Abstract
Frequent use of non-steroidal anti-inflammatory drugs (NSAIDs) has been paralleled by increasing occurrence of adverse reactions, which vary from mild local skin rashes or gastric irritation to severe, generalized symptoms and even life-threatening anaphylaxis. NSAID-induced hypersensitivity reactions may involve both immunological and non-immunological mechanisms and should be differentiated from type A adverse reactions. Clinical diagnosis and effective management of a hypersensitive patient cannot be achieved without identifying the underlying mechanism. In this review, we discuss the current classification of NSAID-induced adverse reactions and propose a practical diagnostic algorithm that involves 7 steps leading to the determination of the type of NSAID-induced hypersensitivity and allows for proper patient management.
Keywords: Nonsteroidal anti-inflammatory drugs, NSAID-induced hypersensitivity, aspirin hypersensitivity, aspirin, drug allergy
INTRODUCTION
Since the first non-steroidal anti-inflammatory drugs (NSAIDs) (antypiryne and aspirin) were synthesized at the end of the XIX century, dozens of new compounds with similar anti-inflammatory activity have been developed and introduced to clinical practice for the treatment of chronic inflammatory disorders, pain, and fever. Frequent use of NSAIDs has been paralleled by increasing occurrence of adverse reactions, which vary from mild local skin rashes or gastric irritation to severe, generalized symptoms and even life-threatening anaphylaxis. NSAIDs are among the most commonly used drugs and are the first or the second to antibiotics (depending on the population studied) as the cause of adverse drug reactions (ADRs).1 Difficulties in the proper diagnosis of NSAID-induced adverse reactions are related to the fact that, as opposed to e.g. antibiotics, most unwanted reactions after NSAIDs result from pharmacological action of the drugs, are dose-related, would occur in any treated patient, and should be differentiated from hypersensitivity reactions. Furthermore, NSAID-induced hypersensitivity reactions are characterized by a wide pattern of symptoms, which may involve both immunological and non-immunological mechanisms, thus creating one of the biggest diagnostic challenges in allergy. Over the last decade, it has become obvious that clinical diagnosis and effective management of drug-induced hypersensitivity reactions cannot be achieved without identifying and understanding underlying mechanisms and that history alone may not be sufficient for accurate diagnosis of drug hypersensitivity.2,3 This applies specifically to NSAID-induced hypersensitivity reactions. Suspected mechanisms may prompt a choice of the appropriate diagnostic tool, and identification of the mechanism will guide employment of appropriate avoidance strategy and management modalities.
At the beginning of this century, Stevenson et al.4 proposed the first classification of acute NSAID hypersensitivity based on the understanding of pathomechanisms underlying various clinical patterns of hypersensitivity. More recently, the European Academy of Allergy and Clinical Immunology (EAACI) "Task Force on NSAID Hypersensitivity" presented a modified classification and new nomenclature of acute and delayed NSAID-induced hypersensitive reactions and offered evidence-based recommendations and algorithms for diagnosis and management.2,5,6 In this review, we will attempt to convince the readers that implementing this classification in clinical practice is not very difficult and may facilitate proper diagnosis and management.
Pharmacological mechanisms for NSAID-induced hypersensitivity reactions
The mechanism of action of NSAIDs was discovered in 1971 by Sir John Vane,7 who employed original bioassay, demonstrated that these drugs share common pharmacologic activity, namely inhibition of prostaglandin synthesis. Later, it was documented that NSAIDs inhibit enzymes responsible for synthesis of prostanoids (prostaglandins, prostacyclin, and thromboxane) and cyclooxygenase (COX, previously named prostaglandin G/H-synthase), existing in 2 isoforms (COX-1 and COX-2). COX-1 is constitutively expressed by most cells, leading to the production of prostanoids (like prostacyclin PGI2) that play a housekeeping role in the maintenance of normal renal function, platelet aggregation, and gastric mucosal integrity.8 COX-2 can be expressed both constitutively and in response to inflammatory stimuli and is responsible for the generation of prostanoids important for inflammation. COX-2-derived prostanoids are also involved in physiological responses: reproduction, renal function, bone resorption, and neurotransmission. The expression level of COX-2 is normally low in cells, but may be significantly increased during inflammation or upon cell activation by a number of factors, including cytokines and intracellular messengers. NSAIDs differ markedly in their potency to inhibit COX-1 and COX-2, which not only affects their clinical effectiveness, but explains different capacity to generate side effects and to induce hypersensitivity reactions. Aspirin and most of the "classical" NSAIDs (e.g. indomethacin, naproxen, and diclofenac) predominantly inhibit COX-1 and to lesser extent COX-2, which inhibit the production of protective prostanoids leading to common adverse symptoms involving the gastrointestinal tract. Newly developed compounds that predominantly inhibit COX-2 (e.g. nimesulide and meloxicam) or selective COX-2 inhibitors (e.g. celecoxib, rofecoxib) are strong inhibitors of inflammatory prostanoids, but only slightly affect the production of protective prostanoids generated. However, COX-1 results in much better gastric safety profile. In 1975, Szczeklik et al. documented that some respiratory and cutaneous NSAID-induced hypersensitivity reactions are related to the pharmacological activity of these drugs i.e. to the inhibition of prostaglandin synthesis providing the explanation for cross-reactivity among NSAIDs. Although all NSAIDs share the property of COX (prostaglandins) inhibition, they may have diverse chemical structures (Table 1), allowing some of them to act as antigens with potential to induce a drug-specific immune response. Understanding the mechanism of pharmacological activity, potency, and selectivity in inhibition of COX1/COX-2 of different NSAIDs as well as structural diversity is crucial for a proper diagnosis of NSAID-induced reactions.9
Table 1. Classification of NSAIDs according to chemical structure.
| Group | Drugs |
|---|---|
| Salicylic acid derivates | Acetylsalicylic acid (Aspirin) |
| Sodium salicylate | |
| Diflunisal | |
| Salicylsalicylic acid | |
| Sulfasalazine | |
| Olsalazine | |
| Para-aminophenol derivatives | Acetaminophen |
| Indol and indene acetic acid | Indomethacin |
| Sulindac | |
| Etodolac | |
| Heteroaryl acetic acid | Ibuprofen |
| Neproxen | |
| Flurbiprofen | |
| Ketoprofen | |
| Fenoprofen | |
| Oxaprozin | |
| Anthranilic acid (fenemates) | Mefenamic acid |
| Meclofenamic acid | |
| Enolic acid derivatives (oxicams) | Piroxicam |
| Tenoxicam | |
| Meloxicam |
Spectrum and mechanisms of adverse reactions to NSAIDs
"ADR" is the umbrella term for all unwanted reactions associated with drug intake. According to WHO nomenclature, ADR is "a response to a drug that is noxious and unintended and occurs at doses normally used in humans for the prophylaxis, diagnosis or therapy of disease, or for the modification of physiological function."
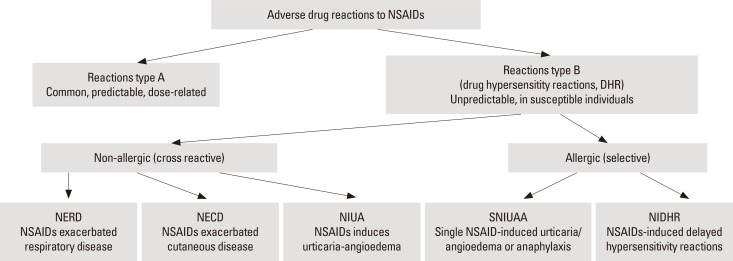
Thus, the term ADR covers all reactions irrespective to pathomechanism, provided there is a casual link between drug intake and signs or symptoms. Two major types of ADRs have been distinguished: types A and B (Fig. 1). Type A reactions which are the most common, are predictable i.e. may occur in any treated patient if a patient takes higher than usually recommended or cumulative doses of a drug. For NSAIDs, the most common type A side effects are gastrointestinal symptoms, such as stomachache and cramps). Other symptoms often seen in patients regularly treated with aspirin (which is a prototypic NSAID) include bleeding and tinnitus. NSAIDs are also the leading drugs causing chronic renal failure resulting from vasoconstriction of the afferent arteriole and decreased glomerular filtration rate.10
Fig. 1. NSAIDs-induced adverse reactions.
In contrast to type A ADRs, type B reactions occur only in some susceptible individuals in response to a small dose of the drug (non-dose-related). Type B reactions fulfill the definition of drug hypersensitivity reaction as proposed by EAACI/WAO consensus: objectively reproducible signs or symptoms initiated by a drug at a dose tolerated by normal subjects. Most NSAID-induced hypersensitivity reactions do not involve immunological mechanisms (thus are non-allergic), but are related to inhibition by NSAIDs of COX-1, which triggers inflammatory cell activation and mediators release. Non-allergic drug hypersensitivity reactions have previously been called "pseudoallergic or idiosyncratic reactions," but these terms are not advised to be used anymore. If the mechanism of the reactions is not clear, the umbrella term drug hypersensitivity should be used. When a definite immunological mechanism (either drug-specific antibody or T cells) is demonstrated, these reactions should be classified as allergic hypersensitivity (drug allergy). Allergic reactions can be further divided into IgE-mediated and non-IgE-mediated hypersensitivity reactions based on a specific immunological mechanism involved.
Classification of NSAID hypersensitivity reactions
Current classification of NSAID hypersensitivity was originally proposed by Stevenson et al.4 and more recently has been modified by ENDA/EAACI Task Force.2,5 This classification can be implemented in allergy practice, since it stems from ease in determining clinical patterns of the reactions (Table 2). Application of the classification allows for identification of the pathomechanism of NSAID hypersensitivity in a given patient, with important implications for patient management. Hypersensitivity reactions to NSAIDs may manifest with a variety of symptoms (respiratory, cutaneous, anaphylactic, or other organ-specific) which may appear with different onset times after drug intake. The reactions can be divided into cross-reactive (which involve reactions to several, chemically non-related NSAIDs) and selective, when a patient reacts only to a single drug (reactions to other NSAIDs may occur only if they have very similar chemical structures). Cross-reactivity to several NSAIDs results from the mechanism of hypersensitivity associated with inhibition of COX-1, which is a common property of the NSAID pharmacological activity. In contrast, selective-type NSAID-induced hypersensitivity is a result of immunological (allergic) reaction to a culprit drug, which is mediated either by IgE (acute reactions) or by T cells (delayed reactions). Three out of 5 distinguished by current classification types of hypersensitivity (1 respiratory and 2 cutaneous) are cross-reactive and 2 (1 cutaneous/anaphylactic and 1 delayed) are selective and immunologically mediated.
Table 2. Classification of NSAID-induced hypersensitivity reactions.
| Type of Reaction | Name of reaction | Abbreviation | Definition | Previously used names |
|---|---|---|---|---|
| Cross-reactive - non allergic (Non-immunologically mediated reactions) | NSAIDs exacerbated respiratory disease | NERD | Reaction manifesting primarily as bronchial obstruction, dyspnea and nasal congestion/rhinorrhea, occurring in patients with an underlying chronic airway respiratory disease (asthma/rhinosinusitis/nasal polyps). | aspirin triad, asthma triad, Samter's syndrome, Widal syndrome, aspirin-induced asthma or aspirin-sensitive rhinosinusits/asthma syndrome, aspirin-intolerant asthma, aspirin-exacerbated respiratory disease |
| NSAIDs exacerbated cutaneous disease | NECD | Reaction manifesting as wheals and/or angioedema occurring in patients with a history of chronic spontaneous urticaria. | aspirin-induced urticaria; aspirin-exacerbated cutaneous disease | |
| NSAIDs induced urticaria-angioedema | NIUA | Reaction manifesting as wheals and/or angioedema occurring in otherwise healthy subjects (without history of chronic spontaneous urticaria). Symptoms are induced by at least two NSAIDs with different chemical structure (not belonging to the same chemical group). | aspirin-induced urticaria; multiple drug-induced urticarial angioedema | |
| Selective-allergic (Immunologically mediated reactions) | Single NSAID-induced urticaria/angioedema or anaphylaxis | SNIUAAA | Immediate hypersensitivity reactions to a single NSAID or to several NSAIDs belonging to the same chemical group, manifesting as urticaria, angioedema and/or anaphylaxis. These subjects tolerate other chemically non-related NSAIDs, and usually do not have a history of chronic urticaria or asthma. | single drug-induced reactions, allergic reactions |
| NSAIDs-induced delayed hypersensitivity reactions | NIDHR | Reactions to a single NSAID developing more than 24 hours after drug administration and manifesting by either skin symptoms (exanthema, fixed drug eruption), other organ specific symptoms (e. g. renal, pulmonary) or severe cutaneous adverse reactions (SCAR). |
Cross-reactive types
NSAIDs exacerbate respiratory disease (NERD)
This hypersensitivity reaction mainly manifests with respiratory symptoms and occurs in patients with underlying chronic airway disease like asthma and/or rhinosinusitis with nasal polyps. Bronchial obstruction induced by aspirin/NSAIDs usually develops within 30-180 minutes after drug ingestion and may be accompanied by extrabronchial symptoms: nasal (rhinorrhea, nasal congestion), ocular, cutaneous (flushing of the upper thorax, urticaria and/or angioedema) or gastric.11 Symptoms of underlying disease (chronic rhinosinusitis with polyps and/or asthma) usually precede the development of hypersensitivity to aspirin, although in some patients ASA/NSAID intake may precipitate the first asthma attack.12 Important from the practical point of view is observation that NSAID hypersensitivity in patients with asthma is a risk factor for severe course of the underlying asthma,13 and near-fatal or fatal outcome of asthma occurs more often in this group as compared to non-sensitive asthmatics.14 Chronic rhinosinusitis in NERD patients is usually severe, often complicated by recurrent nasal polyp formation that responds less to surgical treatment.12 Chronic eosinophilic inflammation of higher than usual intensity is present in both the lower and upper airway mucosae of NERD patients. NERD represents a non-immunological, cross-reactive type of hypersensitivity, since patients hypersensitive to one NSAIDs would react to aspirin and other NSAIDs that are COX-1 inhibitors, while weak COX-1 inhibitors or preferential COX-2 inhibitors are usually well tolerated.15,16 It has been postulated that, in susceptible individuals, COX-1 inhibition and decreased prostaglandin generation trigger activation of mast cells and eosinophils, leading to release of inflammatory mediators responsible for developing symptoms. Major mediators generated during NSAID-induced reactions are cysteinyl-leukotrienes (cysLTs),17 and pharmacological inhibition of cysLT type 1 receptors alleviates NSAID-induced symptoms.18 Also polymorphisms of cysteinyl leukotriene pathway were associated with development of NERD.19
NSAIDs exacerbate cutaneous disease (NECD)
This type of hypersensitivity manifesting with cutaneous symptoms appears in patients with a history of chronic spontaneous urticaria. Symptoms of urticaria and/or angioedema usually appear usually 0.5 to 6 hours after NSAID ingestion, although both immediate (within 15 minutes of ingestion) and late (within several hours) reactions have been described. Skin lesions subside within few hours, but may persist for several days.20,21 The magnitude of drug-induced symptoms is dose-dependent and greater when chronic urticaria is active. NSAID-induced reactions are less frequent and less intense when chronic urticaria is in remission or under control. Chronic spontaneous urticaria in patients with NECD can also be exacerbated by triggers other than NSAIDs (infections, antibiotics, physical factors, and stress), further complicating the clinical picture and diagnosis.22 Cutaneous reactions induced by aspirin or other NSAIDs in patients with chronic urticaria are not immunologically mediated but represent a cross-reactive type of non-allergic drug hypersensitivity NECD. Similar to NERD, the mechanism of reactions to NSAIDs seems to be related to inhibition of cyclooxygenase -1 and increased generation of cysLTs.23 Thus, patients with NECD would cross-react to COX-1 inhibitors, while selective COX-2 inhibitors are tolerated by the majority of them.24
NSAID-induced urticaria/angioedema (NIUA)
In these patients, aspirin and NSAIDs evoke a similar spectrum of cutaneous manifestations: wheals and/or angioedema with similar onset times. In contrast to NECD, NIUA is diagnosed if hypersensitivity reactions occur in otherwise healthy subjects (without a history of chronic spontaneous urticaria). The mechanism is thought to represent COX-1 inhibition, but it has not been well documented.21,24 The next 2 subtypes of NSAID hypersensitivity represent immunologically mediated reactions.
Selective types of hypersensitivity reactions
Single NSAIDs induce urticaria/angioedema/anaphylaxis (SNIUAA)
A subpopulation of patients is reporting immediate hypersensitivity reactions to a single NSAID (or to several NSAIDs but belonging to the same chemical group). Patients usually present with a history of good tolerance to other chemically unrelated NSAIDs, including aspirin.21 A range of symptoms from mild urticaria and localized angioedema to laryngeal edema and anaphylaxis usually develop within the first hour after a single NSAID intake.21 However, in some instances symptoms may develop within minutes or even seconds (e.g. after intravenous injection of metamizol).25 These subjects are usually otherwise healthy individuals without any specific underlying chronic diseases. The clinical spectrum of symptoms and timing of reactions suggest an allergic type I mechanism. Only for some NSAIDs, however, specific IgE can be detected in the skin test or in the serum (e.g. patients hypersensitive to pyrazolones ).26,27
NSAIDs induce delayed hypersensitivity reactions (NIDHR)
Reactions developing usually more than 24 hours after drug intake are considered to represent the delayed type of immunological hypersensitivity. Delayed cutaneous manifestation is most common and involves maculopapular eruptions (MPE), fixed drug eruptions (FDE), photosensitivity reactions, delayed urticaria,28,29,30 and contact dermatitis.31 Severe drug hypersensitivity reactions (drug-induced hypersensitivty syndrome, acute generalized exanthematous pustulosis, and severe cutaneous adverse reaction [SCAR])32,33,34 as well as organ-specific injury (pneumonitis and nephritis) may occur.35 Presumed immunological mechanisms involve the stimulation of drug-specific CD4+ and CD8+ T cells through their T cell receptors and represents a delayed-type hypersensitivity. T cell-dependent mechanisms have been documented in delayed urticaria, MPE induced by aceclophenac36 and metamizol, and SCAR induced by ibuprofen.37,38
Practical algorithm for the diagnosis of NSAID hypersensitivity reactions
The diagnosis of NSAID adverse reaction is based on clinical history, physical examination of a patient and, if possible and appropriate, in vitro or in vivo tests, followed by drug challenge procedures. Clinical history is crucial for choosing further diagnostic tools and should be carefully collected; however, it can rarely be sufficient and in most cases should be followed by other diagnostic modalities. History should include detailed information on recent drug-induced reactions: culprit medication, pattern and chronology of symptoms, time interval between the last dose and symptoms onset, and disappearance of symptoms after drug withdrawal. Previous exposure to a culprit drug and other medications taken should be recorded. The presence of comorbidities like asthma, rhinosinusitis, nasal polyposis, or chronic urticaria, is documented and confirmed. The detailed history of previous NSAID intake (names and doses) both before and after adverse reactions occurred should be collected. Specifically, patients should be asked about intake and tolerance of acetylsalicylic acid (aspirin). Drug allergy questionnaires already available (e.g. EAACI) may be helpful at the early stage of the diagnostic process. In some cases, history alone is not sufficient to identify the culprit drug and in most circumstances to determine the type and mechanism of hypersensitivity that may be crucial for deciding further diagnostic steps. Furthermore, history is not considered a reliable predictor of a future reaction to the same drug, and the predictive value of history depends on the type of the reaction.
Seven steps to diagnosis
Here we propose a practical diagnostic algorithm involving 7 steps that helps determine the type of NSAID hypersensitivity and allows for choosing proper patient management (Fig. 2). Steps 1-4 that include a detailed analysis of patient history can be done by a non-specialist and may be sufficient to a proper diagnosis for some patients. In most cases of NSAID hypersensitivity, however, information acquired from history is not sufficient to confirm the diagnosis, thus further steps including in vitro testing and oral provocation challenges may be necessary to perform. In our opinion, steps 5-7 should be undertaken by an experienced allergy specialist.
Fig. 2. Seven steps to the diagnosis of NSAID hypersensitivity reactions.
Step 1: Assess if this is a predictable (type A, intolerance) or unpredictable (type B, hypersensitivity) adverse reaction
The history should enable physicians to discriminate type A from type B of NSAID-induced adverse drug reactions. Type A adverse reactions may typically involve gastric symptoms, increased bleeding, or nephrotoxicity, and is associated with chronic treatment with a NSAID, often in higher than usual doses. If type A adverse reactions are suspected, in some circumstances (e.g. with gastric symptoms), the drug may be reintroduced in lower doses, and/or accompanied by gastroprotective agents (e.g. proton pump inhibitors). If type B reactions are suspected, the culprit drug should be strictly avoided as it can evoke severe reactions. However, the physician should follow the next diagnostic steps to determine the type of hypersensitivity and eventually propose to the patient safe drug alternative or desensitization procedures if appropriate.
Step 2: Ask for the onset time of the reaction
If the reaction started to develop within hours (up to 24 hours, but usually 1-2 hours) after drug intake, the acute type of the reaction can be suspected. Unfortunately, the onset time of the acute reaction does not allow for the determination of the type and underlying pathomechanism. Reactions developing within minutes or during the first hour after drug intake may represent immunologically IgE-mediated reactions (SNIUAA) or (in highly susceptible patients challenged with the dose of NSAIDs significantly exceeding their threshold) may belong to the cross-reactive type (NERD, NECD). Most reactions of cross-reactive type develop within 1 to 2 hours, but in some patients may occur several hours later. Reactions occurring more than 24 hours after drug intake, with high probability, represent immunologically mediated delayed-type hypersensitivity.
Step 3: Analyze the clinical pattern of drug-induced symptoms and underlying chronic diseases
If bronchial (dyspnea, wheezing, and cough) or nasal (rhinorrhea and nasal congestion) symptoms occur within the first hour after NSAID ingestion, a cross-reactive respiratory type of NSAID hypersensitivity can be suspected (NERD). The history of chronic bronchial asthma and chronic rhinosinusitis with/without nasal polyposis supports cross-reactive, NSAID-exacerbated respiratory disease. In some instances, respiratory bronchial symptoms should be differentiated from laryngeal angioedema symptoms representing the IgE-mediated type of hypersensitivity (SINUAA). Patients with acute cutaneous symptoms (urticaria and/or angioedema) after NSAID intake represent either the cross-reactive type of hypersensitivity (NECD or NIUA) or the SNIUAA. The presence of chronic spontaneous urticaria speaks for a diagnosis of NECD, while lack of chronic urticaria history suggests a diagnosis of either NECD or SNIUAA. If skin symptoms develop rapidly and are accompanied by concomitant systemic/anaphylactic signs (hypotension), a diagnosis of SNIUAA is very likely. It is worth noting that, in patients with NERD, skin symptoms (rash and urticaria) may accompany respiratory symptoms in about 10% reactions induced by NSAIDs. The symptomatology of delayed-type hypersensitivity (NIDHR) is very diverse ranging from skin symptoms to severe organ-specific or systemic symptoms. In summary, while NERD and NECD are associated with underlying chronic disorders (respiratory and skin, respectively), underlying diseases are not typical for NIUA, SINUAA, or NIDHR.
Step 4: Ask about history of tolerance/intolerance to other NSAIDs
This step is critical for assessing if the patient's hypersensitivity is cross-reactive (NERD, NECD, or NIUA) or belongs to the selective type (SNIUAA or NIDHR, depending on the onset time of the reaction). A history of similar symptoms evoked in the past by another NSAID (either COX-1 or COX-2 inhibitors) e.g. by aspirin in a patient reporting respiratory or cutaneous symptoms after ibuprofen speaks in favor of cross-reactive type of hypersensitivity. NERD is suspected if respiratory symptoms occur and NECD or NIUA if cutaneous symptoms are evoked. History of good tolerance to strong cyclooxygenase-1 inhibitors (aspirin, ibuprofen, ketorolac etc.) after previous reaction to another NSAID suggests non-cross-reactive or selective, immunologically mediated type of hypersensitivity (either SINUAA or NIDHR). However, such patients may report reactions to another NSAID belonging to the same chemical group, a sharing common epitope. For example, a patient with SNIUAA and history of anaphylactic reaction to metamizol will tolerate ibuprofen but may react to aminophenazone that belongs to pyrazlones, the same chemical group as metamizol. It has been proposed that history of 3 or more episodes of reaction to 2 different NSAIDs is predictive for the cross-reactive type of hypersensitivity, while 2 or more reactions to the same NSAID with concomitant history of good tolerance to another NSAID with strong potency speaks for the selective type.39
Step 5: Confirm/exclude cross-reactivity to other NSAIDs by the challenge
The presence of the cross-reactive or selective type of hypersensitivity has serious implications for patient's management and thus should be confirmed by oral challenge tests. If a patient previously reacted to NSADs other than aspirin, oral challenge with aspirin is the gold standard. A positive response to aspirin will confirm and n exclude the cross-reactive type of hypersensitivity. If aspirin is the culprit drug, the patient should be challenged with an alternative strong COX-1 inhibitor, and the conclusions are the same as the above, depending on a positive or negative challenge result.2 Confirming cross-reactivity with respiratory symptoms allows for a diagnosis of NERD. If skin symptoms are evoked by cross-reactive drugs, NECD or NIUA is suspected, and further distinguished on the basis of the presence (NECD) or lack of underlying chronic urticaria (NIUA). If the challenge test with aspirin or other strong COX-1 inhibitors is negative SNIUAA that is selective, the immunologically mediated type of acute NSAID hypersensitivity can be suspected.
If the cross-reactive type of hypersensitivity (NERD, NECD, and NIUA) is diagnosed and documented, the patient is prompted to avoid all NSAIDs with strong COX-1 inhibitory activity, but selective COX-2 inhibitors can be recommended if anti-inflammatory treatment is indicated. In the case of the selective (allergic) type of hypersensitivity (SNIUAA, SNIDHR), a patient would usually react to a single drug, and thus other NSAIDs, even potent COX-1 inhibitors, may be well tolerated. However, NSAIDs may belong to several chemical classes, and structural similarity among drugs within each class may result in immunological cross-reactivity between NSAIDs (e.g. IgE-mediated hypersensitivity reactions to pyrazolones) (Table 1). Thus, NSAIDs belonging to the same chemical class should be avoided by these patients.
Step 6: Consider skin testing or in vitro testing
Although the diagnosis of NSAID hypersensitivity is mainly based on clinical history, in some circumstances skin testing with a culprit drug or in vitro tests may be applied, depending on the suspected type of hypersensitivity. Intradermal skin testing with a culprit drug is indicated only if a clinical history suggests SNIUAA, a selective type of hypersensitivity. The usefulness of skin testing has been documented for pyrazolones, although sensitivity is not optimal, and the risk of systemic responses after intradermal testing exists.25,27 If the cross-reactive type is suspected (NECD, NIUA), intradermal skin testing is not indicated, since it has a rather limited negative predictive value.2 In patients with a history of NIDHR like delayed cutaneous reactions, skin patch tests can be useful for contact dermatitis, fixed drug eruption, and maculopapular rash (and some cases of Stevens-Johnson syndrome/toxic epidermal necrolysis). Alternatively used, intradermal tests with delayed reading have high specificity but low sensitivity. Although several in vitro cell activation tests have been used to confirm a diagnosis of the acute type of NSAID hypersensitivity, they have not been validated and are not recommended for clinical practice.2 Similarly, in patients with delayed-type NSAID hypersensitivity, lymphocyte activation tests have a limited diagnostic value and are not recommended as well.2,40
Step 7: Consider oral provocation challenge
An oral provocation test with a culprit drug remains the gold standard for the diagnosis of hypersensitivity to NSAIDs and should be performed, particularly if the history is unclear or when a definite diagnosis is required.3 The decision to perform a provocation test should be taken separately for an individual patient after careful consideration of the benefit of confirming the diagnosis and the potential risk and effort associated with a challenge. Both sensitivity and specificity of oral provocation tests with NSAIDs are very high, exceeding 90%. In specific clinical circumstances, however, a negative result of oral tests in a patient with a positive history does not exclude NSAID-induced hypersensitivity e.g. if NERD or NECD is suspected and a patient is on long-term corticosteroid therapy and/or has well-controlled underlying disease (asthma or urticaria) over a longer period of time.41,42 In patients with suspected NERD, an inhalation provocation test or a nasal test with lysine aspirin can be considered as they are safer and faster to perform than oral challenges.43,44 Oral provocation with a culprit drug in NIDHR is usually not recommended.
CONCLUSIONS
Current classification of hypersensitivity to NSAIDs is based on understanding of the complexity of clinical presentations and diversity of immunological/non-immunological mechanisms of reactions. This classification has practical implications for allowing a physician to assess, based on the history, the type of reaction and for considering further rational steps to confirm the diagnosis. If the type of NSAID hypersensitivity is confirmed, recommendations based on the current classification for drug avoidance, use of alternative NSAIDs, and other management modalities, including aspirin desensitization, can be implemented.2
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Conaghan PG. A turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol Int. 2012;32:1491–1502. doi: 10.1007/s00296-011-2263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowalski ML, Asero R, Bavbek S, Blanca M, Blanca-Lopez N, Bochenek G, et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy. 2013;68:1219–1232. doi: 10.1111/all.12260. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson DD, Kowalski ML. An epidemic of over diagnosing drug allergies. Allergy Asthma Proc. 2014;35:92–94. doi: 10.2500/aap.2014.35.1015. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson DD, Sanchez-Borges M, Szczeklik A. Classification of allergic and pseudoallergic reactions to drugs that inhibit cyclooxygenase enzymes. Ann Allergy Asthma Immunol. 2001;87:177–180. doi: 10.1016/S1081-1206(10)62221-1. [DOI] [PubMed] [Google Scholar]
- 5.Kowalski ML, Makowska JS, Blanca M, Bavbek S, Bochenek G, Bousquet J, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) - classification, diagnosis and management: review of the EAACI/ENDA(#) and GA2LEN/HANNA*. Allergy. 2011;66:818–829. doi: 10.1111/j.1398-9995.2011.02557.x. [DOI] [PubMed] [Google Scholar]
- 6.Torres MJ, Barrionuevo E, Kowalski M, Blanca M. Hypersensitivity reactions to nonsteroidal anti-inflammatory drugs. Immunol Allergy Clin North Am. 2014;34:507–524. vii–viii. doi: 10.1016/j.iac.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Vane JR. The mode of action of aspirin and similar compounds. J Allergy Clin Immunol. 1976;58:691–712. doi: 10.1016/0091-6749(76)90181-0. [DOI] [PubMed] [Google Scholar]
- 8.Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- 9.Park HS, Kowalski ML, Sanchez-Borges M. Hypersensitivity to aspirin and other nonsteroidal antiinflammatory drugs. In: Adkinson NF Jr, Bochner BS, Burks W, Busse WW, Holgate ST, Lemanske RF Jr, O'Hehir RE, editors. Middleton's allergy: principles and practice. 8th ed. Philadelphia (PA): Elsevier/Saunders; 2014. pp. 1296–1309. [Google Scholar]
- 10.Hoitsma AJ, Wetzels JF, Koene RA. Drug-induced nephrotoxicity. Aetiology, clinical features and management. Drug Saf. 1991;6:131–147. doi: 10.2165/00002018-199106020-00004. [DOI] [PubMed] [Google Scholar]
- 11.Samter M, Beers RF., Jr Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med. 1968;68:975–983. doi: 10.7326/0003-4819-68-5-975. [DOI] [PubMed] [Google Scholar]
- 12.Szczeklik A, Nizankowska E, Duplaga M AIANE Investigators; European Network on Aspirin-Induced Asthma. Natural history of aspirin-induced asthma. Eur Respir J. 2000;16:432–436. doi: 10.1034/j.1399-3003.2000.016003432.x. [DOI] [PubMed] [Google Scholar]
- 13.Kowalski ML, Cieślak M, Pérez-Novo CA, Makowska JS, Bachert C. Clinical and immunological determinants of severe/refractory asthma (SRA): association with Staphylococcal superantigen-specific IgE antibodies. Allergy. 2011;66:32–38. doi: 10.1111/j.1398-9995.2010.02379.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimine F, Hasegawa T, Suzuki E, Terada M, Koya T, Kondoh A, et al. Contribution of aspirin-intolerant asthma to near fatal asthma based on a questionnaire survey in Niigata Prefecture, Japan. Respirology. 2005;10:477–484. doi: 10.1111/j.1440-1843.2005.00740.x. [DOI] [PubMed] [Google Scholar]
- 15.Senna G, Bilò MB, Antonicelli L, Schiappoli M, Crivellaro MA, Bonadonna P, et al. Tolerability of three selective cyclo-oxygenase-2 inhibitors, meloxicam, celecoxib and rofecoxib in NSAID-sensitive patients. Eur Ann Allergy Clin Immunol. 2004;36:215–218. [PubMed] [Google Scholar]
- 16.Quiralte J, Delgado J, Sáenz de San Pedro B, López-Pascual E, Nieto MA, Ortega N, et al. Safety of the new selective cyclooxygenase type 2 inhibitors rofecoxib and celecoxib in patients with anaphylactoid reactions to nonsteroidal anti-inflammatory drugs. Ann Allergy Asthma Immunol. 2004;93:360–364. doi: 10.1016/s1081-1206(10)61395-6. [DOI] [PubMed] [Google Scholar]
- 17.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy Asthma Immunol Res. 2014;6:288–295. doi: 10.4168/aair.2014.6.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson DD, Simon RA, Mathison DA, Christiansen SC. Montelukast is only partially effective in inhibiting aspirin responses in aspirin-sensitive asthmatics. Ann Allergy Asthma Immunol. 2000;85:477–482. doi: 10.1016/S1081-1206(10)62575-6. [DOI] [PubMed] [Google Scholar]
- 19.Park SM, Park JS, Park HS, Park CS. Unraveling the genetic basis of aspirin hypersensitivity in asthma beyond arachidonate pathways. Allergy Asthma Immunol Res. 2013;5:258–276. doi: 10.4168/aair.2013.5.5.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szczeklik A, Niżankowska-Mogilnicka E, Sanak M. Hypersensitivity to aspirin and other NSAIDs. In: Kay AB, Bousquet J, Holt PG, Kaplan AP, editors. Allergy and allergic diseases. 2nd ed. Hoboken (NJ): Wiley-Blackwell; 2008. pp. 1966–1979. [Google Scholar]
- 21.Doña I, Blanca-López N, Cornejo-García JA, Torres MJ, Laguna JJ, Fernández J, et al. Characteristics of subjects experiencing hypersensitivity to non-steroidal anti-inflammatory drugs: patterns of response. Clin Exp Allergy. 2011;41:86–95. doi: 10.1111/j.1365-2222.2010.03651.x. [DOI] [PubMed] [Google Scholar]
- 22.Kozel MM, Bossuyt PM, Mekkes JR, Bos JD. Laboratory tests and identified diagnoses in patients with physical and chronic urticaria and angioedema: a systematic review. J Am Acad Dermatol. 2003;48:409–416. doi: 10.1067/mjd.2003.142. [DOI] [PubMed] [Google Scholar]
- 23.Mastalerz L, Setkowicz M, Sanak M, Szczeklik A. Hypersensitivity to aspirin: common eicosanoid alterations in urticaria and asthma. J Allergy Clin Immunol. 2004;113:771–775. doi: 10.1016/j.jaci.2003.12.323. [DOI] [PubMed] [Google Scholar]
- 24.Doña I, Blanca-López N, Jagemann LR, Torres MJ, Rondón C, Campo P, et al. Response to a selective COX-2 inhibitor in patients with urticaria/angioedema induced by nonsteroidal anti-inflammatory drugs. Allergy. 2011;66:1428–1433. doi: 10.1111/j.1398-9995.2011.02684.x. [DOI] [PubMed] [Google Scholar]
- 25.Kowalski ML, Bienkiewicz B, Woszczek G, Iwaszkiewicz J, Poniatowska M. Diagnosis of pyrazolone drug sensitivity: clinical history versus skin testing and in vitro testing. Allergy Asthma Proc. 1999;20:347–352. doi: 10.2500/108854199778251799. [DOI] [PubMed] [Google Scholar]
- 26.Rutkowski K, Nasser SM, Ewan PW. Paracetamol hypersensitivity: clinical features, mechanism and role of specific IgE. Int Arch Allergy Immunol. 2012;159:60–64. doi: 10.1159/000335213. [DOI] [PubMed] [Google Scholar]
- 27.Himly M, Jahn-Schmid B, Pittertschatscher K, Bohle B, Grubmayr K, Ferreira F, et al. IgE-mediated immediate-type hypersensitivity to the pyrazolone drug propyphenazone. J Allergy Clin Immunol. 2003;111:882–888. doi: 10.1067/mai.2003.163. [DOI] [PubMed] [Google Scholar]
- 28.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gebhardt M, Wollina U. Cutaneous side-effects of nonsteroidal anti-inflammatory drugs (NSAID) Z Rheumatol. 1995;54:405–412. [PubMed] [Google Scholar]
- 30.Patel RM, Marfatia YS. Clinical study of cutaneous drug eruptions in 200 patients. Indian J Dermatol Venereol Leprol. 2008;74:430. doi: 10.4103/0378-6323.42883. [DOI] [PubMed] [Google Scholar]
- 31.Barbaud A. Contact dermatitis due to topical drugs. G Ital Dermatol Venereol. 2009;144:527–536. [PubMed] [Google Scholar]
- 32.Ward KE, Archambault R, Mersfelder TL. Severe adverse skin reactions to nonsteroidal antiinflammatory drugs: A review of the literature. Am J Health Syst Pharm. 2010;67:206–213. doi: 10.2146/ajhp080603. [DOI] [PubMed] [Google Scholar]
- 33.Mockenhaupt M, Kelly JP, Kaufman D, Stern RS SCAR Study Group. The risk of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with nonsteroidal antiinflammatory drugs: a multinational perspective. J Rheumatol. 2003;30:2234–2240. [PubMed] [Google Scholar]
- 34.Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) Br J Dermatol. 2007;157:989–996. doi: 10.1111/j.1365-2133.2007.08156.x. [DOI] [PubMed] [Google Scholar]
- 35.Mihovilovic K, Ljubanovic D, Knotek M. Safe administration of celecoxib to a patient with repeated episodes of nephrotic syndrome induced by NSAIDs. Clin Drug Investig. 2011;31:351–355. doi: 10.1007/BF03256934. [DOI] [PubMed] [Google Scholar]
- 36.Ameen KH, Pinninti R, Jami S. Aceclofenac induced Stevens-Johnson/toxic epidermal necrolysis overlap syndrome. J Pharmacol Pharmacother. 2013;4:69–71. doi: 10.4103/0976-500X.107691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rozieres A, Vocanson M, Saïd BB, Nosbaum A, Nicolas JF. Role of T cells in nonimmediate allergic drug reactions. Curr Opin Allergy Clin Immunol. 2009;9:305–310. doi: 10.1097/ACI.0b013e32832d565c. [DOI] [PubMed] [Google Scholar]
- 38.Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139:683–693. doi: 10.7326/0003-4819-139-8-200310210-00012. [DOI] [PubMed] [Google Scholar]
- 39.Doña I, Blanca-López N, Torres MJ, García-Campos J, García-Núñez I, Gómez F, et al. Drug hypersensitivity reactions: response patterns, drug involved, and temporal variations in a large series of patients. J Investig Allergol Clin Immunol. 2012;22:363–371. [PubMed] [Google Scholar]
- 40.Bavbek S, Ikincioğullari A, Dursun AB, Guloğlu D, Arikan M, Elhan AH, et al. Upregulation of CD63 or CD203c alone or in combination is not sensitive in the diagnosis of nonsteroidal anti-inflammatory drug intolerance. Int Arch Allergy Immunol. 2009;150:261–270. doi: 10.1159/000222678. [DOI] [PubMed] [Google Scholar]
- 41.Nizankowska-Mogilnicka E, Bochenek G, Mastalerz L, Swierczynska M, Picado C, Scadding G, et al. EAACI/GA2LEN guideline: aspirin provocation tests for diagnosis of aspirin hypersensitivity. Allergy. 2007;62:1111–1118. doi: 10.1111/j.1398-9995.2007.01409.x. [DOI] [PubMed] [Google Scholar]
- 42.Macy E, Bernstein JA, Castells MC, Gawchik SM, Lee TH, Settipane RA, et al. Aspirin challenge and desensitization for aspirin-exacerbated respiratory disease: a practice paper. Ann Allergy Asthma Immunol. 2007;98:172–174. doi: 10.1016/S1081-1206(10)60692-8. [DOI] [PubMed] [Google Scholar]
- 43.Nizankowska E, Bestyńska-Krypel A, Cmiel A, Szczeklik A. Oral and bronchial provocation tests with aspirin for diagnosis of aspirin-induced asthma. Eur Respir J. 2000;15:863–869. doi: 10.1034/j.1399-3003.2000.15e09.x. [DOI] [PubMed] [Google Scholar]
- 44.Milewski M, Mastalerz L, Nizankowska E, Szczeklik A. Nasal provocation test with lysine-aspirin for diagnosis of aspirin-sensitive asthma. J Allergy Clin Immunol. 1998;101:581–586. doi: 10.1016/S0091-6749(98)70163-0. [DOI] [PubMed] [Google Scholar]