Abstract
The rise of human populations and the growth of cities contribute to the depletion of natural resources, increase their cost, and create potential climatic changes. To overcome difficulties in supplying populations and reducing the resource cost, a search for alternative pharmaceutical, nanotechnology, and energy sources has begun. Among the alternative sources, microalgae are the most promising because they use carbon dioxide (CO2) to produce biomass and/or valuable compounds. Once produced, the biomass is ordinarily harvested and processed (downstream program). Drying, grinding, and extraction steps are destructive to the microalgal biomass that then needs to be renewed. The extraction and purification processes generate organic wastes and require substantial energy inputs. Altogether, it is urgent to develop alternative downstream processes. Among the possibilities, milking invokes the concept that the extraction should not kill the algal cells. Therefore, it does not require growing the algae anew. In this review, we discuss research on milking of diatoms. The main themes are (a) development of alternative methods to extract and harvest high added value compounds; (b) design of photobioreactors; (c) biodiversity and (d) stress physiology, illustrated with original results dealing with oleaginous diatoms.
Keywords: diatom, biotechnology, milking, physiology, stress, biofuel, secondary metabolites
1. Introduction
The rise of human populations and the expansion of cities into the countryside contribute to an accelerated depletion of natural resources [1], thereby increasing the prices of these resources in commercial markets and potentially changing the Earth’s climate. To overcome difficulties in supplying populations and reducing the resource cost, an intensive search for alternative pharmaceutical, food, high value molecules (HVM), and energy sources, including via genetic engineering and nanotechnology, has started [2,3,4,5,6,7,8,9,10]. Among the alternative sources, microalgae are promising because using their efficient capacity to photosynthesize, microalgae convert light energy into chemical energy into organic molecules such as carbohydrates and lipids. These molecules are synthesized using the carbon dioxide (CO2) from the environment. Among algae, diatoms (Bacillariophyceae) are responsible for a large part (up to 41%–50%) of the CO2 fixed in oceans [11,12]. In some circumstances, microalgae synthesize secondary metabolites [2,10,13,14,15] that can have important applications for food, health, cosmetic, energy or pharmaceutical industries. These compounds are called high value molecules (HVM). For instance, the price of the natural carotenoid astaxanthin is expected to reach $14,000 US kg−1 in 2018 [16]. Due to the diversified evolutionary history, the chemical diversity of HVM synthesized by microalgae is broad. Table 1 presents the main storage HVM, including oil content, of some microalgae.
Table 1.
Main storage compounds and oil percentage of some microalgae.
| Phylum | Class | Taxonomy | Oil Content (% d.w.) | High Value Molecules | Reference |
|---|---|---|---|---|---|
| Chlorophyta | Chlorodendrophyceae | Tetraselmis suecica | 15–32 | Carotenoids, chlorophyll, tocopherol, lipids | [40] |
| Chlorophyta | Chlorophyceae | Ankistrodesmus sp. | 28–40 | Mycosporine-like amino acids, polysaccharides | [17] |
| Chlorophyta | Chlorophyceae | Dunaliella salina | 10 | Carotenoid, β carotene, mycosporine-like amino acids, sporopollenin |
[41] |
| Chlorophyta | Chlorophyceae | Dunaliella tertiolecta | 36–42 | Carotenoid, β carotene, mycosporine-like amino acids | [42] |
| Chlorophyta | Chlorophyceae | Neochloris oleoabundans | 35–65 | Fatty acids, starch | [43] |
| Chlorophyta | Trebouxiophyceae | Botryococcus braunii | 29–75 | Isobotryococcene, botryococcene, triterpenes | [44] |
| Chlorophyta | Trebouxiophyceae | Chlorella vulgaris | 58 | Neutral lipids | [45] |
| Chlorophyta | Trebouxiophyceae | Chlorella emersonii | 34 | Neutral lipids | [46] |
| Chlorophyta | Trebouxiophyceae | Chlorella protothecoides | 15–55 | Eicosapentaenoic acid (EPA), ascorbic acid | [47] |
| Chlorophyta | Trebouxiophyceae | Chlorella minutissima | 57 | C16- and C18-lipids | [48] |
| Heterokontophyta | Bacillariophyceae | Nitzschia laevi | 28–69 | EPA | [49] |
| Heterokontophyta | Coscinodiscophyceae | Thalassiosira pseudonana | 21–31 | Glycosylglycerides, neutral lipids, TAG | [50] |
| Heterokontophyta | Labrynthulomycetes | Schizochytrium limacinum | 50–77 | Docosahexaenoic acid (DHA) | [51] |
| Myzozoa | Peridinea | Crypthecodinium cohnii | 20 | DHA, Starch | [52] |
| Ochrophyta | Coscinodiscophyceae | Cyclotella sp. | 42 | Neutral lipids | [53] |
| Ochrophyta | Eustigmatophyceae | Nannochloropsis sp. | 46–68 | EPA, TAG, ω-3 LC-PUFA | [54] |
Nowadays, algae are important biotechnological tools with applications in various industrial fields [17,18,19]. Examples are:
manufacture of fertilizers [28],
production of secondary metabolites for pharmaceutical products [2,29,30],
Another reason to be interested in microalga-based biotechnological processes is that they are expected to be more productive per unit surface area of Earth than any cultivated agricultural plant [55,56]. For instance, diatom oil production is predicted to be 6–200 times higher than oilseed crops per unit surface area [27,57,58].
Although the use of microalgae for the production of HVM is very recent, microalgae played a crucial role in the formation of crude oil deposits in ocean floors, which are a rich natural source of fossil fuel [59]. This contribution continues today because algae are responsible for a large fraction of the organic carbon being buried on continental margins [60] and oceanic diatoms may even alleviate predicted global warming [61].
In current processes, the algal biomass is harvested, water is removed and HVM are extracted and purified. Milking processes aim to keep the algae alive while HVM are extracted, allowing indefinite repetition of the process. The milking approach will be more environmentally and economically friendly, if it comes to commercial fruition.
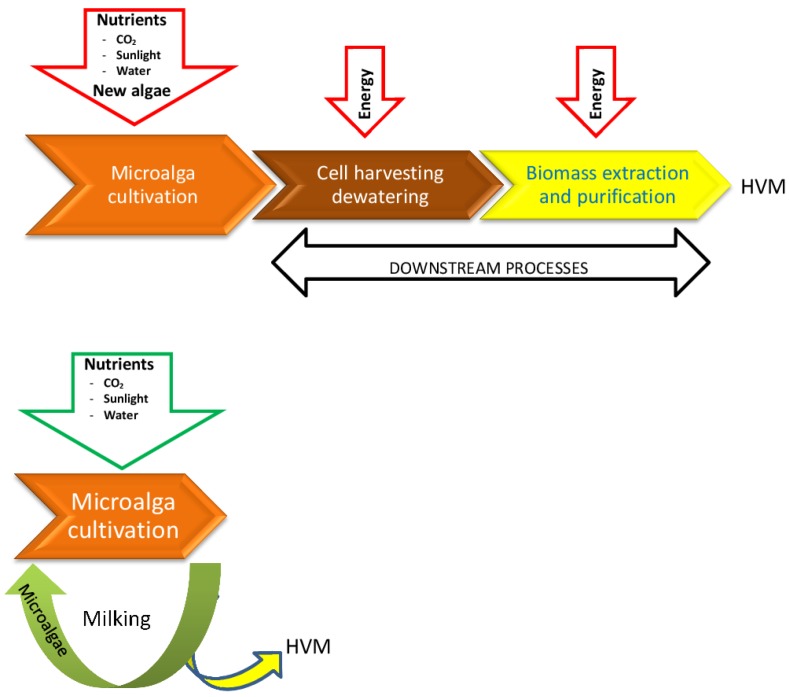
Whatever the biotechnological process used and the final products obtained from the algae, they all start with growing algae to obtain biomass. It is usually considered that microalga growth presents low productivity both in terms of biomass and product formation [62]. Therefore, a first critical issue in developing alga biotechnology is the optimization of culture parameters [63,64,65] (Figure 1). Once produced, the biomass is harvested and processed according to a dedicated downstream program generally involving dewatering, drying, grinding, extraction, and purification steps [65]. Grinding [66] and extraction [67] are the most popular steps for separation of the HVM from the organisms [65,68] (Figure 1). Obviously, drying and grinding require energy inputs and kill the microalgal biomass that then needs to be repeatedly regrown. In addition, extraction and purification processes generate organic wastes. A critical assessment of current microalga bioprocessing technology reveals that downstream processing also poses a number of important technical and economic challenges ([65,69,70] and references therein).
Figure 1.
Scheme presenting milking as an alternative to current processes. (Top): Current processes are systems that require starting new microalgal cultures for each batch of HVM, with fresh nutrients; (Bottom): Milking only requires inputs of carbon dioxide and water used up in producing HVM, and thus is closer to a closed system.
“No harvesting method has yet been identified as being efficient, reliable and at reasonable cost”.
[71]
For instance, it is estimated that downstream operations costs usually represent 50%–80% of the total processing costs [72,73,74,75,76,77,78]. Moreover, some of the currently proposed downstream processes are not economically sustainable, because they depend on waste CO2 [79,80,81,82,83], which is a nonstarter for large-scale production. This can be seen as follows. In 2011, 21% of CO2 emissions were industrial, while 22% were due to transport, and the latter figure is projected to reach 40% by 2035 [84]. This means that even with 100% utilization of industrial CO2 waste, there is not enough to be converted to biofuel for all transport now, let alone by 2035. So although the use of atmospheric CO2 may slow HVM production [85], the use of waste CO2 is at best a stopgap.
Altogether, it seems urgent to develop alternative downstream processes. Among the possibilities, the concept of milking was introduced by Frenz and collaborators in 1989 [86] to describe the extraction of hydrocarbons from the green algae Botryococcus braunii Kützing (Trebouxiophyceae) by exposing the cells for a short time to hexane (cf. [87]). Ramachandra et al. [27] have pointed out the analogy to grinding up cows to extract dairy milk, which has been acknowledged, but regarded as inevitable:
“Plenty of organisms use those same inputs—All photosynthetic microalgae, for example. But you can’t milk them like a cow. You have to crush them”.
[79]
The milking concept specifies that the extraction should not kill the cells. Consequently, milking does not require the constant need of culturing and re-growing the entire stock of algae, which has a typical timescale of a few hours to weeks. If only hydrocarbon HVM were milked, then the need for nitrogen/phosphorus fertilizers would be reduced [88] or eliminated.
In some of the literature the distinction that we are now making between extraction and milking was not considered, so that what we are calling extraction was labeled as “milking” [67]. Similarly, we previously lumped secretion and extraction under “milking” [27,89]. We think that careful distinctions between these approaches will make for better dialogue. Thus we define:
Milking as the removal of a specific set of products without killing the organism, in such a way that it can be milked again at a later time.
Extraction as the removal of a specific set of products without concern about the survival of the organism, generally leaving an organic residue.
Secretion or spontaneous oozing as the active dumping of a specific set of products by an organism into its surrounding environment.
In this manuscript, we propose food for thought toward the development of milking of microalgae, particularly diatoms. To reach our goal, the main grounds for thought that we have identified are (a) development of alternative methods to extract and harvest HMV; (b) design and functioning of photobioreactors; (c) biochemodiversity and (d) diatom (stress) physiology. They are discussed separately in this contribution and, when possible, illustrated with original results dealing with diatoms accumulating lipids. These algae are referred to as oleaginous diatoms. Although recent reviews [90,91] addressed several points discussed in this contribution, none of them focused on diatoms and milking.
2. Development of Alternative Processes to Extract and Harvest High Value Molecules (HVM)
The key element of milking is finding a way to extract HVM without killing the HVM-producing cells. This concept was first applied to higher plants by milking, for instance, rubber 2000 years ago [92]; maple syrup historically [93] and prehistorically [94,95]; turpentine as long ago as Hippocrates [96]; and, more recently, halophilic bacteria [97,98] and microalgae. The first applications with algae used biocompatible solvents to extract HVM (reviewed in [27,62]). Recently, Gillet [99] calculated the molecular dynamics of the plasma membrane during milking using organic solvents. The conclusion of this theoretical study agreed with earlier experimental results obtained by Zhang et al. [67] and showed that hexadecane is the best organic solvent to milk lipids from Nannochloropsis sp. (Eustigmatophyceae) (cf. [100]). However, solvents alone are usually not efficient for HVM extraction if the cell wall is too robust [101]. In this case, HVM extraction should be assisted by other methods. It is important to recall here that the efficiency of these processes may depend on the growth stage of the cells. Table 2 presents an overview of these different potential technologies with a summary of their strengths and weaknesses, although without any pretention of being exhaustive. The aim of this section is to provide a detailed analysis of these possibilities in the framework of a milking strategy. We do not consider destructive processes, such as microwaving and heating [102], though in moderation they might also serve to induce milking.
Table 2.
Extraction processes potentially applicable for milking of microorganisms.
| Milking Process | Microorganism | Advantages | Disadvantages | Ability to Keep Cells Alive |
|---|---|---|---|---|
| Biocompatible organic solvents | Microalgae [67,100] | Improvement of lipid production Positive effect on growth |
Not environmentally friendly Possible toxic mechanism |
Yes, when using hydrophobic solvents |
| Pulsed electric field (PEF) | Yeast [103] Microalgae [104,105,106,107] Cyanobacteria [108] |
High extraction yield Adjustable PEF parameters Not an energy-intensive process Large-scale process demonstrated Continuous process |
Effect of electric pulsation is size dependent | Yes, but depends on the PEF parameters |
| Spontaneous oozing | Microalgae [109] Bacteria [110,111,112] Cyanobacteria [113,114,115] |
Not an energy-intensive process Possibility of scaling up Application in solar panels |
Slow oozing of HVM | Yes, it is a natural mechanism |
| Mechanical methods | ||||
| -sonication | Microalgae [116,117] Cyanobacteria [118,119,120] |
Improvement of lipid recovery | Cellular damage apoptosis Thickness of the cell wall |
No |
| -pressure | Microalgae [this work] | Not an energy-intensive process Weak pressure to be used (below 750 µN) |
Large-scale process not demonstrated Process needs to be improved | Yeswhen using low pressure (< 750 µN) |
| -centrifugation | Diatoms [work in progress] | Continuous process Application in solar panels |
Requires energy | Not yet tested |
| Membrane-bound protein pumps | Bacteria [121,122] | Oozing of HVM Lower toxicity of overexpressed HVM High rate growth Possibility of scaling up |
Metabolism engineering Organic phase needed for solubilization of water insoluble HVM |
Yes |
2.1. Pulsed Electric Field
Electroporation has been applied to diatoms and other microalgae, albeit primarily to allow genetic transformation [123,124,125,126,127,128]. However, the application of trains of electric pulses can also be used to favor the release of HVM from cells. In this method, cell membranes are punctured by electric pulses, subsequently allowing the cell components to ooze out. The method was first established with yeast [103,129] and more recently applied to photosynthetic organisms such as cyanobacteria [108] and microalgae [104,105]. The “punctured” cells then heal and remain viable and the same batch of algae can be reused for further extraction of HVM [106]. Because electric pulses can have deleterious effects in bacteria [130], algae [131] and mammal cells [132], such as irreversible electroporation, the choice of the characteristics of the electric pulses such as length, intensity, polarity, frequency of repetition, etc. require a compromise between milking efficiency and algal survival. The tuning of physical parameters is of importance because the effect of the pulsed electric field is cell size dependent: the smaller the cell, the stronger the electric treatment [105,133,134]. Repetitive 2 ms long electric pulses of alternative polarities with field strength of 3 and 6 kV/cm were capable of significantly inducing the extraction of cytoplasmic proteins from two freshwater microalgae, Chlorella vulgaris Beyerinck (Trebouxiophyceae) and Haematococcus pluvialis Flotow (Chlorophyceae), both with rigid cell walls [105]. A post-pulse incubation step was found to be necessary in order to allow oozing, but also to permit the algae to recover between trains of electric pulses [105,107,135].
2.2. Spontaneous Oozing
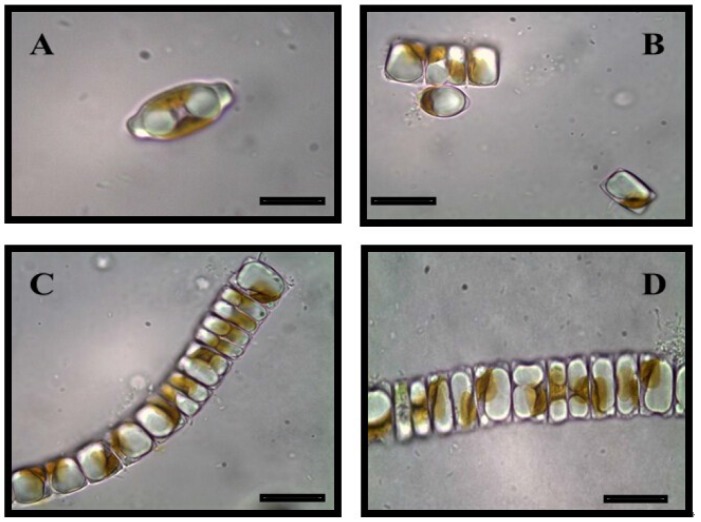
Currently, there are proprietary reports that some bacteria [110,111,112], green algae [62,86] and genetically engineered cyanobacteria [113,114,115,136,137] can secrete lipids [82] from their cytoplasm to the external environment. Botryococcus secretes its oil into a kind of cell wall called the outer matrix [87]. Surface properties may prove important in secretion [138]. Very recently, Vinayak et al. [109] reported that a small diatom strain (14–18 µm long and 6–7 µm width), Diadesmis confervaceae Kützing (Figure 2), producing 14.6% lipid content, exudes lipid droplets into the culture medium. The mechanisms involved in oozing are not yet determined. The droplets accumulate either in the chloroplasts (plastoglobules) or/and in the cytoplasm (oleosomes) (see also Section 4).
Figure 2.
Diadesmis confervaceae in solitary and chain forms as observed under 100× oil immersion. Note oozed oil droplets in panel C. Cf. [109]. Scale bar: 10 µm.
2.3. Mechanical Pressure
In algae lacking a natural oozing mechanism, one could assume that exerting a mechanical pressure, such as ultrasound or touch, could force HVM to come out of the cells. Similarly to electric pulse treatment (Section 2.1), ultrasound has been used in processes for improving the extraction of carotenoids (Haematococcus pluvialis [139,140]), chlorophyll (Chlorella sp. [141], Dunaliella [142,143]), and lipid (Chlorella vulgaris [116]) (for a review, see [144]). Because ultrasound effects can be harmful (death [118], nonviable cellular damage [119], or induction of programmed cell death [120]), the characteristics of the ultrasound treatment should be chosen to keep the cells alive and, thus, be qualified for inclusion in a milking process. For instance, Araujo et al. [116] reported that ultrasound treatment of Chlorella vulgaris produced a significant improvement in lipid recovery when compared to the yield reached in the absence of the treatment. However, the efficiency of the treatment depends on the cell wall strength as only a weak improvement was recorded with Scenedesmus, a Chlorophyceae taxon with tougher cell walls [117,118]. For instance a 20 min, 20 kHz treatment causes death of the cyanobacterium Microcystis aeruginosa Kützing [118].
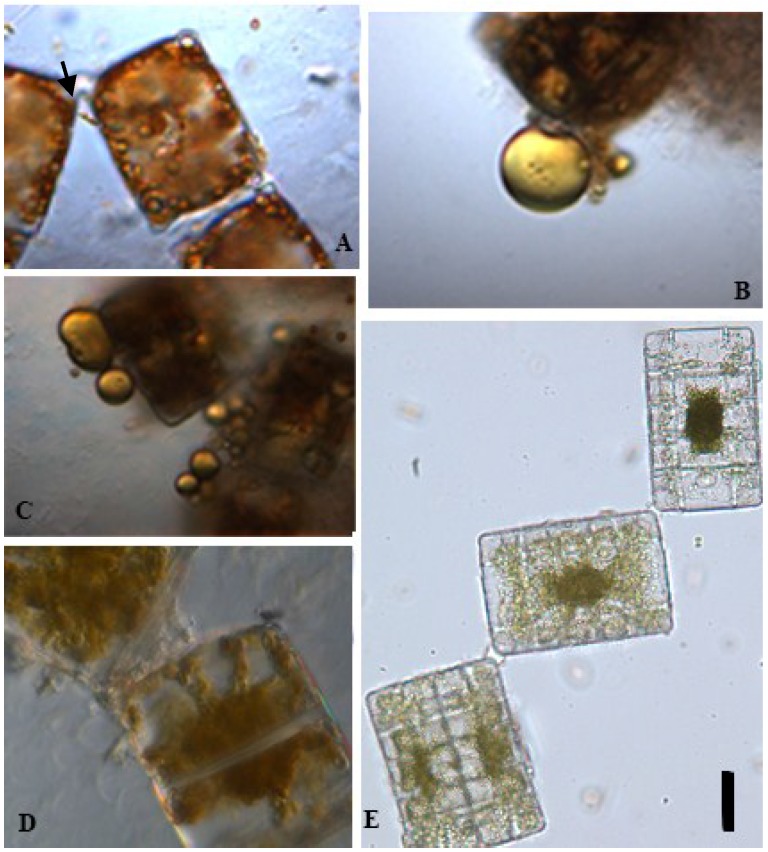
Diatom cells present the unique feature of being enclosed in a hydrated silicon dioxide cellwall denoted the frustule. Frustule shape and decorations are widely diversified [145,146]. In addition, each diatom has an imperfect bilateral symmetry since one of the frustules is slightly larger than the other, allowing one valve to fit inside the edge of the other. Because of this and the robustness of the frustule, mechanical methods could provide a very powerful method favoring the release of HVM. To test this possibility, diatoms harvested in Georgia, USA (see Section 3) were placed in water on a rigid surface and covered with an 18×18 mm2 coverslip and pressed with a microbial needle. Pressure was applied in the middle of the coverslip until water flow out from under the coverslip ceased. All expressed water remained on the slide at the edges of the coverslip. The population remaining under the coverslip was then visually scanned and changes such as breaking of the cells or release of oil were documented. Where possible to identify them, the same cells were scrutinized. With the mechanical pressure on the cover slip live diatoms were not visibly broken or harmed and the release of oil from Terpsinoë musica Ehrenberg was recorded at least twice. Interestingly, Terpsinoë musica naturally produce zigzag colonies (Figure 3, panel A,E) in which the individual cells are attached by organic pads secreted from the terminal apical pore fields (Figure 3A arrow and 3B) [147]. The observations reported here suggest that the oil is also released through the apical pore field, which is also known to be used for the release of carbohydrates [148]. It would be interesting to test other diatoms with an apical pore field [149] for their oozing capacity. Although the force that has to be exerted on a live diatom for expressing oil has not yet been measured, we can assume that it is considerably less than the force required to break an isolated diatom valve. Hamm et al. [150] presented mechanical properties of various diatoms under tensile and compressive stresses, and reported the breaking force for an isolated diatom valve to be 750 μN.
Figure 3.
Terpsinoë musica is a freshwater diatom that releases oil under mechanical pressure on the cover slip. Scale bar = 10 µm. (A) Freshly collected Terpsinoë musica; (B,C) Terpsinoë musica cells kept 7 days in an incubator released oil when mechanically pressed; (D) Terpsinoë musica divides 10 days after oil release; (E) Zigzag colony of Terpsinoë musica.
2.4. Centrifugation
At the time of writing, we have just started work on release of oil from diatoms via centrifugation, which will be reported later. We have yet to determine if the centrifugal force that releases oil is lower than that which kills them. If so, the centrifugation could become a milking technique. Centrifugation is presently used for algal separation [151]. In sea urchin eggs centrifuged at 9000× g the lipids rise to the centripetal end and exert enough buoyancy to divide the egg in two [152].
3. The Design and Functioning of Photobioreactors
In microalgal biotechnology, the cultivation of biological material is considered as one of the major constraints to commercial development, despite the fact that maximum doubling rates between 2 and 5 day−1 have been reported [27,153]. The simplest method for algae production is the open raceway pond: shallow, circulating, open bodies of water that are exposed to the elements and use the natural energy of the sun [154,155]. There are several advantages to this design, such as less material and capital being required for operation, but there are many drawbacks [65,156,157,158]. Primarily, the open nature of a raceway pond leaves it vulnerable to contamination, low volumetric productivity and poor temperature and light intensity control [159]. Photobioreactors (PBRs) allow much more control than open raceway ponds due to their closed systems [73,74,160,161]. They are usually made of glass or plastic and can be used outside with natural sunlight as well as indoors with artificial light such as light-emitting diodes (LEDs) [162,163]. The three main designs, annular PBRs, which are large columns, flat panel PBRs, and tubular PBRs, allow for laboratory conditions at all times. Yet, this approach is considerably more expensive than the open raceway pond [65,76,164,165]. As far as oil production is concerned, for all methods to be economically viable, they either have to be cheaper than traditional gasoline and be seamlessly introduced into an infrastructure based on fossil fuels, or they need to be protected by tariffs or embargos on imported oil [166] and/or subsidized relative to fossil oil. To involve the milking philosophy into microalgal biotechnology, downstream processes have to be modified to allow milking operations such as electric pulses to occur.
One can also propose innovative photobioreactors such as the live diatom solar panel [27]. Such a panel may require sandwiching diatoms in a series of polymeric microchannels that can be squeezed by increasing pressure on the backside of the channel. Forces of this magnitude can be produced in a microfluidic environment by electrical and mechanical means. The former mechanism can utilize Maxwell stresses induced by specifically designed electrode systems [167,168]. One suitable material for such polymeric channels is polydimethylsiloxane (PDMS), which can covalently bond to glass during the solar panel manufacturing process, and can be prepared with a long-lasting hydrophilic surface suitable for cell culture [169]. PDMS is impermeable to water but would transmit air including much needed CO2 [170] to diatoms. The front of the glass-PDMS chambers can provide a fluidic environment to diatoms, while the backside of the PDMS chambers can be interfaced with gas channels. Increasing pressure in the gas channels would push the flexible PDMS and squeeze diatoms between the PDMS and glass substrates, possibly releasing oil. Large-scale systems with thousands of individually controlled PDMS micro-valves have been demonstrated before [171]. The diatom solar panel envisioned would require only several valves and the entire system could be fabricated cheaply, making this proposed technology cost effective. Separation of milked oil from the diatoms could perhaps be facilitated by buoyancy, pumping, centrifugation and/or the use of spatial gradients or step functions of the surface hydrophobicity of the PDMS channels [172]. If the diatoms used in diatom biofuel solar panels do not spontaneously secrete HVM, then a power source to apply force to the live diatoms to milk them for biofuel (see Section 2) would be needed (possibly using solar electric power generated in the same panel). Instead of bulk solvent extraction, the algal cells could be left in place and, lipids and/or other HVM removed by microfluidics [173].
In this review our focus is on the use of living diatoms to produce biofuel and other compounds. It is important to distinguish this from the use of nonliving diatom frustules in novel electricity-generating solar panels [174]. Of course, both could be combined in a single panel.
Solar panels containing living diatoms or other algae could go on rooftops, for example, and we have estimated that surface areas of 10 square meters per person might suffice to match USA rates of gasoline consumption [175], especially if the algae could be selectively bred [176] or genetically engineered for octane production [177] or other low carbon and volatile compounds [27]. Diatom solar panels also have the advantage of local gasoline production, reducing the need for a distribution system, storage of energy overnight in liquid form instead of in heavy batteries that last only a few years [178] and could make use of species adapted to local conditions [179]. While developing new processes, especially algal solar panels [27,180], one should keep in mind that algae that are in use must stay alive. Species should therefore be identified as, for instance, from geothermal waters that are thermo-resistant (as are some green microalgae [181]) or thermophilic [27] (as some diatoms are [182,183,184,185]).
4. Diatom Chemobiodiversity
Tens of thousands of strains of microalgae have been described, millions of species are expected to exist [186,187], and new species, including diatoms, are continuously being described. In contrast to this amazing biodiversity, no more than 10 species are commonly used at the commercial level, regardless of the geographical location of the producing company, which is often done outdoors [65,188]! These species include the diatoms Phaeodactylum tricornutum and Odontella aurita [16]. Thus another way forward is the development of more strains of industrial interest [173]. This is especially important if HVM production units are widely distributed from the geographic point of view as prospective studies have predicted [189]. In other words, promising strains must be optimized for local climatic conditions, and media optimized for those strains [190]. Bloom diatoms, for example, may be particularly good oil sources [191], and perhaps invasive diatoms [192] would be worth considering. There are several nonexclusive possibilities for finding such species/strains: (a) strain selection, (b) synthetic biology and (c) exploring biodiversity. Study of biodiversity can also lead to the discovery of new HVM.
4.1. Strain Selection
In the introductory paragraph of this section, we underlined how it will be quite important to use dedicated species for the production of HVM. Because methods for synthetic biology are still under development for microalgae (see Section 4.3), screening remains one of the most promising methods for the selection of dedicated species provided a selection criterion has been determined and a fast and low cost detection method is available [193]. For instance, Kopecky et al. [194] screened algal collections for the capacity of green algae to synthesize secondary carotenoids. Using high light stress as a selection pressure and thin-layer chromatography as the analytical method several putative, interesting strains were characterized for their secondary pigment content. Recently, nondestructive methods such as Fast-Fourier InfraRed (FT-IR) spectroscopy have been developed to characterize the biochemical contents of microalgae [195,196,197].
The selection pressure need not be a natural factor. Bougaran et al. [198] developed a nongenetically modified organism mutation-selection method based on UVC irradiation (at 254 nm wavelength) followed by flow cytometry selection to isolate an Isochrysis affinis galbana population overaccumulating neutral lipid (with a productivity increase of 80%). Robert et al. [199] used the herbicide metolachlor, a member of the chloroacetamide family of compounds, to select diatom strains with hyperaccumulation of long-chain polyunsaturated fatty acids (PUFA). PUFA are health-benefitting molecules [2,200]. This herbicide was chosen because it is an inhibitor of the fatty acid ester-type (FAE) elongase biosynthesis of very long-chain fatty acids in microalgae [201]. For a review on the metabolic pathways leading to very long chain fatty acids, see [14].
4.2. Exploring Biodiversity
It is usually admitted that biodiversity constitutes the richest source of bioactive molecules [202]. Unfortunately, since World War II, the search for such compounds from natural sources has been gradually replaced by synthetic and combinatorial chemistry (for a review, see [203]). In the recent past, many different scale bioprospecting programs such as the Aquatic Species Program [204] or Tara Oceans Cruise [205] have aimed to find new HVM from nature [206,207,208]. The Aquatic Species Program identified 50 promising microalgal strains for biofuel production among which 60% are diatoms. This number is far greater than the number of diatom species that have been used so far in microalga biotech. This shift back to bioprospecting programs partly relies on the assumption that living organisms are a storehouse for HVM that are just waiting to be discovered. Theoretical investigations of the relationship between biological and chemical diversities revealed that it is unlikely that a fine combing of global resources sharing similar biological niches will discover interesting species unless they are distant from the phylogenetic point of view, as shown for the plant–herbivore relationship [209]. Therefore, bioprospecting would be more successful in screening biological resources, including microalga collections, from diversified ecological niches such as hot sources [181,185], depths of the ocean [181,210], or very saline or polluted waters [145] with a search for organisms that are phylogenetically distant from one another [211]. Once new strains have been collected from the natural environment, they should be selected according to defined criteria through a screening program as explained in Section 4.1 (phytohormones [212], lipids [213]). For instance, various places in Georgia were sampled for diatoms from March to July 2011 in triplicate (Table 3).
Table 3.
Site locality and identification for sites collection within southeastern USA, temperature (T), pH, percent dissolved oxygen (DO %) and conductivity (µS cm−1), mean ± SE.
| Location | No. | T (°C) | pH | DO % | Conductivity | Latitude | Longitude |
|---|---|---|---|---|---|---|---|
| Lake Sinclair Power Plant | 1 | 23.5 ± 4.2 | 7.0 ± 0.2 | 9 ± 2.52 | 32 ± 3.6 | 33.20 | −83.30 |
| Lake Sinclair, Goat Island | 2 | 21.8 ± 1.3 | 8.5 ± 0.1 | 110 ± 14.1 | 67 ± 6.2 | 33.16 | −83.23 |
| Lake Sinclair at Dam | 3 | 19.9 ± 6.1 | 7.8 ± 0.8 | 69 ± 8.2 | 46 ± 7 | 33.14 | −83.20 |
| Oconee River at Dam | 4 | 23.8±5.1 | 7.1± 2.1 | 61 ± 11.1 | 82.6 ± 1.4 | 33.14 | −83.20 |
| Oconee River Greenway | 5 | 20.2 ± 9 | 7.2 ± 1.6 | 75 ± 2.6 | 78.3 ± 6.9 | 33.08 | −83.21 |
| Fishing Creek | 6 | 22.4 ± 3.2 | 6.3 ± 0.4 | 89 ± 8.2 | 29.2 ± 11.4 | 33.08 | −83.22 |
| Tobler Creek | 7 | 22.1 ± 2.1 | 7.2 ± 0.2 | 45 ± 10.6 | 85 ± 5.3 | 33.12 | −83.27 |
| Andalusia pond | 8 | 23.5 ± 1.8 | 6.8 ± 0.5 | 76 ± 9.6 | 24.3 ± 1.8 | 33.13 | −83.27 |
| Bartram forest pond | 9 | 19.5 ± 3.2 | 7.4 ± 1.3 | 58 ± 12.6 | 78.5 ± 1.6 | 33.02 | −83.21 |
| Savannah River at Port Wentworth, GA | 10 | 25.5 ± 4.4 | 7.6 ± 0.4 | 86 ± 9.7 | 8204 ± 125.4 | 32.17 | −81.16 |
Five lentic (three locations at Lake Sinclair, ponds at Andalusia Farm and Bartram Forest, Baldwin County) and five lotic locations were sampled; two on Oconee River and one each at Savannah River, Tobler and Fishing Creeks, in southeastern Georgia, USA (Table 3), all locations being freshwater with the exception of the samples from the Savanna River at Port Wentworth, GA with conductivity higher than 2000 µS cm−1. Each habitat sample was taken as composite vegetation; water and sediment samples were taken following the loose sediment collection technique in EPA’s periphyton protocols [214]. Representatives of 24 diatom genera were identified from the natural samples with 314 taxa identified to species from all samples. The brackish sample from the Savannah River estuary was the most diverse with 167 diatom species. The freshwater diatom community was dominated by representatives of pennate genera including Nitzschia, Fragilariforma, Synedra and Eunotia (see Supplementary Table S1).
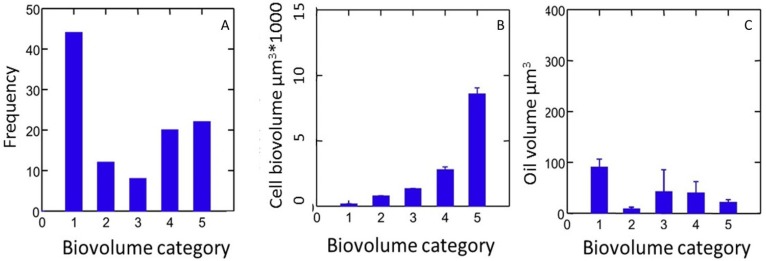
Cell size and the relationship between size and oil production were tested with the data. Only 106 individual cells out of thousands visually scanned (representing 341 species) had visible lipid droplets. Small diatoms dominated the size distribution of those diatoms containing lipid droplets and the frequency numbers above the highest potential average of oil content within that category (Figure 4). Only representatives of the genera Craticula, Pinnularia, and Eunotia from natural freshwater conditions and Pleurosira and Terpsinoë from the brackish environment had visible lipid droplets. None of the araphid diatoms observed had visible lipid droplets. Some pennate diatoms such as Pinnularia and Craticula representatives were shown to have less than 10% volume concentrations of oil within the first 7 days (see also Supplementary Data S2).
Figure 4.
Frequency of cells in five diatom cell biovolume categories, for cells that contained lipid droplets. (A) Frequency of cells in 5 diatom cell biovolume categories, for cells that contained lipid droplets; (B) Average biovolume within each cell biovolume category; (C) Oil content within each cell biovolume category.
4.3. Toward Dedicated Producers Using Synthetic Biology
Despite the promises of studying chemobiodiversity for industrial processes, the chance of finding, in a short time, perfect strain(s) adapted to the ad hoc industrial environment, growing quickly and producing high amounts of specific compounds is as difficult as finding a needle in a haystack. This reasoning led to the concept of engineered cells as HVM factories. In this concept, such cells would be tailored to be capable of producing dedicated HVM and could be used to tackle some of society’s most important and/or toughest challenges [215].
Diatoms have been successfully transformed by microprojectile bombardment (Chaetoceros sp. [216], Cyclotella cryptica [217], Cylindrotheca fusiformis [218,219], Fistulifera sp. [220], Navicula saprophila [217], Phaeodactylum tricornutum [221,222], Thalassiosira pseudonana [219]. Unfortunately, microprojectile bombardment is costly and does not allow a high transformation efficiency. To overcome these difficulties, electroporation transformation protocols have been developed for Phaeodactylum tricornutum [126,128,223] and Chaetoceros gracilis [224]. They allow a maximum transformation efficiency (2.8 × 10−5 cells) comparable to the ones obtained with Chlamydomonas sp. (2.0 × 10−5 cells [225]) or Nannochloropsis sp. (1–2 × 10−5 cells [226,227]).
Several publications established the benefit of genetic engineering for enhancing the production of HVM [228,229]. For instance, Trentacoste et al. [230] were successful in increasing the lipid metabolism in the diatom Thalassiosira pseudonana by targeting knockdown of acyltransferase enzyme locus Thaps3_264297 homologous to human CGI-58, having a conserved histidine-glycine dipeptide, without affecting the growth and viability of Thalassiosira pseudonana (whose metabolic network for lipids has been constructed [231]). Using a new dedicated method, Daboussi et al. [232] targeted the diatom model organism Phaeodactylum tricornutum and used zinc finger nucleases, meganucleases, and transcription activator-like effector nucleases to edit Phaeodactylum tricornutum by inducing targeted mutagenesis of certain genes responsible for HVM production. This led to formation of a mutant diatom Tn 19745_1 that produces 45 times more lipid than the control and further increased the HVM production when grown in nutrient-stressed media. However, despite the tremendous reported enhancement, the cost of production of lipids is very high and the process remains uneconomical. In addition, the accumulation of high amounts of HVM inside the cell can prove to be toxic at a level still too low to be relevant for the industry. This is the case for isopentenol, the platform molecule precursor of isoprenoids (for biosynthetic pathways, see [14] for which the lower limit is of the order of 1 g L−1 [122]. Therefore, one should see milking as more than an alternative possibility to get more sustainable blue biotech, i.e., as a mandatory process.
Recently, the concept of the cell factory has been expanded to synthetic biology, a branch of life science aiming to develop new biosynthetic pathways for the production of HVM, programmable logic controls to regulate and optimize complex cellular functions, and robust strains. For instance, Harrison and Dunlop [233] have developed a mathematical model for cell growth and biofuel production that involves a synthetic feedback loop using a biosensor to control efflux pump expression. Such a possibility is obviously critical because the cell toxicity of the accumulated HVM can be high (bacteria [234], cyanobacteria [235]). Thus, the export of accumulated HVM out from the producing cells constitutes a major challenge in achieving high-level production. To reach this goal, transporters facilitating the exportation of HVM should be identified at the molecular level and eventually heterologously expressed in the microalgae to be milked. Of course, the resulting protein(s) have to be addressed to the plasma membrane (see [236]). The proof of concept for such a strategy has been obtained by overexpressing several types of transporting systems in the plasma membrane of Escherichia coli in order to export isoprenoids [121,122] (Table 4).
Table 4.
Cloning of efflux pumps facilitate the excretion of synthesized lipids out from bacteria cells. “Pro/cons” stands for advantages and disadvantages.
| Type of Transporter | Origin | Host Cells | Molecules Transported | Pro/Cons | References |
|---|---|---|---|---|---|
| Resistance-nodulation-cell division (RND) family | Gram-negative bacteria, similarities with cyanobacteria | Escherichia coli | limonene | Pro: increase the excretion of limonene Con: Large tripartite protein complex [239] |
[122] |
| ATP-binding cassette (ABC) | Bacteria | Escherichia coli | carotenoids, squalene, botrycoccene | Pro: present in all 5 kingdoms; import or export molecules and ions across cell membranes | [121,240,241] |
| Formate transporter (focA) | Escherichia coli | Escherichia coli | formate | [242] |
To this end, knowledge of diatom physiology has to be deepened to understand the biochemical, cellular and molecular mechanisms sustaining the production of HVM as well as those that may be triggered by milking. This is especially true for carbon metabolism because HVM are mostly made of carbon and hydrogen atoms [236] (see Section 5). En route to this goal, the generation of robust mathematical models, able to precisely and accurately describe the metabolic state of a cell will constitute a major advantage, especially if they are able to integrate the different levels of complexity that the production of HVM requires [236,237]. Actually, the CO2 concentration and nutrient sources must be investigated and optimized for each microalgal strain [238].
5. Understanding of Diatom (Stress) Biology
Being one of the most important world net primary producers, diatoms play an important ecological role [11,243]. From the evolutionary point of view, the history of diatoms is much more complex than for green algae. It is out of the scope of this paper to detail the different roads through which diatoms have evolved. The interested reader is invited to read recent specialized reviews on this topic [244,245]. To be convinced of this complexity, it is enough to mention that diatom evolution has involved several endosymbiotic events, including with a cyanobacterium and a red alga [246] together with a chlamydial invasion [247]. The gene enrichments provided by these events resulted in a unique set of metabolic and regulation networks [248] that certainly helped diatoms to colonize every type of ecological habitat such as freshwater, brackish, marine and hypersaline environments with wide ranges of temperature, pH, and nutrient availability. Some may be considered extremophiles [145,249]. This colonization capacity reflects a very flexible metabolism allowing them to adapt to various environmental constraints [145,250,251,252,253]. Long-term adaptation mechanisms often involve metabolic shifts, consisting in the production of secondary metabolites [53,163]. Because stress conditions such as salinity [254], nutrient deficiency, temperature and high light stress [255] are “interpreted” by the algae as “dangerous” [91], they accumulate high-energy molecules such as carotenoids and/or lipids [13,53,254,256]. For instance, a nitrogen or silicon depletion stress has been shown to increase oil production in diatoms by a factor of 2 or 3 of their average dry weight [257,258,259]. Desiccation can cause algae to create thick mucus sheaths that are often found to contain starch or oil [260]. Diatoms can store energy in the form of chrysolaminarin or as lipids [261], so we will have to learn how to bias production towards oil. It is usually admitted that microalgal division activity competes with HVM accumulation [53,262,263]. Algae with high oil content, such as Botryococcus, grow slowly and can only be harvested a few times a week whereas those containing low amounts of oil, such as Dunaliella, divide faster and can be harvested daily. For this reason, most industrial applications use algal strains with 20%–40% lipid content [65]. To ensure a high division rate, diatoms can rely on a greater ability to fix CO2 than other phytoplanktonic groups [264]. For instance, under fluctuating light intensities, the diatom Phaeodactylum tricornutum was nearly twice more efficient than the green alga Chlorella vulgaris [60]. This suggests a strong regulation in the fate of photosynthetically fixed carbon [236,265]. This means that any stress impacting negatively on growth should activate HVM production [13,14,53,266]. For instance, lipid production in Chlorella emersonii could be as high as 63% DW when grown under nitrogen stress (Table 1). However, conditions allowing growth and neutral lipid accumulation were found recently for the green microalga Neochloris oleabundans [267] (cf. [230]). In the philosophy of milking, the rate of alga division does not seem very critical because the HVM production will not rely on biomass production but more on the carbon flux through the dedicated pathways, as observed with an Escherichia coli strain secreting isoprenoids thanks to the an overexpressed ABC transporter [121].
In the context of lipid milking, the formation and storage of lipids is of course of particular interest. Diatoms, as other microalgae, store lipids into oleosomes [268] (also called spherosomes, lipid droplets, lipid bodies, oil droplets, etc.), the amount of which increases under stress conditions (green algae [269]; diatoms [109,256]). In diatoms, lipid synthesis involves the chloroplast and the endoplasmic reticulum (for a review, see [14]). However, as in higher plants [14], there is probably no direct pathway for fatty acids to move from chloroplasts to oleosomes as is usually assumed by many complex interconnected reactions involving the endoplasmic reticulum. A first indication for such a view has been deduced from a proteomic study of isolated oleosomes from the diatom Fistulifera solaris JPCC DA0580. Actually, this study has revealed the presence of one specific protein presenting a quinone protein alcohol dehydrogenase-like domain [270]. Using a fluorescent tag, the protein was found to be targeted to the endoplasmic reticulum where it could be involved in the formation of oleosomes [256]. Transportation of oleosomes could occur by exocytosis as observed with the Chlorophyceae alga Dunaliella salina [271].
Formation and composition of oleosomes is of course of particular interest in the context of lipid production because they appear to be a vehicle for oozing (see Section 4). Hildebrand et al. [90] assumed that the larger the size of a diatom, the larger its lipid droplets. This expectation was not confirmed by our measurements as no correlation was found between biovolume and total lipid droplet volume within a cell (Figure 4B,C) and biovolume category #1 in this study had the largest visible oil contents (Figure 4C). This lack of correlation extends even to individual diatoms in chains, which can be presumed to be clonal (Manoylov, unpublished observations, cf. [109]), i.e., genetically identical, and to have experienced the same microenvironment. Time lapse observations of lipid droplets in unicellular and colonial diatoms might help understand these variations and may be important for elucidating the optimal conditions for oil production, and for recovering oil synthesis after mechanical milking cf. [272,273,274,275,276,277,278,279].
6. Conclusions and Perspectives
The study of HVM from microalgae is still in its infancy [204]. Microalgae are fast growing, common, and readily available. The commercial exploitation of microalgae to produce HVM is not new but so far is a niche market because these HVM have no crucial importance for human society. For instance, the ambition to replace a significant part, if not all, of the fossil oil consumption by lipids produced by microalgae, not only constitutes a change of paradigm but also a tremendous change in the market, and consequently, in production. To maximize the benefits of this possibility, it is desirable to have environmentally benign production processes. We have reviewed alternative possibilities to reach this goal via milking of diatoms and illustrated them with original data.
At the time of writing, crude oil prices have dipped, perhaps due to economics [280,281], market manipulation [282], or increased production due to fracking [283,284]. In the short term this volatility is hard on biofuel startups [285], whose cost per US gallon presently varies from $9 to $40 [286]. Nevertheless, we anticipate that in the long run biofuels, perhaps obtained via diatom milking, could technologically fit smoothly into our present transportation and energy sectors, maybe at lower cost, while indeed disrupting many current economic and geopolitical arrangements [287]. The ongoing debate over global warming/climate change, including astronomical phenomena [288,289,290], serves as a backdrop, providing breathing room [291] or a sense of urgency [292] or despondency [293], depending on which working hypotheses one adopts for human versus cosmic impact. The very act of working on the science of biofuel production puts scientists directly into a position to influence policy [294,295]. Milking approaches to biofuels may alter their economics, increasing their presently low [296] EROI (energy return on investment). One hour of sunshine falling on Earth is equivalent to a year’s worth of human energy consumption, so there is plenty of room for maneuvering, including the prospect of achieving artificial photosynthesis [297,298]. Perhaps we shall someday milk artificial diatoms.
To date, the Chlorophyta algae have been the subjects of most biofuel research. Genera such as Chlorella, Botryococcus, Scenedesmus, Neochloris, and Chlamydomonas are the typically researched organisms. Research using Scenedesmus obliquus has yielded phenomenal increases of oil by manipulating chemical and physical factors. For instance, Mandal and Mallick [299] were able to increase the oil content by 43% (cf. [179]). Algal fuels based on diatoms are beginning to show promise [27,90,157,158,176,300,301], which is reasonable, given their contribution to natural oil deposits [59]. Thus diatoms have the ability to produce large amounts of oil. For example, planktonic diatoms are reported to produce lipids in quantities up to 40% dry cell weight [302]. Cultured marine diatoms had 30%–45% dry weight as lipids [303]. The amount of oil in a single diatom is reported as up to 25% of the algal biomass [90] and could perhaps reach 60% of the nonsilica diatom dry mass [27]. For some diatom species, the amount of oil that could be produced has been projected to reach up to 200 times more oil per hectare than soybeans (reviewed in [27]). Diatoms also have the ability to reproduce quickly and can create very large biovolumes. Diatoms can reproduce and double in as little as five hours under laboratory conditions, and in as little as two days in the environment [27]. How fast milked, stationary diatom cultures will replace their oil is as yet untested.
There is no “puzzling underrepresentation of diatoms in the microalgal biofuels arena” [90], as they have been a focus since 1978 [204]. Biodiesel from microalgae or third generation renewable biofuels could meet the demand [55,56] because they are projected to be capable of generating up to 6–200 times more crude oil per surface area than higher plants [27,57,58]. CO2 will be available either from the atmosphere or directly from industries. However, our calculations here indicate that there is not enough industrial CO2 waste to convert to biofuel to replace fossil energies used for transport.
Unlike crops like rice, wheat, corn, or sugarcane, which when used for ethanol biodiesels compete with food production, algae can be grown without impacting food production [166]. Algae also have other advantages: (a) algae can be cultivated on non-arable land; (b) they can use non-potable water such as municipal/agricultural waste, which also helps to bioremediate the water [20,21,22,23,24,76,165,197,297,304,305,306,307,308]; and (c) they produce a high per-acre yield [189,303,304].
In a recent technico-economic analysis of alga-derived biofuel under both technical and economic uncertainties associated with the development of biorefinery processes, Brownbridge et al. [309] concluded that it is unlikely that algal biodiesel as a primary product can be commercially feasible unless other HVM products are also extracted as exemplified by the new mobile algae refinery [310]. However, it is a well-known engineering dictum that it is difficult to maximize more than one function simultaneously without compromise [311]. Therefore, a better approach is to concentrate on optimizing one function only: for instance, milking diatoms or other algae for biofuel production. Microalga HVM come from the cellular process of photosynthesis and carbon metabolism. Many basic questions about microalgae, such as how the complex, interconnecting metabolic and regulation networks work, are as yet still unanswered. Getting this information requires not only genomic and transcriptomic analyses, but also more integrated omics analyses [236].
Acknowledgments
V.V. would like to thank Department of Biotechnology, Ministry of Science and Technology, Government of India for the financial support. K.M. would like to express gratitude to the many students measuring biovolumes and lipid droplets in her lab. R.G. thanks the Gulf Specimen Aquarium & Marine Laboratory and Birds Hill Provincial Park for congenial natural environments for writing. B.S., J.M., H.G. thank the University of Le Mans for their financial support. H.G. thanks the “Le Mans Métropole” and “Le Conseil Général de la Sarthe” for funding her PhD research. V.B., G.P. and J.H. thank the University of Le Mans and the Mayenne Council for financial support.
Abbreviations
- DW
dry weight
- EPA
Eicosapentaenoic acid
- EROI
energy return on investment
- FAE
Fatty Acid Ester-type
- FT-IR
Fast-Fourier InfraRed spectroscopy
- HVM
high value molecules
- LED
light emitting diode
- PBR
photobioreactor
- PDMS
polydimethylsiloxane
- PEF
pulsed electric field
- PUFA
PolyUnsaturated Fatty Acids
- SE
standard error
- UVC
ultraviolet C band
- TAG
TriAcylGlycerol
Supplementary Information
Supplementary Table S1: Diatom taxa reported per site with samples from 10 different locations in Georgia.
Supplementary Data S2: Measurement of lipid droplets.
Author Contributions
Conceived and designed the manuscript: R.G., B.S. Performed the experiments: K.M.M. Analyzed the data: K.M.M. Wrote the paper: V.V., K.M.M, H.G., V.B., J.H., G.P., J.M., R.G., B.S.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Alexandratos N., Bruinsma J. World Agriculture towards 2030/2050: The 2012 Revision. FAO; Rome, Italy: 2012. ESA Working paper Rome. [Google Scholar]
- 2.Mimouni V., Ulmann L., Pasquet V., Mathieu M., Picot L., Bougaran G., Cadoret J.-P., Morant-Manceau A., Schoefs B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012;13:2733–2750. doi: 10.2174/138920112804724828. [DOI] [PubMed] [Google Scholar]
- 3.Schoefs B. Chlorophyll and carotenoid analysis in food products. A practical case-by-case view. TrAC Trends Anal. Chem. 2003;22:335–339. doi: 10.1016/S0165-9936(03)00602-2. [DOI] [Google Scholar]
- 4.Plaza M., Herrero M., Cifuentes A., Ibanez E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009;57:7159–7170. doi: 10.1021/jf901070g. [DOI] [PubMed] [Google Scholar]
- 5.Chisti Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008;26:126–131. doi: 10.1016/j.tibtech.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Drum R.W., Gordon R. Star Trek replicators and diatom nanotechnology. Trends Biotechnol. 2003;21:325–328. doi: 10.1016/S0167-7799(03)00169-0. [DOI] [PubMed] [Google Scholar]
- 7.Gordon R., Sterrenburg F.A.S., Sandhage K. A Special Issue on Diatom Nanotechnology. J. Nanosci. Nanotechnol. 2005;5:1–4. doi: 10.1166/jnn.2005.017. [DOI] [Google Scholar]
- 8.Gordon R., Losic D., Tiffany M.A., Nagy S.S., Sterrenburg F.A.S. The Glass Menagerie: Diatoms for novel applications in nanotechnology. Trends Biotechnol. 2009;27:116–127. doi: 10.1016/j.tibtech.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Gordon R. Diatoms and nanotechnology: Early history and imagined future as seen through patents. In: Smol J.P., Stoermer E.F., editors. The Diatoms: Applications for the Environmental and Earth Sciences. Volume 2. Cambridge University Press; Cambridge, UK: 2010. pp. 585–602. [Google Scholar]
- 10.Gordon R., Seckbach J. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. [Google Scholar]
- 11.Field C.B., Behrenfeld M.J., Randerson J.T., Falkowski P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science. 1998;281:237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 12.Williams P.J.le B., Laurens L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010;3:554–590. doi: 10.1039/b924978h. [DOI] [Google Scholar]
- 13.Lemoine Y., Schoefs B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010;106:155–177. doi: 10.1007/s11120-010-9583-3. [DOI] [PubMed] [Google Scholar]
- 14.Heydarizadeh P., Poirier I., Loizeau D., Ulmann L., Mimouni V., Schoefs B., Bertrand M. Plastids of marine phytoplankton produce bioactive pigments and lipids. Mar. Drugs. 2013;11:3425–3471. doi: 10.3390/md11093425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spolaore P., Joannis-Cassan C., Duran E., Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006;101:87–96. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- 16.Leu S., Boussiba S. Advances in the production of high-value products by microalgae. Ind. Biotechnol. 2014;10:169–183. doi: 10.1089/ind.2013.0039. [DOI] [Google Scholar]
- 17.Priyadarshani I., Rath B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012;3:89–100. [Google Scholar]
- 18.Henrikson R., Edwards M. Imagine Our Algae Future: Visionary Algae Architecture and Landscape Designs: International Algae Competition. CreateSpace Independent Publishing Platform; Seattle, WA, USA: 2012. [Google Scholar]
- 19.Henrikson R., Edwards M. Algae Microfarms: For Home, School, Community and Urban Gardens, Rooftop, Mobile and Vertical Farms and Living Buildings. CreateSpace Independent Publishing Platform; Seattle, WA, USA: 2013. [Google Scholar]
- 20.Oswald W.J. Micro-algae and waste-water treatment. In: Borowitzka M.A., Borowitzka L.J., editors. Micro-Algal Biotechnology. Cambridge University Press; New York, NY, USA: 1988. pp. 305–328. [Google Scholar]
- 21.Oswald W.J. The role of microalgae in liquid waste treatment and reclamation. In: Lembi C.A., Waaland J.R., editors. Algae and Human Affairs. Cambridge University Press; Cambridge, UK: 1988. pp. 255–281. [Google Scholar]
- 22.De la Noüe J., Laliberté G., Proulx D. Algae and wastewater. J. Appl. Phycol. 1992;4:247–254. doi: 10.1007/BF02161210. [DOI] [Google Scholar]
- 23.Shilton A.N., Powell N., Guieysse B. Plant based phosphorus recovery from wastewater via algae and macrophytes. Curr. Opin. Biotechnol. 2012;23:884–889. doi: 10.1016/j.copbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandra T.V., Madhab M.D., Shilpi S., Joshi N.V. Algal biofuel from urban wastewater in India: Scope and challenges. Renew. Sust. Energ. Rev. 2013;21:767–777. doi: 10.1016/j.rser.2012.12.029. [DOI] [Google Scholar]
- 25.Koussa J., Chaiboonchoe A., Salehi-Ashtiani K. Computational approaches for microalgal biofuel optimization: A review. BioMed Res. Int. 2014;2014:649453. doi: 10.1155/2014/649453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitan O., Dinamarca J., Hochman G., Falkowski P.G. Diatoms: A fossil fuel of the future. Trends Biotechnol. 2014;32:117–124. doi: 10.1016/j.tibtech.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandra T.V., Mahapatra D.M., Karthick B., Gordon R. Milking diatoms for sustainable energy: Biochemical engineering versus gasoline-secreting diatom solar panels. Ind. Eng. Chem. Res. 2009;48:8769–8788. doi: 10.1021/ie900044j. [DOI] [Google Scholar]
- 28.Metting F.B., Jr., Rayburn W.R., Reynaud P.A. Algae and agriculture. In: Lembi C.A., Waaland J.R., editors. Algae and Human Affairs. Cambridge University Press; Cambridge, UK: 1988. pp. 335–370. [Google Scholar]
- 29.Li S., Glombitza K.W., Koch M. Phlorotannins from the brown alga Carpophyllum maschalocarpum. Planta Med. 1989;55:610–611. doi: 10.1055/s-2006-962160. [DOI] [PubMed] [Google Scholar]
- 30.Rengasamy K.R.R., Kulkarni M.G., Stirk W.A., van Staden J. Advances in algal drug research with emphasis on enzyme inhibitors. Biotechnol. Adv. 2014;32:1364–1381. doi: 10.1016/j.biotechadv.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Becker E.W. Nutritional properties of microalgae: Potentials and constraints. In: Richmond A., editor. CRC Handbook of Microalgal Mass Culture. CRC Press; Boca Raton, FL, USA: 1986. pp. 339–419. [Google Scholar]
- 32.Klok A.J., Lamers P.P., Martens D.E., Draaisma R.B., Wijffels R.H. Edible oils from microalgae: Insights in TAG accumulation. Trends Biotechnol. 2014;32:521–528. doi: 10.1016/j.tibtech.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Lane K., Derbyshire E., Li W., Brennan C. Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: A review of the literature. Crit. Rev. Food Sci. Nutr. 2014;54:572–579. doi: 10.1080/10408398.2011.596292. [DOI] [PubMed] [Google Scholar]
- 34.De Pauw N., Persoone G. Micro-algae for aquaculture. In: Borowitzka M.A., Borowitzka L.J., editors. Micro-Algal Biotechnology. Cambridge University Press; New York, NY, USA: 1988. pp. 197–221. [Google Scholar]
- 35.Benemann J.R. Microalgae aquaculture feeds. J. Appl. Phycol. 1992;4:233–245. doi: 10.1007/BF02161209. [DOI] [Google Scholar]
- 36.Becker E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007;25:207–210. doi: 10.1016/j.biotechadv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Hawkins R.L., Nakamura M. Expression of human growth hormone by the eukaryotic alga, Chlorella. Curr. Microbiol. 1999;38:335–341. doi: 10.1007/PL00006813. [DOI] [PubMed] [Google Scholar]
- 38.Gregory J.A., Mayfield S.P. Developing inexpensive malaria vaccines from plants and algae. Appl. Microbiol. Biotechnol. 2014;98:1983–1990. doi: 10.1007/s00253-013-5477-6. [DOI] [PubMed] [Google Scholar]
- 39.Rosales-Mendoza S. Future directions for the development of Chlamydomonas-based vaccines. Expert Rev. Vaccines. 2013;12:1011–1019. doi: 10.1586/14760584.2013.825455. [DOI] [PubMed] [Google Scholar]
- 40.Pérez-López P., González-García S., Ulloa R.G., Sineiro J., Feijoo G., Teresa Moreira M. Life cycle assessment of the production of bioactive compounds from Tetraselmis suecica at pilot scale. J. Cleaner Prod. 2014;64:323–331. doi: 10.1016/j.jclepro.2013.07.028. [DOI] [Google Scholar]
- 41.Moulin P., Lemoine Y., Schoefs B. Modifications of the carotenoid metabolism in plastids: A response to stress conditions. In: Pessarakli M., editor. Handbook of Plant and Crop Stress. 3rd ed. CRC Press; Boca Raton, FL, USA: 2010. pp. 407–433. [Google Scholar]
- 42.Li Y., Horsman M., Wu N., Lan C.Q., Dubois-Calero N. Biofuels from microalgae. Biotechnol. Prog. 2008;24:815–820. doi: 10.1021/bp070371k. [DOI] [PubMed] [Google Scholar]
- 43.Garibay-Hernández A., Vazquez-Duhalt R., Serrano-Carreón L., Martinez A. Nitrogen limitation in Neochloris oleoabundans: A reassessment of its effect on cell growth and biochemical composition. Appl. Biochem. Biotechnol. 2013;171:1775–1791. doi: 10.1007/s12010-013-0454-1. [DOI] [PubMed] [Google Scholar]
- 44.Ge Y., Liu J., Tian G. Growth characteristics of Botryococcus braunii 765 under high CO2 concentration in photobioreactor. Bioresour. Technol. 2011;102:130–134. doi: 10.1016/j.biortech.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 45.Illman A.M., Scragg A.H., Shales S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Technol. 2000;27:631–635. doi: 10.1016/S0141-0229(00)00266-0. [DOI] [PubMed] [Google Scholar]
- 46.Scragg A.H., Illman A.M., Carden A., Shales S.W. Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass Bioenergy. 2002;23:67–73. doi: 10.1016/S0961-9534(02)00028-4. [DOI] [Google Scholar]
- 47.Liang Y., Sarkany N., Cui Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009;31:1043–1049. doi: 10.1007/s10529-009-9975-7. [DOI] [PubMed] [Google Scholar]
- 48.Li Z., Yuan H., Yang J., Li B. Optimization of the biomass production of oil algae Chlorella minutissima UTEX2341. Bioresour. Technol. 2011;102:9128–9134. doi: 10.1016/j.biortech.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Wen Z.Y., Chen F. Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol. Adv. 2003;21:273–294. doi: 10.1016/S0734-9750(03)00051-X. [DOI] [PubMed] [Google Scholar]
- 50.Bromke M.A., Giavalisco P., Willmitzer L., Hesse H. Metabolic analysis of adaptation to short-term changes in culture conditions of the marine diatom Thalassiosira pseudonana. PLoS ONE. 2013;8:e67340. doi: 10.1371/journal.pone.0067340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ethier S., Woisard K., Vaughan D., Wen Z. Continuous culture of the microalgae Schizochytrium limacinum on biodiesel-derived crude glycerol for producing docosahexaenoic acid. Bioresour. Technol. 2011;102:88–93. doi: 10.1016/j.biortech.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Deschamps P., Guillebeault D., Devassine J., Dauvillée D., Haebel S., Steup M., Buléon A., Putaux J.-L., Slomianny M.-C., Colleoni C., et al. The heterotrophic dinoflagellate Crypthecodinium cohnii defines a model genetic system to investigate cytoplasmic starch synthesis. Eukaryot. Cell. 2008;7:872–880. doi: 10.1128/EC.00461-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma K.K., Schuhmann H., Schenk P.M. High lipid induction in microalgae for biodiesel production. Energies. 2012;5:1532–1553. doi: 10.3390/en5051532. [DOI] [Google Scholar]
- 54.Pal D., Khozin-Goldberg I., Cohen Z., Boussiba S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011;90:1429–1441. doi: 10.1007/s00253-011-3170-1. [DOI] [PubMed] [Google Scholar]
- 55.Cadoret J.-P., Bernard O. La production de biocarburant lipidique avec des microalgues: Promesses et defis/Lipid biofuel production with microalgae: Potential and challenges. J. Soc. Biol. 2008;202:201–211. doi: 10.1051/jbio:2008022. [DOI] [PubMed] [Google Scholar]
- 56.Chisti Y. Biodiesel from microalgae. Biotechnol. Adv. 2007;25:294–306. doi: 10.1016/j.biotechadv.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Hu Q., Sommerfeld M., Jarvis E., Ghirardi M., Posewitz M., Seibert M., Darzins A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008;54:621–639. doi: 10.1111/j.1365-313X.2008.03492.x. [DOI] [PubMed] [Google Scholar]
- 58.Demirbas A. Progress and recent trends in biodiesel fuels. Energy Conv. Manag. 2009;50:14–34. doi: 10.1016/j.enconman.2008.09.001. [DOI] [Google Scholar]
- 59.Shukla S.K., Mohan R. The contribution of diatoms to worldwide crude oil deposits. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 355–382. [Google Scholar]
- 60.Smetacek V. Diatoms and the ocean carbon cycle. Protist. 1999;150:25–32. doi: 10.1016/S1434-4610(99)70006-4. [DOI] [PubMed] [Google Scholar]
- 61.Wu Y., Campbell D.A., Irwin A.J., Suggett D.J., Finkel Z.V. Ocean acidification enhances the growth rate of larger diatoms. Limnol. Oceanogr. 2014;59:1027–1034. doi: 10.4319/lo.2014.59.3.1027. [DOI] [Google Scholar]
- 62.Hejazi M.A., Wijffels R.H. Milking of microalgae. Trends Biotechnol. 2004;22:189–194. doi: 10.1016/j.tibtech.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Orosa M., Franqueira D., Cid A., Abalde J. Analysis and enhancement of astaxanthin accumulation in Haematococcus pluvialis. Bioresour. Technol. 2005;96:373–378. doi: 10.1016/j.biortech.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Kamath B.S., Vidhyavathi R., Sarada R., Ravishankar G.A. Enhancement of carotenoids by mutation and stress induced carotenogenic genes in Haematococcus pluvialis mutants. Bioresour. Technol. 2008;99:8667–8673. doi: 10.1016/j.biortech.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 65.Bahadar A., Khan M.B. Progress in energy from microalgae: A review. Renew. Sust. Energy Rev. 2013;27:128–148. doi: 10.1016/j.rser.2013.06.029. [DOI] [Google Scholar]
- 66.Greenwell H.C., Laurens L.M.L., Shields R.J., Lovitt R.W., Flynn K.J. Placing microalgae on the biofuels priority list: A review of the technological challenges. J. R. Soc. Interface. 2010;7:703–726. doi: 10.1098/rsif.2009.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang F., Cheng L.H., Xu X.H., Zhang L., Chen H.L. Screening of biocompatible organic solvents for enhancement of lipid milking from Nannochloropsis sp. Process Biochem. 2011;46:1934–1941. doi: 10.1016/j.procbio.2011.06.024. [DOI] [Google Scholar]
- 68.Mercer P., Armenta R.E. Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol. 2011;113:539–547. doi: 10.1002/ejlt.201000455. [DOI] [Google Scholar]
- 69.Benemann J.R., Weissman J.C., Koopman B.L., Oswald W.J. Energy production by microbial photosynthesis. Nature. 1977;268:19–23. doi: 10.1038/268019a0. [DOI] [Google Scholar]
- 70.De Boer K., Moheimani N.R., Borowitzka M.A., Bahri P.A. Extraction and conversion pathways for microalgae to biodiesel: A review focused on energy consumption. J. Appl. Phycol. 2012;24:1681–1698. doi: 10.1007/s10811-012-9835-z. [DOI] [Google Scholar]
- 71.Montagne X., Porot P., Aymard C., Querleu C., Bouter A., Lorne D., Cadoret J.-P., Lombaert-Valot I., Petillon O. Algogroup: Towards a shared vision of the possible deployment of algae to biofuels. Oil Gas Sci. Technol. 2013;68:875–898. doi: 10.2516/ogst/2013164. [DOI] [Google Scholar]
- 72.Ahmed F., Li Y., Schenk P.M. Algal biorefinery: Sustainable production of biofuels and aquaculture feed? In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 21–42. [Google Scholar]
- 73.Bajhaiya A.K., Suseela M.R., Ramteka P.W. Approaches and prospectives for algal fuel. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 269–282. [Google Scholar]
- 74.Pattarkine M., Pattarkine V.M. Nanotechnology for algal biofuels. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 147–164. [Google Scholar]
- 75.Riosmena-Rodríguez R., Arredondo-Vega B.O., Reynoso-Granados T., Cordoba M., López-Vivas J.M., López-Calderon J.M. Approaches to and perspectives on biodiesel and oil production using algae in Mexico. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 269–282. [Google Scholar]
- 76.Craggs R.J., Lundquist T., Benemann J. Wastewater treatment pond algal production for biofuel. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 425–446. [Google Scholar]
- 77.Kumar S. Sub-and supercritical water based processes for microalgae to biofuels. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 467–494. [Google Scholar]
- 78.Richardson J.W., Johnson M.D., Outlaw J.L. Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res. Biomass Biofuels Bioprod. 2012;1:93–100. [Google Scholar]
- 79.Lane J. Joule’s quest for fuels from CO2, sunlight and water. [(accessed on 21 April 2015)]. Available online: http://www.biofuelsdigest.com/bdigest/2014/07/03/joules-quest-for-fuels-from-co2-sunlight-and-water/
- 80.Wilkinson S. Curb global warming: Make waste CO2 into fuel. Chem. Eng. News. 1997;75:6. doi: 10.1021/cen-v075n033.p006. [DOI] [Google Scholar]
- 81.Jiang Z., Xiao T., Kuznetsov V.L., Edwards P.P. Turning carbon dioxide into fuel. Phil. Trans. R. Soc. Lond. A: Math. Phys. Eng. Sci. 2010;368:3343–3364. doi: 10.1098/rsta.2010.0119. [DOI] [PubMed] [Google Scholar]
- 82.Ladd C., Venter J.C. 10 Big Questions for Maverick Geneticist J. Craig Venter on America’s Energy Future. [(accessed on 8 August 2010)]. Available online: http://www.popularmechanics.com/blogs/science_news/4275738.html.
- 83.Wang X.W., Liang J.R., Luo C.S., Chen C.P., Gao Y.H. Biomass, total lipid production, and fatty acid composition of the marine diatom Chaetoceros muelleri in response to different CO2 levels. Bioresour. Technol. 2014;161:124–130. doi: 10.1016/j.biortech.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 84.Van der Hoeven M. CO2 Emissions from Fuel Combustion: Highlights. 2013 ed. International Energy Agency; Paris, France: 2013. [Google Scholar]
- 85.Moheimani N. Microalgae Culture (3) Algae R&D Center, Murdoch University; Perth, Australia: 2014. [Google Scholar]
- 86.Frenz J., Largeau C., Casadevall E. Hydrocarbon recovery by extraction with a biocompatible solvent from free and immobilized cultures of Botryococcus braunii. Enzyme Microb. Technol. 1989;11:717–724. doi: 10.1016/0141-0229(89)90120-8. [DOI] [Google Scholar]
- 87.Zhang F., Cheng L.-H., Xu X.-H., Zhang L., Chen H.-L. Application of membrane dispersion for enhanced lipid milking from Botryococcus braunii FACHB 357. J. Biotechnol. 2013;165:22–29. doi: 10.1016/j.jbiotec.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 88.Rickman M., Davis R.H., Pellegrino J. Fractionation of organic fuel precursors from electrolytes with membranes. Ind. Eng. Chem. Res. 2013;52:10530–10539. doi: 10.1021/ie4008908. [DOI] [Google Scholar]
- 89.Yadugiri V.T. Milking diatoms—A new route to sustainable energy. Curr. Sci. 2009;97:748–750. [Google Scholar]
- 90.Hildebrand M., Davis A.K., Smith S.R., Traller J.C., Abbriano R. The place of diatoms in the biofuels industry. Biofuels. 2012;3:221–240. doi: 10.4155/bfs.11.157. [DOI] [Google Scholar]
- 91.Ho S.-H., Ye X., Hasunuma T., Chang J.-S., Kondo A. Perspectives on engineering strategies for improving biofuel production from microalgae—A critical review. Biotechnol. Adv. 2014;32:1448–1459. doi: 10.1016/j.biotechadv.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 92.Ciesielski A., Limited R.T. An Introduction to Rubber Technology. Rapra Technology Limited; Shrewsbury, UK: 1999. [Google Scholar]
- 93.Svanberg I., Sõukand R., Łuczaj Ł., Kalle R., Zyryanova O., Dénes A., Papp N., Nedelcheva A., Šeškauskaite D., Kołodziejska-Degórska I., et al. Uses of tree saps in northern and eastern parts of Europe. Acta Soc. Bot. Pol. 2012;81:343–357. doi: 10.5586/asbp.2012.036. [DOI] [Google Scholar]
- 94.Nearing H., Nearing S. The Maple Sugar Book: Together with Remarks on Pioneering as a Way of Living in the Twentieth Century. Chelsea Green Publishing; White River Junction, VT, USA: 2000. [Google Scholar]
- 95.Munson P.J. Still more on the antiquity of maple sugar and syrup in aboriginal eastern North America. J. Ethnobiol. 1989;9:159–170. [Google Scholar]
- 96.Haller J.S., Jr. Sampson of the terebinthinates: Medical history of turpentine. South. Med. J. 1984;77:750–754. doi: 10.1097/00007611-198406000-00021. [DOI] [PubMed] [Google Scholar]
- 97.Sauer T., Galinski E.A. Bacterial milking: A novel bioprocess for production of compatible solutes. Biotechnol. Bioeng. 1998;57:306–313. [PubMed] [Google Scholar]
- 98.Van-Thuoc D., Guzmán H., Quillaguamán J., Hatti-Kaul R. High productivity of ectoines by Halomonas boliviensis using a combined two-step fed-batch culture and milking process. J. Biotechnol. 2010;147:46–51. doi: 10.1016/j.jbiotec.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 99.Gillet J.-N. Ultrafast molecular dynamics of biofuel extraction for microalgae and bacteria milking: Blocking membrane folding pathways to damaged lipid-bilayer conformations with nanomicelles. J. Biomol. Struct. Dyn. 2015;33:690–705. doi: 10.1080/07391102.2014.907544. [DOI] [PubMed] [Google Scholar]
- 100.Zhang F., Cheng L.H., Xu X.H., Zhang L., Chen H.L. Technologies of microalgal harvesting and lipid extraction. Prog. Chem. 2012;24:2062–2072. [Google Scholar]
- 101.Wijffels R.H., Barbosa M.J. An outlook on microalgal biofuels. Science. 2010;329:796–799. doi: 10.1126/science.1189003. [DOI] [PubMed] [Google Scholar]
- 102.Ghasemi Naghdi F., Thomas-Hall S.R., Durairatnam R., Pratt S., Schenk P.M. Comparative effects of biomass pre-treatments for direct and indirect transesterification to enhance microalgal lipid recovery. Front. Energy Res. 2014;2:57. doi: 10.3389/fenrg.2014.00057. [DOI] [Google Scholar]
- 103.Ganeva V., Galutzov B., Teissie J. High yield electroextraction of proteins from yeast by a flow process. Anal. Biochem. 2003;315:77–84. doi: 10.1016/S0003-2697(02)00699-1. [DOI] [PubMed] [Google Scholar]
- 104.Coustets M., Al-Karablieh N., Thomsen C., Teissie J. Flow process for electroextraction of total proteins from microalgae. J. Membr. Biol. 2013;246:751–760. doi: 10.1007/s00232-013-9542-y. [DOI] [PubMed] [Google Scholar]
- 105.Coustets M., Joubert-Durigneux V., Hérault J., Schoefs B., Blanckaert V., Garnier J.-P., Teissié J. Optimization of protein electroextraction from microalgae by a flow process. Bioelectrochemistry. 2015;103:74–81. doi: 10.1016/j.bioelechem.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 106.Reep P., Green M.P. Procedure for extracting of lipids from algae without cell sacrifice. 20120040428 A1. US Patent. 2012 Feb 16;
- 107.Gateau H., Marchand J., Schoefs B. Abstracts, Functional Studies on Model Organisms (EFOR), 6th Annual Meeting, Paris, France. EFOR; Paris, France: 2015. Pulse electric fields allow the biocompatible extraction of molecules from the microalgae Haematococcus pluvialis. [Google Scholar]
- 108.Sheng J., Vannela R., Rittmann B.E. Evaluation of cell-disruption effects of pulsed-electric-field treatment of Synechocystis PCC 6803. Environ. Sci. Technol. 2011;45:3795–3802. doi: 10.1021/es103339x. [DOI] [PubMed] [Google Scholar]
- 109.Vinayak V., Gordon R., Gautam S., Rai A. Discovery of a diatom that oozes oil. Adv. Sci. Lett. 2014;20:1256–1267. doi: 10.1166/asl.2014.5591. [DOI] [Google Scholar]
- 110.Tsukagoshi N., Yoshida H., Katsurayama M., Udaka S. Uncoupled release of protein and lipid in a protein-secreting bacterium, Bacillus brevis-47. Biochim. Biophys. Acta. 1983;759:278–285. doi: 10.1016/0304-4165(83)90324-0. [DOI] [PubMed] [Google Scholar]
- 111.Raetz C.R.H. Biosynthesis, secretion and function of lipid A in Gram-negative bacteria. Glycobiology. 2001;11:872–872. [Google Scholar]
- 112.Wald M.L. Biotech Company to Patent Fuel-Secreting Bacterium. [(accessed on 21 April 2015)]. Available online: http://www.nytimes.com/2010/09/14/science/earth/14fuel.html.
- 113.Liu X., Sheng J., Curtiss R., III Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. USA. 2011;108:6899–6904. doi: 10.1073/pnas.1103014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.MB-BigB Joule Unlimited’s bacteria secretes diesel fuel. [(accessed on 29 August 2012)]. Available online: http://www.alt-energy.info/biofuel/joule-unlimiteds-bacteria-secretes-diesel-fuel/
- 115.Robertson D.E., Jacobson S.A., Morgan F., Berry D., Church G.M., Afeyan N.B. A new dawn for industrial photosynthesis. Photosynth. Res. 2011;107:269–277. doi: 10.1007/s11120-011-9631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Araujo G.S., Matos L.J., Fernandes J.O., Cartaxo S.J., Goncalves L.R., Fernandes F.A., Farias W.R. Extraction of lipids from microalgae by ultrasound application: Prospection of the optimal extraction method. Ultrason. Sonochem. 2013;20:95–98. doi: 10.1016/j.ultsonch.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 117.Ranjan A., Patil C., Moholkar V.S. Mechanistic assessment of microalgal lipid extraction. Ind. Eng. Chem. Res. 2010;49:2979–2985. doi: 10.1021/ie9016557. [DOI] [Google Scholar]
- 118.Rajasekhar P., Fan L., Thang N., Roddick F.A. Impact of sonication at 20 kHz on Microcystis aeruginosa, Anabaena circinalis and Chlorella sp. Water Res. 2012;46:1473–1481. doi: 10.1016/j.watres.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 119.Joyce E.M., King P.M., Mason T.J. The effect of ultrasound on the growth and viability of microalgae cells. J. Appl. Phycol. 2014;26:1741–1748. doi: 10.1007/s10811-013-0202-5. [DOI] [Google Scholar]
- 120.Broekman S., Pohlmann O., Beardwood E.S., de Meulenaer E.C. Ultrasonic treatment for microbiological control of water systems. Ultrason. Sonochem. 2010;17:1041–1048. doi: 10.1016/j.ultsonch.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 121.Doshi R., Tuan N., Chang G. Transporter-mediated biofuel secretion. Proc. Natl. Acad. Sci. USA. 2013;110:7642–7647. doi: 10.1073/pnas.1301358110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dunlop M.J., Dossani Z.Y., Szmidt H.L., Chu H.C., Lee T.S., Keasling J.D., Hadi M.Z., Mukhopadhyay A. Engineering microbial biofuel tolerance and export using efflux pumps. Mol. Syst. Biol. 2011;7:487. doi: 10.1038/msb.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dunahay T.G., Jarvis E.E., Zeiler K.G., Roessler P.G., Brown L.M. Genetic engineering of microalgae for fuel production: Scientific note. Appl. Biochem. Biotechnol. 1992;34–5:331–339. doi: 10.1007/BF02920556. [DOI] [Google Scholar]
- 124.Coll J.M. Methodologies for transferring DNA into eukaryotic microalgae. Span. J. Agric. Res. 2006;4:316–330. doi: 10.5424/sjar/2006044-209. [DOI] [Google Scholar]
- 125.León R., Fernández E. Nuclear transformation of eukaryotic microalgae: Historical overview, achievements and problems. Adv. Exp. Med. Biol. 2007;616:1–11. doi: 10.1007/978-0-387-75532-8_1. [DOI] [PubMed] [Google Scholar]
- 126.Miyahara M., Aoi M., Inoue-Kashino N., Kashino Y., Ifuku K. Highly efficient transformation of the diatom Phaeodactylum tricornutum by multi-pulse electroporation. Biosci. Biotechnol. Biochem. 2013;77:874–876. doi: 10.1271/bbb.120936. [DOI] [PubMed] [Google Scholar]
- 127.Li F.J., Gao D.W., Hu H.H. High-Efficiency nuclear transformation of the oleaginous marine Nannochloropsis species using PCR product. Biosci. Biotechnol. Biochem. 2014;78:812–817. doi: 10.1080/09168451.2014.905184. [DOI] [PubMed] [Google Scholar]
- 128.Zhang C.Y., Hu H.H. High-efficiency nuclear transformation of the diatom Phaeodactylum tricornutum by electroporation. Mar. Genom. 2014;16:63–66. doi: 10.1016/j.margen.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 129.Stirke A., Zimkus A., Balevicius S., Stankevic V., Ramanaviciene A., Ramanavicius A., Zurauskiene N. Permeabilization of yeast Saccharomyces cerevisiae cell walls using nanosecond high power electrical pulses. Appl. Phys. Lett. 2014;105:253701. doi: 10.1063/1.4905034. [DOI] [Google Scholar]
- 130.Joubert V., Cheype C., Bonnet J., Packan D., Garnier J.-P., Teissie J., Blanckaert V. Inactivation of Bacillus subtilis var. niger of both spore and vegetative forms by means of corona discharges applied in water. Water Res. 2013;47:1381–1389. doi: 10.1016/j.watres.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 131.Antezana Zbinden M.D., Sturm B.S.M., Nord R.D., Carey W.J., Moore D., Shinogle H., Stagg-Williams S.M. Pulsed electric field (PEF) as an intensification pretreatment for greener solvent lipid extraction from microalgae. Biotechnol. Bioeng. 2013;110:1605–1615. doi: 10.1002/bit.24829. [DOI] [PubMed] [Google Scholar]
- 132.Deipolyi A.R., Golberg A., Yarmush M.L., Arellano R.S., Oklu R. Irreversible electroporation: Evolution of a laboratory technique in interventional oncology. Diagn. Interv. Radiol. 2014;20:147–154. doi: 10.5152/dir.2013.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sixou S., Teissié J. Specific electropermeabilization of leukocytes in a blood sample and application to large volumes of cells. Biochim. Biophys. Acta. 1990;1028:154–160. doi: 10.1016/0005-2736(90)90149-I. [DOI] [PubMed] [Google Scholar]
- 134.Bellard E., Teissié J. Double-pulse approach of electrogenotherapy: An analysis at the single cell level. IEEE Trans. Plasma Sci. 2009;37:538–544. doi: 10.1109/TPS.2009.2014954. [DOI] [Google Scholar]
- 135.Rols M.P., Teissie J. Ionic-strength modulation of electrically induced permeabilization and associated fusion of mammalian cells. Eur. J. Biochem. 1989;179:109–115. doi: 10.1111/j.1432-1033.1989.tb14527.x. [DOI] [PubMed] [Google Scholar]
- 136.Liu X., Fallon S., Sheng J., Curtiss R., III CO2-limitation-inducible Green Recovery of fatty acids from cyanobacterial biomass. Proc. Natl. Acad. Sci. USA. 2011;108:6905–6908. doi: 10.1073/pnas.1103016108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reppas N.B., Ridley C.P. Methods and Compositions for the Recombinant Biosynthesis of N-Alkanes. 7,794,969. United States Patent. 2010 Sep 14;
- 138.Ozkan A., Berberoglu H. Physico-chemical surface properties of microalgae. Colloids Surf. B Biointerfaces. 2013;112:287–293. doi: 10.1016/j.colsurfb.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 139.Ruen-ngam D., Shotipruk A., Pavasant P. Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2010;46:64–70. doi: 10.1080/01496395.2010.493546. [DOI] [Google Scholar]
- 140.Zou T.-B., Jia Q., Li H.-W., Wang C.-X., Wu H.-F. Response surface methodology for ultrasound-assisted extraction of astaxanthin from Haematococcus pluvialis. Mar. Drugs. 2013;11:1644–1655. doi: 10.3390/md11051644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kong W., Liu N., Zhang J., Yang Q., Hua S., Song H., Xia C. Optimization of ultrasound-assisted extraction parameters of chlorophyll from Chlorella vulgaris residue after lipid separation using response surface methodology. J. Food Sci. Technol.-Mysore. 2014;51:2006–2013. doi: 10.1007/s13197-012-0706-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Macias-Sánchez M.D., Mantell C., Rodríguez M., Martinez de la Ossa E., Lubián L.M., Montero O. Comparison of supercritical fluid and ultrasound-assisted extraction of carotenoids and chlorophyll a from Dunaliella salina. Talanta. 2009;77:948–952. doi: 10.1016/j.talanta.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 143.Pasquet V., Chérouvrier J.-R., Farhat F., Thiéry V., Piot J.-M., Bérard J.-B., Kaas R., Serive B., Patrice T., Cadoret J.-P., et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011;46:59–67. doi: 10.1016/j.procbio.2010.07.009. [DOI] [Google Scholar]
- 144.Roselló-Soto E., Galanakis C.M., Brnčić M., Orlien V., Trujillo F.J., Mawson R., Knoerzer K., Tiwari B.K., Barba F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015;42:134–149. doi: 10.1016/j.tifs.2015.01.002. [DOI] [Google Scholar]
- 145.Sterrenburg F.A.S., Gordon R., Tiffany M.A., Nagy S.S. Diatoms: Living in a constructal environment. In: Seckbach J., editor. Algae and Cyanobacteria in Extreme Environments. Series: Cellular Origin, Life in Extreme Habitats and Astrobiology, Volume 11. Springer; Dordrecht, The Netherlands: 2007. pp. 141–172. [Google Scholar]
- 146.Round F.E., Crawford R.M., Mann D.G. The Diatoms, Biology & Morphology of the Genera. Cambridge University Press; Cambridge, UK: 1990. [Google Scholar]
- 147.Stoermer E.F., Julius M.L. Centric diatoms. In: Wehr J.D., Sheath R.G., editors. Freshwater Algae of North America. Ecology and Classification. Academic Press; New York, NY, USA: 2003. pp. 559–594. [Google Scholar]
- 148.Bahulikar R.A., Kroth P.G. Localization of EPS components secreted by freshwater diatoms using differential staining with fluorophore-conjugated lectins and other fluorochromes. Eur. J. Phycol. 2007;42:199–208. doi: 10.1080/09670260701289779. [DOI] [Google Scholar]
- 149.Kociolek J.P., Stoermer E.F. A preliminary investigation of the phylogenetic relationships among the freshwater, apical pore field-bearing cymbelloid and gomphonemoid diatoms (Bacillariophyceae) J. Phycol. 1988;24:377–385. doi: 10.1111/j.1529-8817.1988.tb00187.x. [DOI] [Google Scholar]
- 150.Hamm C.E., Merkel R., Springer O., Jurkojc P., Maier C., Prechtel K., Smetacek V. Architecture and material properties of diatom shells provide effective mechanical protection. Nature. 2003;421:841–843. doi: 10.1038/nature01416. [DOI] [PubMed] [Google Scholar]
- 151.Abodeely J., Stevens D., Ray A., Schaller K., Newby D. Algal Supply System Design—Harmonized Version [Report INL/EXT-13–28890] Idaho National Laboratory (INL); Idaho Falls, ID, USA: 2013. [Google Scholar]
- 152.Anderson E. A cytological study of the centrifuged whole, half, and quarter eggs of the sea urchin Arbacia punctulata. J. Cell Biol. 1970;47:711–733. doi: 10.1083/jcb.47.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Furnas M.J. Net in situ growth rates of phytoplankton in an oligotrophic, tropical shelf ecosystem. Limnol. Oceanogr. 1991;36:13–29. doi: 10.4319/lo.1991.36.1.0013. [DOI] [Google Scholar]
- 154.Ketheesan B., Nirmalakhandan N. Development of a new airlift-driven raceway reactor for algal cultivation. Appl. Energy. 2011;88:3370–3376. doi: 10.1016/j.apenergy.2010.12.034. [DOI] [Google Scholar]
- 155.Matsumoto H., Shioji N., Hamasaki A., Ikuta Y. Basic study on optimization of raceway-type algal cultivator. J. Chem. Eng. Jpn. 1996;29:541–543. doi: 10.1252/jcej.29.541. [DOI] [Google Scholar]
- 156.Cohen B. Changes Going on in the Algae Production Industry. [(accessed on 23 April 2015)]. Available online: http://www.nationalalgaeassociation.com/Changes_going_on_in_the_algae_production_industry.docx.
- 157.Day J.G., Stanley M.S. Biological constraints on the production of microalgal-based biofuels. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 101–130. [Google Scholar]
- 158.McNichol J., McGinn P. Adapting mass algaculture for a northern climate. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 131–146. [Google Scholar]
- 159.Rhodes C.J. Making fuel from algae: Identifying fact amid fiction. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 177–192. [Google Scholar]
- 160.Bhargava P., Mohan M.K. From isolation of potential microalgal strains to strain engineering for biofuel. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 63–82. [Google Scholar]
- 161.Zittelli G.C., Rodolfi L., Bassi N., Biondi N., Tredici M.R. Algae for Biofuels and Energy. Springer; Dordrecht, The Netherlands: 2013. Photobioreactors for microalgal biofuel production; pp. 115–131. [Google Scholar]
- 162.Schulze P.S.C., Barreira L.A., Pereira H.G.C., Perales J.A., Varela J.C.S. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014;32:423–431. doi: 10.1016/j.tibtech.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 163.Darko E., Heydarizadeh P., Schoefs B., Sabzalian M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014;369:20130243. doi: 10.1098/rstb.2013.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reijnders L. Foreword: The production of algal biofuels. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. xiii–xix. [Google Scholar]
- 165.Dahiya A., Todd J., McInnis A. Wastewater treatment integrated with algae production for biofuel. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 447–466. [Google Scholar]
- 166.Gordon R., Poulin B.J. Quitting cold turkey: Rapid oil independence for the USA. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 3–20. [Google Scholar]
- 167.Wang X.J., Wang X.B., Gascoyne P.R.C. General expressions for dielectrophoretic force and electrorotational torque derived using the Maxwell stress tensor method. J. Electrost. 1997;39:277–295. doi: 10.1016/S0304-3886(97)00126-5. [DOI] [Google Scholar]
- 168.Beskok Ali. (Department of Mechanical Engineering, Southern Methodist University, Dallas, TX, USA). Personal communication. 2014.
- 169.Zhou J.W., Ellis A.V., Voelcker N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis. 2010;31:2–16. doi: 10.1002/elps.200900475. [DOI] [PubMed] [Google Scholar]
- 170.Forry S.P., Locascio L.E. On-chip CO2 control for microfluidic cell culture. Lab Chip. 2011;11:4041–4046. doi: 10.1039/c1lc20505f. [DOI] [PubMed] [Google Scholar]
- 171.Thorsen T., Maerkl S.J., Quake S.R. Microfluidic large-scale integration. Science. 2002;298:580–584. doi: 10.1126/science.1076996. [DOI] [PubMed] [Google Scholar]
- 172.Zhang T., Cui T.H. Tunable wetting properties of patterned silicon microchannels with varied surface free energy based on layer-by-layer nano self-assembly. J. Micromech. Microeng. 2011;21:045015. doi: 10.1088/0960-1317/21/4/045015. [DOI] [Google Scholar]
- 173.Lim H.S., Kim J.Y., Kwak H.S., Sim S.J. Integrated microfluidic platform for multiple processes from microalgal culture to lipid extraction. Anal. Chem. 2014;86:8585–8592. doi: 10.1021/ac502324c. [DOI] [PubMed] [Google Scholar]
- 174.Jeffryes C., Campbell J., Li H.Y., Jiao J., Rorrer G. The potential of diatom nanobiotechnology for applications in solar cells, batteries, and electroluminescent devices. Energy Environ. Sci. 2011;4:3930–3941. doi: 10.1039/c0ee00306a. [DOI] [Google Scholar]
- 175.Gordon R., Witkowski A., Gebeshuber I.C., Allen C.S. The diatoms of Antarctica and their potential roles in nanotechnology. In: Masó M., editor. Antarctica: Time of Change. Editions ACTAR; Barcelona, Spain: 2010. pp. 84–95. [Google Scholar]
- 176.Chepurnov V.A., Chaerle P., Mann D.G. How to breed diatoms: Examination of two species with contrasting reproductive biology. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 323–340. [Google Scholar]
- 177.Dodson V.J., Leblond J.D. Now you see it, now you don’t: Differences in hydrocarbon production in the diatom Phaeodactylum tricornutum due to growth temperature. J. Appl. Phycol. 2014 doi: 10.1007/s10811-014-0464-6. [DOI] [Google Scholar]
- 178.Poulin Bryan. (Faculty of Business Administration, Lakehead University, Thunder Bay, Canada). Personal communication. 2014.
- 179.Mutanda T., Ramesh D., Karthikeyan S., Kumari S., Anandraj A., Bux F. Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour. Technol. 2011;102:57–70. doi: 10.1016/j.biortech.2010.06.077. [DOI] [PubMed] [Google Scholar]
- 180.Murphy T.E. Ph.D. Thesis. University of Texas; Austin, TX, USA: 2013. Artifcial Leaf for Biofuel Production and Harvesting: Transport Phenomena and Energy Conversion. [Google Scholar]
- 181.Onay M., Sonmez C., Oktem H.A., Yucel A.M. Thermo-resistant green microalgae for effective biodiesel production: Isolation and characterization of unialgal species from geothermal flora of Central Anatolia. Bioresour. Technol. 2014;169:62–71. doi: 10.1016/j.biortech.2014.06.078. [DOI] [PubMed] [Google Scholar]
- 182.Nikulina T.V., Kociolek J.P. Diatoms from hot springs from Kuril and Sakhalin Islands (Far East, Russia) In: Seckbach J., Kociolek J.P., editors. The Diatom World. Springer; Dordrecht, The Netherlands: 2011. pp. 333–363. [Google Scholar]
- 183.Quintela A., Almeida S., Terroso D., Ferreira da Silva E., Forjaz V., Rocha F. Diatom assemblages of thermal and mineral waters from volcanic environments in São Miguel Island, Azores. Diatom Res. 2013;28:407–417. doi: 10.1080/0269249X.2013.822833. [DOI] [Google Scholar]
- 184.Owen R.B., Renaut R.W., Jones B. Geothermal diatoms: A comparative study of floras in hot spring systems of Iceland, New Zealand, and Kenya. Hydrobiologia. 2008;610:175–192. doi: 10.1007/s10750-008-9432-y. [DOI] [Google Scholar]
- 185.Lindemann S.R., Moran J.J., Stegen J.C., Renslow R.S., Hutchison J.R., Cole J.K., Dohnalkova A.C., Tremblay J., Singh K., Malfatti S.A., et al. The epsomitic phototrophic microbial mat of Hot Lake, Washington: Community structural responses to seasonal cycling. Front. Microbiol. 2013;4:article 323. doi: 10.3389/fmicb.2013.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Andersen R.A. Diversity of eukaryotic algae. Biodivers. Conserv. 1992;1:267–292. doi: 10.1007/BF00693765. [DOI] [Google Scholar]
- 187.Guiry M.D. How many species of algae are there? J. Phycol. 2012;48:1057–1063. doi: 10.1111/j.1529-8817.2012.01222.x. [DOI] [PubMed] [Google Scholar]
- 188.Grobbelaar J.U. Physiological and technological considerations for optimising mass algal cultures. J. Appl. Phycol. 2000;12:201–206. doi: 10.1023/A:1008155125844. [DOI] [Google Scholar]
- 189.Pienkos P.T., Darzins A. The promise and challenges of microalgal-derived biofuels. Biofuels Bioprod. Biorefin. 2009;3:431–440. doi: 10.1002/bbb.159. [DOI] [Google Scholar]
- 190.Chagoya J.C., Brown J., Gomez M.S., Zhang J., Jiang Y., Laverty K., Brown L., Quigg A., Burow M.D. Media optimization and lipid formation of two native diatoms for cultivation in the Southwest Texas desert. J. Appl. Phycol. 2014;26:2075–2085. doi: 10.1007/s10811-014-0238-1. [DOI] [Google Scholar]
- 191.Kim J.K., Kottuparambil S., Moh S.H., Lee T.K., Kim Y.-J., Rhee J.-S., Choi E.-M., Kim B.H., Yu Y.J., Yarish C. Potential applications of nuisance microalgae blooms. J. Appl. Phycol. 2014 doi: 10.1007/s10811-014-0410-7. [DOI] [Google Scholar]
- 192.Jellyman P.G., Clearwater S.J., Clayton J.S., Kilroy C., Blair N., Hickey C.W., Biggs B.J.F. Controlling the invasive diatom Didymosphenia geminata: An ecotoxicity assessment of four potential biocides. Arch. Environ. Contam. Toxicol. 2011;61:115–127. doi: 10.1007/s00244-010-9589-z. [DOI] [PubMed] [Google Scholar]
- 193.Valdez-Ojeda R., González-Muñoz M., Us-Vázquez R., Narváez-Zapata J., Chavarria-Hernandez J.C., López-Adrián S., Barahona-Pérez F., Toledano-Thompson T., Garduño-Solórzano G., Escobedo-Gracia Medrano R.M. Characterization of five fresh water microalgae with potential for biodiesel production. Algal Res. 2015;7:33–44. doi: 10.1016/j.algal.2014.11.009. [DOI] [Google Scholar]
- 194.Kopecky J., Schoefs B., Loest K., Stys D., Pulz O. Microalgae as a source for secondary carotenoid production: A screening study. Arch. Hydrobiol. Suppl. 2000;133:153–168. [Google Scholar]
- 195.Meng Y., Yao C., Xue S., Yang H. Application of Fourier transform infrared (FT-IR) spectroscopy in determination of microalgal compositions. Bioresour. Technol. 2014;151:347–354. doi: 10.1016/j.biortech.2013.10.064. [DOI] [PubMed] [Google Scholar]
- 196.Wagner H., Jungandreas A., Fanesi A., Wilhelm C. Surveillance of C-allocation in microalgal cells. Metabolites. 2014;4:453–464. doi: 10.3390/metabo4020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mahapatra D.M., Ramachandra T.V. Algal biofuel: Bountiful lipid from Chlorococcum sp. proliferating in municipal wastewater. Curr. Sci. 2013;105:47–55. [Google Scholar]
- 198.Bougaran G., Rouxel C., Dubois N., Kaas R., Grouas S., Lukomska E., Le Coz J.-R., Cadoret J.-P. Enhancement of neutral lipid productivity in the microalga Isochrysis affinis Galbana (T-Iso) by a mutation-selection procedure. Biotechnol. Bioeng. 2012;109:2737–2745. doi: 10.1002/bit.24560. [DOI] [PubMed] [Google Scholar]
- 199.Robert S., Mansour M.P., Blackburn S.I. Metolachlor-mediated selection of a microalgal strain producing novel polyunsaturated fatty acids. Mar. Biotechnol. 2007;9:146–153. doi: 10.1007/s10126-006-6102-9. [DOI] [PubMed] [Google Scholar]
- 200.Kasbi-Chadli F., Boquien C.-Y., Simard G., Ulmann L., Mimouni V., Leray V., Meynier A., Ferchaud-Roucher V., Champ M., Nguyen P., et al. Maternal supplementation with n-3 long chain polyunsaturated fatty acids during perinatal period alleviates the metabolic syndrome disturbances in adult hamster pups fed a high-fat diet after weaning. J. Nutr. Biochem. 2014;25:726–733. doi: 10.1016/j.jnutbio.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 201.Juneau P., Dewez D., Matsui S., Kim S.G., Popovic R. Evaluation of different algal species sensitivity to mercury and metolachlor by PAM-fluorometry. Chemosphere. 2001;45:589–598. doi: 10.1016/S0045-6535(01)00034-0. [DOI] [PubMed] [Google Scholar]
- 202.Metting F.B., Jr. Biodiversity and application of microalgae. J. Ind. Microbiol. 1996;17:477–489. doi: 10.1007/BF01574779. [DOI] [Google Scholar]
- 203.Ramesha B.T., Gertsch J., Ravikanth G., Priti V., Ganeshaiah K.N., Shaanker R.U. Biodiversity and chemodiversity: Future perspectives in bioprospecting. Curr. Drug Targets. 2011;12:1515–1530. doi: 10.2174/138945011798109473. [DOI] [PubMed] [Google Scholar]
- 204.Sheehan J., Dunahay T., Benemann J., Roessler P. A Look Back at the U.S. Department of Energy’s Aquatic Species Program: Biodiesel from Algae. National Renewable Energy Laboratory; Golden, CO, USA: 1998. Close-Out Report NREL/TP-580-24190. [Google Scholar]
- 205.Tara Expeditions The Oceanomics Project. [(accessed on 21 April 2015)]. Available online: http://oceans.taraexpeditions.org/en/m/science/news/the-oceanomics-project/
- 206.Fujii K., Matsunobu S., Takahashi Y. Characterization of the new microalgal strains, Oogamochlamys spp., and their potential for biofuel production. Algal Res. 2014;5:164–170. doi: 10.1016/j.algal.2014.08.003. [DOI] [Google Scholar]
- 207.Ma Y., Wang Z., Yu C., Yin Y., Zhou G. Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresour. Technol. 2014;167:503–509. doi: 10.1016/j.biortech.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 208.Tale M., Ghosh S., Kapadnis B., Kale S. Isolation and characterization of microalgae for biodiesel production from Nisargruna biogas plant effluent. Bioresour. Technol. 2014;169:328–335. doi: 10.1016/j.biortech.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 209.Becerra P.I., Bustamante R.O. The effect of herbivory on seedling survival of the invasive exotic species Pinus radiata and Eucalyptus globulus in a Mediterranean ecosystem of Central Chile. For. Ecol. Manag. 2008;256:1573–1578. doi: 10.1016/j.foreco.2008.04.011. [DOI] [Google Scholar]
- 210.Boeuf G., Kornprobst J.-M. Biodiversité et chimiodiversité marines/Biodiversity and marine chemodiversity. Biofutur. 2009;301:28–32. [Google Scholar]
- 211.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 212.Jiraskova D., Poulickova A., Novak O., Sedlakova K., Hradecka V., Strnad M. High-throughput screening technology for monitoring phytohormone production in microalgae. J. Phycol. 2009;45:108–118. doi: 10.1111/j.1529-8817.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- 213.Griffiths M.J., Harrison S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009;21:493–507. doi: 10.1007/s10811-008-9392-7. [DOI] [Google Scholar]
- 214.Barbour M.T., Gerritsen J., Snyder B.D., Stribling J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish [EPA 841-B-99-002] 2nd ed. Office of Water, U.S. Environmental Protection Agency; Washington, DC, USA: 1999. [Google Scholar]
- 215.Zhang W., Nielsen D.R. Synthetic biology applications in industrial microbiology. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Miyagawa-Yamaguchi A., Okami T., Kira N., Yamaguchi H., Ohnishi K., Adachi M. Stable nuclear transformation of the diatom Chaetoceros sp. Phycol. Res. 2011;59:113–119. doi: 10.1111/j.1440-1835.2011.00607.x. [DOI] [Google Scholar]
- 217.Dunahay T.G., Jarvis E.E., Roessler P.G. Genetic transformation of the diatoms Cyclotella cryptica and Navicula saprophila. J. Phycol. 1995;31:1004–1012. doi: 10.1111/j.0022-3646.1995.01004.x. [DOI] [Google Scholar]
- 218.Fischer H., Robl I., Sumper M., Kroger N. Targeting and covalent modification of cell wall and membrane proteins heterologously expressed in the diatom Cylindrotheca fusiformis (Bacillariophyceae) J. Phycol. 1999;35:113–120. doi: 10.1046/j.1529-8817.1999.3510113.x. [DOI] [Google Scholar]
- 219.Poulsen N., Kröger N. A new molecular tool for transgenic diatoms. Control of mRNA and protein biosynthesis by an inducible promoter-terminator cassette. FEBS J. 2005;272:3413–3423. doi: 10.1111/j.1742-4658.2005.04760.x. [DOI] [PubMed] [Google Scholar]
- 220.Muto M., Fukuda Y., Nemoto M., Yoshino T., Matsunaga T., Tanaka T. Establishment of a genetic transformation system for the marine pennate diatom Fistulifera sp. strain JPCC DA0580-a high triglyceride producer. Mar. Biotechnol. 2013;15:48–55. doi: 10.1007/s10126-012-9457-0. [DOI] [PubMed] [Google Scholar]
- 221.Apt K.E., Kroth-Pancic P.G., Grossman A.R. Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol. General Genet. 1996;252:572–579. doi: 10.1007/BF02172403. [DOI] [PubMed] [Google Scholar]
- 222.Zaslavskaia L.A., Lippmeier J.C., Kroth P.G., Grossman A.R., Apt K.E. Transformation of the diatom Phaeodactylum tricornutum (Bacillariophyceae) with a variety of selectable marker and reporter genes. J. Phycol. 2000;36:379–386. doi: 10.1046/j.1529-8817.2000.99164.x. [DOI] [Google Scholar]
- 223.Niu Y.-F., Yang Z.-K., Zhang M.-H., Zhu C.-C., Yang W.-D., Liu J.-S., Li H.-Y. Transformation of diatom Phaeodactylum tricornutum by electroporation and establishment of inducible selection marker. BioTechn. Rapid Dispatches. 2012 doi: 10.2144/000113881. [DOI] [PubMed] [Google Scholar]
- 224.Ifuku K., Yan D., Miyahara M., Inoue-Kashino N., Yamamoto Y.Y., Kashino Y. A stable and efficient nuclear transformation system for the diatom Chaetoceros gracilis. Photosynth. Res. 2015;123:203–211. doi: 10.1007/s11120-014-0048-y. [DOI] [PubMed] [Google Scholar]
- 225.Shimogawara K., Fujiwara S., Grossman A., Usuda H. High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics. 1998;148:1821–1828. doi: 10.1093/genetics/148.4.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kilian O., Benemann C.S.E., Niyogi K.K., Vick B. High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc. Natl. Acad. Sci. USA. 2011;108:21265–21269. doi: 10.1073/pnas.1105861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Radakovits R., Jinkerson R.E., Fuerstenberg S.I., Tae H., Settlage R.E., Boore J.L., Posewitz M.C. Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropsis gaditana. Nat. Commun. 2013;4:2356. doi: 10.1038/ncomms3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Radakovits R., Jinkerson R.E., Darzins A., Posewitz M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell. 2010;9:486–501. doi: 10.1128/EC.00364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gong Y., Guo X., Wan X., Liang Z., Jiang M. Characterization of a novel thioesterase (PtTE) from Phaeodactylum tricornutum. J. Basic Microbiol. 2011;51:666–672. doi: 10.1002/jobm.201000520. [DOI] [PubMed] [Google Scholar]
- 230.Trentacoste E.M., Shrestha R.P., Smith S.R., Gle C., Hartmann A.C., Hildebrand M., Gerwick W.H. Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth. Proc. Natl. Acad. Sci. USA. 2013;110:19748–19753. doi: 10.1073/pnas.1309299110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Parker K. M.Sc. Thesis. Moss Landing Marine Labs, San Jose State University; San Jose, CA, USA: 2013. Metabolic Network Construction Based on the Genome of the Marine Diatom Thalassiosira pseudonana and the Analysis of Genome-wide Transcriptome Data to Investigate Triacylglyceride Accumulation. [Google Scholar]
- 232.Daboussi F., Leduc S., Maréchal A., Dubois G., Guyot V., Perez-Michaut C., Amato A., Falciatore A., Juillerat A., Beurdeley M., et al. Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat. Commun. 2014;5 doi: 10.1038/ncomms4831. [DOI] [PubMed] [Google Scholar]
- 233.Harrison M.E., Dunlop M.J. Synthetic feedback loop model for increasing microbial biofuel production using a biosensor. Front. Microbiol. 2012;3:360. doi: 10.3389/fmicb.2012.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Foo J.L., Jensen H.M., Dahl R.H., George K., Keasling J.D., Lee T.S., Leong S., Mukhopadhyay A. Improving microbial biogasoline production in Escherichia coli using tolerance engineering. mBio. 2014;5:e0193214. doi: 10.1128/mBio.01932-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jin H., Chen L., Wang J., Zhang W. Engineering biofuel tolerance in non-native producing microorganisms. Biotechnol. Adv. 2014;32:541–548. doi: 10.1016/j.biotechadv.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 236.Heydarizadeh P., Marchand J., Chenais B., Sabzalian M.R., Zahedi M., Moreau B., Schoefs B. Functional investigations in diatoms need more than a transcriptomic approach. Diatom Res. 2014;29:75–89. doi: 10.1080/0269249X.2014.883727. [DOI] [Google Scholar]
- 237.Kutchan T.M. Predictive metabolic engineering in plants: Still full of surprises. Trends Biotechnol. 2005;23:381–383. doi: 10.1016/j.tibtech.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 238.Wang B., Li Y.Q., Wu N., Lan C.Q. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008;79:707–718. doi: 10.1007/s00253-008-1518-y. [DOI] [PubMed] [Google Scholar]
- 239.Nikaido H., Takatsuka Y. Mechanisms of RND multidrug efflux pumps. BBA-Proteins Proteomics. 2009;1794:769–781. doi: 10.1016/j.bbapap.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Higgins C.F. ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 241.Rees D.C., Johnson E., Lewinson O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009;10:218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Reyes L.H., Almario M.P., Kao K.C. Genomic library screens for genes involved in n-butanol tolerance in Escherichia coli. PLoS ONE. 2011;6:e17678. doi: 10.1371/journal.pone.0017678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tréguer P., Nelson D.M., van Bennekom A.J., DeMaster D.J., Leynaert A., Quéguiner B. The silica balance in the world ocean: A reestimate. Science. 1995;268:375–379. doi: 10.1126/science.268.5209.375. [DOI] [PubMed] [Google Scholar]
- 244.Falkowski P.G., Katz M.E., Knoll A.H., Quigg A., Raven J.A., Schofield O., Taylor F.J. The evolution of modern eukaryotic phytoplankton. Science. 2004;305:354–360. doi: 10.1126/science.1095964. [DOI] [PubMed] [Google Scholar]
- 245.Sims P.A., Mann D.G., Medlin L.K. Evolution of the diatoms: Insights from fossil, biological and molecular data. Phycologia. 2006;45:361–402. doi: 10.2216/05-22.1. [DOI] [Google Scholar]
- 246.Moustafa A., Beszteri B., Maier U.G., Bowler C., Valentin K., Bhattacharya D. Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science. 2009;324:1724–1726. doi: 10.1126/science.1172983. [DOI] [PubMed] [Google Scholar]
- 247.Becker B., Hoef-Emden K., Melkonian M. Chlamydial genes shed light on the evolution of photoautotrophic eukaryotes. BMC Evol. Biol. 2008;8 doi: 10.1186/1471-2148-8-203. Article Number 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Armbrust E.V., Berges J.A., Bowler C., Green B.R., Martinez D., Putnam N.H., Zhou S., Allen A.E., Apt K.E., Bechner M., et al. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science. 2004;306:79–86. doi: 10.1126/science.1101156. [DOI] [PubMed] [Google Scholar]
- 249.Kociolek J.P. Diatoms: Unique eukaryotic extremophiles providing insights into planetary change. Proc. Soc. Photo-Opt. Instrum. Eng. (SPIE) 2007;6694:66940S. [Google Scholar]
- 250.Bertrand M., Schoefs B., Siffel P., Rohacek K., Molnar I. Cadmium inhibits epoxidation of diatoxanthin to diadinoxanthin in the xanthophyll cycle of the marine diatom Phaeodactylum tricornutum. FEBS Lett. 2001;508:153–156. doi: 10.1016/S0014-5793(01)03050-2. [DOI] [PubMed] [Google Scholar]
- 251.Nguyen-Deroche T.L.N., Caruso A., Le T.T., Bui T.V., Schoefs B., Tremblin G., Morant-Manceau A. Zinc affects differently growth, photosynthesis, antioxidant enzyme activities and phytochelatin synthase expression of four marine diatoms. Sci. World J. 2012;2012:982957. doi: 10.1100/2012/982957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Masmoudi S., Nguyen-Deroche N., Caruso A., Ayadi H., Morant-Manceau A., Tremblin G., Bertrand M., Schoefs B. Cadmium, copper, sodium and zinc effects on diatoms: From heaven to hell—A review. Cryptogam. Algol. 2013;34:185–225. doi: 10.7872/crya.v34.iss2.2013.185. [DOI] [Google Scholar]
- 253.Rohacek K., Bertrand M., Moreau B., Jacquette B., Caplat C., Morant-Manceau A., Schoefs B. Relaxation of the non-photochemical chlorophyll fluorescence quenching in diatoms: Kinetics, components and mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 2014;369S1:20130241. doi: 10.1098/rstb.2013.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cheng R.-l., Feng J., Zhang B.-X., Huang Y., Cheng J., Zhang C.-X. Transcriptome and gene expression analysis of an oleaginous diatom under different salinity conditions. Bioenergy Res. 2014;7:192–205. doi: 10.1007/s12155-013-9360-1. [DOI] [Google Scholar]
- 255.Gacheva G.V., Gigova L.G. Biological activity of microalgae can be enhanced by manipulating the cultivation temperature and irradiance. Cent. Eur. J. Biol. 2014;9:1168–1181. doi: 10.2478/s11535-014-0350-x. [DOI] [Google Scholar]
- 256.Maeda Y., Sunaga Y., Yoshino T., Tanaka T. Oleosome-associated protein of the oleaginous diatom Fistulifera solaris contains an endoplasmic reticulum-targeting signal sequence. Mar. Drugs. 2014;12:3892–3903. doi: 10.3390/md12073892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Burrows E., Bennette N., Carrieri D., Dixon J., Brinker A., Frada M., Baldassano S., Falkowski P., Dismukes G. Dynamics of lipid biosynthesis and redistribution in the marine diatom Phaeodactylum tricornutum under nitrate deprivation. BioEnergy Res. 2012;5:876–885. doi: 10.1007/s12155-012-9201-7. [DOI] [Google Scholar]
- 258.Taguchi S., Hirata J.A., Laws E.A. Silicate deficiency and lipid synthesis of marine diatoms. J. Phycol. 1987;23:260–267. doi: 10.1111/j.1529-8817.1987.tb04133.x. [DOI] [Google Scholar]
- 259.Zhang L., Han J.C., Yang G.P., Zhu B.H., Pan K.H. Association of triacylglyceride content and transcript abundance of genes involving in lipid synthesis of nitrogen deficient Phaeodactylum tricornutum. Chin. J. Oceanol. Limnol. 2014;32:397–402. doi: 10.1007/s00343-014-3173-8. [DOI] [Google Scholar]
- 260.Badour S.S., Gergis M.S. Cell division and fat accumulation in Nitzschia sp. grown in continuously illuminated mass cultures. Arch. Mikrobiol. 1965;51:94–102. doi: 10.1007/BF00406853. [DOI] [PubMed] [Google Scholar]
- 261.Beattie A., Percival E., Hirst E.L. Studies on the metabolism of the Chrysophyceae. Comparative structural investigations on leucosin (chrysolaminarin) separated from diatoms and laminarin from the brown algae. Biochem. J. 1961;79:531–537. doi: 10.1042/bj0790531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Huang B., Marchand J., Moreau B., Lukomwska E., Bougaran G., Cadoret J.-P., Schoefs B. Book of Abstracts of the 33ème Colloque de l’Association des Diatomistes de Langue Française. l’Association des Diatomistes de Langue française; Thonon-les-Bains, France: 2014. Impact de l’approvisionnement en CO2 sur l’utilisation du carbone chez la diatomée Phaeodactylum tricornutum. [Google Scholar]
- 263.Ho S.-H., Huang S.-W., Chen C.-Y., Hasunuma T., Kondo A., Chang J.-S. Characterization and optimization of carbohydrate production from an indigenous microalga Chlorella vulgaris FSP-E. Bioresour. Technol. 2013;135:157–165. doi: 10.1016/j.biortech.2012.10.100. [DOI] [PubMed] [Google Scholar]
- 264.Thomas W.H., Dodson A.N., Reid F.M.H. Diatom productivity compared to other algae in natural marine phytoplankton assemblages. J. Phycol. 1978;14:250–253. doi: 10.1111/j.1529-8817.1978.tb00294.x. [DOI] [Google Scholar]
- 265.Jungandreas A., Costa B.S., Jakob T., von Bergen M., Baumann S., Wilhelm C. The acclimation of Phaeodactylum tricornutum to blue and red light does not influence the photosynthetic light reaction but strongly disturbs the carbon allocation pattern. PLoS ONE. 2014;9:e99727. doi: 10.1371/journal.pone.0099727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Schoefs B., Rmiki N.-E., Rachadi J., Lemoine Y. Astaxanthin accumulation in Haematococcus requires a cytochrome P450 hydroxylase and an active synthesis of fatty acids. FEBS Lett. 2001;500:125–128. doi: 10.1016/S0014-5793(01)02596-0. [DOI] [PubMed] [Google Scholar]
- 267.Klok A.J., Martens D.E., Wijffels R.H., Lamers P.P. Simultaneous growth and neutral lipid accumulation in microalgae. Bioresour. Technol. 2013;134:233–243. doi: 10.1016/j.biortech.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 268.Yatsu L.Y., Jacks T.J., Hensarling T.P. Hensarli.Tp Isolation of spherosomes (oleosomes) from onion, cabbage, and cottonseed tissues. Plant Physiol. 1971;48:675–682. doi: 10.1104/pp.48.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Davidi L., Katz A., Pick U. Characterization of major lipid droplet proteins from Dunaliella. Planta. 2012;236:19–33. doi: 10.1007/s00425-011-1585-7. [DOI] [PubMed] [Google Scholar]
- 270.Nojima D., Yoshino T., Maeda Y., Tanaka M., Nemoto M., Tanaka T. Proteomics analysis of oil body-associated proteins in the oleaginous diatom. J. Proteome Res. 2013;12:5293–5301. doi: 10.1021/pr4004085. [DOI] [PubMed] [Google Scholar]
- 271.Zhang X.Q., Dubacq J.P., Alfsen A. Biochemical and cytological evidence for the stimulation of clathrin-coated (vesicle) formation by exogenous folic acid in Dunaliella salina (Chlorophyta) J. Phycol. 1993;29:203–209. doi: 10.1111/j.0022-3646.1993.00203.x. [DOI] [Google Scholar]
- 272.Hamano S. On the oil of the living egg of salmon, Oncorhynchus keta. Bull. Jpn. Soc. Sci. Fish. 1951;17:47–52. doi: 10.2331/suisan.17.47. [DOI] [Google Scholar]
- 273.Targett-Adams P., Chambers D., Gledhill S., Hope R.G., Coy J.F., Girod A., McLauchlan J. Live cell analysis and targeting of the lipid droplet-binding adipocyte differentiation-related protein. J. Biol. Chem. 2003;278:15998–16007. doi: 10.1074/jbc.M211289200. [DOI] [PubMed] [Google Scholar]
- 274.Bostrom P., Rutberg M., Ericsson J., Holmdahl P., Andersson L., Frohman M.A., Boren J., Olofsson S.O. Cytosolic lipid droplets increase in size by microtubule-dependent complex formation. Arterioscler. Thromb. Vasc. Biol. 2005;25:1945–1951. doi: 10.1161/01.ATV.0000179676.41064.d4. [DOI] [PubMed] [Google Scholar]
- 275.Nagayama M., Uchida T., Gohara K. Temporal and spatial variations of lipid droplets during adipocyte division and differentiation. J. Lipid Res. 2007;48:9–18. doi: 10.1194/jlr.M600155-JLR200. [DOI] [PubMed] [Google Scholar]
- 276.Watanabe T., Thayil A., Jesacher A., Grieve K., Debarre D., Wilson T., Booth M., Srinivas S. Characterisation of the dynamic behaviour of lipid droplets in the early mouse embryo using adaptive harmonic generation microscopy. BMC Cell Biol. 2010;11:38. doi: 10.1186/1471-2121-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Dou W., Zhang D., Jung Y., Cheng J.-X., Umulis D.M. Label-free imaging of lipid-droplet intracellular motion in early Drosophila embryos using femtosecond-stimulated Raman loss microscopy. Biophys. J. 2012;102:1666–1675. doi: 10.1016/j.bpj.2012.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Long A.P., Manneschmidt A.K., VerBrugge B., Dortch M.R., Minkin S.C., Prater K.E., Biggerstaff J.P., Dunlap J.R., Dalhaimer P. Lipid droplet de novo formation and fission are linked to the cell cycle in fission yeast. Traffic. 2012;13:705–714. doi: 10.1111/j.1600-0854.2012.01339.x. [DOI] [PubMed] [Google Scholar]
- 279.Dalhaimer P. Lipid droplet organelle distribution in populations of dividing cells studied by simulation. Phys. Biol. 2013;10:036007. doi: 10.1088/1478-3975/10/3/036007. [DOI] [PubMed] [Google Scholar]
- 280.Baffes J., Kose A., Ohnsorge F., Stocker M., Chen D., Cosic D., Gong X., Huidrom R., Vashakmadze E., Zhang J., et al. Global Economic Prospects: Having Fiscal Space and Using It. World Bank Publications; Washington, DC, USA: 2015. Understanding the plunge in oil prices: Sources and implications; pp. 155–168. [Google Scholar]
- 281.Baumeister C., Kilian L. Understanding the decline in the price of oil since June 2014. [(accessed on 21 April 2015)]. Available online: http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2557316.
- 282.Rahemtulla K. World Manipulated Into Buying Saudi Oil. [(accessed on 21 April 2015)]. Available online: http://www.wallstreetdaily.com/2014/11/07/u-s-saudi-arabia-oil/
- 283.Sabino A.M. New York fracking outlook still gloomy. Nat. Gas Electr. 2015;31:20–25. doi: 10.1002/gas.21808. [DOI] [Google Scholar]
- 284.Golden J.M., Wiseman H.J. The fracking revolution: Shale gas as a case study in innovation policy. Emory Law J. 2015;64:955–1040. [Google Scholar]
- 285.Dale B.E., Anderson J.E., Brown R.C., Csonka S., Dale V.H., Herwick G., Jackson R.D., Jordan N., Kaffka S., Kline K.L. Take a closer look: Biofuels can support environmental, economic and social goals. Environ. Sci. Technol. 2014;48:7200–7203. doi: 10.1021/es5025433. [DOI] [PubMed] [Google Scholar]
- 286.Gendy T.S., El-Temtamy S.A. Commercialization potential aspects of microalgae for biofuel production: An overview. Egypt. J. Pet. 2013;22:43–51. doi: 10.1016/j.ejpe.2012.07.001. [DOI] [Google Scholar]
- 287.Aluya J. Biofuels has become disruptive technology to the energy market. Biofuels. 2015;5:457–467. doi: 10.1080/17597269.2014.989136. [DOI] [Google Scholar]
- 288.Kirkby J., Carslaw K.S. Variations of galactic cosmic rays and the Earth’s climate. In: Frisch P.C., editor. Solar Journey: The Significance of Our Galactic Environment for the Heliosphere and Earth: The Significance of Our Galactic Environment for the Heliosphere and Earth. Springer; Dordrecht, The Netherlands: 2006. pp. 349–397. [Google Scholar]
- 289.Haigh J.D. Solar variability and climate. In: Lilensten J., editor. Space Weather. Volume 344. Springer; The Netherlands: 2007. pp. 65–81. [Google Scholar]
- 290.Hanslmeier A. The Sun and Space Weather. Volume 347. Springer; The Netherlands: 2007. Space weather and climate; pp. 143–173. [Google Scholar]
- 291.Previdi M., Polvani L.M. Climate system response to stratospheric ozone depletion and recovery. Q. J. R. Meteorol. Soc. 2014;140:2401–2419. doi: 10.1002/qj.2330. [DOI] [Google Scholar]
- 292.O’Riordan T. Fracking, sustainability, and democracy. Environ. Sci. Policy Sustain. Dev. 2015;57:2–3. [Google Scholar]
- 293.Ipsen D. The lifecycle of oil: Problems and conflicts. In: Hartard S., Liebert W., editors. Competition and Conflicts on Resource Use. Springer; Switzerland: 2015. pp. 61–74. [Google Scholar]
- 294.Upham P., Dendler L. Scientists as policy actors: A study of the language of biofuel research. Environ. Sci. Policy. 2015;47:137–147. doi: 10.1016/j.envsci.2014.11.005. [DOI] [Google Scholar]
- 295.Fresewinkel M., Rosello R., Wilhelm C., Kruse O., Hankamer B., Posten C. Integration in microalgal bioprocess development: Design of efficient, sustainable, and economic processes. Eng. Life Sci. 2014;14:560–573. doi: 10.1002/elsc.201300153. [DOI] [Google Scholar]
- 296.Murphy D.J. The implications of the declining energy return on investment of oil production. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2014;372:20130126. doi: 10.1098/rsta.2013.0126. [DOI] [PubMed] [Google Scholar]
- 297.Shen Y. Carbon dioxide bio-fixation and wastewater treatment via algae photochemical synthesis for biofuels production. RSC Adv. 2014;4:49672–49722. doi: 10.1039/C4RA06441K. [DOI] [Google Scholar]
- 298.Wikipedia Artificial photosynthesis. [(accessed on 21 April 2015)]. Available online: http://en.wikipedia.org/wiki/Artificial_photosynthesis.
- 299.Mandal S., Mallick N. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl. Microbiol. Biotechnol. 2009;84:281–291. doi: 10.1007/s00253-009-1935-6. [DOI] [PubMed] [Google Scholar]
- 300.Dahiya A. Integrated approach to algae production for biofuel utilizing robust algal species. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 83–100. [Google Scholar]
- 301.Topf M., Tavasi M., Kinel-Tahan Y., Iluz D., Dubinsky Z., Yehoshua Y. Algal oils: Biosynthesis and uses. In: Gordon R., Seckbach J., editors. The Science of Algal Fuels: Phycology, Geology, Biophotonics, Genomics and Nanotechnology. Springer; Dordrecht, The Netherlands: 2012. pp. 193–214. [Google Scholar]
- 302.Shifrin N.S., Chisholm S.W. Phytoplankton lipids: Interspecific differences and effects of nitrate, silicate and light-dark cycles. J. Phycol. 1981;17:374–384. doi: 10.1111/j.0022-3646.1981.00374.x. [DOI] [Google Scholar]
- 303.Chen Y.-C. The biomass and total lipid content and composition of twelve species of marine diatoms cultured under various environments. Food Chem. 2012;131:211–219. doi: 10.1016/j.foodchem.2011.08.062. [DOI] [Google Scholar]
- 304.Pittman J.K., Dean A.P., Osundeko O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011;102:17–25. doi: 10.1016/j.biortech.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 305.Mahapatra D.M., Chanakya H., Ramachandra T. Euglena sp. as a suitable source of lipids for potential use as biofuel and sustainable wastewater treatment. J. Appl. Phycol. 2013;25:855–865. doi: 10.1007/s10811-013-9979-5. [DOI] [Google Scholar]
- 306.Mahapatra D.M., Chanakya H.N., Ramachandra T.V. Bioremediation and lipid synthesis through mixotrophic algal consortia in municipal wastewater. Bioresour. Technol. 2014;168:142–150. doi: 10.1016/j.biortech.2014.03.130. [DOI] [PubMed] [Google Scholar]
- 307.Fathi A.A., Azooz M.M., Al-Fredan M.A. Phycoremediation and the potential of sustainable algal biofuel production using wastewater. Am. J. Appl. Sci. 2013;10:189–194. doi: 10.3844/ajassp.2013.189.194. [DOI] [Google Scholar]
- 308.Chanakya H.N., Mahapatra D.M., Sarada R., Abitha R. Algal biofuel production and mitigation potential in India. Mitig. Adapt. Strateg. Glob. Chang. 2013;18:113–136. doi: 10.1007/s11027-012-9389-z. [DOI] [Google Scholar]
- 309.Brownbridge G., Azadi P., Smallbone A., Bhave A., Taylor B., Kraft M. The future viability of algae-derived biodiesel under economic and technical uncertainties. Bioresour. Technol. 2014;151:166–173. doi: 10.1016/j.biortech.2013.10.062. [DOI] [PubMed] [Google Scholar]
- 310.Mobile Algae Refinery: VALORIE, the Versatile ALgae On-site Raw Ingredient Extractor. [(accessed on 21 April 2015)]. Available online: https://www.youtube.com/watch?v=gkoxA74q25c.
- 311.Wikipedia Multi-objective optimization. [(accessed on 21 April 2015)]. Available online: https://en.wikipedia.org/wiki/Multi-objective_optimization.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.