62-3-351. [DOI] [PubMed] [Google Scholar]
- Theodore T. S., Panos C. Protein and fatty acid composition of mesosomal vesicles and plasma membranes of Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):571–576. doi: 10.1128/jb.116.2.571-576.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEAVER J. R., PATTEE P. A. INDUCIBLE RESISTANCE TO ERYTHROMYCIN IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1964 Sep;88:574–580. doi: 10.1128/jb.88.3.574-580.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- van Embden J., Cohen S. N. Molecular and genetic studies of an R factor system consisting of independent transfer and drug resistance plasmids. J Bacteriol. 1973 Nov;116(2):699–709. doi: 10.1128/jb.116.2.699-709.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


