Abstract
There is a current tendency towards bioactive natural products with applications in various industries, such as pharmaceutical, biomedical, cosmetics and food. This has put some emphasis in research on marine organisms, including macroalgae and microalgae, among others. Polysaccharides with marine origin constitute one type of these biochemical compounds that have already proved to have several important properties, such as anticoagulant and/or antithrombotic, immunomodulatory ability, antitumor and cancer preventive, antilipidaemic and hypoglycaemic, antibiotics and anti-inflammatory and antioxidant, making them promising bioactive products and biomaterials with a wide range of applications. Their properties are mainly due to their structure and physicochemical characteristics, which depend on the organism they are produced by. In the biomedical field, the polysaccharides from algae can be used in controlled drug delivery, wound management, and regenerative medicine. This review will focus on the biomedical applications of marine polysaccharides from algae.
Keywords: polysaccharides, algae, bioactive, biomedical, pharmaceuticals, therapeutics, drug delivery, regenerative medicine, wound management
1. Introduction
Contemporary tendency for natural products to be applied in medicine and to promote health has put some emphasis in research on marine organisms, including macro- and microalgae, and cyanobacteria. Extensive literature on the health benefits and uses as food or as drug carriers of brown, red and green seaweeds, and the polysaccharides (PS) they produce, was published in the last decade [1,2,3,4,5,6,7,8]. However, comparatively, there are only a handful of research papers on microalgae [9,10,11,12,13,14], despite the richness of their composition and the ability to make them grow.
Polysaccharides already proved to have several important properties [3,8,12,15,16,17,18,19,20,21,22]. However, the attempts to establish a relationship between the structures of the PS and their bioactivities/actions have been a challenge due to the complexity of this type of polymers. In fact, aside from the homogalactan from Gyrodinium impudicum (a dinoflagellate) [23], the β-glucan from Chlorella vulgaris (a green microalga) [24] and the PS from a few species of seaweeds (Table 1, Table 2 and Table 3), most of these carbohydrates are highly branched heteropolymers with different substituents in the various carbons of their backbone and side-sugar components. Additionally, the monosaccharide composition and distribution within the molecule, and the glycosidic bonds between monosaccharides can be very heterogeneous, which is a real impairment for the study of their structures. Moreover, this heterogeneity also depends on the species, between strains of the same species, and on the time and place of harvest.
Table 1.
Marine species of brown macroalgae (PHAEOPHYTES) producing polysaccharides (PS): some structural features and applications.
| Type of PS | Source | Structure | Action/Application | References | |
|---|---|---|---|---|---|
| Main Mono-Sugars/Disaccharide Units | Glycosidic Bonds of Backbone | ||||
| Chromophyta Dictyotales | |||||
| Heterofucans S-fucans | Canistrocarpus cervicornis a.k.a. Dictyota cervicornis | Fuc | Anticoagulant, antioxidant; anti-proliferative | [2,25] | |
| S-galactofucans | D. menstrualis | Gal, fuc, xyl, glcAc | Peripheral anti-nociceptive, anti-inflammatory, antioxidant; anticoagulant, anti-proliferative | [1,2,26] | |
| D. mertensis | antioxidant; anticoagulant, anti-proliferative | [2] | |||
| Heterofucans | Dictyopteris delicatula | Fuc | Anticoagulant, antioxidant, antitumor, anti-proliferative | [2,27] | |
| D. polypodioides | Fuc | Antitumor | [28] | ||
| S-galactofucans | Lobophora variegata | Gal, fuc | Antioxidant, anticoagulant, anti-inflammatory | [29,30] | |
| Heterofucans | Padina gymnospora | GlcAc, fuc, | (1,3)- and (1,4)-β-d-glcAc | Antioxidant, anticoagulant, anti-thrombotic, antiviral | [2,31,32] |
| S-fucan | P. tetrastromatica | Fuc, gal, xyl, glcAc | (1,2)- and (1,3)-α-fuc | [33] | |
| S-galactofucans; sPS; S-fucans |
Spatoglossum schröederi | Gal, fuc, xyl; Fuc |
(1,4)- and (1,3)-α-fuc | Anti-thrombotic; Peripheral anti-nociceptive; Anti-proliferative, anti-adhesive, antioxidant |
[2,34,35,36,37,38] |
| Ectocarpales | |||||
| S-galactofucans | Adenocystis utricularis | Gal, fuc, rham, uronic acid | (1,3)-α-fuc | Antiviral | [39] |
| S-fucans | Cladosiphon okamuranus a.k.a. Okinawa mozuku | Fuc, glc, glcAc | (1,3)-α-l-fuc | Anti-proliferative, antiviral, anti-inflammatory, antiadhesive, antitumor, immunomodulator; angiogenic, gastroprotective, cardioprotective, restenosis preventive | [15,22,40,41,42,43,44,45,46,47] |
| S-fucoidan | C. novae-caledoniae | Fuc | Antitumor | [48] | |
| Fucans | Leathesia difformis | Fuc | Antiviral | [49] | |
| LMW-S-fucans | Nemacystus decipiens | Fuc | Anticoagulant | [50] | |
| Fucales | |||||
| S-fucans; LMW-sPS; S-Laminaran; or otherwise modified |
Ascophyllum nodosum | Fuc, xyl, gal, glcAc; Glc |
(1,3)- and (1,4)-α-l-fuc (alternating); (1,3)- and (1,6)-β-glc |
Immunomodulatory, anti-inflammatory, anticoagulant, anti-thrombotic, anti-metastatic, antitumor, antiadhesive, restenosis preventive; Anti-thrombotic, anticoagulant, angiogenic Antitumor, anticoagulant; serum hypocholesterolaemic, hypotensive, antibacterial, immunomodulator |
[15,20,51,52,53,54,55,56,57,58,59,60,61] |
| S-fucans | Fucus spp. F. vesiculosus | Fuc, xyl, gal, glcAc | (1,3)- and (1,4)-α-l-fuc (alternating) | Immunostimulant, antiviral, antitumor, antiproliferative, antiadhesive, anticoagulant, antioxidant, anti-metastatic, anti-inflammatory; anti-angiogenic, antithrombotic (except F. vesiculosus) | [2,15,62,63,64,65,66,67,68,69,70] |
| Laminaran; S-laminaran or otherwise modified | Fucus sp. | Glc | (1,3)- and (1,6)-β-glc | Antitumor, decreases liver triglyceride, cholesterol and phospholipid levels; serum hypocholesterolaemic, hypotensive, antibacterial, immunomodulator anticoagulant | [56,59,61] |
| S-fucans | Hizikia fusiforme a.k.a. Sargassum fusiforme | Fuc, gal, man, glcAc | (1,2)-α-d-man alternating with (1,4)-β-d-glcAc; some (1,4)-β-d-gal | Anticoagulant, anti-thrombotic | [71,72] |
| Fucans | Pelvetia fastigiata | Fuc | Antiviral | [73] | |
| LMW-S-fucans | P. canaliculata | Fuc | Antiviral | [74] | |
| S-fucans | Sargassum spp. | Fuc, gal, xyl, uronic acid | Prevent hyperlipidaemia, normalize dislipidaemia | [75,76,77] | |
| S-galactofucans | Sargassum sp. | Gal, fuc, rham, glcAc | (1,6)-β-d-gal and/or (1,2)-β-d-man | Antitumor | [28,62,78,79,80] |
| S-heterofucans | S.filipendula | Fuc | Antioxidant, anti-proliferative | [2,81] | |
| S-fucoidan | S. henslowianum | Fuc | Anti-proliferative, antitumor | [75] | |
| S-fucoidan | S. horneri | Fuc | (1,3)-α-l-fuc, (1,3)- and (1,4)-α-l-fuc | Antitumor, antiviral | [62,80] |
| LMW-fucoidan | S. patens | Fuc | Antiviral | [32] | |
| sPS | Turbinaria conoides | Antioxidant | [82] | ||
| Laminariales | |||||
| S-galactofucan | Costaria costata | Gal, fuc | Antitumor | [16] | |
| S-fucans | Ecklonia cava E. kurome | Fuc, rham, gal, glcAc | (1,3)- or (1,6)-, and (1,4)-α-l-fuc | Anti-proliferative, antitumor, anticoagulant, antioxidant, antithrombotic, anti-inflammatory | [16,83,84,85,86,87,88] |
| Fucoidans; laminarans |
Eisenia bicyclis | Fuc; Glc |
(1,3)- and (1,6)-β-d-glc | Anti-proliferative, antitumor, anticoagulant; Antitumor |
[83,89,90,91] |
| Laminaran; S-laminaran or otherwise modified | Laminaria sp (or Saccharina) | Glc | (1,3)- and (1,6)-β-glc | Antitumor, anticoagulant, decreases liver triglyceride, cholesterol and phospholipid levels; serum hypocholesterolaemic, hypotensive, antibacterial, immunomodulator | [56,59,61] |
| S-fucoidans | Laminaria spp. | Fuc, xyl, man, glcAc | (1,3)-α-l-fuc | Antioxidant, anticoagulant, antithrombotic, anti-adhesive, anti-proliferative, anti-inflammatory, anti-angiogenic, anti-metastatic | [15,52,83,92,93,94,95,96] |
| S-galactofucan | L. japonica a.k.a. Saccharina japonica | Gal, fuc | (1,3)- and (1,4)-α-l-fuc (alternating) | Anti-lipidaemic, increases HDL, antiviral, antitumor, immunomodulator, antioxidant neuroprotective | [3,15,97,98,99,100,101,102] |
| Fucoidans | Lessonia vadosa | Fuc | Anticoagulant | [103] | |
| S-fucoidan | Saccharina cichorioides a.k.a. Laminaria cichorioides | Fuc | Antitumor, anticoagulant, anti-thrombotic | [104,105] | |
| S-galactofucans fucoidan | Undaria pinnatifida | Gal, fuc, xyl, uronic acid | (1,3)- and (1,4)-α-l-fuc (alternating) | Antiviral, anticoagulant, antitumor, anti-proliferative, immunomodulatory, anti-inflammatory induced osteoblastic differentiation | [3,52,69,106,107,108,109,110,111] |
| LMW-S-fucans | Anticoagulant | [112] | |||
| Laminaran; S-laminaran or otherwise modified | Glc | Anticoagulant, antitumor; serum hypocholesterolaemic, hypotensive, antibacterial, immunomodulator | [56,59,61] | ||
Table 2.
Marine species of red macroalgae (RHODOPHYTES) producing PS: some structural features and applications.
| Type of PS | Source | Structure | Action/ Application | References | |
|---|---|---|---|---|---|
| Main mono-Sugars/Disaccharide Units | Glycosidic Bonds of Backbone | ||||
| Rhodophyta Bangiales | |||||
| S-galactan porphyran | Porphyra spp. | Gal | (1,3)-β-d-gal or (1,4)-α-l-gal | Antitumor, hypotensive, regulates blood cholesterol | [113,114] |
| sPS | P. haitanensis | Antioxidant | [115] | ||
| Porphyran | P. yezoensis | Antitumor, immunomodulatory, hypolipidaemic | [116,117,118,119] | ||
| Ceramiales | |||||
| S-agarans | Bostrychia montagnei | Antiviral | [120] | ||
| S-agarans | Cryptopleura ramosa | Antiviral | [121] | ||
| Digenea simplex | Antiviral | [122] | |||
| Corallinales | |||||
| LMW-PS | Corallina sp. | Antiviral | [32] | ||
| Cryptonemiales | |||||
| Cryptonemia crenulata | Gal | Antiviral | [123] | ||
| S-agaran | Gloiopeltis complanata | Gal, Agal | [→3)-β-d-gal-(1→4)-3,6-α-l-Agal-(1→], and [→3)-β-d-gal-(1→4)-α-l-gal-(1→] | [114] | |
| Agaroid-carrageenan | G. furcata | Gal | 6-O-methyl-gal, 3,6Agal(1,3)-β-d-, and (1,4)-α-l-gal or (1,4)-α-l-Agal | [124] | |
| Gelidiales | |||||
| di-S-galactan | Gelidium crinale | Gal | Anticoagulant | [125] | |
| S-agarans and hybrid dl-galactans | Pterocladia capillacea | Gal | Antiviral | [126] | |
| Gigartinales | |||||
| S-agarans S-galactans | Aghardiella tenera |
Gal |
Antiviral | [127,128] | |
| S-λ-carrageenan | Chondrus crispus | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Antiviral, anticoagulant, antithrombotic | [1,5,129,130,131] |
| LMW-sPS | C. ocellatus | Antitumor | [132] | ||
| S-galactans | Euchema cottonii | Gal | Antioxidant | [2] | |
| S-κ-carrageenan | E. spinosa | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Anticoagulant, anti-thrombotic | [5,130,131] |
| LMW-sPS | Furcellaria lumbricalis | Immunostimulant | [133] | ||
| S-galactans | Gigartina acicularis | Gal | Antioxidant | [2] | |
| S-carrageenans | G. skottsbergii | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Antiviral, anticoagulant | [130,131,134,135] |
| Hybrid dl-galactans | Gymnogongrus torulosus | Gal | Antiviral | [136] | |
| LMW-PS | Hypnea charoides | Antiviral | [32] | ||
| LMW-S-carrageenans | Kappaphycus striatus | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Antitumor, immunomodulator | [1,131] |
| S-λ-carrageenan | Phyllophora brodiei | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Anticoagulant, antithrombotic | [130,131,137] |
| LMW-sPS | Soliera chordalis | Immunostimulant | [138] | ||
| S-carrageenans | Stenogramme interrupta | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Antiviral | [130,131,139] |
| Gracilariales | Antioxidant | [2] | |||
| sPS | Gracilaria caudata | ||||
| S-agarans S-galactans | G.corticata | Gal | Antiviral | [140] | |
| sPS | G. verrucosa | Immunomodulator | [141] | ||
| Halymeniales | |||||
|
S-galactan |
Grateloupia indica | Gal | Anticoagulant, antithrombotic | [137] | |
| Nemaliales | |||||
| S-mannans | Nemalion helminthoides | Man | Antiviral | [142] | |
| Xylogalactans S-xylomannans |
Nothogenia fastigiata | Xyl, gal Xyl, man |
Antiviral, anticoagulant | [143,144,145] | |
| Nematomatales | |||||
| S-galactans | Schizymenia dubyi | Gal, uronic acid | Antiviral | [146] | |
| S-λ-carrageenan | S. pacifica | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Antiviral | [130,131,147] |
| S-galactan | S. binderi | Gal | Anticoagulant | [148] | |
| Rhodymeniales | |||||
| di-S-galactan; LMW-sPS | Botryocladia occidentalis | Gal | Anticoagulant; anti-venom | [149,150] | |
| LMW-carrageenans | Champia feldmannii | Gal, Agal | (1,3)-α-d-gal, and (1,4)-β-3,6-Agal or (1,4)-β-d-gal (alternating) | Antitumor | [130,131,151] |
| Sebdeniales | |||||
| S-xylomannans | Sebdenia polydactyla | Xyl, man | Antiviral | [152] | |
Table 3.
Marine species of green macroalgae (CHLOROPHYTES) producing PS: some structural features and applications.
| Type of PS | Source | Structure | Action/ Application | References | |
|---|---|---|---|---|---|
| Main Mono-Sugars/Disaccharide Units | Glycosidic Bonds of Backbone | ||||
| Chlorophyta Bryopsidales | |||||
| sPS, including S-galactans | Caulerpa spp. | Antioxidant, anticoagulant, antithrombotic; antiviral, anti-proliferative, antitumor | [2,153,154] | ||
| sPS and derivatives | C. cupressoides | Gal, man, xyl | Anti-inflammatory, antinociceptive | [8,155,156] | |
| LMW-PS sPS |
C. racemosa | Gal, glc, ara, uronic acid | Antiviral; antitumor | [32,154,157] | |
| S-arabinogalactans | Codium spp. | Gal, ara | (1,3)-β-d-gal | Anticoagulant, antithrombotic, antiviral | [124,153,158,159,160,161] |
| S-pyrulylated-galactans | C. isthmocladum | (1,3)-β-d-gal | Antioxidant, anticoagulant, anti-proliferative | [2,162] | |
| Ulotrichales | |||||
| S-mannans | Capsosiphon fulvescens | Man, glcAc, gal | Immunomodulator | [163] | |
| S-rhamnans and LMW-S-rhamnans | Monostroma latissimum | Rham | (1,3)-α-l-rham, and (1,3)-α-l-rham or (1,2)-α-l-rham or (1→2,3)-α-l-rham | Antiviral, anticoagulant | [164,165,166,167,168] |
| S-rhamnans | M. nitidum | Rham, glc | Anticoagulant, antithrombotic, hepatoprotective, antitumor, immnunomodulator | [165,166,169,170,171] | |
| Ulvales | |||||
| Rhamnans | Enteromorpha intestinalis | Rham, xyl, glcAc | Antitumor, immunomodulator | [172,173] | |
| LMW-sPS | E. linza | Anticoagulant | [174] | ||
| S-ulvans and derivatives | E. prolifera | Immunomodulator, antioxidant, hypolipidaemic | [124,175,176,177] | ||
| S-ulvans and derivatives | Ulva spp. | Rham, xyl, glc, glcAc, IduAc | Anti-adhesive, antiproliferative, hepatoprotective | [178,179] | |
| sPS | U. conglobata | Rham, uronic acid | Anticoagulant | [180] | |
| sPS | U. fasciata | rham | Antioxidant. antitumor | [181] | |
| S-galactans sPS |
U. lactuca |
Rham, xyl, glcAc |
Antioxidant, anti-proliferative, hypocholesterolaemic, hepatoprotective, antitumor; Antiviral, anti-inflammatory, antinociceptive |
[90,182,183,184,185,186,187,188,189] | |
| S-ulvans | U. pertusa | Rham, xyl, glcAc, iduAc | [→4)-β-d-GlcAc-(1,4)-α-l-rham3S-(1→], and [→4)-α-l-IduAc-(1,4)-α-l-rham3S-(1→] | Antioxidant, anti-proliferative, hypocholesterolaemic | [90,182,183,184,185] |
| LMW-S-ulvan or otherwise modified | U. pertusa | Antioxidant, hypotriglyceridaemic, decrease LDL- and increases HDL-cholesterol, immunostimulatory | [166,185,190,191] | ||
| S-PS | U. rigida | Rham, glcAc | β-d-glcAc-(1,4)-l-rham (disacharide) | Immunostimulatory | [178,192] |
The PS produced by algae are presented in Table 1, Table 2 and Table 3, according to the group of macroalga, Phaeophytes, Rhodophytes, Chlorophytes, and in Table 4, which is relative to microalgae. Nevertheless, there are always some similarities between the PS from each group of seaweeds: often, fucoidans are extracted from brown algal species (Table 1), agaroids and carrageenans come from red macroalgae (Table 2), and ulvans are obtained from green seaweeds (Table 3). Regarding microalgae (Table 4), and as far as we know, there are not common names for their PS, to the exception of spirulan from Arthrospira platensis. There are species that, besides producing large amounts of these useful polymers, they secrete them out into the culture medium and these polymers are easily extracted [14].
Table 4.
Marine species of microalgae/blue-green algae producing PS; main neutral sugars.
| Type of PS | Source | Main Neutral Sugars | Action/Application | References |
|---|---|---|---|---|
| MICROALGAE | ||||
| Diatoms | ||||
| sPS | Cylindrotheca closterium | xyl, glc, man, rham | [193,194] | |
| sPS | Navicula salinarum | glc, xyl, gal, man | [193] | |
| s-EPS | Phaeodactylum tricornutum | glc, man, xyl, rham | Anti-adhesive | [195,196,197] |
| EPS | Haslea ostrearia | [198] | ||
| EPS | Nitzschia closterium | [199] | ||
| EPS | Skeletonema costatum | |||
| EPS | Chaetoceros spp. | rham, fuc, gal, man | [200] | |
| EPS | Amphora sp. | [201] | ||
| Chlorophytes | ||||
| sPS | Chlorella stigmatophora | glc, xyl, fuc, | Anti-inflammatory, immunomodulator | [195] |
| sPS | C. autotrophica | [202] | ||
| PS β-(1,3)-glucan |
C. vulgaris | rham, gal, arab, 2-O-methyl-rham glc |
Antitumor, infection preventive agent | [24,203,204] |
| EPS | Dunaliella salina | gal, glc, xyl, fru | [205] | |
| EPS | Ankistrodesmus angustus | [201] | ||
| EPS | Botryococcus braunii | gal, fuc, glc, rham | [206,207] | |
| Prasinophyte | ||||
| sPS | Tetraselmis sp. | Anti-adhesive | [202] | |
| Prymnesiophyte/haptophyte | ||||
| sPS | Isochrysis sp. | [202] | ||
| Rhodophytes | ||||
| sPS | Porphyridium sp. | xyl, gal, glc | Anti-inflammatory, immunomodulator, prevention of tumour cell growth, anti-adhesive, antiviral, biolubricant | [208,209,210,211,212,213] |
| sPS | P. cruentum | xyl, gal, glc, glcAc, 3-O-methyl-xyl | Antioxidant and free radical scavenging, immunomodulator, antiviral, antibacterial, antilipidaemic, antiglycaemic | [214,215,216,217,218,219,220,221,222] |
| sPS | P. purpureum | antiviral | [223] | |
| sPS | Rhodella reticulata | xyl, rham, 3-O-methyl-rham, 4-O-methyl-gal | Antiviral, antilipidaemic, antiglycaemic, prevention of tumour cell growth | [208,213,219], |
| R. maculata | xyl, gal, glc,3-O-methyl-xyl | [224,225] | ||
| Dinoflagellates | ||||
| sPS | Cochlodinium polykrikoides | man, gal, glc | Antiviral | [226] |
| sPS | Gyrodinium impudicum | gal | Antiviral, anti-inflammatory, immunomodulator, anti-proliferative, prevention of tumour cell growth | [23,227,228,229] |
| CYANOBACTERIA | ||||
| EPS | Aphanothece halophytica | glc, fuc, man, arab, glcAc | [230] | |
| EPS s-Spirulan |
Arthrospira platensis | gal, xyl, glc, fru rham, fuc, glc, 3-O-methyl-rham |
Antiviral, antibacterial, prevention of tumour cell growth Anti-proliferative, anti-adhesive, anti-metastatic |
[19,223,231,232,233,234,235] |
| sPS | Anabaena, Gloethece, Nostoc Aphanocapsa, Phormidium, Synechocystis, Cyanothece | [19] |
Both micro- and macroalgae are excellent sources of PS, most of them being sulphated (sPS). They are associated with several biological activities and potential health benefits, making them interesting compounds for the application in pharmaceuticals, therapeutics, and regenerative medicine. Some of the beneficial bioactivities demonstrated by the crude PS and their derivatives, either in vitro or in vivo, upon various kinds of cell-lines and animal models, include anticoagulant and/or antithrombotic properties, immunomodulatory ability, antitumor and cancer preventive activity (as anti-proliferative agents, tumour suppressors or natural cell-killers). They are also good antidislipidaemic and hypoglycaemic agents, and can be powerful antioxidants, antibiotics and anti-inflammatory. For example, the sPS from Enteromorpha and Porphyridium have demonstrated strong antitumor and immunomodulating properties [173,211]; those from Caulerpa cupressoides and Dyctiota menstrualis are good antinociceptive agents [1,155], and the sPS from Cladosiphon okaramanus showed angiogenic, gastro- and cardioprotective bioactivities [15,46,47].
2. Some Structural Characteristics of Polysaccharides Produced by Marine Algae
The chemical structure of PS produced by macro- and microalgae may significantly determine their properties, namely physico-chemical and biochemical, and reflect their physical behavior and biological activities, as will be discussed further on in this review.
2.1. Macroalgae
Seaweeds (or marine macroalgae), whose PS have been studied more often, belong to the groups Chlorophyta (green seaweeds), Phaeophyceae (brown algae, Chromophyta) and Rhodophyta (red macroalgae).
Brown seaweeds usually contain fucoidans; the oligosaccharides obtained from the hydrolysis of fucoidans may often contain gal, glc, uronic acids, and/or other monosaccharides (Table 1), linked together and to the main chain by different types of glycosidic bonds. This is the case, for example, for the laminaran from E. bicyclis (Laminarales), or the galactofucan from Sargassum sp. (Fucales), and the fucan from P. tetrastromatica (Dictyotales) (Table 1). However, the structure complexity of these fucoidans makes difficult to establish a relationship between the PS-chains/composition and their biological actions, and/or some kind of protocols to design universal pharmaceuticals or other drug-like substances to prevent and/or cure specific diseases. This issue will be discussed later in this review.
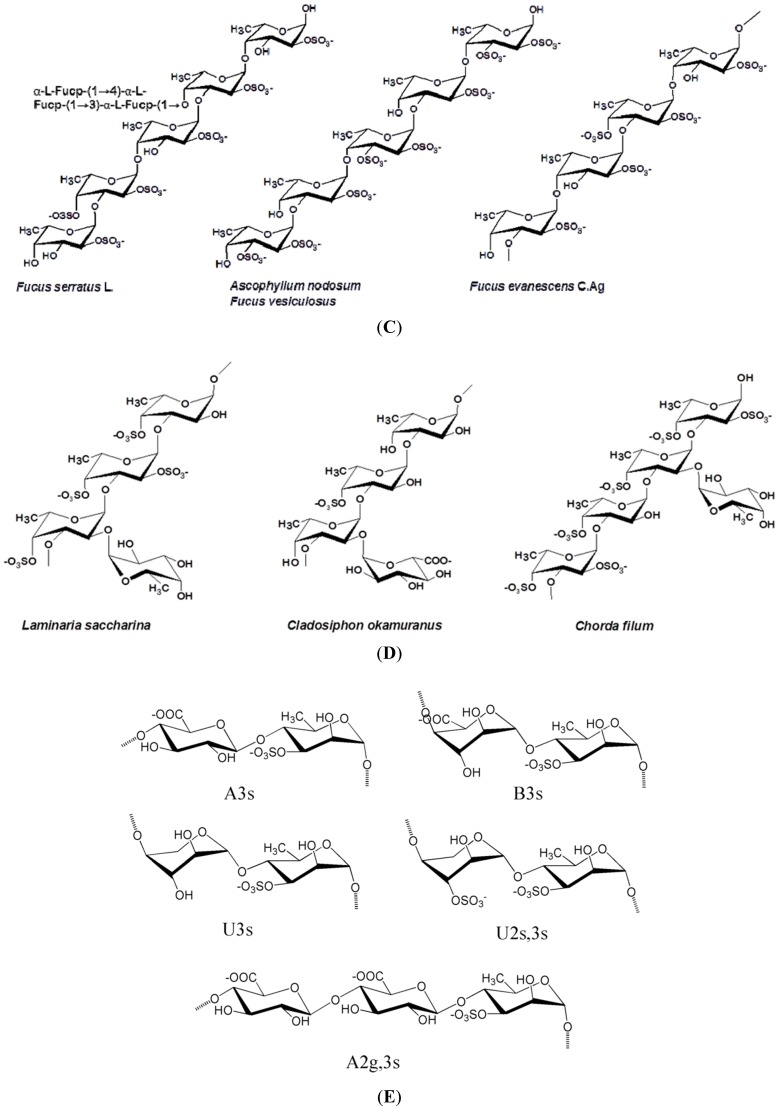
The monosaccharide composition, the linkage types, the overall structure of fucoidans, and some of their di- and oligosaccharides were well explored by Ale et al. [75], Fedorov et al. [3] and Li et al. [103]. For example, Ale’s group [75] showed the difference between sPS from three species of Fucus by focusing on the various substituents at C-2 and C-4 carbons, despite the similarities of their backbones; they also highlighted the possible structures of fucoidans from two species of Sargassum, already suggested by Duarte et al. [78] and Li et al. [71]. Cumashi and coworkers suggested some structures for the backbone chain of several seaweeds [15]. Among them are the schemes for the components of the main chain showing either the (1,3)-, and (1,3)- and (1,4)-linked fuc residues or some di- and trisaccharide repeating units for A. nodosum, C. okamuranus, L. saccharina (a.k.a. Saccharina latissima), and some species of Fucus. On the other hand, Fedorov et al. [3] focused on the structures and bioactivities of different sPS, such as fucoidans (e.g., galactofucan from Laminaria (a.k.a. Saccharina japonica), and laminarans (e.g., the one from E. bicyclis) (Table 1).
Red macroalgae contain large amounts of sPS (Table 2), mostly galactans (agaroids and/or carrageenans), with alternating repeating units of 1,3-α-gal and 1,4-β-d-gal [236], and/or 3,6-anhydrogal (3,6-Agal) [237]. Substituents can be other monosaccharides (man, xyl), sulphate, methoxy and/or pyruvate groups, the pattern of sulphation dividing carrageenans into different families, for example, in C-4 for κ-carrageenan, and in C-2 for λ-carrageenan. In addition, the rotation of gal in 1,3-linked residues divides agaroids (l-isomer) from carrageenans (d-isomer) [18]. Apart from agarans [18], found in species of Porphyra, Polysiphonia, Acanthophora, Goiopeltis, Bostrychia or Cryptopleura (Table 2), red seaweeds are also good sources of κ-carrageenan (E. spinosa, K. alvarezii), λ-carrageenan (Chondrus sp, G. skottsbergii and Phillophora) (Table 2) [238], ι-carrageenan (E. spinosa) [239], and other heterogalactans with man and/or xyl bulding up their backbones. Among these, we may find xylogalactans in N. fastigiata [143], xylomannans in S. polydactyla [152] (Table 2).
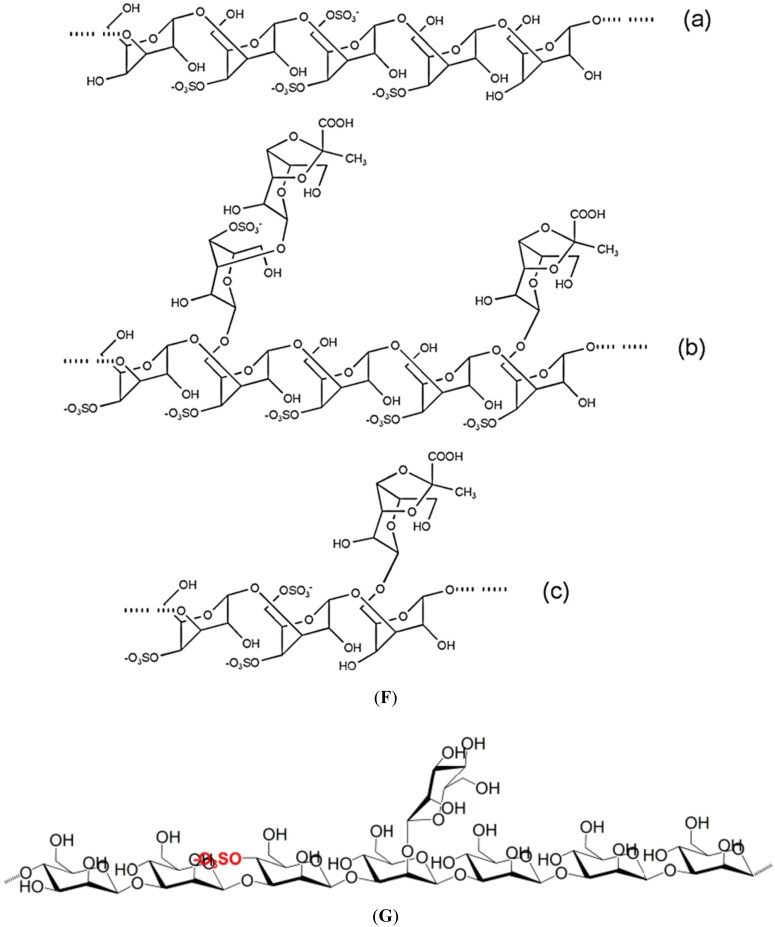
Regarding green macroalgae, the information on their structures and applications is scarce. Nevertheless, Wangs’s group [8] has made an excellent overview on those properties for the sPS from several genera of “macro-chlorophytes”. These sPS are very diverse and complex, with various types of glycosidic bonds between monomers, and include galactans (Caulerpa spp.), rhamnans (C. fulvescens and Enteromorpha), arabino- and pyruvylated galactans (Codium spp.), and the most known ulvans from Ulva spp and E. prolifera (Table 3). Wang and coworkers [8] also included some repeating aldobiuronic di-units for the backbone of ulvans, containing IduAc or glcAc (U. armoricana and U. rigida, respectively), disaccharides (S-)xyl-S-rham, and a trisaccharide unit composed by 1,4-linked glcAc, glcAc, and S-rham. The backbone of rhamnans seems to be somewhat simpler (Table 3), but other types of glycosidic bonds can also appear. For example, four repeating disaccharide units were indicated for the homopolymer of M. latissimum [240] (Table 3). Species from Codium are very interesting: their sPS may include different percentages of arabinose (ara) and gal, giving place to arabinans (C. adhaerens; [153]), galactans (C. yezoense) [241], arabinogalactans [8]. Pyruvylated galactans were also identified in C. yezoense [241], C. isthmocladium [2] and C. fragile [242]. Some other species of Codium present other PS-types such as (1,4)-β-d mannans in C. vermilara [158], or the rare (1,3)-β-d mannans in C. fragile [243], with various sulphation patterns. C. fulvescens contains “vary branched” S-mannan as well [163].
2.2. Microalgae and Cyanobacteria
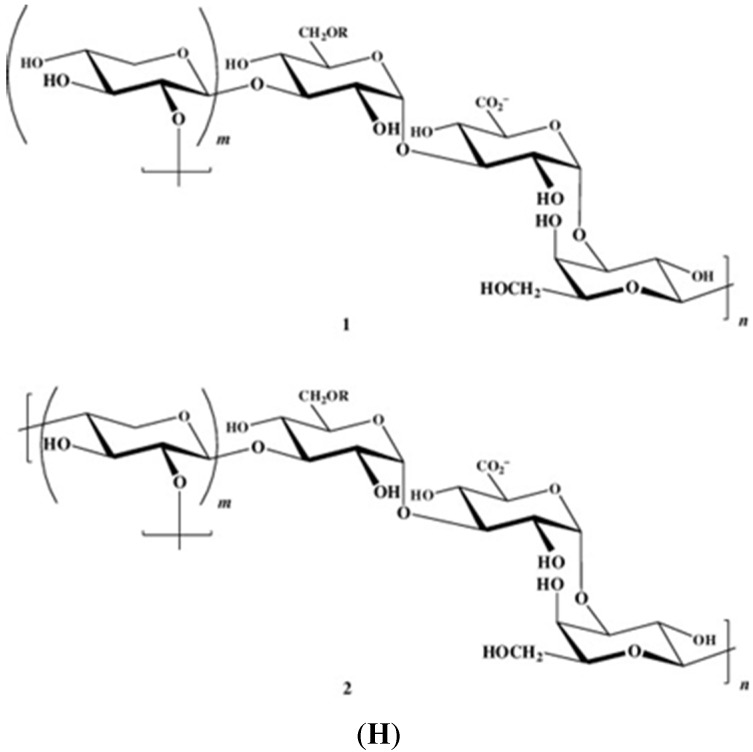
The characteristics of the various PS produced by microalgae, including their composition and structure, were recently discussed [14]. Some particular aspects about these polymers came to light. For example, it seems that concerning microalgae only G. impudicum and C. vulgaris contain homo-PS of galactose (gal) [23] and glucose (glc) [24], respectively, while the PS from the other species are heteropolymers of gal, xylose (xyl) and glc in different proportions. Rhamnose (rham), fuc and fructose can also appear, and some of the microalgal PS present uronic acids as well (Table 4). The glycosidic bonds are described for only a few PS, such as the one from Aphanothece halophytica, whose monosaccharides are mainly 1,3-linked, but linkages of type 1 also appear for glc and glcAc [230], which suggests that these two last molecules are terminal, and some multiple bonds, such as 1,2,4-linked and 1,3,6-linked mannose (man) residues [230], are present as well, suggesting some branches coming out from the backbone of the PS. Further, there are some special features of microalgal PS, as it is the case of acofriose 3-O-methyl-rham in the polymers of Chlorella [203], Botryococcus braunii and calcium-spirulan (CaSp) of Arthrospira platensis [244]. In Porphyridium cruentum, an aldobiuronic acid [3-O-(α-d-glucopyranosyluronic acid)-l-galactopyranose), or glcAc-gal disaccharide], and two hetero-oligosaccharides were also identified [245], and so did two other aldobiuronic acids [246], which were also found in other species of Porphyridium and Rhodella [247]. Furthermore, other repeating disaccharide-units [233,234], and some oligosaccharides were also highlighted [233]. In addition, Ford and Percival [196,197] found that the structure of the sPS from Phaeodactylum tricornutum was a ramified sulphated glucoronomannan, with a backbone composed by β-(1,3)-linked man; a triuronic acid, an aldobiuronic acid and a glucan made of β-(1,3)-linked glc were also identified as being constituents of the side chains of that polymer.
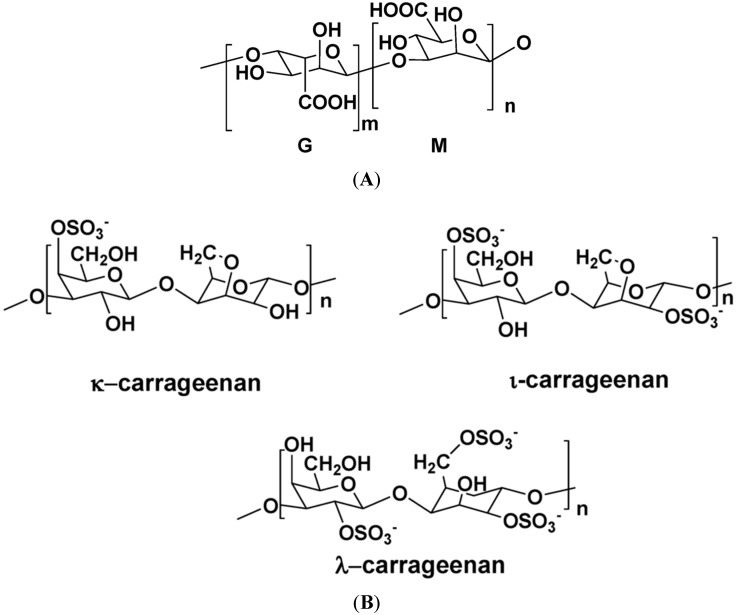
Figure 1 illustrates the structures of some PS from macro- and microalgae.
Figure 1.
Examples of structures of PS from macro- and microalgae. (A) Repeating units suggested for the structure of alginates [3]; (B) Repeating units of some carrageenans [3]; (C) Fucoidan backbone of A. nodosum and three species of Fucus, showing the different distribution pattern of sulphate [75]; (D) Repeating units, sulphation pattern and gycosidic bounds of the backbone structures of PS of three different brown seaweeds [75]; (E) Alternative positions and combinations for the repeating units of ulvans. A3s and B3s are aldobiouronic repeating di-units suggested for U. rigida and U. armoricana. U3s and U2s,3s are, respectively, a xyl-(S-rham) and a (S-xyl)-(S-rham) disaccharides [8]; (F) Galactans of Codium spp. (a) linear (1,3)-β-d-galactan, (b) and (c) pyruvylated branched sulphated galactans [8]; (G) A rare mannan of the PS from C. fragile, with (1,3)-β-man residues and branches at C-2 [8]. Tabarsa et al. [243] referred that either branches or sulphates may be bound at the C-2 and/or C-4 positions along the PS backbone); (H) Models 1 or 2 for the possible acidic repeating unit in polysaccharide II, from Porphyridium sp. R = H, SO2O, terminal gal or terminal xyl, m = 2 or 3 [14].
3. Potential Medical/Biomedical Applications of Polysaccharides from Marine Algae. Relation with Some Chemical Features of Their Structures
The PS are complex and heterogeneous macromolecules, coming from different genera belonging to the larger groups of algae, and species and strains of the same genus. Often, difficulties are found in identifying their chemical structure and therefore, their biological activities not being thoroughly understood. Few researchers have focused on such a challenging task as the exploitation of possible relation chemical structure–activity of PS. One approach to look for structure–biological activity relationships has been to make inferences based on information obtained from studies of invertebrate sulphated polysaccharides that have a regular structure and, thus, could be more easily studied [18].
The types of glycosidic linkages and the contents and positions of the sulphate groups may be significantly different in the various sPS, depending on species, region of the thallus, growing conditions, extraction procedures, and analytical methods [2,186]. The biological and pharmacological activities of sPS normally result from a complex interaction of several structural features, including the sulphation level, distribution of sulphate groups along the polysaccharide backbone, molecular weight, sugar residue composition, and stereochemistry [248,249]. For instance, the general structural features of fucans that are important in their anticoagulation activity include the sugar composition, molecular weight, sulphation level and the position of sulphate groups on the sugar backbone [84,86,250,251]. Also, it has been observed that the antiviral activity of sPS increases with the molecular weight [252]. Galactans, fucans and galactofucans are representative polysaccharides from brown and red seaweeds that differ in structure, sulphation level and molecular weight, and yet all were shown to inhibit HSV-1 and HSV-2 infection [253]. Recently, by using NMR, it was found that branched fucoidan oligosaccharides might present higher imuno-inflammatory activity than linear structures, because they were better at inhibiting the complement system [254]. Usov [236] compared two sulphated galactans from Botryocladia occidentalis and Gelidium crinale. He concluded that the interaction of the sPS with different compounds participating in the coagulation process depends on the differences in the structural features; unfortunately, data on the configuration of galactose in the galactan from G. crinale are not sufficient to fully understand the relationship.
3.1. Antiviral, Antibacterial and Antifungal Activities
An overview on the antiviral activity against several kinds of virus and retrovirus, enveloped or naked was well documented by Carlucci et al. [255] and Wijesekara et al. [21]. These reviews focused on the HIV type 1 and type 2, the human papilloma virus (HPV), the encephalo-myocarditis virus, the hepatitis virus type A and type B and the dengue and yellow fever virus. The inhibition of infection by most of these viruses was explained by the action of sPS, which might block the attachment of virions to the host cell surfaces [140,256]. Another way of exerting their activity is by inhibiting the replication of the enveloped virus, such as the HIV, the human cytomegalovirus (HCMV) and the respiratory syncytial virus (RSV) [18,147,153], either by inhibiting the virus adsorption or the entry into the host cells. Some of the sPS are effective only if applied simultaneously with the virus or immediately after infection [18]. Another mechanism of action of fucoidans and other sPS is through the inhibition of the syncytium formation induced by viruses [21,257].
Some S-xylomannans were reported to present antiviral sulphate-dependent activity, as it was the case of PS from S. polydactyla and S. latifolium, which inhibited the multiplication of HSV-1 in Vero-cells [152,258]. In addition, the molecular weight (MW) seems to play an important role in the antiviral properties of the sPS, the effect increasing with the MW [18]. However, other structural features can be co-responsible for the reinforcement of the antiviral effectiveness, like sulphation patterns, composition and distribution of sugar residues along the backbone, and the complexity of the polymers [18,152,248,253]. Further, the fucoidans from L. japonica already proved their effectiveness in fighting both RNA and DNA viruses [103], such as poliovirus III, adenovirus III, ECHO6 virus, coxsackie B3 and A16 viruses. Moreover, these sPS can protect host cells by inhibiting the cytopathic activity of those viruses [99].
In addition to their virucidal activity against HIV and other viruses associated to sexually transmitted diseases (STD) [5], including HPV, some carrageenans might find application as vaginal lubricant gels and coatings of condoms, with microbicidal activity, for they do not present any significant anticoagulant properties or cytotoxicity [259,260]. Furthermore, some fucoidans, apart from inhibiting attachment of virus particles to host cells, were able to inhibit the attachment of human spermatozoids to the zona pellucida of oocytes [261]; this property could be used for the development of a contraceptive gel with microbicidal characteristics [20].
The polysaccharides produced by some marine microalgae, and which may be released into the culture medium, showed antiviral activity against different kinds of viruses, such as the HIV-1, HSV-1 and HSV-2, VACV and Flu-A (Table 4), as described by Raposo et al. [14]. Sulphated PS, in particular, proved to increase the antiviral capacity [231]. In fact, the antiviral activity of the PS may depend on the culture medium, algal strain and cell line used for testing, but also on the methodology, and the degree of sulphation, as is the case of EPS from P. cruentum [216,262]. Despite the slight toxicity that some PS may present, they could be safely applied in in vivo experiments, decreasing the replication of the virus VACV, for instance [223].
The mechanisms involved in the antiviral activity of sPS may be understood analyzing what happens when cells are infected by a virus. Just before infection, viruses have to interact with some glycosaminoglycan receptors (GAG), such as heparin sulphate (HS) [263]. The GAG to which a protein can be covalently bound are part of the target cell surface and can also be found in the intracellular matrix of various connective and muscle tissues. SPS may impair the attachment of the virus particles by competing for those GAG-receptors, as they are chemically similar to HS [130,255], most of them having a covalently linked core protein [264,265]. Besides, as it happens with GAG, sPS are negatively charged and highly sulphated polymers [40,255,266], whose monosaccharide distribution pattern might influence the specificity of the bound protein, determining several biological functions [263]. For viruses to attach to the host cell surface, the linkage between the basic groups of the glycoproteins of the virus and the anionic components of the PS (sulphate, for example) at the cell surface must be established [248]. In fact, whichever the algal PS is, either from seaweeds or microalgae, by mimicking these GAG, they may induce the formation of a virus-algal PS complex, thus, impairing the cell infection by blocking the interaction virus-host cell receptor. Hidari and coworkers [40], for instance, showed that dengue virus (DENV) establishes an exclusive complex with fucoidan, and viral infection is, therefore, inhibited. They suggested that arginine-323 had a high influence on the interaction between the DENV-2 virus and the fucoidan, in an in vitro experiment with BHK-21 cells. These researchers also found that glucuronic acid seems to be crucial since no antiviral activity was observed when this compound was reduced to glucose.
Sulphated polysaccharides from seaweeds, such as alginates, fucoidans and laminaran appear to have antibacterial activity against E. coli and species from Staphylococcus. A fucoidan from L. japonica and sodium alginate were found to inhibit E. coli [267], for example, by adhering to bacteria and killing those microorganisms [5], thus showing bactericidal properties. This type of PS is also a good antibacterial agent against Helicobacter pylori, eradicating their colonies, restoring the stomach mucosa, in clinical trial studies, and regenerating biocenosis in the intestines [268]. Laminaran from Fucus, Laminaria, A. nodosum and U. pinnatifida demonstrated to have an effect on pathogenic bacteria [56] as well, with the advantage of being unable to promote blood coagulation [269]. An S-galactan from Chaetomorpha aerea inhibited the growth of Staphylococcus aureus (50 mg/mL of extract) but not that of Salmonella enteritidis [270]. In contrast, the carrageenans from some seaweeds [271] and the sulphated exopolysaccharide (sEPS) from the red microalga Porphyridium cruentum, despite the higher concentration used [216], showed a significant inhibitory activity against S. enteritidis. In fact, some PS from microalgae, such as A. platensis (Table 4), may present antibacterial properties against some specific bacteria, the activity depending on the solvent used to extract the polymer, as referred to by Raposo et al. [14].
By stimulating the production and/or expression of ILs, dectin-1 and toll-like receptors-2 on macrophages and dendritic cells, respectively, (1,3)-β-glucans from, e.g., C. vulgaris, and laminarans, also induced antifungal and antibacterial responses in rats [272], and some resistance to mammal organisms towards infections by E. coli [273]. Therefore, these types of PS promise to be good antimicrobial agents.
3.2. Anti-Inflammatory and Immunomodulatory Activities
Polysaccharides from macro- and microalgae have long demonstrated to have biological and pharmaceutical properties, such as anti-inflammatory and immunomodulation (Table 1, Table 2, Table 3 and Table 4) [14]. Neverthless, the anti-inflammatory properties may be shown in several ways, depending on the PS, its source and type/site of inflammation. There is growing evidence that sPS are able to interefere with the migration of leukocytes to the sites of inflammation. For example, the heterofucan from D. menstrualis decreases inflammation by directly binding to the cell surface of leukocytes, especially polymorphonuclear cells (PMNs). It completely inhibits the migration of the leukocytes into the peritoneal cavity of mice where the injured tissue was after being submitted to simulated pain and inflammation, without the production of pro-inflammatory cytokines [1]. Sometimes, the recruitment of these PMNs shows to be dependent on P- and/or L-selectins, as it was demonstrated for fucoidans of some brown seaweeds [15,112].
Some other studies refer the association of the anti-inflammatory activity with the immunomodulatory ability. This seems to be the case in the work by Kang et al. [88], who simulated an inflammation process in RAW 264.7 cells (peritoneal macrophage primary cells) induced by lipopolysaccharides (LPS). They found that the fucoidan from E. cava inhibited, in a dose-dependent manner, the enzyme nitric oxide synthase induced by LPS (iNOS) and the gene expression for the enzyme cyclooxygenase-2 (COX-2) and, as a consequence, the production of nitric oxide (NO) and prostaglandin E2 (PGL2). Li et al. [274] confirmed the anti-inflammation mechanism in vivo via the immunomodulatory system in vivo, since the fucoidan from L. japonica reduced the inflammation of rats’ myocardium damaged cells, by inactivating the cytokines HMG B1 and NF-κB, two groups of proteins secreted by the immune cells during inflammatory diseases. These protective and regenerative effects of fucoidans (from A. nodosum), via the immunomodulatory system, were also verified in the destruction/proteolysis of connective tissue by Senni et al. [275]. These researchers referred to the fact that severe inflammation and the subsequent excessive release of cytokines and matrix proteinases could result in rheumatoid arthritis or chronic wounds and leg ulcers, which could be treated with fucoidans [275].
In addition to the polysaccharide from Ulva rigida, a green seaweed [192], the sPS p-KG03 from the marine dinoflagellate G. impudicum, also activates the production of nitric oxide and immunostimulates the production of cytokines in macrophages [227].
The enhancement of the immunomodulatory system by some sPS from marine algae is also a way for sPS to suppress tumour cell’s growth and their proliferation, and to be natural neoplastic-cell killers (apoptotic effect).
Studies with arabinogalactan and other fucoidans revealed them to be immunostimulators by activating macrophages and lymphocytes, which suggests their effectiveness in the immuno-prevention of cancer [22,276]. The PS from U. pinnatifida was also suggested to treat/relieve the symptoms of pulmonary allergic inflammation as it supresses the activity of Th2 immune responses [111]. On the other hand, fucoidan activated macrophages and splenocytes to produce cytokines and chemokines [277].
Polysaccharides from marine microalgae, such as Porphyridium, Phaeodactylum, and C. stigmatophora (Table 4), showed pharmacological properties, such as anti-inflammatory activity and as immunomodulatory agents, as reported by Raposo et al. [14]. Some of these sPS, for example, the ones from C. stigmatophora and P. tricornutum (Table 4), have revealed anti-inflammatory efficacy in vivo and in vitro [195]. The mechanisms underlying the anti-inflammatory and immunomodulatory activities may be unsderstood by making some considerations at the molecular level. On one side, the protein moiety that is covalently bound to most PS seems to play a critical role in the activation of NF-κB and MAPK pathways involved in the macrophage stimulation [265,278]. This was evidenced in an in vitro experiment performed by Tabarsa and colleagues [265]. They showed that the PS from C. fragile was not able to stimulate RAW264.7 cells to produce NO and the protein alone was also unable to induce NO release, but the complex sPS-protein did inhibit the inflammatory process. On the other side, several other researchers found that proteins were not essential or responsible for the immunostimulatory responses of the cells [192,279]. In addition, Tabarsa and coworkers [265] demonstrated that the sulphate content and the MW were not crucial for the stimulation of murine macrophage cells. In fact, both desulphated and LMW-PS derivatives of C. fragile produced immunomodulatory responses similar to the ones of the original PS. In contrast, the sPS from U. rigida induced a strong sulphate-dependent release of NO [192], thus, the sulphate content showing to be essential for the stimulation of macrophages. These researchers mentioned the possibility of the sulphate interfering in the interaction PS-cell surface receptors.
The interaction of algal sPS with the complement system suggests that they might influence the innate immunity to reduce the pro-inflammatory state [254]. In addition, algal polysaccharides have been shown to regulate the innate immune response directly by binding to pattern recognition receptors (PRRs) [280]. For example, λ-carrageenan stimulated mouse T cell cultures in a toll-like receptor-4 (TLR4) [281].
Different effects were observed in other types of sPS: Zhou et al. [282] proved that carrageenans from Chondrus with lower molecular weights better stimulated the immune system. The same trend was verified for the sEPS from the red microalga Porphyridium [221], a 6.53 kDa LMW-fragment at 100 µg/mL presenting the strongest immunostimulating activity.
It is worth remarking that carrageenans from red seaweeds are recognized for triggering potent inflammatory and carcinogenic effects either in rats and mice cells [130]. However, while some carrageenans stimulate the activity of macrophages, others inhibit macrophage activities [21].
Although PS from various macro- and microalgae do not show anticoagulant and/or antithrombotic activities, attention should be paid to the anticoagulant properties of some PS, since their use could cause severe bleeding complications. This issue will be discussed further on in this review.
3.3. Anti-Proliferative, Tumour Suppressor, Apoptotic and Cytotoxicity Activities
Because of the growing number of individuals suffering from different types of cancer and the secondary effects of synthetic chemicals and other types of treatment used against tumour damages, research was driven towards demand for natural therapeutics with bioactive compounds. In this context, sPS from both macro- and microalgae already proved to have antitumor biological activities.
An S-fucoidan from C. okamuranus exhibited anti-proliferative activity in U937 cells (myeloid cancer cell-line) by inducing cell apoptosis following a pathway dependent of Caspases-3 and -7 [43]. In another study, conducted by Heneji’s group [283], a similar fucoidan induced apoptosis in two different leukaemia cell lines. These results indicate that fucoidans might be good candidates for alternative therapeutics in treating adult T-cell leukaemia [22]. S-fucoidans from E. cava also seem to be promising to treat other types of human leukaemia (monocyte- and promyelocytic-origin) cell-lines [284]. There was some evidence that the fucoidan from L. guryanovae inactivated the epidermal growth factor (tyrosine kinase) receptor (EGFR), which is greatly involved in cell transformation, differentiation and proliferation [285,286]. Therefore, this kind of sPS could be used as antitumor and anti-metastatic therapeutical/preventing agent, which might act either on tumour cells or by stimulating the immune response [287].
Further, the sPS from E. bicyclis and several other seaweeds (Table 1, Table 2 and Table 3) have demonstrated their potent bioactivity against different kinds of tumours, including lung and skin, both in vitro and in vivo [62,83,288,289] causing apoptosis in various tumour cell-lines [62,290,291,292]. The mechanisms involved in this antitumor activity might be associated again with the production of pro-inflammatory interleukins IL-2 and IL-12 and cytokine interferon-gamma (INF-γ) by the immune-stimulated macrophages, together with the increase of the activity of the natural killer cells (NK cells) and the induction of apoptosis [62,293]. NK cells can also upregulate the secretion of IFN-γ, which can activate either the T-cells for the production of IL-2 or the macrophages, which, after being activated, keep on producing IL-12 and activating NK cells [293,294]. The enhancement of the cytotoxicity of these NK cells (lymphocytes and macrophages) can be stimulated by other sPS such as fucoidans and carrageenans from other seaweeds [276,282]. Polysaccharides can also activate some signalling receptors in the membranes of macrophages, such as Toll-like receptor-4 (TLR-4), cluster of differentiation 14 (CD14), competent receptor-3 (CR-3) and scavenging receptor (SR) [295]; these are also activated by other intracellular pathways, involving several other protein-kinases, that enhance the production of NO, which, in turn, plays an important role in causing tumour apoptosis [295]. These immunomodulation properties of S-fucoidans could be used for the protection of the damaged gastric mucosa as it was already demonstrated by using rat-models [296]. More information on the pathways and mechanisms responsible for the immune-inflammatory activities, including the involvement of the complementary system, may be found in Jiao and colleagues’ work [18].
The anti-adhesive properties of some sPS, especially fucoidans might also explain their anti-metastatic activity (Table 1, Table 2 and Table 3), both in vitro and in vivo, in various animal-models [15,297], as they can inhibit the adhesion of tumour cells to platelets, thus decreasing the possibilities of proliferation of neoplastic cells. The mechanisms by which fucoidans and other sPS exert their anti-adhesive ability were well documented by Li’s group [103]. Some researchers also highlighted the mitogenic properties and the cytotoxicity and tumoricidal activity of some arabinogalactans and fucoidans as well [42,276], either in different cell-lines or various animal-models.
The anti-adhesive properties of algal sPS may also be relevant as these polymers can block the adhesion of tumour cells to the basal membrane, thus demonstrating to impair implantation of tumour cells and metastatic activity by binding to the extracellular matrix [37]. For example, the sPS from Cladosiphon was shown to prevent gastric cancer in vivo, since it inhibited the adhesion of H. pylori to the stomach mucosa (mucin) of gerbils [45]. Metastasis appearance could also be reduced in vivo by S-laminaran, a 1,3:1,6-β-d-glucan, because this compound inhibited the activity of heparanase, an endo-β-d-glucuronidase involved in the degradation of the main PS component in the basal membrane and the extracellular matrix. The expression of this enzyme is known to be associated with tumour metastasis [59].
These antitumor properties may also be found in some PS from microalgae, such as A. platensis, which are inhibitors of cell proliferation [234]. Other sPS, such as sPS p-KG03 from G. impudicum, have also anti-proliferative activity in cancer cell lines (in vitro) and inhibitory activity against tumour growth (in vivo) [227,228,298]. Other PS from microalgae, such as C. vulgaris (Table 4), and sPS or LMW-derivatives of sPS from P. cruentum (Table 4), for example, are described as having similar properties in the review performed by Raposo et al. [14].
In some research work, the immunomodulatory activity was associated to the ability of inhibiting carcinogenesis. For example, Jiao’s group [172] found that a sulphated rhamnan and some derivatives from the green seaweed E. intestinalis suppressed tumour cell growth in vivo (mice), but they did not show any toxicity against tumour cells in vitro. The oral administration of the sPS to mice enhanced the spleen and thymus indexes, and also induced the production of TNF-α and NO in macrophages, increased lymphocyte proliferation, and enhanced TNF-α release into serum.
The degree of sulphation may play some role in the carcinogenesis process, although the action of the sPS may also depend on the type of tumour. In fact, an oversulphated PS demonstrated the capacity of inhibiting the growth of L-1210 leukaemia tumour in mice, but, on the other hand, it was unable to inhibit the growth of Sarcoma-180 tumour in mice [83]. In addition to the sulphation level, MW may also influence the anticancer activity. For instance, LMW-PS derivatives showed to enhance antitumor activity [91,299]. However, the increment in the anticancer activity greatly depends on the conditions of the PS depolymerisation [299]. Kaeffer et al. [186] suggested that the in vitro antitumor activity of LMW-PS, sulphated or not, against cancerous colonic epithelial cells (Caco cells) might be associated with the inhibition of tumour cells proliferation and/or differentiation.
3.4. Anticoagulant and Antithrombotic Activities
There are several studies on the anticoagulant properties of PS isolated from seaweeds, presented in a recent review [14] by different groups of researchers: Wang et al. [8], Costa and colleagues [2], Cumashi et al. [15], Athukorala et al. [300] and Wijesekara and coworkers [21].
The main sources of the sPS from green seaweeds with anticoagulant properties are Codium and Monostroma [167,301]. Some of the PS, such as S-rhamnans, showed their action by extending the clotting time via the intrinsic and extrinsic pathways [167]. In fact, Codium spp present strong anticoagulant effects [159,160], but other species from Division/Phyllum Chlorophyta also contain sPS (native, low-molecular or otherwise modified) with anticoagulant properties (Table 3). The mechanism of action of the referred PS is mostly attributed to either a direct inhibition of thrombin or by enhancing the power of antithrombin III [302,303].
Some other PS from green seaweeds also showed potent anticoagulant properties but their mechanisms of action are associated not only to a direct increase in the clotting time (APTT assays) by inhibiting the contact activation pathway (intrinsic pathway), but also by inhibiting the heparin cofactor II-mediated action of thrombin [180,304] thus showing a potent antithrombotic bioactivity.
In addition to their anticoagulant properties demonstrated in vitro by APTT and TT tests, several sPS from algae of different groups (Table 1, Table 2 and Table 3) present antithrombotic qualities in vivo [305,306] by increasing the time of clot formation. In fact, Wang and colleagues [8] published an exhaustive work on this issue by including a summary table with 24 references about both the anticoagulant, and anti- and prothrombotic activities of several sPS from various green seaweeds. In two other studies, Wijesekara et al. [21] and Costa and coworkers [2] also included the sPS from brown and red macroalgae that present effects on the blood clotting time. Wijesekara and colleagues [21] referred to the fact that there are few reports on the interference of PS from algae on the PT (prothrombin) pathway, meaning that most of the marine sPS may not affect the extrinsic pathway of coagulation [21]. As a matter of fact, Costa et al. [2] did not detect any inhibition in the extrinsic coagulation pathway (PT test), for the concentrations used; only C. cupressoides increased the clotting time. In addition, they found no anticoagulant properties (APTT and PT assays) in the sPS from a brown seaweed (S. filipendula) and a red macroalga (G. caudate). Further, in our laboratory we found no anticoagulant properties in the sEPS from different strains of the red microalga P. cruentum, despite the high content in sulphate and molecular weight. As Costa et al. [2] observed, this could be due to the absence of sulphate groups in the monosaccharides at the non-reducing ends of the branches, which impaired the interaction between target proteases and coagulation factors. Nishino et al. [84] and Dobashi et al. [72] defended that there might be no effect above an upper limit for the content in sulphate, since the difference in the anticoagulant and antithrombotic activities decreased with the increase of the sulphate content.
It seems that some of the chemical and structural features of the sPS may have some influence on their anticoagulant and/or antithrombotic activities. The degree and distribution pattern of sulphate, the nature and distribution of monosaccharides and their glycosidic bonds, and also the molecular weight showed to play some role on the coagulation and platelet aggregation processes induced by S-galactans and S-fucoidans [2,307,308]. In fact, at least for some fucoidans, the anticoagulant properties are related to the content in C-2 and C-2,3 (di)sulphate, this last feature being usually common in these PS [52,53,105]. Several other studies documented the anticoagulant activity and inhibition of platelet aggregation [22,103,130], supplying more information on the mechanisms of different sPS for these biological activities. Higher MW-PS usually present stronger anticoagulant activity [309] and if a PS has a more linear backbone, a longer polymer is required to accomplish the same anticoagulant effects [251]. However, both the native PS and LMW-derivatives of the green seaweed M. latissimum presented strong anticoagulant activities [168]. Nishino and colleagues also observed that high molecular weight fucans (e.g., 27 and 58 kDa) showed greater anticoagulant activity than the ones with lower molecular weight (~10 kDa) [85]. They found that a higher content of fucose and sulphate groups coincided with higher anticoagulant activities of sulphated polysaccharide fractions from E. kurome [84]. However, despite its high sulphation level, the galactofucan from U. pinnatifida lacks significant anticoagulation activity [38]. Moreover, an S-galactofucan from the brown seaweed S. schröederi did not present any anticoagulant properties in vitro, but demonstrated a strong antithrombotic activity when administered to an animal-model during an experimental induced venous thrombosis, this effect disappearing with the desulphation of the polymer [38].
As for other PS, the anticoagulant properties of the PS from marine microalgae may not only depend on the percentage of sulphate residues, but rather on the distribution/position of sulphate groups and, probably, on the configuration of the polymer chains [14]. Spirulan from A. platensis (Table 4) is one of the PS from marine microalgae that strongly interferes with the blood coagulation-fibrinolytic system and exhibits antithrombogenic properties [159,310], therefore, promising to be an anti-thrombotic agent in clots’ breakdown, although care should be taken regarding hemorrhagic strokes [14].
It seems that the anticoagulant mechanisms of action of PS may be attributed to: (i) the inhibition of thrombin directly or via antithrombin III (AT-III) [66,302,303,311,312]; (ii) the increment in the activity of thrombin inhibitors, such as AT-III and/or heparin cofactor II (HC-II) [130,304,313], in both the intrinsic (contact activation or normal, measured by APPT test) and extrinsic (Tissue factor, TF, measured by PT test) pathways [314], the activation of HC-II seeming to be sulphate-dependent [315].
One explanation for the sPS to act directly on thrombin may be associated with the ability of those polymers to bind to thrombin, thus, hindering its catalytic activity [15,316]. In addition, some sPS may also inhibit thrombin from linking to their receptors in human platelets (protease activated receptor-1 and GP-1b) [317]. However, a high content of glucuronic acid might render a sPS unable to interfere in the coagulation process [15].
3.5. Antilipidaemic (Hypocholesterolaemic and Hypotriglyreridaemic), Hypoglycaemic and Hypotensive Activities
Sulphated PS from seaweeds are potent inhibitors of human pancreatic cholesterol esterase, an enzyme that promotes its absorption at the intestinal level; this inhibitory effect is enhanced by higher molecular weights and degree of sulphation [6].
An S-ulvan from U. pertusa in an in vivo study using mice-models regulated the ratio HDL/LDL-cholesterol and reduced the levels of triglycerides (TG) in serum [185]. However, in another experiment with rats and mice, using native ulvans from the same species, the animals experienced a hypocholesterolaemic effect but no reduction in the TG profile [318]. An opposite reaction was observed when the PS was acetylated and oversulphated, as TG levels were normalized. It seems that the ability to sequester bile extracts may be involved [185]. The contents in sulphate and acetylate groups play important roles during the dislipidaemia process [191,319]. Ulvans from Ulva spp also showed antiperoxidative properties, preventing liver tissues from hyperlipidaemia, including that induced by toxic chemicals and protecting the injured tissue from the oxidative stress [189], and improving antioxidant performance of the animal models. In fact, these sPS regulated superoxide dismutase (SOD) and catalase, increased vitamins E and C, and reduced-glutathione, and had some role in reducing the levels of aspartate and alanine transaminases in the rats’ liver [179,185]. Further, the sPS from M. nitidum also demonstrated hepatoprotective activity by increasing the expression of liver detoxifying enzymes, and, therefore, showed to be good agents for chemoprevention medicine [171]. The activity of these PS may be related to their uronic acid and sulphate content, which are able to sequester and bind to bile acids [320], reducing their levels. Other sPS from green seaweeds also revealed hypolipidaemic properties, such as that from E. prolifera. This PS regulated the lipidic profile both in plasma and liver, increasing HDL-cholesterol, in rats [177]. Fucoidans from L. japonica, the native or LMW-derivate, have hypolipidaemic effects, decreasing total and LDL-cholesterol in the serum and TG in rats [321], and they prevented hypercholesterolaemia in mice [322]. Another mechanism to reduce blood cholesterol in humans by sPS is associated to their high capacity to inhibit pancreatic cholesterol esterase, which is responsible for the absorption of cholesterol and fatty acids at the intestine [6]. It seems that the presence of sulphate at the C-3 position of the sugar residues greatly enhances that inhibition [6]. Porphyran from P. yezoensis has anti-hyperlipidaemic properties [119,323] by reducing the release of apolipoprotein-B100 (apoB100) and decreasing the synthesis of lipids in human liver cultured cells [324]. By reducing the secretion of apoB100, porphyran has the potential to be used as a therapeutic agent to treat CVD. In addition, some types of carrageenans have already proved to decrease blood cholesterol in humans [325] and in rats fed on a diet enriched with a mixture of κ/λ-carrageenans from G. radula [326].
Most of the PS from marine microalgae are naturally highly sulphated, with high molecular weights, making them not-easily absorbable and thus enabling them to be used as anticholesterolaemic agents. Few studies were carried out in this area, namely focusing on Porphyridium, P. cruentum, R. reticulata (Table 4) [327,328,329,330], but these suggest a strong potential of sulphated polysaccharides from unicellular algae to be used as hypolipidaemic and hypoglycaemic agents, and as promising agents for reducing coronary heart disease, due to their hypocholesterolaemic effects [14].
As far as we know, scarce research was performed on the mechanisms underlying the antihyperlipidaemic activity. However, the sequestration and disruption of the enterophatic circulation of the bile acids may be involved [185,331,332]. For example, ulvans and their LMW-derivatives, and also the sEPS from Porphyridium showed to increase the excretion of bile [185,333]. Another explanation for the antihyperlipidaemic activity of sPS may be associated to the fact that they can effectively increase the anionic charges on the cell surface, which improve the removal of cholesterol excess from the blood, thus, resulting in a decrease of serum cholesterol [103]. In addition, most PS have ion exchange capacity, such as those from Porphyridium and Rhodella [334], and they can function as dietary fibres. This could also explain the ability to lower down cholesterol [335]. PS may act as dietary fibres, immunostimulating the goblet cells in the intestine to increase the release and effects of mucin [336]. Moreover, the administration of PS may increase the viscosity of the intestinal contents, interfering with the formation of micelles and nutrient absorption, thus, lowering lipid absorption, and reducing gastrointestinal transit time (GTT) [333,337].
Other PS have the ability to inhibit the enzyme α-glucosidase, thus improving the postprandial hyperglycaemia [338], and another can also reduce the blood pressure by inhibiting the release of plasma angiotensin II [339].
3.6. Antiaging (Antioxidant) Activity
The main mechanism by which sPS from green seaweeds exert their primary antioxidant action is by scavenging free-radicals (superoxide, hydroxyl, 1,1-diphenyl-2-picrylhydrazyl (DPPH)-radicals) or by inhibiting their appearance [8]. They also demonstrated to have total antioxidant capacity, and a strong ability as reducing agents and as ferrous chelators [8]. However, some other sPS, such as S-heterogalactan (C. cupressoides) do not show a good scavenging power, but they are rather powerful against reactive oxygen species (ROS) [340]. It is interesting to note that fucoidans from brown seaweeds seem to exert a reducing power bigger than the sPS from other groups [2]; the PS from S. filipendula has an effect even stronger than vitamin C. Moreover, the fucoidan from L. japonica has a great potential to be used in medicine in order to prevent free-radical-mediated diseases, as it successfully prevented peroxidation of lipids in plasma, liver and spleen in vivo (mice), despite showing no effects in vitro [100]. The sPS from another species of Sargassum (S. fulvellum) has shown a NO scavenging activity higher than some commercial antioxidants [341]. In addition, the sPS from the red macroalga P. haitanensis demonstrated to decrease antioxidant damages in aging mice [115].
It seems that LMW-sPS may present higher antioxidant activity than the native polymers, as it was verified with the PS from U. pertusa and E. prolifera [166,342]. It is probably related with the ability of PS to be incorporated in the cells and to donate protons [21].
As noted by Raposo et al. [14], sulphated PS produced and secreted out by marine microalgae have shown the capacity to prevent the accumulation and the activity of free radicals and reactive chemical species. Therefore, sPS might act as protecting systems against these oxidative and radical stress agents. The sPS from Porphyridium and Rhodella reticulata (Table 4) exhibited antioxidant activity [343,344], although some research revealed no scavenging activity and no ability to inhibit the oxidative damage in cells and tissues for the crude sPS with high molecular weight from Porphyridium cruentum, while the EPS-derived products after microwave treatment showed antioxidant activity [220]. In all cases, the antioxidant activity was dose-dependent. Methanolic extracts of EPS from A. platensis also exhibit a very high antioxidant capacity [235].
Due to their strong antioxidant properties, most of the sPS from marine macro- and microalgae are promising since they may protect human health from injuries induced by ROS, which can result in cancer, diabetes, some inflammatory and neurodegenerative diseases, and some other aging-related disorders, such as Alzheimer and CVD.
The influence of sulphate content on the antioxidant activity depends rather on the origin of the PS. For example, the PS from U. fasciata and other macro- and microalgae with lower sulphate content demonstrated a strong antioxidative power [165,181,220,343], while the antioxidant activity observed in PS from E. linza and other seaweeds showed to be sulphate-dependent [174,345]. Furthermore, high sulphated PS was shown to have an enhanced scavenging power [97,182], this property being also dependent on the sulphate distribution pattern [2]. It seems, in addition, that the protein moiety of PS may play some role on the antioxidative power. For example, Tannin-Spitz et al. [343] reported a stronger antioxidant activity for the crude PS of Porphyridium than for the denatured PS.
Zhao et al. [346] found that the antioxidant activity of sPS was apparently related, not only to molecular weight and sulphated ester content, but also to glucuronic acid and fructose content. This antioxidant activity seems to be attributable to metal chelating, free radical and hydroxyl radical scavenging activities of the sPS.
3.7. Nutritional Applications: Fibres (Dietary), Prebiotic and Probiotic
As already mentioned by Raposo et al. [14], PS can find applications in the food industry as emulsifying and gelling agents, as flocculant and hydrating agents, emulsifiers, stabilizers, thickening agents, i.e., food additives [347], like agar E406, alginates E400-404, carrageenan E407. The sPS from marine microalgae could be used as nutraceuticals due to their content in fibres, the ability of acid binding and for cation exchange, and the properties of faecal bulking as well, being also good candidates as prebiotics [348]. The PS alone or in combination with other compounds have a great potential to be used in edible films and coatings of foods, while carriers of flavors, colorants, spices and nutraceuticals [349]. In our laboratory, experiments have already been carried out with based EPS from P. cruentum-coatings applied to fresh-cut apple. These polymers also have the potential to be used in low-fat or fat-free food products, as fat substitutes in mayonnaises [350,351], salad dressings and other food emulsions [352].
3.8. Other Biological Activities
As it happens in relation to the fucoidan from S. schröederi (Dictyotales) [36], a heterofucan-derivative from D. menstrualis, another member of Dictyotales, also presented antinociceptive activity. It acted as a peripheral analgesic agent, reaching 61.2% of pain reduction (4 mg/kg) in mice, this effect being as potent as dipyrone’s, and it was dose-dependent [1]. This suggests that this kind of S-fucans and some S-galactans can act as analgesic agents but not as anaesthetic ones, as they do not decrease pain when it involves the CNS. S-galactan from G. cornea is another sPS with analgesic characteristics, but at a higher concentration (9 mg/kg) [353]. A S-galactan from C. feldmannii is a more potent antinociceptive agent (80% reduction in contractions), but it also presents good anticoagulant properties [354]. Sulphated PS from C. cupressoides [155,156], at a dose of 27 mg/kg/day, reduced by 90% the writhes induced in mice by acetic acid, but they also showed analgesic effects only via peripheral mechanisms [156]. It seems that these sPS act by binding to the surface of the leukocytes, hindering their migration to the focus of tissue injury [1,355], therefore, demonstrating anti-inflammatory properties as well. Thus, all these sPS promise to be good peripheral antinociceptive agents, with some special care in relation to the galactan from Champia feldmannii due to its anticoagulant properties.
The angiogenic (neovascularization) properties of PS can be considered according to two angles. When dealing with treatment/prevention of neoplasias it is very important that the PS in question does not show that ability, so that the tumour will be reduced, and cells might die if not irrigated. Therefore, sPS, such as fucoidans may function as tumour supressors by inhibiting angiogenesis induced by tumour cells [3]. However, if the disorder we are dealing with is the result of an ischaemic issue, a PS with angiogenic activity should be used in order to re-establish the blood flow of the injured tissues, thus, acting as cardioprotective after ischaemia. The angiogenic mechanisms of fucoidans and glucans were well explained by Fedorov et al. [3] and Cumashi et al. [15].
Angiogenesis involves the differentiation of mature endothelial cells, their proliferation and migration. In fact, some sPS demonstrated the capacity to promote therapeutic revascularization in animal models, increasing the vessel formation when administered by injection in rats with ischaemic hind limb [57]. The mechanisms involved in the angiogenic properties of modified fucoidans are associated with the ability of these polymers to interact with endothelial cells, modulating the activity of proangiogenic growth factors, such as fibroblast growth factor-2 (FGF-2). The latter is mitogenic for that type of cells, fibroblasts and smooth muscle cells [103], and extracellular matrix components [58,356,357]. In fact, there is a correlation of the reduction of plasminogen-activator inhibitor (PAI-1) secretion with the upregulation of cell-surface α-6 integrin sub-unit. This could be an explanation for the proangiogenic ability, including the induction in vitro of tube formation by human endothelial cells. The fucoidans of C. okamuranus and F. vesiculosus are promising in treatment of ischaemic disorders, including infarcted myocardium, as they did not show to inhibit tubulogenesis in HUVEC cells. This cardioprotective activity was confirmed in animal models by enhancing creatinine phosphokinase, lactate dehydrogenase, and alanine and aspartate transaminases [47].
Fucoidans from two species of Laminaria and three species of Fucus revealed antiangiogenic properties, through the inhibition of the in vitro neogenesis of tubules in human umbilical vein endothelial cells (HUVEC), while a decrease in PAI-1 in HUVEC supernatants was also observed [15]. It is worth noting that these sPS revealed anticoagulant and antithrombotic activities, and some of these fucoidans inhibited the adhesion of breast cancer cells to platelets, as well, thus showing anti-adhesive and anti-metastatic properties. These features suggest that this type of polymers could be used as complementary agents in the therapeutical treatment of cancer.
In addition to the cardioprotective effects, the fucoidan from C. okamuranus Tokida demonstrated a great potential to be used as a gastroprotective agent [46]. It was used as a component of a new drug to treat/prevent gastric ulcers, and to inhibit Helicobacter pylori from adhering to the mucosa of the stomach [358], and also inhibited stomach cancer [44].
The fucoidans from other seaweeds are promising as well, not only as hepatoprotective agents against chemical damages, stimulating the release of IL-10 and inhibiting proinflammatory cytokines [359,360], but also against hepatic fibrosis, protecting hepatocytes and inhibiting the proliferation of hepatic stellate cells, which are co-responsible in the process [361].
Being an antioxidant against free radicals, fucoidan from F. vesiculosus might be an alternative or complementary therapeutic in uropathy and renalpathy, since it could prevent from the injuries caused by oxalate-induced free radicals [362] and from the mitochondrial damages associated to the process [363]. Several other disorders of the urinary system, including Heymann nephritis, are also liable to treatment or complementary therapeutics through the use of fucoidans [293,364,365,366,367].
The PS from other seaweeds demonstrated either stimulatory or inhibitory effects on some enzymes as was reported in the review by Smit [20], and inhibited cytotoxic and myotoxic effects against snake venoms as well, thus protecting the muscle from necrosis [368].
3.9. Biomedical Applications
Biomedical field is constantly demanding for new biomaterials with innovative properties. Natural polymers appear as materials of election for this objective due to their biocompatibility and biodegradability [369].
Alongside their biological activity and potential pharmaceutical use, as has already been addressed in this review, PS may be used as biomaterials, as such, or in combination with other synthetic or natural substances. There are several potential biomedical applications for PS in: regenerative medicine, such as wound management products, drug delivery systems (DDSs), tissue engineering, and medical fibres and biotextiles [369,370] (Table 5).
Table 5.
Some applications of algal PS in biomedicine.
| Groups of PSs | Possible Sources | Applications | References |
|---|---|---|---|
| Alginates | Laminaria spp, A. nodosum, Ecklonia sp., M. pyrifera, Durvillaea, Lessonia | Drugs carriers | [371] |
| Encapsulation | [372,373,374] | ||
| Scaffolds for ligaments and tissue engineering | |||
| Regeneration of tissues | |||
| Moulding in dentistry | |||
| Wound healing and dressings | [375,376,377] | ||
| Agaroids | B. montaignei, Goiopeltis spp., A. tenera, P. capillacea | Cell encapsulation | |
| Scaffolds for tissue engineering | [378] | ||
| Wound healing and dressings | [379] | ||
| Revascularization | [380] | ||
| Ulvans | Ulva rigida, Ulva spp. | Drug carriers | [381] |
| Wound dressings | [382,383] | ||
| Tissue engineering | [384] | ||
| β-glucans | A. nodosum, E. bicyclis, Fucus sp., Laminaria sp., U. pinnatifida (laminaran); C. vulgaris | Wound healing | [385,386,387] |
| Burn-wound dressings | |||
| Tissue regeneration | [388,389,390] | ||
| fucoidans | U. pinnatifida | Vaccines for immunotherapy | [299] |
| PSs from microalgae | A. platensis | Production of nanofibers | [391] |
| Gluing and soft tissue closure after surgery | [6] | ||
| Porphyridium | Lubricants for bone joints | [212,392] |
Alginates from macroalgae have been most used in several applications: controlled drug release [371], cell encapsulation, scafold in ligament, tissue engineering and regeneration of almost all tissues in the human organism, or even preparation of moulds in dentistry [372,373,374]. A review recently made available was devoted to the processing of alginate fibres for use as wound management materials [375,376]. PS have been widely applied as hydrogels, eventually combined with other substances, for: the encapsulation and delivery of Langerhans islets [393], ovarian follicles [394] and stem cells in neural tissue engineering [395], skin tissue engineering [396], bone tissue engineering [397] and skeletal muscle regeneration [398]; regenerating the osteochondral interface [399]; capturing of endothelial progenitor cells from the human blood [400]. Kaltostat® is a commercially available alginate dressing that promotes haemostasis and manages exudate in low to moderately exuding wounds. In the form of porous scaffolds, alginate has been used for creating a capillary bed in newly reconstructed tissues [401], and in the form of electrospin nanofibrous scaffolds, for constructing vascular replacements containing endothelial cells and smooth muscle cells (SMC) [402]. Alginate may also be used as a component of scaffolds for heart valve engineering [403], and for cardiac tissue engineering [404].
Similarly to alginate, agar can be used for cell encapsulation in tissue engineering applications. Due to its chondrogenic potential, agar was selected to entrap chondrocytes within poly-l-lactide scaffolds [378]. Composite membranes with agar proved to be promising wound dressings for healing burns or ulcers [379]. Agar gel supported the formation of in vivo autologous vascular prosthetic tissues, called “biotubes” [380].
However, not all polysaccharides are suitable for tissue engineering, mainly due to their jelly-like consistency and insufficient mechanical properties. As referred above, even PS used in tissue engineering usually need to be combined with other natural or synthetic polymers, or reinforced with inorganic substances [405].
A new generation of medical textiles incorporated with PS, such as alginate, is growing with respect to, in wound management products [406]. The main qualities of fibres and wound dressing products include antiviral, fungistatic, non-toxic, highly absorbent, non-allergic, breathable, haemostatic, and biocompatible. Such products with good mechanical properties may incorporate medication [377].
sPS are capable of binding to protein and may be involved in the cell development, cell differentiation, cell adhesion, cell signaling and cell matrix interactions. These bioactive molecules present a great potential for medical, pharmaceutical and biotechnological applications, such as wound dressings, biomaterials, tissue regeneration and 3D culture scaffolds, and even drugs [19]. Their biological activities and their resemblance to glycosaminoglycans (GAGs), which have been most studied, might position these PS in advantage. For example, ulvans from green algae, may be processed into porous structures, including nanofibres [381], particles [382], membranes [383] and hydrogels [384], which make them good candidates for medical applications, such as drug delivery, wound dressing and bone tissue engineering [381,382,383,384]. Carrageenans, besides being hydrogels, may also be processed into fibres, membranes or porous structures for several biomedical applications [407].
Fucoidans from brown algae besides having application in the biopharmaceutical industry (immunomodulatory, antiviral and anticoagulant agents), have found new applications in biomedicine, for instance, as nanoparticles of fucoidan and chitosan [408], and more recently in the synthesis of biohybrid glycopolymers [409].
The (1,3)-β-glucans have been used to help healing wounds, by inducing the migration of macrophages to the wound site [385], to accelerate the healing process as a constituent in composites [386,410], and in burn-wound dressings, therefore, reducing the need for analgesics [387]. Fucoidan-chitosan films and/or gels may be used to treat dermal burns and regenerate epithelial tissue [388]. The mechanism of action of hydrocolloids may be associated to the fact that wound dressings with sPS decrease wound secretions and scars, reducing the bacterial inflammation [389,390], with the advantage of being free from prions or other animal contaminants.
Native sPS, LMW- or otherwise modified derivatives, are also promising in the prevention of arteriosclerosis after cardiac transplantation [411,412], in coating encapsulates for controlled drug delivering, as new materials for cell immobilization and tissue engineering [7,413], and as carrier-materials for transplantation of chondrocytes and osteoblasts, improving neo-cartilage and neo-bone formation [414].
The use of fucoidans in dendritic cell-based vaccines for cancer immunotherapy has been also suggested [299].
Another promising and emerging application of the PS from microalgae might be associated to the production of nanofibres from the biomass of A. platensis (Table 4), to be used as extracellular matrices for the culture of stem cells in order to treat spinal cord injuries [391].
Their gluing and adhesive capacities, as well as their strong cohesive and binding strength, allied to their non-toxic and non-irritating properties, make the bioadhesive PS produced by marine microalgae good candidates as mucobioadhesives or glues for bone gluing and soft tissue closure after surgery. They might also replace the metallic screws and traditional wound closure methods, respectively [6].
The sEPS from Porphyridium (Table 4) has already shown a good lubrication capacity [355], being an excellent candidate to substitute for hyaluronic acid as a biolubricant. Another promising application could be as a component of a joint-lubricating solution, as it was demonstrated by injecting the EPS from Porphyridium into the joints of rabbits’ knees [212], thus mitigating degenerative joint disorders caused by arthritis.
4. Conclusions
Polysaccharides may be regarded as key ingredients for the production of bio-based materials in life sciences (e.g., medical devices, pharmaceutics, food, cosmetics). There are an enormous variety of polysaccharides that can be synthetized and/or released by marine macro- and microalgae. Both these marine organisms are excellent sources of PS, most of them sulphated (sPS). Although some similarities may be found between the PS from each group of organisms, they can be very heterogeneous and structurally different. The biological source and biodegradability of these biopolymers, coupled to the large variety of functionalities they encompass, make them promising compounds for the application in pharmaceuticals, therapeutics, and regenerative medicine. Some of the beneficial bioactivities demonstrated by the crude PS and their derivatives, either in vitro or in vivo, include anticoagulant and/or antithrombotic properties, immunomodulatory ability, antitumor and cancer preventive activity. They are also good antilipidaemic and hypoglycaemic agents, and can be powerful antioxidants, antibiotics and anti-inflammatory. Other biomedical properties of PS have been discussed, such as antinociceptive, angiogenic, cardioprotective, gastroprotective and hepatoprotective activities. The biomedical applications and potentialities of PS in this area were listed, such as healing wound agents, mucobioadhesives for bone and soft tissue closure, biolubricants to mitigate joint disorders caused by arthritis, vaccines for cancer immunotherapy, or in a new generation of biotextiles and medical fibres, in drug delivery systems, and scaffolds in regenerative medicine.
From the extensive list above, the importance of this type of compounds—PS from macro- and microalgae—for medical use is quite obvious. However, despite all the interesting properties and potentialities for human health, the use of these PS, especially those from microalgae need to be further explored. In particular, the toxicity and bioavailability of some of these polymers are yet to be studied on humans.
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia (FCT) of Portuguese Republic Government, in the frame of the project PEst-OE/EQB/LA0016/2013.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Albuquerque I.R.L., Cordeiro S.L., Gomes D.L., Dreyfuss J.L., Filgueira L.G.A., Leite E.L., Nader H.B., Rocha H.A.O. Evaluation of anti-nociceptive and anti-inflammatory activities of a heterofucan from Dictyota menstrualis. Mar. Drugs. 2013;11:2722–2740. doi: 10.3390/md11082722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa L.S., Fidelis G.P., Cordeiro S.L., Oliveira R.M., Sabry D.A., Câmara R.B.G., Nobre L.T.D.B., Costa M.S.S.P., Almeida-Lima J., Farias E.H.C., et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010;64:21–28. doi: 10.1016/j.biopha.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Fedorov S.N., Ermakova S.P., Zvyagintseva T.N., Stonik V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs. 2013;11:4876–4901. doi: 10.3390/md11124876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiménez-Escrig A., Gómez-Ordóñez E., Rupérez P. Seaweed as a source of novel nutraceuticals: Sulfated polysaccharides and peptides. Adv. Food Nutr. Res. 2011;64:325–337. doi: 10.1016/B978-0-12-387669-0.00026-0. [DOI] [PubMed] [Google Scholar]
- 5.Kraan S. Carbohydrates-Comprehensive Studies on Glycobiology and Glycotechnology. InTech; Rijeka, Croatia: 2012. Algal polysaccharides, novel applications and outlook; pp. 489–524. Chapter 22. [Google Scholar]
- 6.Laurienzo P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs. 2010;8:2435–2465. doi: 10.3390/md8092435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez d’Ayala G., Malinconico M., Laurienzo P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules. 2008;13:2069–2106. doi: 10.3390/molecules13092069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L., Wang X., Wu H., Liu R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs. 2014;12:4984–5020. doi: 10.3390/md12094984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burja A.M., Banaigs B., Abou-Mansor E., Burgess J.G., Wright P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron. 2001;57:9347–9377. doi: 10.1016/S0040-4020(01)00931-0. [DOI] [Google Scholar]
- 10.Pulz O., Gross W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- 11.De Jesus Raposo M.F., de Morais A.M.M.B. Microalgae for the prevention of cardiovascular disease and stroke. Life Sci. 2015;125:32–41. doi: 10.1016/j.lfs.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 12.De Jesus Raposo M.F., de Morais R.M.S.C., de Morais A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs. 2013;11:233–252. doi: 10.3390/md11010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Jesus Raposo M.F., de Morais R.M.S.C., de Morais A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013;93:479–486. doi: 10.1016/j.lfs.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 14.De Jesus Raposo M.F., de Morais A.M.M.B., de Morais R.M.S.C. Bioactivity and Applications of polysaccharides from marine microalgae. In: Merillon J.-M., Ramawat K.G., editors. Polysaccharides: Bioactivity and Biotechnology. Springer; Cham, Switzerland: 2014. [DOI] [Google Scholar]
- 15.Cumashi A., Ushakova N.A., Preobrazhenskaya M.E., D’Incecco A., Piccoli A., Totani L., Tinari N., Morozevich G.E., Berman A.E., Bilan M.I., et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;5:541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- 16.Ermakova S., Sokolova R., Kim S.-M., Um B.-H., Isakov V., Zvyagintseva T. Fucoidans from Brown seaweeds Sargassum hornery, Ecklonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011;164:841–850. doi: 10.1007/s12010-011-9178-2. [DOI] [PubMed] [Google Scholar]
- 17.Fujitani N., Sakari S., Yamagushi Y., Takenaka H. Inhibitory effects of microalgae on activation of hyaluronidase. J. Appl. Phycol. 2001;13:489–492. doi: 10.1023/A:1012592620347. [DOI] [Google Scholar]
- 18.Jiao G., Yu G., Zhang J., Ewart H.S. Chemical structures and bioactivities of sulphated polysaccharides from marine algae. Mar. Drugs. 2011;9:196–223. doi: 10.3390/md9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senni K., Pereira J., Gueniche F., Delbarre-Ladrat C., Sinquin C., Ratiskol J., Godeau G., Fisher A.M., Helley D., Colliec-Jouault S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs. 2011;9:1664–1681. doi: 10.3390/md9091664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smitt A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004;16:245–262. doi: 10.1023/B:JAPH.0000047783.36600.ef. [DOI] [Google Scholar]
- 21.Wijesekara I., Pangestuti R., Kim S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011;84:14–21. doi: 10.1016/j.carbpol.2010.10.062. [DOI] [Google Scholar]
- 22.Wijesinghe W.A.J.P., Jeon Y.-J. Biological activities and potential industrial applications of fucose rich sulphated polysaccharides and fucoidans from brown seaweeds: A review. Carbohydr. Polym. 2012;88:13–20. doi: 10.1016/j.carbpol.2011.12.029. [DOI] [Google Scholar]
- 23.Yim J.H., Kim S.J., Ahn S.H., Lee H.K. Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03. Bioresour. Technol. 2007;98:361–367. doi: 10.1016/j.biortech.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Nomoto K., Yokokura T., Satoh H., Mutai M. Anti-tumor effect by oral administration of Chlorella extract, PCM-4 by oral admission. Gan To Kagaku Zasshi. 1983;10:781–785. (In Japanese) [PubMed] [Google Scholar]
- 25.Camara R.B.G., Costa L.S., Fidelis G.P., Nobre L.T.D.B., Dantas-Santos N., Cordeiro S.L., Costa M.S.S.P., Alves L.G., Rocha H.A.O. Heterofucans from the brown seaweed Canistrocarpus cervicornis with anticoagulant and antioxidant activities. Mar. Drugs. 2011;9:124–138. doi: 10.3390/md9010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albuquerque I.R.L., Queiroz K.C.S., Alves L.G., Santos E.A., Leite E.L., Rocha H.A.O. Heterofucans from Dictyota menstrualis have anticoagulant activity. Braz. J. Med. Biol. Res. 2004;37:167–171. doi: 10.1590/S0100-879X2004000200002. [DOI] [PubMed] [Google Scholar]
- 27.Bilan M.I., Usov A.I. Structural analysis of fucoidans. Nat. Prod. Commun. 2008;3:1639–1648. [Google Scholar]
- 28.Sokolova R.V., Ermakova S.P., Awada S.M., Zvyagintseva T.N., Kanaan H.M. Composition, structural characteristics, and antitumor properties of polysaccharides from the brown algae Dictyopteris polypodioides and Sargassum sp. Chem. Nat. Comp. 2011;47:329–334. doi: 10.1007/s10600-011-9925-1. [DOI] [Google Scholar]
- 29.Medeiros V.P., Queiroz K.C., Cardoso M.L., Monteiro G.R., Oliveira F.W., Chavante S.F., Guimarães L.A., Rocha H.A., Leite E.L. Sulfated galactofucan from Lobophora variegata: Anticoagulant and anti-inflammatory properties. Biochemistry (Mosc.) 2008;73:1018–1024. doi: 10.1134/S0006297908090095. [DOI] [PubMed] [Google Scholar]
- 30.Paiva A.A., Castro A.J., Nascimento M.S., Will L.S., Santos N.D., Araújo R.M., Xavier C.A., Rocha F.A., Leite E.L. Antioxidant and anti-inflammatory effect of polysaccharides from Lobophora variegata on zymosan-induced arthritis in rats. Int. Immunopharmacol. 2011;11:1241–1250. doi: 10.1016/j.intimp.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Silva T.M., Alves L.G., de Queiroz K.C., Santos M.G., Marques C.T., Chavante S.F., Rocha H.A.O., Leite E.L. Partial characterization and anticoagulant activity of a heterofucan from the brown seaweed Padina gymnospora. Braz. J. Med. Biol. Res. 2005;38:523–533. doi: 10.1590/s0100-879x2005000400005. [DOI] [PubMed] [Google Scholar]
- 32.Zhu W., Ooi V.E.C., Chan P.K.S., Ang P.O., Jr. Inhibitory effect of extracts of marine algae from Hong Kong against Herpes simplex viruses. In: Chapman A.R.O., Anderson R.J., Vreeland V.J., Davison I.R., editors. Proceedings of the 17th International Seaweed Symposium; Oxford, UK: Oxford University Press; 2003. pp. 159–164. [Google Scholar]
- 33.Karmakar P., Ghosh T., Sinha S., Saha S., Mandal P., Ghosal P.K., Ray B. Polysaccharides from the brown seaweed Padina tetrastromatica: Characterization of a sulfated fucan. Carbohyd. Polym. 2009;78:416–421. doi: 10.1016/j.carbpol.2009.04.039. [DOI] [Google Scholar]
- 34.Almeida-Lima J., Dantas-Santos N., Gomes D.L., Cordeiro S.L., Sabry D.A., Costa L.S., Freitas M.L., Silva N.B., Moura C.E.B., Lemos T.M.A.M., et al. Evaluation of acute and subchronic toxicity of a non-anticoagulant, but antithrombotic algal heterofucan from the Spatoglossum schröederi in wistar rats. Rev. Bras. Farmacogn. 2011;21:674–679. doi: 10.1590/S0102-695X2011005000098. [DOI] [Google Scholar]
- 35.Almeida-Lima J., Costa L.S., Silva N.B., Melo-Silveira R.F., Silva F.V., Felipe M.B.M.C., Medeiros S.R.B.M., Leite E.L., Rocha H.A.O. Evaluating the possible genotoxic, mutagenic and tumor cell proliferation-inhibition effects of a non-anticoagulant, but antithrombotic algal heterofucan. J. Appl. Toxicol. 2010;30:708–715. doi: 10.1002/jat.1547. [DOI] [PubMed] [Google Scholar]
- 36.Farias W.R., Lima P.C., Rodrigues N.V., Siqueira R.C., Amorim R.M., Pereira M.G., Assreuy A.M. A novel antinociceptive sulphated polysaccharide of the brown marine alga Spatoglossum schröederi. Nat. Prod. Commun. 2011;6:863–866. [PubMed] [Google Scholar]
- 37.Rocha H.A.O., Franco C.R.C., Trindade E.S., Veiga S.S., Leite E.L., Dietrich C.P., Nader H.B. Fucan inhibits Chinese hamster ovary cell (CHO) adhesion to fibronectin by binding to the extracellular matrix. Planta Med. 2005;71:628–633. doi: 10.1055/s-2005-871268. [DOI] [PubMed] [Google Scholar]
- 38.Rocha H.A.O., Moraes F.A., Trindade E.S., Franco C.R.C., Torquato R.J.S., Veiga S.S., Valente A.P., Mourão P.A., Leite E.L., Nader H.B., et al. Structural and haemostatic activities of a sulfated galactofucan from the brown alga Spatoglossum schröederi. An ideal antithrombotic agent? J. Biol. Chem. 2005;280:41278–41288. doi: 10.1074/jbc.M501124200. [DOI] [PubMed] [Google Scholar]
- 39.Ponce N.M.A., Pujol C.A., Damonte E.B., Flores M.L., Stortz C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003;338:153–165. doi: 10.1016/S0008-6215(02)00403-2. [DOI] [PubMed] [Google Scholar]
- 40.Hidari K.I.P.J., Takahashi N., Arihara M., Nagaoka M., Morita K., Suzuki T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem. Biophys. Res. Commun. 2008;376:91–95. doi: 10.1016/j.bbrc.2008.08.100. [DOI] [PubMed] [Google Scholar]
- 41.Nagaoka M., Shibata H., Kimura-Takagi I., Hashimoto S., Kimura K., Makino T., Aiyama R., Ueyama S., Yokokura T. Structural study of fucoidan from Cladosiphon okamuranus Tokida. Glycoconj. J. 1999;16:19–26. doi: 10.1023/A:1006945618657. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu J., Wada-Funada U., Mano H., Matahira Y., Kawaguchi M., Wada M. Proportion of murine cytotoxic T cells is increased by high molecular-weight fucoidan extracted from Okinawa mozuku (Cladosiphon okamuranus) J. Health Sci. 2005;51:394–397. doi: 10.1248/jhs.51.394. [DOI] [Google Scholar]
- 43.Teruya T., Konishi T., Uechi S., Tamaki H., Tako M. Anti-proliferative activity of oversulfated fucoidan from commercially cultured Cladosiphon okamuranus Tokida in U937 cells. Int. J. Biol. Macromol. 2007;41:221–226. doi: 10.1016/j.ijbiomac.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Kawamoto H., Miki Y., Kimura T., Tanaka K., Nakagawa T., Kawamukai M., Matsuda H. Effects of fucoidan from Mozuku on human stomach cell lines. Food Sci. Technol. Res. 2006;12:218–222. doi: 10.3136/fstr.12.218. [DOI] [Google Scholar]
- 45.Shibata H., Iimuro M., Uchiya N., Kawamori T., Nagaoka M., Ueyama S., Hashimoto S., Yokokura T., Sugimura T., Wakabayashi K. Preventive effects of Cladosiphon fucoidan against Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2003;8:59–65. doi: 10.1046/j.1523-5378.2003.00124.x. [DOI] [PubMed] [Google Scholar]
- 46.Shibata H., Kimura-Takagi I., Nagaoka M., Hashimoto S., Aiyama R., Iha M., Ueyama S., Yokokura T. Properties of fucoidan from Cladosiphon okamuranus Tokida in gastric mucosal protection. Biofactors. 2000;11:235–245. doi: 10.1002/biof.5520110402. [DOI] [PubMed] [Google Scholar]
- 47.Thomes P., Rajendran M., Pasanban B., Rengasamy R. Cardioprotective activity of Cladosiphon okamuranus against isoproterenol induced myocardial infraction in rats. Phytomedicine. 2010;18:52–57. doi: 10.1016/j.phymed.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z., Teruya K., Eto H., Shirahata S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS ONE. 2011;6:e27441. doi: 10.1371/journal.pone.0027441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feldman S.C., Reynaldi S., Stortz C.A., Cerezo A.S., Damonte E.B. Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine. 1999;6:335–340. doi: 10.1016/S0944-7113(99)80055-5. [DOI] [PubMed] [Google Scholar]
- 50.Kim K.J., Lee O.H., Lee H.H., Lee B.Y. A 4-week repeated oral dose toxicity study of fucoidan from the sporophyll of Undaria pinnatifida in Sprague-Dawley rats. Toxicology. 2010;267:154–158. doi: 10.1016/j.tox.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Anastase-Ravion S., Carreno M.P., Blondin C., Ravion O., Champion J., Chaubet F., Haeffner-Cavaillon N., Letourneur D. Heparin-like polymers modulate proinflammtory cytokine production by lipopolysaccharide-stimulated human monocytes. J. Biomed. Mat. Res. 2002;60:375–383. doi: 10.1002/jbm.10112. [DOI] [PubMed] [Google Scholar]
- 52.Chevolot L., Foucault A., Chaubet F., Kervarec N., Sinquin C., Fisher A.M., Boisson-Vidal C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999;319:154–165. doi: 10.1016/S0008-6215(99)00127-5. [DOI] [PubMed] [Google Scholar]
- 53.Chevolot L., Mulloy B., Racqueline J. A disaccharide repeat unit is the structure structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001;330:529–535. doi: 10.1016/S0008-6215(00)00314-1. [DOI] [PubMed] [Google Scholar]
- 54.Colliec-Jouault S., Millet J., Helley D., Sinquin C., Fischer A.M. Effect of low-molecular-weight fucoidan on experimental arterial thrombosis in the rabbit and rat. J. Thromb. Haemost. 2003;1:1114–1115. doi: 10.1046/j.1538-7836.2003.t01-1-00215.x. [DOI] [PubMed] [Google Scholar]
- 55.Foley S.A., Szegezdi E., Mulloy B., Samali A., Tuohy M.G. An unfractionated fucoidan from Ascophyllum nodosum: Extraction, characterization, and apoptotic effects in vitro. J. Nat. Prod. 2011;74:1851–1861. doi: 10.1021/np200124m. [DOI] [PubMed] [Google Scholar]
- 56.Hoffman R., Paper D.H., Donaldson J., Alban S., Franz G. Characterization of a laminarin sulfate which inhibits basic fibroblast growth-factor binding and endothelial-cell proliferation. J. Cell Sci. 1995;108:3591–3598. doi: 10.1242/jcs.108.11.3591. [DOI] [PubMed] [Google Scholar]
- 57.Luyt C.E., Meddahi-Pellé A., Ho-Tin-Noe B., Colliec-Jouault S., Guezennec J., Louedec L., Prats H., Jacob M.P., Osborne-Pellegrin M., Letourneur D., et al. Low-molecular-weight fucoidan promotes therapeutic revascularization in a rat model of critical hindlimb ischemia. J. Pharmacol. Exp. Therapeut. 2003;305:24–30. doi: 10.1124/jpet.102.046144. [DOI] [PubMed] [Google Scholar]
- 58.Matou S., Helley D., Chabut D., Bros A., Fischer A.M. Effect of fucoidan on fibroblast growth factor-2-induced angiogenesis in vitro. Thromb. Res. 2002;106:213–221. doi: 10.1016/S0049-3848(02)00136-6. [DOI] [PubMed] [Google Scholar]
- 59.Miao H.Q., Elkin M., Aingorn E., Ishai-Michaeli R., Stein C.A., Vlodavsky I. Inhibition of heparanase activity and tumor metastasis by laminarin sulfate and synthetic phosphorothioate oligodeoxynucleotides. Int. J. Cancer. 1999;83:424–431. doi: 10.1002/(SICI)1097-0215(19991029)83:3<424::AID-IJC20>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 60.Percival E. Glucuronoxylofucan, a cell-wall component of Ascophyllum nodosum. Carbohydr. Res. 1968;7:272–277. doi: 10.1016/S0008-6215(00)81200-8. [DOI] [Google Scholar]
- 61.Renn D.W., Noda H., Amano H., Nishino T., Nishizana K. Antihypertensive and antihyperlipidemic effects of funoran. Fisch. Sci. 1994;60:423–427. [Google Scholar]
- 62.Ale M.T., Maruyama H., Tamauchi H., Mikkelsen J.D., Meyer A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011;49:331–336. doi: 10.1016/j.ijbiomac.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 63.Beress A., Wassermann O., Tahhan S., Bruhn T., Beress L., Kraiselburd E.N., Gonzalez L.V., de Motta G.E., Chavez P.I. A new procedure for the isolation of anti-HIV compounds (polysaccharides and polyphenols) from the marine alga Fucus vesiculosus. J. Nat. Prod. 1993;56:478–488. doi: 10.1021/np50094a005. [DOI] [PubMed] [Google Scholar]
- 64.Bilan M.I., Grachev A.A., Ustuzhanina N.E., Shashkov A.S., Nifantiev N.E., Usov A.I. Structure of a fucoidan from brown seaweed Fucus evanescens. Carbohydr. Res. 2002;337:719–730. doi: 10.1016/S0008-6215(02)00053-8. [DOI] [PubMed] [Google Scholar]
- 65.Mourão P.A., Pereira M.S. Searching for alternatives to heparin: Sulfated fucans from marine invertebrates. Trends Cardiovasc. Med. 1999;9:225–232. doi: 10.1016/S1050-1738(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 66.Pereira M.S., Mulloy B., Mourão P.A. Structure and anticoagulant activity of sulfated fucans. Comparison between the regular, repetitive, and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae. J. Biol. Chem. 1999;274:7656–7667. doi: 10.1074/jbc.274.12.7656. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura T., Suzuki H., Wada Y., Kodama T., Doi T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-κB-dependent signaling pathways through macrophage scavenger receptors. Biochem. Biophys. Res. Commun. 2006;343:286–294. doi: 10.1016/j.bbrc.2006.02.146. [DOI] [PubMed] [Google Scholar]
- 68.Park H.S., Kim G.Y., Nam T.J., Deuk Kim N., Hyun Choi Y. Antiproliferative activity of fucoidan was associated with the induction of apoptosis and autophagy in AGS human gastric cancer cells. J. Food Sci. 2011;76:T77–T83. doi: 10.1111/j.1750-3841.2011.02099.x. [DOI] [PubMed] [Google Scholar]
- 69.Synytsya A., Kim W.J., Kim S.M., Pohl R., Synytsya A., Kvasnicka F., Copikova J., Park Y.I. Structure and antitumor activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010;81:41–48. doi: 10.1016/j.carbpol.2010.01.052. [DOI] [Google Scholar]
- 70.Yang J.W., Yoon S.Y., Oh S.J., Kim S.K., Kang K.W. Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem. Biophys. Res. Commun. 2006;346:345–350. doi: 10.1016/j.bbrc.2006.05.135. [DOI] [PubMed] [Google Scholar]
- 71.Li B., Wei X.J., Sun J.L., Xu S.Y. Structural investigation of a fucoidan containing a fucose-free core from the brown seaweed, Hizikia fusiforme. Carbohydr. Res. 2006;341:1135–1146. doi: 10.1016/j.carres.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 72.Dobashi K., Nishino T., Fujihara M. Isolation and preliminary characterization of fucose-containing sulfated polysaccharides with blood-anticoagulant activity from seaweed Hizikia fusiforme. Carbohydr. Res. 1989;194:315–320. doi: 10.1016/0008-6215(89)85032-3. [DOI] [PubMed] [Google Scholar]
- 73.Venkateswaran P.S., Millman I., Blumberg B.S. Interaction of fucoidan from Pelvetia fastigiata with surface antigens of hepatitis B and woodchuck hepatitis viruses. Planta Med. 1989;55:265–270. doi: 10.1055/s-2006-962000. [DOI] [PubMed] [Google Scholar]
- 74.Klarzynski O., Descamps V., Plesse B., Yvin J.C., Kloareg B., Fritig B. Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol. Plant Microbe Interact. 2003;16:115–122. doi: 10.1094/MPMI.2003.16.2.115. [DOI] [PubMed] [Google Scholar]
- 75.Ale M.T., Mikkelsen J.D., Meyer A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs. 2011;9:2106–2130. doi: 10.3390/md9102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raghavendran H.R., Sathivel A., Devaki T. Effect of Sargassum polycystum (Phaeophyceae)-sulphated polysaccharide extract against acetaminophen-induced hyperlipidemia during toxic hepatitis in experimental rats. Mol. Cell. Biochem. 2005;276:89–96. doi: 10.1007/s11010-005-3194-x. [DOI] [PubMed] [Google Scholar]
- 77.Josephine A., Veena C.K., Amudha G., Preetha S.P., Varalakshmi P. Protective role of sulphated polysaccharides in abating the hyperlipidemic nephropathy provoked by cyclosporine A. Arch. Toxicol. 2007;81:371–379. doi: 10.1007/s00204-006-0151-8. [DOI] [PubMed] [Google Scholar]
- 78.Duarte M.E., Cardoso M.A., Noseda M.D., Cerezo A.S. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr. Res. 2001;333:281–293. doi: 10.1016/S0008-6215(01)00149-5. [DOI] [PubMed] [Google Scholar]
- 79.Ale M.T., Mikkelsen J.D., Meyer A.S. Designed optimization of a single-step extraction of fucose-containing sulfated polysaccharides from Sargassum sp. J. Appl. Phycol. 2011;24:715–723. doi: 10.1007/s10811-011-9690-3. [DOI] [Google Scholar]
- 80.Hoshino T., Hayashi T., Hayashi K., Hamada J., Lee J.B., Sankawa U. An antivirally active sulfated polysaccharide from Sargassum horneri (Turner) C. Agardh. Biol. Pharm. Bull. 1998;21:730–734. doi: 10.1248/bpb.21.730. [DOI] [PubMed] [Google Scholar]
- 81.Costa L.S., Fidelis G.P., Telles C.B.S., Dantas-Santos N., Camara R.B.G., Cordeiro S.L., Costa M.S.S.P., Almeida-Lima J., Melo-Silveira R.F., Albuquerque I.R.L., et al. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs. 2011;9:952–966. doi: 10.3390/md9060952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chattopadhyay N., Ghosh T., Sinha S., Chattopadhyay K., Karmakar P., Ray B. Polysaccharides from Turbinaria conoides: Structural features and antioxidant capacity. Food Chem. 2010;11:823–829. doi: 10.1016/j.foodchem.2009.05.069. [DOI] [Google Scholar]
- 83.Yamamoto I., Takahashi M., Tamura E., Maruyama H., Mori H. Antitumor activity of edible marine algae: Effect of crude fucoidan fractions prepared from edible brown seaweed against L-1210 leukemia. Hydrobiology. 1984;116/117:145–148. doi: 10.1007/BF00027653. [DOI] [Google Scholar]
- 84.Nishino T., Yokoyama G., Dobahi K. Isolation, purification and characterization of fucose-containing sulfated polysaccharides from the brown seaweed Ecklonia kurome and their blood-anticoagulant activities. Carbohydr. Res. 1989;186:119–129. doi: 10.1016/0008-6215(89)84010-8. [DOI] [PubMed] [Google Scholar]
- 85.Nishino T., Aizu Y., Nagumo T. The influence of sulfate content and molecular weight of a fucan sulfate from the brown seaweed Ecklonia kurome on its antithrombin activity. Thromb. Res. 1991;64:723–731. doi: 10.1016/0049-3848(91)90072-5. [DOI] [PubMed] [Google Scholar]
- 86.Nishino T., Kiyohara H., Yamada H., Nagumo T. An anticoagulant fucoidan from the brown seaweed Ecklonia kurome. Phytochemistry. 1991;30:535–539. doi: 10.1016/0031-9422(91)83722-W. [DOI] [PubMed] [Google Scholar]
- 87.Hu J.F., Geng M.Y., Zhang J.T., Jiang H.D. An in vitro study of the structure-activity relationships of sulfated polysaccharide from brown algae to its antioxidant effect. J. Asian Nat. Prod. Res. 2001;3:353–358. doi: 10.1080/10286020108040376. [DOI] [PubMed] [Google Scholar]
- 88.Kang S.M., Kim K.N., Lee S.H., Ahn G., Cha S.H., Kim A.D., Yang X.-D., Kang M.-C., Jeon Y.-J. Anti-inflammatory activity of polysaccharide purified from AMG-assistant extract of Ecklonia cava in LPS-stimulated RAW264.7 macrophages. Carbohydr. Polym. 2011;85:80–85. doi: 10.1016/j.carbpol.2011.01.052. [DOI] [Google Scholar]
- 89.Takahashi M. Studies on the mechanism of host mediated antitumor action of fucoidan from a brown alga Eisenia bicyclis. J. Jpn. Soc. Reticuloendothel. Syst. 1983;22:269–283. [Google Scholar]
- 90.Usui T., Asari K., Mizuno T. Isolation of highly purified fucoidan from Eisenia bicyclis and its anticoagulant and antitumor activities. Agric. Biol. Chem. 1980;44:2. doi: 10.1271/bbb1961.44.1965. [DOI] [Google Scholar]
- 91.Rioux L.E., Turgeon S.L., Beaulieu M. Structural characterization of laminaran and galactofucan extracted from the brown seaweed Saccharina longicruris. Phytochemistry. 2010;71:1586–1595. doi: 10.1016/j.phytochem.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 92.Usov A.I., Smirnova G.P., Bilan M.I., Shashkov A.S. Polysaccharides of algae: 53. Brown alga Laminaria saccharina (L.) Lam. as a source of fucoidan. Bioorg. Khim. 1998;24:382–389. [Google Scholar]
- 93.Maruyama H., Yamamoto I. An antitumor fraction from an edible brown seaweed Laminaria religiosa. Hydrobiologia. 1984;116/177:534–536. doi: 10.1007/BF00027740. [DOI] [Google Scholar]
- 94.Kitamura K., Matsuo M., Yasui T. Enzymic degradation of fucoidan by fucoidanase from the hepatopancreas of Patinopecten yessoensis. Biosci. Biotechnol. Biochem. 1992;56:490–494. doi: 10.1271/bbb.56.490. [DOI] [PubMed] [Google Scholar]
- 95.Huang L., Wen K., Gao X., Liu Y. Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm. Biol. 2010;48:422–426. doi: 10.3109/13880200903150435. [DOI] [PubMed] [Google Scholar]
- 96.Xue C.H., Chen L., Li Z.J., Cai Y.P., Lin H., Fang Y. Antioxidative activities of low molecular fucoidans from kelp Laminaria japonica. Dev. Food Sci. 2004;42:139–145. [Google Scholar]
- 97.Wang J., Zhang Q., Zhang Z., Li Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008;42:127–132. doi: 10.1016/j.ijbiomac.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Vishchuk O.S., Ermakova S.P., Zvyagintseva T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011;346:2769–2776. doi: 10.1016/j.carres.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 99.Li F., Tian T.C., Shi Y.C. Study on antivirus effect of fucoidan in vitro. J. N. Bethune Univ. Med. Sci. 1995;21:255–257. [Google Scholar]
- 100.Li D.Y., Xu R.Y., Zhou W.Z., Sheng X.B., Yang A.Y., Cheng J.L. Effects of fucoidan extracted from brown seaweed on lipid peroxidation in mice. Acta Nutrim. Sin. 2002;24:389–392. [Google Scholar]
- 101.Wang W.T., Zhou J.H., Xing S.T., Guan H.S. Immunomodulating action of marine algae sulfated polysaccharides on normal and immunosuppressed mice. Chin. J. Pharm Toxicol. 1994;8:199–202. [Google Scholar]
- 102.Luo D., Zhan Q., Wang H., Cui Y., Sun Z., Yang J., Zheng Y., Jia J., Yu F., Wang X. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009;617:33–40. doi: 10.1016/j.ejphar.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 103.Li B., Lu F., Wei X., Zhao R. Fucoidan: Structure and bioactivity. Molecules. 2008;13:1671–1695. doi: 10.3390/molecules13081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee S.H., Athukorala Y., Lee J.S., Jeon Y.J. Simple separation of anticoagulant sulfated galactan from red algae. J. Appl. Phycol. 2008;20:1053–1059. doi: 10.1007/s10811-007-9306-0. [DOI] [Google Scholar]
- 105.Yoon S.J., Pyun Y.R., Hwang J.K., Mourão P.A.S. A sulfated fucan from the brown alga Laminaria cichorioides has mainly heparin cofactor II-dependent anticoagulant activity. Carbohydr. Res. 2007;342:2326–2330. doi: 10.1016/j.carres.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 106.Thompson K.D., Dragar C. Antiviral activity of Undaria pinnatifida against herpes simplex virus. Phytother. Res. 2004;18:551–555. doi: 10.1002/ptr.1487. [DOI] [PubMed] [Google Scholar]
- 107.Hemmingson J.A., Falshow R., Furneaux R.H., Thompsom K. Structure and antiviral activity of the galactofucans sulfates extracted from Undaria pinnatifida (Phaeophyta) J. Appl. Phycol. 2006;18:185–193. doi: 10.1007/s10811-006-9096-9. [DOI] [Google Scholar]
- 108.Maruyama H., Tamauchi H., Hashimoto M., Nakano T. Antitumor activity and immune response of Mekabu fucoidan extracted from Sporophyll of Undaria pinnatifida. Vivo. 2003;17:245–249. [PubMed] [Google Scholar]
- 109.Cho Y.S., Jung W.K., Kim J.A., Choi I.W., Kim S.K. Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem. 2009;116:990–994. doi: 10.1016/j.foodchem.2009.03.051. [DOI] [Google Scholar]
- 110.Cho M.L., Lee B.Y., You S.G. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules. 2011;16:291–297. doi: 10.3390/molecules16010291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maruyamaa H., Tamauchib H., Hashimotoc M., Nakano T. Suppression of Th2 immune responses by Mekabu fucoidan from Undaria pinnatifida sporophylls. Int. Arch. Allergy Immunol. 2005;137:289–294. doi: 10.1159/000086422. [DOI] [PubMed] [Google Scholar]
- 112.Preobrazhenskaya M.E., Berman A.E., Mikhailov V.I., Ushakova N.A., Mazurov A.V., Semenov A.V., Usov A.I., Nifant’ev N.E., Bovin N.V. Fucoidan inhibits leukocyte recruitment in a model peritoneal inflammation in rat and blocks interaction of P-selectin with its carbohydrate ligand. Biochem. Mol. Biol. Int. 1997;43:443–451. doi: 10.1080/15216549700204231. [DOI] [PubMed] [Google Scholar]
- 113.Noda H. Health benefits and nutritional properties of nori. J. Appl. Phycol. 1993;5:255–258. doi: 10.1007/BF00004027. [DOI] [Google Scholar]
- 114.Takano R., Hayashi K., Hara S., Hirase S. Funoran from the red seaweed Gloiopeltis complanata: Polysaccharides with sulphated agarose structure and their precursor structure. Carbohydr. Polym. 1995;27:305–311. doi: 10.1016/0144-8617(95)00070-4. [DOI] [Google Scholar]
- 115.Zhang Q.B., Li N., Zhou G.F., Lu X.L., Xu Z.H., Li Z. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanensis (Rhodophyta) in aging mice. Pharmacol. Res. 2003;48:151–155. doi: 10.1016/S1043-6618(03)00103-8. [DOI] [PubMed] [Google Scholar]
- 116.Kwon M.J., Nam T.J. Porphyran induces apoptosis related signal pathway in AGS gastric cancer cell lines. Life Sci. 2006;79:1956–1962. doi: 10.1016/j.lfs.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 117.Yoshizawa Y., Enomoto A., Todoh H., Ametani A., Kaminogawa S. Activation of murine macrophages by polysaccharide fractions from marine algae (Porphyra yezoensis) Biosci. Biotechnol. Biochem. 1993;57:1862–1866. doi: 10.1271/bbb.57.1862. [DOI] [PubMed] [Google Scholar]
- 118.Yoshizawa Y., Ametani A., Tsunehiro J., Nomura K., Itoh M., Fukui F., Kaminogawa S. Macrophage stimulation activity of the polysaccharide fraction from a marine alga (Porphyra yezoensis): Structure-function relationships and improved solubility. Biosci. Biotechnol. Biochem. 1995;59:1933–1937. doi: 10.1271/bbb.59.1933. [DOI] [PubMed] [Google Scholar]
- 119.Tsuge K., Okabe M., Yoshimura T., Sumi T., Tachibana H., Yamada K. Dietary effect of porphyran from Porphyra yezoensis on growth and lipid metabolism of Sprague-Dawley rats. Food Sci. Technol. Res. 2004;10:147–151. doi: 10.3136/fstr.10.147. [DOI] [Google Scholar]
- 120.Duarte M.E., Noseda D.G., Noseda M.D., Tulio S., Pujol C.A., Damonte E.B. Inhibitory effect of sulfated galactans from the marine alga Bostrychia montagnei on herpes simplex virus replication in vitro. Phytomedicine. 2001;8:53–58. doi: 10.1078/0944-7113-00007. [DOI] [PubMed] [Google Scholar]
- 121.Carlucci M.J., Scolaro L.A., Matulewicz M.C., Damonte E.B. Antiviral activity of natural sulphated galactans on herpes virus multiplication in cell culture. Planta Med. 1997;63:429–432. doi: 10.1055/s-2006-957727. [DOI] [PubMed] [Google Scholar]
- 122.Sekine H., Ohonuki N., Sadamasu K., Monma K., Kudoh Y., Nakamura H., Okada Y., Okuyama T. The inhibitory effect of the crude extract from the seaweed Dygenea simplex C. Agardh on the in vitro cytopathic activity of HIV-1 and its antigen production. Chem. Pharm. Bull. (Tokyo) 1995;43:1580–1584. doi: 10.1248/cpb.43.1580. [DOI] [PubMed] [Google Scholar]
- 123.Talarico L.B., Zibetti R.G.M., Faria P.C.S., Scolaro L.A., Duarte M.E.R., Noseda M.D., Pujol C.A., Damonte E.B. Anti-herpes simplex virus activity of sulfated galactans from the red seaweed Gymnogongrus griffithsiae and Cryptonemia crenulata. Int. J. Biol. Macromol. 2004;34:63–71. doi: 10.1016/j.ijbiomac.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 124.Takano R., Iwane-Sakata H., Hayashi K., Hara S., Hirase S. Concurrence of agaroid and carrageenan chains in funoran from the red seaweed Gloiopeltis furcata Post. Et Ruprecht (Cryptonemiales, Rhodophyta) Carbohydr. Polym. 1998;35:81–87. doi: 10.1016/S0144-8617(97)00230-0. [DOI] [Google Scholar]
- 125.Pereira M.G., Benevides N.M.B., Melo M.R.S., Valente A.P., Melo F.R., Mourão P.A.S. Structure and anticoagulant activity of a sulfated galactan from the red alga, Gelidium crinale. Is there a specific structural requirement for the anticoagulant action? Carbohydr. Res. 2005;340:2015–2023. doi: 10.1016/j.carres.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 126.Pujol C.A., Errea M.I., Matulewicz M.C., Damonte E.B. Antiherpetic activity of S1, an algal derived sulphated galactan. Phytother Res. 1996;10:410–413. doi: 10.1002/(SICI)1099-1573(199608)10:5<410::AID-PTR875>3.0.CO;2-K. [DOI] [Google Scholar]
- 127.De Clercq E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med. Res. Rev. 2000;20:323–349. doi: 10.1002/1098-1128(200009)20:5<323::AID-MED1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 128.Witvrouw M., Este J.A., Mateu M.Q., Reymen D., Andrei G., Snoeck R., Ikeda S., Pauwels R., Bianchini N.V., Desmyter J., de Clercq E. Activity of a sulfated polysaccharide extracted from the red seaweed Aghardhiella tenera against human immunodeficiency virus and other enveloped viruses. Antivir. Chem. Chemother. 1994;5:297–303. doi: 10.1177/095632029400500503. [DOI] [Google Scholar]
- 129.Luescher-Mattli M. Algae, a possible source for new drugs in the treatment of HIV and other viral diseases. Curr. Med. Chem. 2003;2:219–225. [Google Scholar]
- 130.Prajapati V.D., Mahereriya P.M., Jani G.K., Soalnki H.K. Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014;105:97–112. doi: 10.1016/j.carbpol.2014.01.067. [DOI] [PubMed] [Google Scholar]
- 131.Campo V.L., Kawano D.F., da Silva D.B., Carvalho I. Carrageenans: Biological properties, chemical modifications and structural analysis. Carbohydr. Polym. 2009;77:167–180. doi: 10.1016/j.carbpol.2009.01.020. [DOI] [Google Scholar]
- 132.Mou H., Xiaolu J., Huashi G. A kappa-carrageenan derived oligosaccharide prepared by enzymatic degradation containing anti-tumor activity. J. Appl. Phycol. 2003;15:297–303. doi: 10.1023/A:1025103530534. [DOI] [Google Scholar]
- 133.Yang B., Yu G., Zhao X., Ren W., Jiao G., Fang L., Wang Y., Du G., Tiller C., Girouard G., et al. Structural characterisation and bioactivities of hybrid carrageenan-like sulphated galactan from red alga Furcellaria lumbricalis. Food Chem. 2011;124:50–57. doi: 10.1016/j.foodchem.2010.05.102. [DOI] [Google Scholar]
- 134.Carlucci M.J., Pujol C.A., Ciancia M., Noseda M.D., Matulewicz M.C., Damonte E.B., Cerezo A.S. Antiherpetic and anticoagulant properties of carrageenans from the red seaweed Gigartina skottsbergii and their cyclized derivatives: Correlation between structure and biological activity. Int. J. Biol. Macromol. 1997;20:97–105. doi: 10.1016/S0141-8130(96)01145-2. [DOI] [PubMed] [Google Scholar]
- 135.Carlucci M.J., Ciancia M., Matulewicz M.C., Cerezo A.S., Damonte E.B. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antivir. Res. 1999;43:93–102. doi: 10.1016/S0166-3542(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 136.Pujol C.A., Estevez J.M., Carlucci M.J., Ciancia M., Cerezo A.S., Damonte E.B. Novel DL-galactan hybrids from the red seaweed Gymnogongrus torulosus are potent inhibitors of herpes simplex virus and dengue virus. Antivir. Chem. Chemother. 2002;13:83–89. doi: 10.1177/095632020201300202. [DOI] [PubMed] [Google Scholar]
- 137.Sen A.K., Das A.K., Banerji N., Siddhanta A.K., Mody K.H., Ramavat B.K., Chauhan V.D., Vedasiromoni J.R., Ganguly D.K. A new sulfated polysaccharide with potent blood anti-coagulant activity from the red seaweed Grateloupia indica. Int. J. Biol. Macromol. 1994;16:279–280. doi: 10.1016/0141-8130(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 138.Bondu S., Deslandes E., Fabre M.S., Berthou C., Yu G. Carrageenan from Solieria chordalis (Gigartinales): Structural analysis and immunological activities of the low molecular weight fractions. Carbohydr. Polym. 2010;81:448–460. doi: 10.1016/j.carbpol.2010.02.046. [DOI] [Google Scholar]
- 139.Caceres P.J., Carlucci M.J., Damonte E.B., Matsuhiro B., Zuniga E.A. Carrageenans from chilean samples of Stenogramme interrupta (Phyllophoraceae): Structural analysis and biological activity. Phytochemistry. 2000;53:81–86. doi: 10.1016/S0031-9422(99)00461-6. [DOI] [PubMed] [Google Scholar]
- 140.Mazumder S., Ghosal P.K., Pujol C.A., Carlucci M.J., Damonte E.B., Ray B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta) Int. J. Biol. Macromol. 2002;31:87–95. doi: 10.1016/S0141-8130(02)00070-3. [DOI] [PubMed] [Google Scholar]
- 141.Yoshizawa Y., Tsunehiro J., Nomura K., Itoh M., Fukui F., Ametani A., Kaminogawa S. In vivo macrophage-stimulation activity of the enzyme-degraded water-soluble polysaccharide fraction from a marine alga (Gracilaria verrucosa) Biosci. Biotechnol. Biochem. 1996;60:1667–1671. doi: 10.1271/bbb.60.1667. [DOI] [PubMed] [Google Scholar]
- 142.Recalde M.P., Noseda M.D., Pujol C.A., Carlucci M.J., Matulewicz M.C. Sulfated mannans from the red seaweed Nemalion helminthoides of the South Atlantic. Phytochemistry. 2009;70:1062–1068. doi: 10.1016/j.phytochem.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 143.Damonte E., Neyts J., Pujol C.A., Snoeck R., Andrei G., Ikeda S., Witvrouw M., Reymen D., Haines H., Matulewicz M.C., et al. Antiviral activity of a sulphated polysaccharide from the red seaweed Nothogenia fastigiata. Biochem Pharmacol. 1994;47:2187–2192. doi: 10.1016/0006-2952(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 144.Damonte E.B., Matulewicz M.C., Cerezo A.S., Coto C.E. Herpes simplex virus-inhibitory sulfated xylogalactans from the red seaweed Nothogenia fastigiata. Chemotherapy. 1996;42:57–64. doi: 10.1159/000239422. [DOI] [PubMed] [Google Scholar]
- 145.Kolender A.A., Pujol C.A., Damonte E.B., Matulewicz M.C., Cerezo A.S. The system of sulfated alpha-(1→3)-linked D-mannans from the red seaweed Nothogenia fastigiata: Structures, antiherpetic and anticoagulant properties. Carbohydr. Res. 1997;304:53–60. doi: 10.1016/S0008-6215(97)00201-2. [DOI] [PubMed] [Google Scholar]
- 146.Bourgougnon N., Roussakis C., Kornprobst J.M., Lahaye M. Effects in vitro of sulfated polysaccharide from Schizymenia dubyi (Rhodophyta, Gigartinales) on a non-small-cell bronchopulmonary carcinoma line (NSCLC-N6) Cancer Lett. 1994;85:87–92. doi: 10.1016/0304-3835(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 147.Nakashima H., Kido Y., Kobayashi N., Motoki Y., Neushul M., Yamamoto N. Purification and characterization of an avian myeloblastosis and human immunodeficiency virus reverse transcriptase inhibitor, sulfated polysaccharides extracted from sea algae. Antimicrob. Agents Chemother. 1987;31:1524–1528. doi: 10.1128/AAC.31.10.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zuniga E.A., Matsuhiro B., Mejias E. Preparation of a low-molecular weight fraction by free radical depolymerization of the sulfated galactan from Schizymenia binderi (Gigartinales, Rhodophyta) and its anticoagulant activity. Carbohydr. Polym. 2006;66:208–215. doi: 10.1016/j.carbpol.2006.03.007. [DOI] [Google Scholar]
- 149.Farias W.R.L., Valente A.P., Pereira M.S., Mourão P.A.S. Structure and anticoagulant activity of sulfated galactans. Isolation of a unique sulfated galactan from the red alga Botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J. Biol. Chem. 2000;275:29299–29307. doi: 10.1074/jbc.M002422200. [DOI] [PubMed] [Google Scholar]
- 150.Toyama M.H., Toyama D.O., Torres V.M., Pontes G.C., Farias W.R.L., Melo F.R., Oliveira S.C.B., Fagundes F.H.R., Diz Filho E.B.S., Cavada B.S. Effects of Low Molecular Weight Sulfated Galactan Fragments From Botryocladia occidentalis on the Pharmacological and Enzymatic Activity of Spla2 from Crotalus durissus cascavella. Protein J. 2010;29:567–571. doi: 10.1007/s10930-010-9294-9. [DOI] [PubMed] [Google Scholar]
- 151.Lins K.O., Bezerra D.P., Alves A.P., Alencar N.M., Lima M.W., Torres V.M., Farias W.R., Pessoa C., de Moraes M.O., Costa-Lotufo L.V. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer) J. Appl. Toxicol. 2009;29:20–26. doi: 10.1002/jat.1374. [DOI] [PubMed] [Google Scholar]
- 152.Ghosh T., Pujol C.A., Damonte E.B., Sinha S., Ray B. Sulfated xylomannans from the red seaweed Sebdenia polydactyla: Structural features, chemical modification and antiviral activity. Antivir. Chem. Chemother. 2009;19:235–242. doi: 10.1177/095632020901900603. [DOI] [PubMed] [Google Scholar]
- 153.Lee J.B., Hayashi K., Maeda M., Hayashi T. Antiherpetic activities of sulfated polysaccharides from green algae. Planta Med. 2004;70:813–817. doi: 10.1055/s-2004-827228. [DOI] [PubMed] [Google Scholar]
- 154.Ji H., Shao H., Zhang C., Hong P., Xiong H. Separation of the polysaccharides in Caulerpa racemosa and their chemical composition and antitumor activity. J. Appl. Polym. Sci. 2008;110:1435–1440. doi: 10.1002/app.28676. [DOI] [Google Scholar]
- 155.Rodrigues J.A.G., Oliveira Vanderlei E.D.S., Silva L.M., de Araujo I.W., de Queiroz I.N., de Paula G.A., Abreu T.M., Ribeiro N.A., Bezerra M.M., Chaves H.V., et al. Antinociceptive and anti-inflammatory activities of a sulfated polysaccharide isolated from the green seaweed Caulerpa cupressoides. Pharmacol. Rep. 2012;64:282–292. doi: 10.1016/S1734-1140(12)70766-1. [DOI] [PubMed] [Google Scholar]
- 156.Rodrigues J.A.G., Oliveira Vanderlei E.D.S., Gomes Quindere A.L., Monteiro V.S., Mendes de Vasconcelos S.M., Barros Benevides N.M. Antinociceptive activity and acute toxicological study of a novel sulfated polysaccharide from Caulerpa cupressoides var. lycopodium (Chlorophyta) in Swiss mice. Acta Sci. Technol. 2013;35:417–425. [Google Scholar]
- 157.Chattopadhyay K., Adhikari U., Lerouge P., Ray B. Polysaccharides from Caulerpa racemosa: Purification and structural features. Carbohydr. Polym. 2007;68:407–415. doi: 10.1016/j.carbpol.2006.12.010. [DOI] [Google Scholar]
- 158.Fernández P.V., Ciancia M., Miravalles A.B., Estevez J.M. Cell wall polymer mapping in the coenocytic macroalga Codium vermilara. J. Phycol. 2010;46:456–465. doi: 10.1111/j.1529-8817.2010.00821.x. [DOI] [Google Scholar]
- 159.Hayakawa Y., Hayashi T., Hayashi K., Osawa T., Niiya K., Sakuragawa N. Activation of heparin cofactor II by calcium spirulan. J. Biol. Chem. 2000;275:11379–11382. doi: 10.1074/jbc.275.15.11379. [DOI] [PubMed] [Google Scholar]
- 160.Shanmugam M., Mody K.H., Ramavat B.K., Murthy A.S.K., Siddhanta A.K. Screening of Codiacean algae (Chlorophyta) of the Indian coasts for blood anticoagulant activity. Indian J. Mar. Sci. 2002;31:33–38. [Google Scholar]
- 161.Ciancia M., Quintana I., Vizcarguenaga M.I., Kasulin L., de Dios A., Estevez J.M., Cerezo A.S. Polysaccharides from the green seaweeds Codium fragile and C. vermilara with controversial effects on hemostasis. Int. J. Biol. Macromol. 2007;41:641–649. doi: 10.1016/j.ijbiomac.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 162.Farias E.H., Pomin V.H., Valente A.P., Nader H.B., Rocha H.A., Mourão P.A. A preponderantly 4-sulfated, 3-linked galactan from the green alga Codium isthmocladum. Glycobiology. 2008;18:250–259. doi: 10.1093/glycob/cwm139. [DOI] [PubMed] [Google Scholar]
- 163.Na Y.S., Kim W.J., Kim S.M., Park J.K., Lee S.M., Kim S.O., Synytsya A., Park Y.I. Purification, characterization and immunostimulating activity of water-soluble polysaccharide isolated from Capsosiphon fulvescens. Int. Immunopharmacol. 2010;10:364–370. doi: 10.1016/j.intimp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 164.Lee J.B., Hayashi K., Hayashi T., Sankawa U., Maeda M. Antiviral activities against HSV-1, HCMV, and HIV-1 of rhamnan sulfate from Monostroma latissimum. Planta Med. 1999;65:439–441. doi: 10.1055/s-2006-960804. [DOI] [PubMed] [Google Scholar]
- 165.Qi H.M., Zhang Q.B., Zhao T.T., Chen R., Zhang H., Niu X.Z., Li Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005;37:195–199. doi: 10.1016/j.ijbiomac.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 166.Qi H.M., Zhao T.T., Zhang Q.B., Li Z., Zhao Z.Q., Xing R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta) J. Appl. Phycol. 2005;17:527–534. doi: 10.1007/s10811-005-9003-9. [DOI] [Google Scholar]
- 167.Mao W., Li H., Li Y., Zhang H., Qi X., Sun H., Chen Y., Guo S. Chemical characteristic and anticoagulant activity of the sulfated polysaccharide isolated from Monostroma latissimum (Chlorophyta) Int. J. Biol. Macromol. 2009;44:70–74. doi: 10.1016/j.ijbiomac.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 168.Zhang H.J., Mao W.J., Fang F., Li H.Y., Sun H.H., Chen Y., Qi X.H. Chemical characteristics and anticoagulant activities of a sulfated polysaccharide and its fragments from Monostroma latissimum. Carbohydr. Polym. 2008;71:428–434. doi: 10.1016/j.carbpol.2007.06.012. [DOI] [Google Scholar]
- 169.Hayakawa Y., Hayashi T., Lee J.B., Srisomporn P., Maeda M., Ozawa T., Sakuragawa N. Inhibition of thrombin by sulfated polysaccharides isolated from green algae. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000;1543:86–94. doi: 10.1016/S0167-4838(00)00193-X. [DOI] [PubMed] [Google Scholar]
- 170.Karnjanapratum S., You S. Molecular characteristics of sulfated polysaccharides from Monostroma nitidum and their in vitro anticancer and immunomodulatory activities. Int. J. Biol. Macromol. 2011;48:311–318. doi: 10.1016/j.ijbiomac.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 171.Charles A.L., Chang C.K., Wu M.L., Huang T.C. Studies on the expression of liver detoxifying enzymes in rats fed seaweed (Monostroma nitidum) Food Chem. Toxicol. 2007;45:2390–2396. doi: 10.1016/j.fct.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 172.Jiao L., Li X., Li T., Jiang P., Zhang L., Wu M., Zhang L. Characterization and anti-tumor activity of alkali-extracted polysaccharide from Enteromorpha intestinalis. Int. Immunopharmacol. 2009;9:324–329. doi: 10.1016/j.intimp.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 173.Jiao L., Jiang P., Zhang L., Wu M. Antitumor and immunomodulating activity of polysaccharides from Enteromorpha intestinalis. Biotechnol. Biopro. Eng. 2010;15:421–428. doi: 10.1007/s12257-008-0269-z. [DOI] [Google Scholar]
- 174.Wang X., Zhang Z., Yao Z., Zhao M., Qi H. Sulfation, anticoagulant and antioxidant activities of polysaccharide from green algae Enteromorpha linza. Int. J. Biol. Macromol. 2013;58:225–230. doi: 10.1016/j.ijbiomac.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 175.Kim J.K., Cho M.L., Karnjanapratum S., Shin I.S., You S.G. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011;49:1051–1058. doi: 10.1016/j.ijbiomac.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 176.Li B., Liu S., Xing R., Li K., Li R., Qin Y., Wang X., Wei Z., Li P. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr. Polym. 2013;92:1991–1996. doi: 10.1016/j.carbpol.2012.11.088. [DOI] [PubMed] [Google Scholar]
- 177.Teng Z., Qian L., Zhou Y. Hypolipidemic activity of the polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013;62:254–256. doi: 10.1016/j.ijbiomac.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 178.Lahaye M., Robic A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules. 2007;8:1765–1774. doi: 10.1021/bm061185q. [DOI] [PubMed] [Google Scholar]
- 179.Rao H.B.R., Sathivel A., Devaki T. Antihepatotoxic nature of Ulva reticulata (Chlorophyceae) on acetaminophen-induced hepatoxicity in experimental rats. J. Med. Food. 2004;7:495–497. doi: 10.1089/jmf.2004.7.495. [DOI] [PubMed] [Google Scholar]
- 180.Mao W., Zang X., Li Y., Zhang H. Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J. Appl. Phycol. 2006;18:9–14. doi: 10.1007/s10811-005-9008-4. [DOI] [Google Scholar]
- 181.Shao P., Chen X., Sun P. In vitro antioxidant and antitumor activities of different sulfated polysaccharides isolated from three algae. Int. J. Biol. Macromol. 2013;62:155–161. doi: 10.1016/j.ijbiomac.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 182.Xing R.G., Liu S., Yu H.H., Guo Z.Y., Li Z., Li P.C. Preparation of high-molecular weight and high-sulfate content chitosans and their potential antioxidant activity in vitro. Carbohydr. Polym. 2005;61:148–154. doi: 10.1016/j.carbpol.2005.04.007. [DOI] [Google Scholar]
- 183.Lahaye M., Ray B. Cell-wall polysaccharides from the marine green alga Ulva rigida (Ulvales, Chlorophyta)-NMR analysis of ulvan oligosaccharides. Carbohydr. Res. 1996;283:161–173. doi: 10.1016/0008-6215(95)00407-6. [DOI] [PubMed] [Google Scholar]
- 184.Lahaye M., Brunel M., Bonnin E. Fine chemical structure analysis of oligosaccharides produced by an ulvan-lyase degradation of the water-soluble cell-wall polysaccharides from Ulva sp. (Ulvales, Chlorophyta) Carbohydr. Res. 1997;304:325–333. doi: 10.1016/S0008-6215(97)00270-X. [DOI] [PubMed] [Google Scholar]
- 185.Yu P.Z., Li N., Liu X.G., Zhou G.F., Zhang Q.B., Li P.C. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta) Pharmacol. Res. 2003;48:543–549. doi: 10.1016/S1043-6618(03)00215-9. [DOI] [PubMed] [Google Scholar]
- 186.Kaeffer B., Benard C., Lahaye M., Blottiere H.M., Cherbut C. Biological properties of ulvan, a new source of green seaweed sulfated polysaccharides, on cultured normal and cancerous colonic epithelial tells. Planta Med. 1999;65:527–531. doi: 10.1055/s-1999-14009. [DOI] [PubMed] [Google Scholar]
- 187.Margret R.J., Kumaresan S., Ravikumar S. A preliminary study on the anti-inflammatory activity of methanol extract of Ulva lactuca in rat. J. Environ. Biol. 2009;30:899–902. [PubMed] [Google Scholar]
- 188.Chiu Y.H., Chan Y.L., Li T.L., Wu C.J. Inhibition of Japanese encephalitis virus infection by the sulfated polysaccharide extracts from Ulva lactuca. Mar. Biotechnol. 2012;14:468–478. doi: 10.1007/s10126-011-9428-x. [DOI] [PubMed] [Google Scholar]
- 189.Sathivel A., Raghavendran H.R.B., Srinivasan P., Devaki T. Anti-peroxidative and anti-hyperlipidemic nature of Ulva lactuca crude polysaccharide on D-Galactosamine induced hepatitis in rats. Food Chem. Toxicol. 2008;46:3262–3267. doi: 10.1016/j.fct.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 190.Tabarsa M., Han J.H., Kim C.Y., You S.G. Molecular characteristics and immunomodulatory activities of water-soluble sulfated polysaccharides from Ulva pertusa. J. Med. Food. 2012;15:135–144. doi: 10.1089/jmf.2011.1716. [DOI] [PubMed] [Google Scholar]
- 191.Qi H., Liu X., Zhang J., Duan Y., Wang X., Zhang Q. Synthesis and antihyperlipidemic activity of acetylated derivative of ulvan from Ulva pertusa. Int. J. Biol. Macromol. 2012;50:270–272. doi: 10.1016/j.ijbiomac.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 192.Leiro J.M., Castro R., Arranz J.A., Lamas J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2007;7:879–888. doi: 10.1016/j.intimp.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 193.Staats N., de Winder B., Stal L.J., Mur L.R. Isolation and characterization of extracellular polysaccharides from the epipelic diatoms Cylindrotheca closterium and Navicula salinarum. Eur. J. Phycol. 1999;34:161–169. doi: 10.1080/09670269910001736212. [DOI] [Google Scholar]
- 194.Pletikapic G., Radic T.M., Zimmermann A.H., Svetlicic V., Pfannkuchen M., Maric D., Godrjan J., Zutic V. AFM imaging of extracellular polymer release by marine diatom Cylindrotheca closterium (Ehrenberg) Reiman & JC Lewin. J. Mol. Recogn. 2011;24:436–445. doi: 10.1002/jmr.1114. [DOI] [PubMed] [Google Scholar]
- 195.Guzman S., Gato A., Lamela M., Freire-Garabal M., Calleja J.M. Anti-Inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003;17:665–670. doi: 10.1002/ptr.1227. [DOI] [PubMed] [Google Scholar]
- 196.Ford C.W., Percival E. The carbohydrates of Phaeodactylum tricornutum. Part I. Preliminary examination of the organism and characterization of low molecular weight material and of a glucan. J. Chem. Soc. 1965;1298:7035–7041. doi: 10.1039/jr9650007035. [DOI] [Google Scholar]
- 197.Ford C.W., Percival E. The carbohydrates of Phaeodactylum tricornutum. Part II. A sulphated glucuronomannan. J. Chem. Soc. 1965;1299:7042–7046. doi: 10.1039/jr9650007042. [DOI] [Google Scholar]
- 198.Rincé Y., Lebeau T., Robert J.M. Artificial cell-immobilization: A model simulating immobilization in natural environments? J. Appl. Phycol. 1999;11:263–272. doi: 10.1023/A:1008144307248. [DOI] [Google Scholar]
- 199.Penna A., Berluti S., Penna N., Magnani M. Influence of nutrient ratios on the in vitro extracellular polysaccharide production by marine diatoms from Adriatic Sea. J. Plankton Res. 1999;21:1681–1690. doi: 10.1093/plankt/21.9.1681. [DOI] [Google Scholar]
- 200.Urbani R., Sist P., Pletikapić G., Radić T.M., Svetličić V., Žutic V. Diatom Polysaccharides: Extracellular Production, Isolation and Molecular Characterization. In: Karunaratne D.N., editor. The Complex World of Polysaccharides. InTech; Rijeka, Croatia: 2012. Chapter 12. [DOI] [Google Scholar]
- 201.Chen C.-S., Anaya J.M., Zhang S., Spurgin J., Chuang C.-Y., Xu C., Miao A.-J., Chen E.Y.-T., Schwehr K.A., Jiang Y., et al. Effects of engineered nanoparticles on the assembly of exopolymeric substances from phytoplankton. PLoS ONE. 2011;6:e21865. doi: 10.1371/journal.pone.0021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Guzmán-Murillo M.A., Ascencio F. Anti-adhesive activity of sulphated exopolysaccharides of microalgae on attachment of the red sore disease-associated bacteria and Helicobacter pylori to tissue culture cells. Lett. Appl. Microbiol. 2000;30:473–478. doi: 10.1046/j.1472-765x.2000.00751.x. [DOI] [PubMed] [Google Scholar]
- 203.Ogawa K., Yamaura M., Maruyama I. Isolation and identification of 2-O-methyl-l-rhamnose and 3-O-methyl-l-rhamnose as constituents of an acidic polysaccharide of Chlorella vulgaris. Biosci. Biotechnol. Biochem. 1997;61:539–540. doi: 10.1271/bbb.61.539. [DOI] [Google Scholar]
- 204.Ogawa K., Ikeda Y., Kondo S. A new trisaccharide, α-d-glucopyranuronosyl-(1→3)-α-l-rhamnopyranosyl-(1→2)-α-l-rhamopyranose from Chlorella vulgaris. Carbohydr. Res. 1999;321:128–131. doi: 10.1016/S0008-6215(99)00176-7. [DOI] [Google Scholar]
- 205.Mishra A., Kavita K., Jha B. Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr. Polym. 2011;83:852–857. doi: 10.1016/j.carbpol.2010.08.067. [DOI] [Google Scholar]
- 206.Allard B., Guillot J.P. The production of extracellular polysaccharides by fresh-water microalgae. Investigation of the polysaccharide components. In: Grassi G., Delmon B., Molle J.F., Zibetta H., editors. Biomass for Energy and Industry. Elsevier Applied Science; London, UK: 1987. pp. 603–607. [Google Scholar]
- 207.Allard B., Casadeval E. Carbohydrate composition and characterization of sugars from the green alga Botryococcus braunii. Phytochemistry. 1990;29:1875–1878. doi: 10.1016/0031-9422(90)85031-A. [DOI] [Google Scholar]
- 208.Geresh S., Arad S.M. The extracellular polysaccharides of the red microalgae: Chemistry and rheology. Bioresour. Technol. 1991;38:195–201. doi: 10.1016/0960-8524(91)90154-C. [DOI] [Google Scholar]
- 209.Dubinsky O., Barak Z., Geresh S., Arad S.M. Composition of the cell-wall polysaccharide of the unicellular red alga Rhodella reticulata at two phases of growth; Recent Advances in Algal Biotechnology, Proceedings of the 5th International Conference of the Society of Applied Algology; Tiberias, Israel. 28 January–2 February 1990; London, UK: US Office of Naval Research; 1990. p. 17. [Google Scholar]
- 210.Arad S.M. Production of sulphated polysaccharides from red unicellular algae. In: Stadler T., Mollion J., Verdus M.C., Karamanos Y., Morvan H., Christiaen D., editors. Algal Biotechnology. Elsevier Applied Science; London, UK: 1988. pp. 65–87. [Google Scholar]
- 211.Matsui S.M., Muizzudin N., Arad S.M., Marenus K. Sulfated polysaccharides from red microalgae anti-inflammatory properties in vitro and in vivo. Appl. Biochem. Biotechnol. 2003;104:13–22. doi: 10.1385/ABAB:104:1:13. [DOI] [PubMed] [Google Scholar]
- 212.Arad S.M., Atar D. Viscosupplementation with Algal Polysaccharides in the Treatment of Arthritis. WO/2007/066340. Patent. 2007
- 213.Talyshinsky M.M., Souprun Y.Y., Huleihel M.M. Antiviral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int. 2002;2:8–14. doi: 10.1186/1475-2867-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Garcia D., Morales E., Dominguez A., Fábregas J. Communicaciones del III Congreso Ibérico de Biotecnología—Biotec’96. Universidad de Valladolid; Valladolid, Spain: 1996. Productividad mixotrófica del exopolisacárido sulfatado com la microalga marina Porphyridium cruentum; pp. 591–592. [Google Scholar]
- 215.Heaney-Kieras J.H. Ph.D. Thesis. University of Chicago; Chicago, IL, USA: 1972. Study of the Extracellular Polysaccharide of Porphyridium cruentum. [Google Scholar]
- 216.Raposo M.F.J., Morais A.M.M.B., Morais R.M.S.C. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014;101:56–63. doi: 10.1016/j.lfs.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 217.Gloaguen V., Ruiz G., Morvan H., Mouradi-Givernaud A., Maes E., Krausz P., Srecker G. The extracelular polysaccharide of Porphtyridium sp.: An NMR study of lithium-resistant oligosaccharidic fragments. Carbohydr. Res. 2004;339:97–103. doi: 10.1016/j.carres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 218.Geresh S., Adin I., Yarmolinsky E., Karpasas M. Characterization of the extracellular polysaccharide of Porphyridium sp.: Molecular weight determination and rheological properties. Carbohydr. Polym. 2002;50:183–189. doi: 10.1016/S0144-8617(02)00019-X. [DOI] [Google Scholar]
- 219.Dubinsky O., Simon B., Karamanos Y., Geresh S., Barak Z., Arad S.M. Composition of the cell wall polysaccharide produced by the unicellular red alga Rhodella reticulata. Plant Physiol. Biochem. 1992;30:409–414. [Google Scholar]
- 220.Sun L., Wang C., Shi Q., Ma C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009;45:42–47. doi: 10.1016/j.ijbiomac.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 221.Sun L., Wang L., Zhou Y. Immunomodulation and antitumor activities of different molecular weight polysaccharides from Porphyridium cruentum. Carbohydr. Polym. 2012;87:1206–1210. doi: 10.1016/j.carbpol.2011.08.097. [DOI] [Google Scholar]
- 222.Huang J., Chen B., You W. Studies on separation of extracellular polysaccharide from Porphyridium cruentum and its anti-HBV activity in vitro. Chin. J. Mar. Drugs (Chin.) 2005;24:18–21. [Google Scholar]
- 223.Radonic A., Thulke S., Achenbach J., Kurth A., Vreemann A., König T., Walter C., Possinger K., Nitsche A. Anionic polysaccharides from phototrophic microorganisms exhibit antiviral activities to Vaccinia virus. J. Antivir. Antiretrovir. 2010;2:51–55. [Google Scholar]
- 224.Evans L.V., Callow M.E., Percival E., Fareed V.S. Studies on the synthesis and composition of extracellular mucilage in the unicellular red alga Rhodella. J. Cell Sci. 1974;16:1–21. doi: 10.1242/jcs.16.1.1. [DOI] [PubMed] [Google Scholar]
- 225.Fareed V.S., Percival E. The presence of rhamnose and 3-O-methylxylose in the extracellular mucilage from the red alga Rhodella maculata. Carbohydr. Res. 1977;53:276–277. doi: 10.1016/S0008-6215(00)88099-4. [DOI] [Google Scholar]
- 226.Hasui M., Matsuda M., Okutani K., Shigeta S. In vitro antiviral activities of sulphated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped virus. Int. J. Biol. Macromol. 1995;17:293–297. doi: 10.1016/0141-8130(95)98157-T. [DOI] [PubMed] [Google Scholar]
- 227.Bae S.Y., Yim J.H., Lee H.K., Pyo S. Activation of murine peritoneal macrophages by sulphated exopolysaccharide from marine microalga Gyrodinium impudicum (strain KG03): Involvement of the NF-kappa B and JNK pathway. Int. Immunopharmacol. 2006;6:473–484. doi: 10.1016/j.intimp.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 228.Yim J.H., Son E., Pyo S., Lee H.K. Novel sulfated polysaccharide derived from red-tide microalga Gyrodinium impudicum strain KG03 with immunostimulating activity in vivo. Mar. Biotechnol. 2005;7:331–338. doi: 10.1007/s10126-004-0404-6. [DOI] [PubMed] [Google Scholar]
- 229.Yim J.H., Kim S.J., Ahn S.H., Lee C.K., Rhie K.T., Lee H.K. Antiviral effects of sulphated polysaccharide from the marine microalga Gyrodinium impudicum strain KG03. Mar. Biotech. 2004;6:17–25. doi: 10.1007/s10126-003-0002-z. [DOI] [PubMed] [Google Scholar]
- 230.Li P., Liu Z., Xu R. Chemical characterization of the released polysaccharides from the cyanobacterium Aphanothece halophytica GR02. J. Appl. Phycol. 2001;13:71–77. doi: 10.1023/A:1008109501066. [DOI] [Google Scholar]
- 231.Hayashi T., Hayashi K., Maeda M., Kojima I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. 1996;59:83–87. doi: 10.1021/np960017o. [DOI] [PubMed] [Google Scholar]
- 232.Martinez M.J.A., del Olmo L.M.B., Benito P.B. Antiviral activities of polysaccharides from natural sources. Stud. Nat. Prod. Chem. 2005;30:393–418. [Google Scholar]
- 233.Lee J.-B., Hayashi T., Hayashi K., Sankawa U. Structural analysis of calcium spirulan (Ca-SP)-derived oligosaccharides using electrospray ionization mass spectrometry. J. Nat. Prod. 2000;63:136–138. doi: 10.1021/np990348b. [DOI] [PubMed] [Google Scholar]
- 234.Kaji T., Okabe M., Shimada S., Yamamoto C., Fujiwara Y., Lee J.-B., Hayashi T. Sodium spirulan as a potent inhibitor of arterial smooth muscle cell proliferation in vitro. Life Sci. 2004;74:1–9. doi: 10.1016/j.lfs.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 235.Challouf R., Trabelsi L., Dhieb R.B., El Abed O., Yahia A., Ghozzi K., Ammar J.B., Omran H., Ouada H.B. Evaluation of cytotoxicity and biological activities in extracellular polysaccharides released by cyanobacterium Arthrospira platensis. Braz. Arch. Biol. Technol. 2011;54:831–838. doi: 10.1590/S1516-89132011000400024. [DOI] [Google Scholar]
- 236.Usov A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011;65:115–217. doi: 10.1016/B978-0-12-385520-6.00004-2. [DOI] [PubMed] [Google Scholar]
- 237.Pomin V.H. Fucanomics and Galactanomics: Marine Distribution, Medicinal Impact, Conceptions, and Challenges. Mar. Drugs. 2012;10:793–811. doi: 10.3390/md10040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Anderson N.S., Dolan T.C.S., Rees D.A. Carrageenans. Part VII. Polysaccharides from Eucheuma spinosum and Eucheuma cottonii. The covalent structure of λ-carrageenan. J. Chem. Soc. Perkin. Trans. I. 1973;19:2173–2176. doi: 10.1039/p19730002173. [DOI] [PubMed] [Google Scholar]
- 239.Funami T., Hiroe M., Noda S., Asai I., Ikeda S., Nishinari K. Influence of molecular structure imaged with atomic force microscopy on the rheological behavior of carrageenan aqueous systems in the presence or absence of cations. Food Hydrocolloid. 2007;21:617–629. doi: 10.1016/j.foodhyd.2006.07.013. [DOI] [Google Scholar]
- 240.Li H., Mao W., Zhang X., Qi X., Chen Y., Chen Y., Chena Y., Xua J., Zhaoa C., Houa Y., et al. Structural characterization of an anticoagulant-active sulfated polysaccharide isolated from green alga Monostroma latissimum. Carbohydr. Polym. 2011;85:394–400. doi: 10.1016/j.carbpol.2011.02.042. [DOI] [Google Scholar]
- 241.Bilan M.I., Vinogradova E.V., Shashkov A.S., Usov A.I. Structure of a highly pyruvylated galactan sulfate from the Pacific green alga Codium yezoense (Bryopsidales, Chlorophyta) Carbohydr. Res. 2007;342:586–596. doi: 10.1016/j.carres.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 242.Ohta Y., Lee J.B., Hayashi K., Hayashi T. Isolation of sulfated galactan from Codium fragile and its antiviral effect. Biol. Pharm. Bull. 2009;32:892–898. doi: 10.1248/bpb.32.892. [DOI] [PubMed] [Google Scholar]
- 243.Tabarsa M., Karnjanapratum S., Cho M., Kim J.K., You S. Molecular characteristics and biological activities of anionic macromolecules from Codium fragile. Int. J. Biol. Macromol. 2013;59:1–12. doi: 10.1016/j.ijbiomac.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 244.Lee J.-B., Hayashi T., Hayashi K., Sankawa U., Maeda M., Nemoto T., Nakanishi H. Further purification and structural analysis of calcium spirulan from Spirulina platensis. J. Nat. Prod. 1998;61:1101–1104. doi: 10.1021/np980143n. [DOI] [PubMed] [Google Scholar]
- 245.Pignolet O., Jubeau S., Vaca-Garcia C., Michaud P. Highly valuable microalgae: Biochemical and topological aspects. J. Ind. Microbiol. Biotechnol. 2013;40:781–796. doi: 10.1007/s10295-013-1281-7. [DOI] [PubMed] [Google Scholar]
- 246.Heaney-Kieras J.H., Chapman D. Structural studies on the extracellular polysaccharide of the red alga Porphyridium cruentum. Carbohydr. Res. 1976;52:169–177. doi: 10.1016/S0008-6215(00)85957-1. [DOI] [PubMed] [Google Scholar]
- 247.Geresh S., Dubinsky O., Arad S.M., Christian D., Glaser R. Structure of 3-O-(α-d-glucopyranosyluronic acid)-l-galactopyranose, an aldobiuronic acid isolated from the polysaccharides of various unicellular red algae. Carbohydr. Res. 1990;208:301–305. doi: 10.1016/0008-6215(90)80116-K. [DOI] [PubMed] [Google Scholar]
- 248.Damonte E.B., Matulewicz M.C., Cerezo A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004;11:2399–2419. doi: 10.2174/0929867043364504. [DOI] [PubMed] [Google Scholar]
- 249.Ghosh T., Chattopadhyay K., Marschall M., Karmakar P., Mandal P., Ray B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology. 2009;19:2–15. doi: 10.1093/glycob/cwn092. [DOI] [PubMed] [Google Scholar]
- 250.Nishino T., Nagumo T. The sulfate-content dependence of the anticoagulant activity of a fucan sulfate from the brown seaweed Ecklonia kurome. Carbohydr. Res. 1991;214:193–197. doi: 10.1016/S0008-6215(00)90542-1. [DOI] [PubMed] [Google Scholar]
- 251.Pomin V.H., Pereira M.S., Valente A.P., Tollefsen D.M., Pavao M.S.G., Mourao P.A.S. Selective cleavage and anticoagulant activity of a sulfated fucan: Stereospecific removal of a 2-sulfate ester from the polysaccharide by mild acid hydrolysis, preparation of oligosaccharides, and heparin cofactor II-dependent anticoagulant activity. Glycobiology. 2005;15:369–381. doi: 10.1093/glycob/cwi021. [DOI] [PubMed] [Google Scholar]
- 252.Witvrouw M., de Clercq E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 1997;29:497–511. doi: 10.1016/S0306-3623(96)00563-0. [DOI] [PubMed] [Google Scholar]
- 253.Harden E.A., Falshaw R., Carnachan S.M., Kern E.R., Prichard M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir. Res. 2009;83:282–289. doi: 10.1016/j.antiviral.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Clement M.J., Tissot B., Chevolot L., Adjadj E., Du Y., Curmi P.A., Daniel R. NMR characterization and molecular modeling of fucoidan showing the importance of oligosaccharide branching in its anticomplementary activity. Glycobiology. 2010;20:883–894. doi: 10.1093/glycob/cwq046. [DOI] [PubMed] [Google Scholar]
- 255.Carlucci M.J., Mateu C.G., Artuso M.C., Scolaro L.A. Polysaccharides from red algae: Genesis of a renaissance. In: Karunaratne D.N., editor. The Complex World of Polysaccharides. InTech; Rijeka, Croatia: 2012. [(accessed on 2 February 2015)]. pp. 535–554. Chapter 20. Available online: http://www.intechopen.com/books/the-complex-world-of-polysaccharides/polysaccharides-from-red-algae-genesis-of-a-renaissance. [DOI] [Google Scholar]
- 256.Baba M., Snoeck R., Pauwels R., de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988;32:1742–1745. doi: 10.1128/AAC.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mandal P., Mateu C.G., Chattopadhyay K., Pujol C.A., Damonte E.B., Ray B. Structural features and antiviral activity of sulphated fucans from the brown seaweed Cystoseira indica. Antivir. Chem. Chemother. 2007;18:153–162. doi: 10.1177/095632020701800305. [DOI] [PubMed] [Google Scholar]
- 258.Mohsen M.S.A., Mohamed S.F., Ali F.M., El-Sayed O.H. Chemical Structure and Antiviral Activity of Water-soluble Sulfated Polysaccharides from Sargassum latifolium. J. Appl. Sci. Res. 2007;3:1178–1185. [Google Scholar]
- 259.Buck C.B., Thompson C.D., Roberts J.N., Muller M., Lowy D.R., Schiller J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2:671–680. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zeitlin L., Whaley K.J., Hegarty T.A., Moench T.R., Cone R.A. Tests of vaginal microbicides in the mouse genital herpes model. Contraception. 1997;56:329–335. doi: 10.1016/S0010-7824(97)00154-6. [DOI] [PubMed] [Google Scholar]
- 261.Oehninger S., Clark G.F., Acosta A.A., Hodgen G.D. Nature of the inhibitory effect of complex saccharide moieties on the tight binding of human spermatozoa to the human zona pellucida. Fertil. Steril. 1991;55:165–169. [PubMed] [Google Scholar]
- 262.Huleihel M., Ishanu V., Tal J., Arad S.M. Antiviral effect of the red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J. Appl. Phycol. 2001;13:127–134. doi: 10.1023/A:1011178225912. [DOI] [Google Scholar]
- 263.Esko J.D., Selleck S.B. Order out of chaos: Assembly of ligand binding sites in heparin sulfate. Annu. Rev. Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 264.Heaney-Kieras J., Roden L., Chapman D.J. The covalent linkage of protein to carbohydrate in the extracellular protein-polysaccharide from the red alga Porphyridium cruentum. Biochemistry. 1977;165:1–9. doi: 10.1042/bj1650001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tabarsa M., Park G.M., Shin I.S., Lee E., Kim J.K., You S. Structure-activity relationships of sulphated glycoproteins from Codium fragile on nitric oxide releasing capacity from RAW264.7 cells. Mar. Biotechnol. 2015;17:266–276. doi: 10.1007/s10126-015-9615-2. [DOI] [PubMed] [Google Scholar]
- 266.Huleihel M., Ishanu V., Tal J., Arad S.M. Activity of Porphyridium sp. polysaccharide against Herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Met. 2002;50:189–200. doi: 10.1016/S0165-022X(01)00186-5. [DOI] [PubMed] [Google Scholar]
- 267.Li L.-Y., Li L.-Q., Guo C.-H. Evaluation of in vitro antioxidant and antibacterial activities of Laminaria japonica polysaccharides. J. Med. Plants Res. 2010;4:2194–2198. [Google Scholar]
- 268.Miroshnichenko V.A., Yansons T.Y., Polushin O.G. Differentiated approach to the treatment of gastroduodenal pathology with the use of bioactive substances of marine hydrocele. In: Ivanov E.M., editor. New Biomedical Technologies to Use of Dietary Supplements. IMKVL Siberian Branch, Ross. Akad. Med. Nank.; Vladivostok, Russia: 1998. pp. 146–150. [Google Scholar]
- 269.Shanmugam M., Mody K.H. Heparinoid-active sulfated polysaccharides from marine algae as potential blood anticoagulant agents. Curr. Sci. 2000;79:1672–1683. [Google Scholar]
- 270.Pierre G., Sopena V., Juin C., Mastouri A., Graber M., Mangard T. Antibacterial activity of a sulphated galactan extracted from the marine alga Chaetomorpha aerea against Staphylococcus aureus. Biotechnol. Bioproc. Eng. 2011;16:937–945. doi: 10.1007/s12257-011-0224-2. [DOI] [Google Scholar]
- 271.Yamashita S., Sugita-Konishi Y., Shimizu M. In vitro bacteriostatic effects of dietary polysaccharides. Food Sci. Technol. Res. 2001;7:262–264. doi: 10.3136/fstr.7.262. [DOI] [Google Scholar]
- 272.Rice P.J., Adams E.L., Ozment-Skelton T., Gonzalez A.J., Goldman M.P., Lockhart B.E., Barker L.A., Breuel K.F., Deponti W.K., Kalbfleisch J.H., et al. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exper. Ther. 2005;314:1079–1086. doi: 10.1124/jpet.105.085415. [DOI] [PubMed] [Google Scholar]
- 273.Verma M.S., Gu F.X. 1,3-β-Glucans: Drug delivery and pharmacology. In: Karunaratne D.N., editor. The Complex World of Polysaccharides. InTech; Rijeka, Croatia: 2012. pp. 555–572. Chapter 21. [DOI] [Google Scholar]
- 274.Li C., Gao Y., Xing Y., Zhu H., Shen J., Tian J. Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia–reperfusion injury in rats via regulating the inflammation response. Food Chem. Toxicol. 2011;49:2090–2095. doi: 10.1016/j.fct.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 275.Senni K., Gueniche F., Bertaud A.F., Tchen S.I., Fioretti F., Jouault S.C., Durand P., Guezennec J., Godeau G., Letourneur D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006;445:56–64. doi: 10.1016/j.abb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 276.Choi E.M., Kim A.J., Kim Y.O., Hwang J.K. Immunomodulating activity of arabinogalactan and fucoidan in vitro. J. Med. Food. 2005;8:446–453. doi: 10.1089/jmf.2005.8.446. [DOI] [PubMed] [Google Scholar]
- 277.Yoo Y.C., Kim W.J., Kim S.Y., Kim S.M., Chung M.K., Park J.W., Suh H.H., Lee K.B., Park Y.I. Immunomodulating activity of a fucoidan isolated from Korean Undaria pinnatifida sporophyll. Algae. 2007;22:333–338. doi: 10.4490/ALGAE.2007.22.4.333. [DOI] [Google Scholar]
- 278.Chen Z., Soo M.Y., Srinivasan N., Tan B.K.H., Chan S.H. Activation of macrophages by polysaccharide-protein complex from Licium barbarum. Phytother. Res. 2009;23:1116–1122. doi: 10.1002/ptr.2757. [DOI] [PubMed] [Google Scholar]
- 279.Karnjanapratum S., Tabarsa M., Cho M., You S.G. Characterization and immunomodulatory activities of sulfated polysaccharides from Capsosiphon fulvescens. Int. J. Biol. Macromol. 2012;51:720–729. doi: 10.1016/j.ijbiomac.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 280.Chen D., Wu X.Z., Wen Z.Y. Sulfated polysaccharides and immune response: Promoter or inhibitor? Panminerva Med. 2008;50:177–183. [PubMed] [Google Scholar]
- 281.Tsuji R.F., Hoshino K., Noro Y., Tsuji N.M., Kurokawa T., Masuda T., Akira S., Nowak B. Suppression of allergic reaction by lambda-carrageenan: Toll-like receptor 4/MyD88-dependent and -independent modulation of immunity. Clin. Exp. Allergy. 2003;33:249–258. doi: 10.1046/j.1365-2222.2003.01575.x. [DOI] [PubMed] [Google Scholar]
- 282.Zhou G., Sun Y., Xin H., Zhang Y., Li Z., Xu Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004;50:47–53. doi: 10.1016/j.phrs.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 283.Heneji K., Matsuda T., Tomita M., Kawakami H., Ohshiro K., Masuda M., Takasu N., Tanaka Y., Ohta T., et al. Fucoidan extracted from Cladosiphon okamuranus Tokida induces apoptosis of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Nutr. Cancer. 2005;52:189–201. doi: 10.1207/s15327914nc5202_9. [DOI] [PubMed] [Google Scholar]
- 284.Athukorala Y., Ahn G.N., Jee Y.H., Kim G.Y., Kim S.H., Ha J.H., Kang J.-S., Lee K.-W., Jeon Y.-J. Antiproliferative activity of sulfated polysaccharide isolated from an enzymatic digest of Ecklonia cava on the U-937 cell line. J. Appl. Phycol. 2009;21:307–314. doi: 10.1007/s10811-008-9368-7. [DOI] [Google Scholar]
- 285.Chen W.S., Lazar C.S., Poenie M., Tsien R.Y., Gill G.N., Rosenfeld M.G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. Nature. 1987;328:820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- 286.Lee N.Y., Ermakova S.P., Choi H.K., Kusaykin M.I., Shevchenko N.M., Zvyagintseva T.N., Choi H.S. Fucoidan from Laminaria cichorioides inhibits AP-1 transactivation and cell transformation in the mouse epidermal JB6 cells. Mol. Carcinog. 2008;47:629–637. doi: 10.1002/mc.20428. [DOI] [PubMed] [Google Scholar]
- 287.Khotimchenko Y.S. Antitumor properties of non-starch polysaccharides: Fucoidans and Chitosans. Rus. J. Mar. Biol. 2010;36:321–330. doi: 10.1134/S1063074010050019. [DOI] [Google Scholar]
- 288.Yamamoto I., Nagumo T., Yagi K., Tominaga H., Aoki M. Antitumor effect of seaweeds, 1. Antitumor effect of extract from Sargassum and Laminaria. Jpn. J. Exp. Med. 1974;44:543–546. [PubMed] [Google Scholar]
- 289.Yamamoto I., Nagumo T., Takahashi M., Fujihara M., Suzuki Y., Iizima N. Antitumor effect of seaweeds, 3. Antitumor effect of an extract from Sargassum kjellmanianum. Jpn. J. Exp. Med. 1981;51:187–189. [PubMed] [Google Scholar]
- 290.Aisa Y., Miyakawa Y., Nakazato T., Shibata H., Saito K., Ikeda Y., Kizaki M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005;78:7–14. doi: 10.1002/ajh.20182. [DOI] [PubMed] [Google Scholar]
- 291.Kim E.J., Park S.Y., Lee J.Y., Park J.H. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010;10:96–106. doi: 10.1186/1471-230X-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yamasaki-Miyamoto Y., Yamasaki M., Tachibana H., Yamada K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009;57:8677–8682. doi: 10.1021/jf9010406. [DOI] [PubMed] [Google Scholar]
- 293.Zhang Q., Li N., Zhao T., Qi H., Xu Z., Li Z. Fucoidan inhibits the development of proteinuria in active Heymann nephritis. Phytother. Res. 2005;19:50–53. doi: 10.1002/ptr.1623. [DOI] [PubMed] [Google Scholar]
- 294.Maruyama H., Tamauchi H., Iizuka M., Nakano T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu) Planta Med. 2006;72:1415–1417. doi: 10.1055/s-2006-951703. [DOI] [PubMed] [Google Scholar]
- 295.Teruya T., Tatemoto H., Konishi T., Tako M. Structural characteristics and in vitro macrophage activation of acetyl fucoidan from Cladosiphon okamuranus. Glycoconj. J. 2009;26:1019–1028. doi: 10.1007/s10719-008-9221-x. [DOI] [PubMed] [Google Scholar]
- 296.Raghavendran H.R., Srinivasan P., Rekha S. Immunomodulatory activity of fucoidan against aspirin-induced gastric mucosal damage in rats. Int. Immunopharmacol. 2011;11:157–163. doi: 10.1016/j.intimp.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 297.Alekseyenko T.V., Zhanayeva S.Y., Venediktova A.A., Zvyagintseva T.N., Kuznetsova T.A., Besednova N.N., Korolenko T.A. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk sea Fucus evanescens brown alga. Bull. Exper. Biol. Med. 2007;143:730–732. doi: 10.1007/s10517-007-0226-4. [DOI] [PubMed] [Google Scholar]
- 298.Namikoshi M. Bioactive compounds produced by cyanobacteria. J. Int. Microbiol. Biotechnol. 1996;17:373–384. doi: 10.1007/BF01574768. [DOI] [Google Scholar]
- 299.Yang C., Chung D., Shin I.S., Lee H.Y., Kim J.C., Lee Y.J., You S.G. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008;43:433–437. doi: 10.1016/j.ijbiomac.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 300.Athukorala Y., Lee K.W., Kim S.K., Jeon Y.J. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Technol. 2007;98:1711–1716. doi: 10.1016/j.biortech.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 301.Maeda M., Uehara T., Harada N., Sekiguchi M., Hiraoka A. Heparinoid-active sulfated polysaccharide from Monostroma-nitidum and their distribution in the Chlorophyta. Phytochemistry. 1991;30:3611–3614. doi: 10.1016/0031-9422(91)80076-D. [DOI] [Google Scholar]
- 302.Matsubara K., Matsuura Y., Hori K., Miyazawa K. An anticoagulant proteoglycan from the marine green alga, Codium pugniformis. J. Appl. Phycol. 2000;12:9–14. doi: 10.1023/A:1008174115350. [DOI] [Google Scholar]
- 303.Matsubara K., Matsuura Y., Bacic A., Liao M.L., Hori K., Miyazawa K. Anticoagulant properties of a sulfated galactan preparation from a marine green alga, Codium cylindricum. Int. J. Biol. Macromol. 2001;28:395–399. doi: 10.1016/S0141-8130(01)00137-4. [DOI] [PubMed] [Google Scholar]
- 304.Li H., Mao W., Hou Y., Gao Y., Qi X., Zhao C., Chen Y., Chen Y., Li N., Wang C. Preparation, structure and anticoagulant activity of a low molecular weight fraction produced by mild acid hydrolysis of sulfated rhamnan from Monostroma latissimum. Bioresour. Technol. 2012;114:414–418. doi: 10.1016/j.biortech.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 305.Rodrigues J.A.G., Oliveira Vanderlei E.D.S., Bessa E.F., Magalhaes F.D.A., Monteiro de Paula R.C., Lima V., Barros Benevides N.M. Anticoagulant activity of a sulfated polysaccharide isolated from the green seaweed Caulerpa cupressoides. Braz. Arch. Biol. Technol. 2011;54:691–700. doi: 10.1590/S1516-89132011000400007. [DOI] [Google Scholar]
- 306.Rodrigues J.A.G., Lino de Queiroz I.N., Gomes Quindere A.L., Vairo B.C., de Souza Mourao P.A., Barros Benevides N.M. An antithrombin-dependent sulfated polysaccharide isolated from the green alga Caulerpa cupressoides has in vivo anti- and prothrombotic effects. Cienc. Rural. 2011;41:634–639. doi: 10.1590/S0103-84782011000400014. [DOI] [Google Scholar]
- 307.Melo F.R., Pereira M.S., Foguel D., Mourão P.A.S. Antithrombin-mediated anticoagulant activity of sulfated polysaccharides. J. Biol. Chem. 2004;279:20824–20835. doi: 10.1074/jbc.M308688200. [DOI] [PubMed] [Google Scholar]
- 308.Silva F.R.F., Dore C.M.P.G., Marques C.T., Nascimento M.S., Benevides N.M.B., Rocha H.A.O., Chavante S.F., Leite E.L. Anticoagulant activity, paw edema and pleurisy induced carrageenan: Action of major types of commercial carrageenans. Carbohydr. Polym. 2010;79:26–33. doi: 10.1016/j.carbpol.2009.07.010. [DOI] [Google Scholar]
- 309.Chandía N.P., Matsuhiro B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008;42:235–240. doi: 10.1016/j.ijbiomac.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 310.Hayakawa Y., Hayashi T., Hayashi K., Osawa T., Niiya K., Sakuragawa N. Heparin cofactor II-dependent antithrombin activity of calcium spirulan. Blood Coagul. Fibrinol. 1996;7:554–560. doi: 10.1097/00001721-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 311.Grauffel V., Koareg B., Mabeau S., Duran P., Jozefonvicz J. New natural polysaccharides with potent antithrombotic activity: Fucans from brown algae. Biomaterials. 1989;10:363–368. doi: 10.1016/0142-9612(89)90127-0. [DOI] [PubMed] [Google Scholar]
- 312.Kindness G., Williamson F.B., Long W.F. Effect of polyanetholesulfonic acid and xylan sulphate on antithrombin III activity. Biochem. Biophys. Res. Com. 1979;13:1062–1068. doi: 10.1016/0006-291X(79)91516-X. [DOI] [PubMed] [Google Scholar]
- 313.Qiu X., Amarasekara A., Doctor V. Effect of oversulfation on the chemical and biological properties of fucoidan. Carbohydr. Polym. 2006;63:224–228. doi: 10.1016/j.carbpol.2005.08.064. [DOI] [Google Scholar]
- 314.Jung W.K., Athukorala Y., Lee Y.J., Cha S.H., Lee C.H., Vasanthan T., Choi K.-S., Yoo S.-H., Kim S.-K., Jeon Y.J. Sulfated polysaccharide purified from Ecklonia cava accelerates antithrombin III-mediated plasma proteinase inhibition. J. Appl. Phycol. 2007;19:425–430. doi: 10.1007/s10811-006-9149-0. [DOI] [Google Scholar]
- 315.Nishino T., Nagumo T. Anticoagulant and antithrombotic activities of oversulfated fucans. Carbohydr. Res. 1992;229:355–362. doi: 10.1016/S0008-6215(00)90581-0. [DOI] [PubMed] [Google Scholar]
- 316.Minix R., Doctor V.M. Interaction of fucoidan with proteases and inhibitors of coagulation and fibrinolysis. Thromb. Res. 1997;87:419–429. doi: 10.1016/S0049-3848(97)00158-8. [DOI] [PubMed] [Google Scholar]
- 317.De Candia E., de Cristofaro R., Landolfi R. Thrombin-induced platelet activation is inhibited by high- and low-molecular-weight heparin. Circulation. 1999;99:3308–3314. doi: 10.1161/01.CIR.99.25.3308. [DOI] [PubMed] [Google Scholar]
- 318.Yu P.Z., Zhang Q.B., Li N., Xu Z.H., Wang Y.M., Li Z.E. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003;15:21–27. doi: 10.1023/A:1022946700883. [DOI] [Google Scholar]
- 319.Qi H., Huang L., Liu X., Liu D., Zhang Q., Liu S. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta) Carbohydr. Polym. 2012;87:1637–1640. doi: 10.1016/j.carbpol.2011.09.073. [DOI] [Google Scholar]
- 320.Lahaye M. Marine algae as sources of fibres: Determination of soluble and insoluble dietary fibre contents in some “sea vegetables”. J. Sci. Food Agric. 1991;54:587–594. doi: 10.1002/jsfa.2740540410. [DOI] [Google Scholar]
- 321.Li Z.J., Xue C.H., Lin H. The hypolipidemic effects and antioxidative activity of sulfated fucan on the experimental hyperlipidemia in rats. Acta Nutrim. Sin. 1999;21:280–283. [Google Scholar]
- 322.Li D.Y., Xu Z., Zhang S.H. Prevention and cure of fucoidan of L. japonica on mice with hypercholesterolemia. Food Sci. 1999;20:45–46. [Google Scholar]
- 323.Ren D., Noda H., Amano H., Nishino T., Nishizawa K. Study on antihypertensive and antihyperlipidemic effects of marine algae. Fish. Sci. 1994;60:33–40. [Google Scholar]
- 324.Inoue N., Yamano N., Sakata K., Nagao K., Hama Y., Yanagita T. The sulfated polysaccharide porphyran reduces apolipoprotein B100 secretion and lipid synthesis in HepG2 cells. Biosci. Biotechnol. Biochem. 2009;73:447–449. doi: 10.1271/bbb.80688. [DOI] [PubMed] [Google Scholar]
- 325.Panlasigui L.N., Baello O.Q., Dimatangal J.M., Dumelod D.B. Blood cholesterol and lipid-lowering effects of carrageenan on human volunteers. Asia Pac. J. Clin. Nutr. 2003;12:209–214. [PubMed] [Google Scholar]
- 326.Reddy B.S., Watanabe K., Sheinfil A. Effect of dietary wheat bran, alfalfa, pectin and carrageenan on plasma cholesterol and fecal bile acid and neutral sterol excretion in rats. J. Nutr. 1980;110:1247–1254. doi: 10.1093/jn/110.6.1247. [DOI] [PubMed] [Google Scholar]
- 327.Ginzberg A., Cohen M., Sod-Moriah U.A., Shany S., Rosenshtrauch A., Arad S.M. Chickens fed with biomass of the red microalga Porphyridium sp. have reduced blood cholesterol levels and modified fatty acids composition in egg yolk. J. Appl. Phycol. 2000;12:325–330. doi: 10.1023/A:1008102622276. [DOI] [Google Scholar]
- 328.Dvir I., Maislos M., Arad S.M. Feeding rodents with red microalgae. In: Cherbut C., Barry J.L., Lairon D., Durand M., editors. Dietary Fiber, Mechanisms of Action in Human Physiology and Metabolism. John Libbey Eurotext; Paris, France: 1995. pp. 86–91. [Google Scholar]
- 329.Dvir I., Stark A.H., Chayoth R., Madar Z., Arad S.M. Hycholesterolemic effects of nutraceuticals produced from the red microalga Porphyridium sp. in rats. Nutrients. 2009;1:156–167. doi: 10.3390/nu1020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Huang J., Liu L., Yu Y., Lin W., Chen B., Li M. Reduction in the blood glucose level of exopolysaccharide of Porphyridium cruentum in alloxan-induced diabetic mice. J. Fujian Norm. Univ. 2006;22:77–80. (In Chinese) [Google Scholar]
- 331.Glore S.R., van Treeck D., Knehans A.W., Guild M. Soluble fiber in serum lipids: A literature review. J. Am. Diet. Assoc. 1994;94:425–436. doi: 10.1016/0002-8223(94)90099-X. [DOI] [PubMed] [Google Scholar]
- 332.Marlett J. Dietary fibre and cardiovascular disease. In: Cho S.S., Dreher M.D., editors. Handbook of Dietary Fibers. Marcel Dekker; New York, NY, USA: 2001. pp. 17–30. [Google Scholar]
- 333.Dvir I., Chayoth R., Sod-Moriah U., Shany S., Nyska A., Stark A.H., Madar Z., Arad S.M. Soluble polysaccharide of red microalga Porphyridium sp. alters intestinal morphology and reduces serum cholesterol in rats. Br. J. Nutr. 2000;84:469–476. [PubMed] [Google Scholar]
- 334.Lupescu N., Geresh S., Arad S.M., Bernstein M., Glaser R. Structure of some sulfated sugars isolated after acid hydrolysis of the extracellular polysaccharide of Porphyridium sp. unicellular red alga. Carbohydr. Res. 1991;210:349–352. doi: 10.1016/0008-6215(91)80136-B. [DOI] [Google Scholar]
- 335.Guillon F., Champ M. Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res. Int. 2000;33:233–245. doi: 10.1016/S0963-9969(00)00038-7. [DOI] [Google Scholar]
- 336.Barcelo A., Claustre J., Moro F., Chayvaille J.A., Cuber J.C., Plaisancie P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46:218–224. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Oakenfull D. Physicochemical properties of dietary fiber: Overview. In: Cho S.S., Dreher M.D., editors. Handbook of Dietary Fibers. Marcel Dekker; New York, NY, USA: 2001. pp. 195–206. [Google Scholar]
- 338.Ohta T., Sasaki S., Oohori T., Yoshikawa S., Kurihara H. β-Glucosidase inhibitory activity of a 70% methanol extract from ezoishe (Pelvetia babingtonii de Toni) and its effect on the elevation of blood glucose level in rats. Biosci. Biotechnol. Biochem. 2002;66:1552–1554. doi: 10.1271/bbb.66.1552. [DOI] [PubMed] [Google Scholar]
- 339.Fu X.Y., Xue C.H., Ning Y., Li Z.J., Xu J.C. Acute antihypertensive effects of fucoidan oligosaccharides prepared from Laminaria japonica on renovascular hypertensive rat. J. Ocean Univ. Qingdao. 2004;34:560–564. [Google Scholar]
- 340.Costa M.S.S.P., Costa L.S., Cordeiro S.L., Almeida-Lima J., Dantas-Santos N., Magalhaes K.D., Sabry D.A., Lopes Albuquerque I.R., Pereira M.R., Leite E.L. Evaluating the possible anticoagulant and antioxidant effects of sulfated polysaccharides from the tropical green alga Caulerpa cupressoides var. flabellata. J. Appl. Phycol. 2012;24:1159–1167. doi: 10.1007/s10811-011-9745-5. [DOI] [Google Scholar]
- 341.Kim S.H., Choi D.S., Athukorala Y., Jeon Y.J., Senevirathne M., Rha C.K. Antioxidant activity of sulfated polysaccharides isolated from Sargassum fulvellum. J. Food Sci. Nutr. 2007;12:65–73. doi: 10.3746/jfn.2007.12.2.065. [DOI] [Google Scholar]
- 342.Cho M.L., Lee H.-S., Kang I.-J., Won M.-H., You S.G. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011;127:999–1006. doi: 10.1016/j.foodchem.2011.01.072. [DOI] [PubMed] [Google Scholar]
- 343.Tannin-Spitz T., Bergman M., van Moppes D., Grossman S., Arad S.M. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005;17:215–222. doi: 10.1007/s10811-005-0679-7. [DOI] [Google Scholar]
- 344.Chen B., You B., Huang J., Yu Y., Chen W. Isolation and antioxidant property of the extracellular polysaccharide from Rhodella reticulata. World J. Microbiol. Biotechnol. 2010;26:833–840. doi: 10.1007/s11274-009-0240-y. [DOI] [Google Scholar]
- 345.Zhang Z., Wang X., Yu S., Yin L., Zhao M., Han Z. Synthesized oversulfated and acetylated derivatives of polysaccharide extracted from Enteromorpha linza and their potential antioxidant activity. Int. J. Biol. Macromol. 2011;49:1012–1015. doi: 10.1016/j.ijbiomac.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 346.Zhao X., Xue C.H., Li B.F. Study of antioxidant activities of sulfated polysaccharides from Laminaria japonica. J. Appl. Phycol. 2008;20:431–436. doi: 10.1007/s10811-007-9282-4. [DOI] [Google Scholar]
- 347.Bernal P., Llamas M.A. Promising biotechnological applications of antibiofilm exopolysaccharides. Microb. Biotechnol. 2012;5:670–673. doi: 10.1111/j.1751-7915.2012.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Ciferri O. Spirulina, the edible microorganism (algae, single-cell protein) Microbiol. Rev. 1983;47:551–578. doi: 10.1128/mr.47.4.551-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Marceliano M.B. Structure and Function of Polysaccharide Gum-Based Edible Films and Coatings. In: Embuscado M.E., Huber K.C., editors. Edible Films and Coatings for Food Applications. Springer; London, UK: 2009. [Google Scholar]
- 350.Franco J.M., Raymundo A., Sousa I., Gallegos C. Influence of Processing Variables on the Rheological and Textural Properties of Lupin Protein-Stabilized Emulsions. J. Agric. Food Chem. 1998;46:3109–3115. doi: 10.1021/jf980284v. [DOI] [Google Scholar]
- 351.Raymundo A., Franco J., Gallegos C., Empis J., Sousa I. Effect of thermal denaturation of lupin protein on its emulsifying properties. Nahrung. 1998;42:220–224. doi: 10.1002/(SICI)1521-3803(199808)42:03/04<220::AID-FOOD220>3.0.CO;2-Q. [DOI] [Google Scholar]
- 352.Raymundo A., Gouveia L., Batista A.P., Empis J., Sousa I. Fat mimetic capacity of Chlorella vulgaris biomass in oil-in-water food emulsions stabilized by pea protein. Food Res. Int. 2005;38:961–965. doi: 10.1016/j.foodres.2005.02.016. [DOI] [Google Scholar]
- 353.Coura C.O., de Araújo I.W., Vanderlei E.S., Rodrigues J.A., Quinderé A.L., Fontes B.P., de Queiroz I.N., de Menezes D.B., Bezerra M.M., e Silva A.A., et al. Antinociceptive and anti-inflammatory activities of sulphated polysaccharides from the red seaweed Gracilaria cornea. Basic Clin. Pharmacol. Toxicol. 2012;110:335–341. doi: 10.1111/j.1742-7843.2011.00811.x. [DOI] [PubMed] [Google Scholar]
- 354.Assreuy A.M.S., Gomes D.M., Silva M.S.J., Torres V.M., Siqueira R.C.L., Pires A.F., Criddle D.N., Alencar N.M.N., Cavada B.S., Sampaio A.H., et al. Biological effects of a sulfated-polysaccharide isolated from the marine red algae Champia feldmannii. Biol. Pharm. Bull. 2008;31:691–695. doi: 10.1248/bpb.31.691. [DOI] [PubMed] [Google Scholar]
- 355.Ribeiro R.A., Vale M.L., Thomazzi S.M., Paschoalato A.B., Poole S., Ferreira S.H., Cunha F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000;387:111–118. doi: 10.1016/S0014-2999(99)00790-6. [DOI] [PubMed] [Google Scholar]
- 356.Chabut D., Fischer A.M., Colliec-Jouault S., Laurendeau I., Matou S., Le Bonniec B., Helley D. Low molecular weight fucoidan and heparin enhance the basic fibroblast growth factor-induced tube formation of endothelial cells through heparan sulfate-dependent alpha 6 overexpression. Mol. Pharmacol. 2003;64:696–702. doi: 10.1124/mol.64.3.696. [DOI] [PubMed] [Google Scholar]
- 357.Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 358.Itsuko K., Hideyuki S., Masato N., Shusuke H., Haruji S., Teruo Y. Antiulcer agent and adhesion inhibitor for Helicobacter pylori. EP0645143 B1. Patent. 1994 Sep 21;
- 359.Saito A., Yoneda M., Yokohama S., Okada M., Haneda M., Nakamura K. Fucoidan prevents concanavalin A-induced liver injury through induction of endogenous IL-10 in mice. Hepatol. Res. 2006;35:190–198. doi: 10.1016/j.hepres.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 360.Kawano N., Egashira Y., Sanada H. Effect of dietary fiber in edible seaweeds on the development of d-galactosamine-induced hepatopathy in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2007;53:446–450. doi: 10.3177/jnsv.53.446. [DOI] [PubMed] [Google Scholar]
- 361.Hayashi K., Nakano T., Hashimoto M., Kanekiyo K., Hayashi T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008;8:109–116. doi: 10.1016/j.intimp.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 362.Veena C.K., Josephine A., Preetha S.P., Varalakshmi P., Sundarapandiyan R. Renal peroxidative changes mediated by oxalate: The protective role of fucoidan. Life Sci. 2006;79:1789–1795. doi: 10.1016/j.lfs.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 363.Veena C.K., Josephine A., Preetha S.P., Rajesh N.G., Varalakshmi P. Mitochondrial dysfunction in an animal model of hyperoxaluria: A prophylactic approach with fucoidan. Eur. J. Pharmacol. 2008;579:330–336. doi: 10.1016/j.ejphar.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 364.Veena C.K., Josephine A., Preetha S.P., Varalakshmi P. Effect of sulphated polysaccharides on erythrocyte changes due to oxidative and nitrosative stress in experimental hyperoxaluria. Hum. Exp. Toxicol. 2007;26:923–932. doi: 10.1177/0960327107087792. [DOI] [PubMed] [Google Scholar]
- 365.Veena C.K., Josephine A., Preetha S.P., Varalakshmi P. Physico-chemical alterations of urine in experimental hyperoxaluria: A biochemical approach with fucoidan. J. Pharm. Pharmacol. 2007;59:419–527. doi: 10.1211/jpp.59.3.0012. [DOI] [PubMed] [Google Scholar]
- 366.Veena C.K., Josephine A., Preetha S.P., Varalakshmi P. Beneficial role of sulfated polysaccharides from edible seaweed Fucus vesiculosus in experimental hyperoxaluria. Food Chem. 2007;100:1552–1559. doi: 10.1016/j.foodchem.2005.12.040. [DOI] [Google Scholar]
- 367.Liu J.C., Zheng F.L., Liu Y.P. Effect of fucoidan on renal interstitial fibrosis in adenine-induced chronic renal failure in rats. Nephrology. 2008;13:158. doi: 10.1111/j.1440-1797.2008.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Angulo Y., Lomonte B. Inhibitory effect of fucoidan on the activities of crotaline snake venom myotoxic phospholipases A(2) Biochem. Pharmacol. 2003;66:1993–2000. doi: 10.1016/S0006-2952(03)00579-3. [DOI] [PubMed] [Google Scholar]
- 369.Silva T.H., Alves A., Popa E.G., Reys L.L., Gomes M.E., Sousa R.A., Silva S.S., Mano J.F., Reis R.L. Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter. 2012;2:278–289. doi: 10.4161/biom.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Ige O.O., Umoru L.E., Aribo S. Natural Products: A Minefield of Biomaterials. ISRN Mater. Sci. 2012;2012 doi: 10.5402/2012/983062. [DOI] [Google Scholar]
- 371.Fundueanu G., Esposito E., Mihai D., Carpov A., Desbrieres J., Rinaudo M., Nastruzzi C. Preparation and characterization of Ca-alginate microspheres by a new emulsification. Int. J. Pharm. 1998;170:11–21. doi: 10.1016/S0378-5173(98)00063-5. [DOI] [Google Scholar]
- 372.Lee K.Y., Mooney D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012;7:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Draget K.I., Taylor C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocolloid. 2011;25:251–256. doi: 10.1016/j.foodhyd.2009.10.007. [DOI] [Google Scholar]
- 374.Kong H., Mooney D. Polysaccharide- based hydrogels in tissue engineering. In: Dumitriu S., editor. Polysacharides. Structural Diversity and Functional Versatility. 2nd ed. Marcel Dekker; New York, NY, USA: 2005. pp. 817–837. [Google Scholar]
- 375.Qin Y. The characterization of alginate wound dressing with different fiber and textile structures. J. Appl. Polym. Sci. 2006;100:2516–2520. doi: 10.1002/app.23668. [DOI] [Google Scholar]
- 376.Qin Y. Alginate fibbers: An overview of the production processes and applications in wound management. Polym. Int. 2008;57:171–108. doi: 10.1002/pi.2296. [DOI] [Google Scholar]
- 377.Rinaudo M. Biomaterials based on a natural polysaccharide: Alginate. TIP. Revista Especializada en Ciencias Químico-Biológicas. 2014;17:92–96. [Google Scholar]
- 378.Gong Y., He L., Li J., Zhou Q., Ma Z., Gao C., Shen J. Hydrogel-filled polylactide porous scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2007;82:192–204. doi: 10.1002/jbm.b.30721. [DOI] [PubMed] [Google Scholar]
- 379.Bao L., Yang W., Mao X., Mou S., Tang S. Agar/collagen membrane as skin dressing for wounds. Biomed. Mater. 2008;3:1–7. doi: 10.1088/1748-6041/3/4/044108. [DOI] [PubMed] [Google Scholar]
- 380.Nakayama Y., Tsujinaka T. Acceleration of robust “biotube” vascular graft fabrication by in-body tissue architecture technology using a novel eosin Y-releasing mold. J. Biomed. Mater. Res. B Appl. Biomater. 2014;102:231–238. doi: 10.1002/jbm.b.32999. [DOI] [PubMed] [Google Scholar]
- 381.Toskas G., Hund R.-D., Laourine E., Cherif C., Smyrniotopoulos V., Roussis V. Nanofibers based on polysaccharides from the green seaweed Ulva rigida. Carbohydr. Polym. 2011;84:1093–1102. doi: 10.1016/j.carbpol.2010.12.075. [DOI] [Google Scholar]
- 382.Alves A., Duarte A.R.C., Mano J.F., Sousa R.A., Reis R.L. PDLLA enriched with ulvan particles as a novel 3D porous scaffold targeted for bone engineering. J. Supercrit. Fluids. 2012;65:32–38. doi: 10.1016/j.supflu.2012.02.023. [DOI] [Google Scholar]
- 383.Alves A., Sousa R.A., Reis R.L. Processing of degradable ulvan 3D porous structures for biomedical applications. J. Biomed. Mater. Res. A. 2013;101:998–1006. doi: 10.1002/jbm.a.34403. [DOI] [PubMed] [Google Scholar]
- 384.Morelli A., Chiellini F. Ulvan as a New Type of Biomaterial from Renewable Resources: Functionalization and Hydrogel Preparation. Macromol. Chem. Phys. 2010;211:821–832. doi: 10.1002/macp.200900562. [DOI] [Google Scholar]
- 385.Browder W., Williams D., Lucore P., Pretus H., Jones E., Mcnamee R. Effect of Enhanced Macrophage Function on Early Wound-Healing. Surgery. 1988;104:224–230. [PubMed] [Google Scholar]
- 386.Kofuji K., Huang Y., Tsubaki K., Kokido F., Nishikawa K., Isobe T., Murata Y. Preparation and evaluation of a novel wound dressing sheet comprised of beta-glucan-chitosan complex. React. Funct. Polym. 2010;70:784–789. doi: 10.1016/j.reactfunctpolym.2010.07.014. [DOI] [Google Scholar]
- 387.Delatte S.J., Evans J., Hebra A., Adamson W., Othersen H.B., Tagge E.P. Effectiveness of beta-glucan collagen for treatment of partial-thickness burns in children. J. Ped. Sur. 2001;36:113–118. doi: 10.1053/jpsu.2001.20024. [DOI] [PubMed] [Google Scholar]
- 388.Sezer A.D., Hatipoğlu F., Cevher E., Oğurtan Z., Baş A.L., Akbuğa J. Chitosan films containing fucoidan as a wound dressing for dermal burn healing: Preparation and in vitro/in vivo evaluation. AAPS PharmSciTech. 2007;8:E94–E101. doi: 10.1208/pt0802039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Gilchrist T., Martin A.M. Wound treatment with Sorban-an alginate fibre dressing. Biomaterials. 1983;4:317–320. doi: 10.1016/0142-9612(83)90036-4. [DOI] [PubMed] [Google Scholar]
- 390.Motta G.J. Calcium alginate topical wound dressings: A new dimension in the cost-effective treatment for exudating dermal wounds and pressure sores. Ostomy Wound Manag. 1989;25:52–56. [PubMed] [Google Scholar]
- 391.De Morais M.G., Stillings C., Dersch R., Rudisile M., Pranke P., Costa J.A.V., Wendorff J. Preparation of nanofibers containing the microalga Spirulina (Arthrospira) Bioresour. Technol. 2010;101:2872–2876. doi: 10.1016/j.biortech.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 392.Arad S.M., Rapoport L., Moshkovich A., van Moppes D., Karpasan M., Golan R., Golan Y. Superior biolubricant from a species of red microalga. Langmuir. 2006;2:7313–7317. doi: 10.1021/la060600x. [DOI] [PubMed] [Google Scholar]
- 393.Johnson A.S., O'Sullivan E., D’Aoust L.N., Omer A., Bonner-Weir S., Fisher R.J., Weir G.C., Colton C.K. Quantitative assessment of islets of langerhans encapsulated in alginate. Tissue Eng. Part C Methords. 2011;17:435–449. doi: 10.1089/ten.tec.2009.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Tagler D., Tu T., Smith R.M., Anderson N.R., Tingen C.M., Woodruff T.K., Shea L.D. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng. Part A. 2012;18:1229–1238. doi: 10.1089/ten.tea.2011.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Banerjee A., Arha M., Choudhary S., Ashton R.S., Bhatia S.R., Schaffer D.V., Kane R.S. The Influence of Hydrogel Modulus on the Proliferation and Differentiation of Encapsulated Neural Stem Cells. Biomaterials. 2009;30:4695–4699. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Jeong S.I., Jeon O., Krebs M.D., Hill M.C., Alsberg E. Biodegradable photo-crosslinked alginate nanofibre scaffolds with tuneable physical properties, cell adhesivity and growth factor release. Eur. Cells Mater. 2012;24:331–343. doi: 10.22203/ecm.v024a24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Zhou H., Xu H.H.K. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials. 2011;32:7503–7513. doi: 10.1016/j.biomaterials.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Liu J., Xu H.H.K., Zhou H., Weir M.D., Chen Q., Trotman C.A. Human umbilical cord stem cell encapsulation in novel macroporous and injectable fibrin for muscle tissue engineering. Acta Biomater. 2013;9:4688–4697. doi: 10.1016/j.actbio.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Khanarian N.T., Jiang J., Wan L.Q., Mow V.C., Lu H.H. A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Eng. Part A. 2012;18:533–545. doi: 10.1089/ten.tea.2011.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Hatch A., Hansmann G., Murthy S.K. Engineered alginate hydrogels for effective microfluidic capture and release of endothelial progenitor cells from whole blood. Langmuir. 2011;27:4257–4264. doi: 10.1021/la105016a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Yamamoto M., James D., Li H., Butler J., Rafii S., Rabbany S. Generation of stable co-cultures of vascular cells in a honeycomb alginate scaffold. Tissue Eng. Part A. 2010;16:299–308. doi: 10.1089/ten.tea.2009.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Hajiali H., Shahgasempour S., Naimi-Jamal M.R., Petrovi H. Electrospun PGA/gelatin nanofibrous scaffolds and their potential application in vascular tissue engineering. Int. J. Nanomed. 2011;6:2133–2141. doi: 10.2147/IJN.S24312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Hockaday L.A., Kang K.H., Colangelo N.W., Cheung P.Y., Duan B., Malone E., Wu J., Girardi L.N., Bonassar L.J., Lipson H., et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4 doi: 10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Dvir T., Timko B.P., Brigham M.D., Naik S.R., Karajanagi S.S., Levy O., Jin H., Parker K.K., Langer R., Kohane D.S. Nanowired three dimensional cardiac patches. Nat. Nanotechnol. 2011;6:720–725. doi: 10.1038/nnano.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Bacakova L., Novotna K., Parizek M. Polysaccharides as Cell Carriers for Tissue Engineering: The Use of Cellulose in Vascular Wall Reconstruction. Physiol. Res. 2014;63:29–47. doi: 10.33549/physiolres.932644. [DOI] [PubMed] [Google Scholar]
- 406.Petrulyte S. Advanced textile materials and biopolymers in wound management. Dan. Med. Bull. 2008;55:72–77. [PubMed] [Google Scholar]
- 407.Popa E.G., Gomes M.E., Reis R.L. Cell delivery systems using alginate-carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules. 2011;12:3952–3961. doi: 10.1021/bm200965x. [DOI] [PubMed] [Google Scholar]
- 408.Yoon H.Y., Son S., Lee S.J., You D.G., Yhee J.Y., Park J.H., MSwierczewska M., Lee S., Kwon I.C., Kim S.H., et al. Glycol chitosan nanoparticles as specialized cancer therapeutic vehicles: Sequential delivery of doxorubicin and Bcl-2 siRNA. Sci. Rep. 2014;4 doi: 10.1038/srep06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Ghadban A.1., Albertin L., Rinaudo M., Heyraud A. Biohybrid glycopolymer capable of ionotropic gelation. Biomacromolecules. 2012;13:3108–3119. doi: 10.1021/bm300925j. [DOI] [PubMed] [Google Scholar]
- 410.Huang M., Yang M. Evaluation of glucan/poly(vinyl alcohol) blend wound dressing using rat models. Int. J. Pharm. 2008;346:38–46. doi: 10.1016/j.ijpharm.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 411.Alkhatib B., Freguin-Bouilland C., Lallemand F., Henry J.P., Litzler P.Y., Marie J.P., Richard V., Thuillez C., Plissonnier D. Low molecular weight fucan prevents transplant coronaropathy in rat cardiac allograft model. Transpl. Immunol. 2006;16:14–19. doi: 10.1016/j.trim.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 412.Fréguin-Bouilland C., Alkhatib B., David N., Lallemand F., Henry J.P., Godin M., Thuillez C., Plissonnier D. Low molecular weight fucoidan prevents neointimal hyperplasia after aortic allografting. Transplantation. 2007;83:1234–1241. doi: 10.1097/01.tp.0000261109.97928.9c. [DOI] [PubMed] [Google Scholar]
- 413.Yang J.-S., Xie Y.-J., He W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011;84:33–39. doi: 10.1016/j.carbpol.2010.11.048. [DOI] [Google Scholar]
- 414.Augst A.D., Kong H.J., Mooney D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006;6:623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]