Abstract
The aim of this study was to evaluate probiotic characteristics of Lactobacillus salivarius LS01 and Bifidobacterium breve BR03 alone and in combination and their immunomodulatory activity in asthmatic subjects. Subjects affected by allergic asthma were recruited. Initially, LS01 and BR03 were analyzed for their growth compatibility by a broth compatibility assay. To study the antimicrobial activity of probiotic strains, an agar diffusion assay was performed. Finally, cytokine production by peripheral blood mononuclear cells (PBMCs) stimulated with LS01 and BR03 was determined by means of specific quantitative enzyme-linked immunosorbent assay (ELISA). The growth of some clinical pathogens were slightly inhibited by LS01 and LS01-BR03 co-culture supernatant not neutralized to pH 6.5, while only the growth of E. coli and S. aureus was inhibited by the supernatant of LS01 and LS01-BR03 neutralized to pH 6.5. Furthermore, LS01 and BR03 combination was able to decrease the secretion of proinflammatory cytokines by PBMCs, leading to an intense increase in IL-10 production. L. salivarius LS01 and B. breve BR03 showed promising probiotic properties and beneficial immunomodulatory activity that are increased when the 2 strains are used in combination in the same formulation.
Keywords: Asthma, probiotics, immune system
INTRODUCTION
In the last decades, there was a significant increase in the prevalence of allergic diseases in industrialized countries due to multifactorial causes.1,2,3 In particular, allergic asthma is a complex and heterogeneous disease characterized by intermittent reversible obstruction and chronic inflammation of the airways, bronchial hyperactivity, and an infiltration of lymphocytes. The allergic reaction is due to the disruption of the Th1/Th2 balance toward a pronounced Th2 profile; indeed, the production of IL-4, Il-5, or IL-13 by Th2 lymphocytes is involved in the development and maintenance of the allergic response.4 Several studies showed a reduced incidence of allergy in children treated with specific probiotic strains that were able to decrease the production of Th2 cytokines, keeping the production of Th1 stable.5,6 This evidence strengthens the hypothesis that probiotic strains could be used in the treatment of allergic diseases.7 Probiotics are defined as "live microorganisms that when administered in adequate amounts confer a health benefit on the host."8 Probiotics play a pivotal role in controlling intestinal microbiota homeostasis by checking pathogenic bacteria growth and in the development of tolerance against ingested food antigens.9 Moreover, probiotics seem to be able to modulate mucosal immune responses and reduce gastrointestinal inflammation in infants and adults affected by allergic diseases,10 having additional immunomodulatory action by exerting stimulatory effects on the intestinal innate and adaptive immune system, enhancing mucosal barrier functions, inducing the production of anti-inflammatory cytokines, and facilitating the maintenance of immune tolerance.11,12,13,14 However, much evidence showed that each probiotic effect is strictly strain-dependent: different strains belonging to the same species can have opposite effects when administered in the same protocol study and under the same conditions.15 Also, the ability to influence the immune response by modulating the release of pro- and anti-inflammatory cytokines varies among different strains.16 Probiotic microorganisms have been demonstrated to stimulate peripheral blood monocytes cells (PBMCs), leading to a restriction of proinflammatory cytokines and a significant activation of monocytes and IL-10 production.14 The aim of this study was to analyze the effect of 2 probiotic strains (Lactobacillus salivarius LS01 and Bifidobacterium breve BR03), tested alone and in combination, on the cytokine production by PBMCs from subjects affected by allergic asthma. Moreover, we studied the growth compatibility and antibacterial activity of the aforementioned strains.
MATERIALS AND METHODS
Asthmatic patients and blood samples
Samples from subjects with allergic asthma (blood donors; median age 30 years, range 20-35 years) were collected at IRCCS Galeazzi Orthopedic Institute, Milan. The patients with allergic asthma presented with a clinical diagnosis of allergic asthma. Allergic patients enrolled in the study were subjected to radioallergosorbent test (RAST) and skin prick test. All subjects showed a positive RAST test for at least one of the following allergens: dust mite, ragweed, grass, dust, and pollen. Twenty milliliters of heparinized venous peripheral blood was collected from each patient. The study protocol was approved by the internal scientific committee of the Institute, and all patients gave informed consent when assessed for eligibility.
Bacterial strains and culture conditions
L. salivarius LS01 and B. breve BR03 were used in the study. All strains were obtained from commercial products (Bifiderm, Bayer, Milan, Italy) containing about 1×109 CFU (colony forming unit) of each strain. BR03 and LS01 were revitalized prior to each experiment in Wilkins-Chalgren (WC) broth (Sigma-Aldrich, Milan, Italy) and de Man, Rogosa and Sharpe (MRS) broth (Biolife, Milan, Italy), respectively. All strains were grown at 37℃ in anaerobiosis for 48 hours. The following pathogenic bacteria isolated from clinical samples were used in the study: Escherichia coli (n=2), Klebsiella pneumoniae (n=2), Staphylococcus aureus (n=2), Staphylococcus epidermidis (n=2), Streptococcus agalactiae (n=2), Streptococcus pyogenes (n=2), and Pseudomonas aeruginosa (n=2). These strains were grown in Brain-Heart Infusion (BHI) broth (Sigma-Aldrich) at 37℃ for 24 hours.
Growth compatibility test
LS01 and BR03, alone and in combination (ratio 1:1), were inoculated in 10 mL of WC broth in order to have an initial concentration of about 105 CFU/mL. Then, bacteria were incubated at 37℃ in anaerobiosis. After 24, 48, and 72 hours of incubation, an aliquot was taken from each sample and 10-fold dilutions were seeded into Bifidus Selective Agar (BSM) (Sigma-Aldrich) and MRS plates (Biolife). Subsequently, BSM plates were incubated in anaerobiosis at 37℃ for 48 hours, while MRS plates was incubated in 10% CO2 enriched atmosphere at 37℃ for 48 hours for bacterial counts. Experiments were performed in duplicate and repeated 3 times.
Agar well diffusion assay
The ability of LS01 and BR03 to inhibit bacterial growth was assessed by an agar-well diffusion assay estimating the inhibitory effect of cell-free supernatant obtained from the cultured probiotics LS01 and BR03, alone and in combination (mixture), after overnight growth in MRS and WC broth, respectively. Cultured probiotics were centrifuged at 4,000 g for 10 minutes, and the supernatants were collected and divided into 2 aliquots, one of which was neutralized to pH 6.5 using 1 M NaOH to exclude the role of pH on the antibacterial activity. Pathogens were grown in BHI broth at 37℃ in aerobiosis for 24 hours. Overnight cultures of pathogens were diluted 10-fold to achieve semi-confluent growth. Then, 2 mL of the diluted suspension were applied to Mueller-Hinton plates and the surface covered by rotating the plate. Consequently, 9-mm wells were made in each dish by removal of the agar, and 100 µL of probiotic supernatant were added to each well. Free-cell broth was used as a negative control. The presence of an inhibition zone was assessed visually following 24 hours of incubation at 37℃ in aerobiosis. Each test was performed in triplicate.
Isolation of PBMCs
PBMCs were obtained from heparinized blood of subjects affected by allergic asthma, by Ficoll (Sigma-Aldrich) gradient centrifugation, as previously described.17,18 Mononuclear cells were resuspended in culture medium made up of RPMI 164 (Sigma-Aldrich) supplemented with 5% heat-inactivated fetal calf serum (Sigma-Aldrich).
Stimulation of PBMCs
Freshly isolated PBMCs were diluted in culture medium to a final concentration of 2×106/mL and transferred in a volume of 500 µL to flat-bottomed 24-well microtiter trays (Biosigma). Subsequently, 100 µL of culture medium containing LS01 and BR03, alone and in combination, or containing phosphate buffered saline (PBS) (Life Technologies, Monza, Italy) as a negative control, were added. After 24 hours of incubation, a supernatant sample was collected by centrifuging samples at 2,000 g for 10 minutes and stored at -20℃ for further ELISA analysis.
Measurement of cytokines in PBMCs
The production of IL-10, IL-4, IL-5, IL-13, TGF-β, and IL-17 was determined by means of specific ELISA (Life Technologies), according to the manufacturer's instructions. Absorbance was measured at 450 nm using a Biorad spectrophotometer (mod 680; Biorad, Segrate, Italy). For each cytokine, a standard curve was constructed in duplicate and used to quantify the amount of cytokine (pg) per milliliter in culture medium. All assays were performed in triplicate.
Statistical analysis
Non-parametric statistical analyses were performed with the Mann-Whitney U test. Differences were considered statistically significant when P values were less than 0.05.
RESULTS
Growth compatibility assay
The compatibility assay showed that a single strain did not inhibit the growth of the other one when cultured together. Indeed, there was no significant difference in growth curve when strains were grown alone and together.
Antimicrobial activity assay
All pathogens were slightly inhibited by supernatants of LS01 and LS01-BR03 co-culture not neutralized to pH 6.5, and an inhibition halo of 1-2 mm was observed around each well. No inhibition effect was observed when BR03 supernatant alone was tested. Moreover, using supernatants of LS01 and LS01-BR03 co-culture neutralized to pH 6.5, only the growth of E. coli and S. aureus was inhibited. No inhibition effect was observed when BR03 supernatant neutralized to pH 6.5 was tested.
Pattern of cytokine expression in stimulated PBMCs
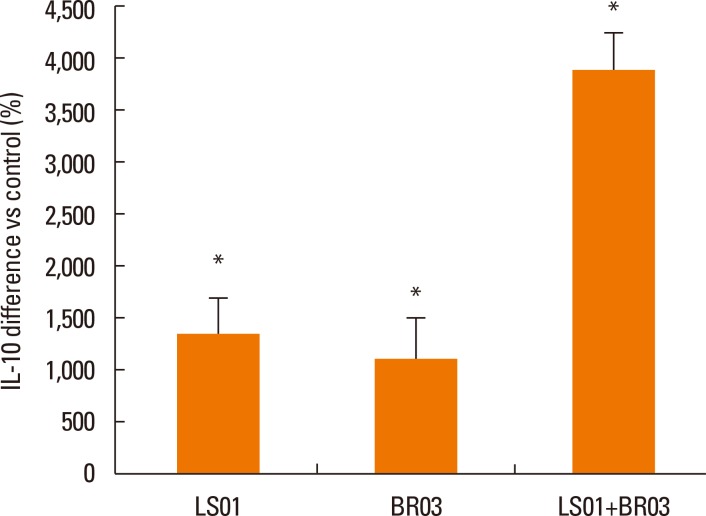
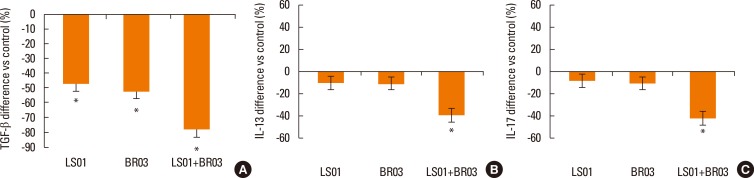
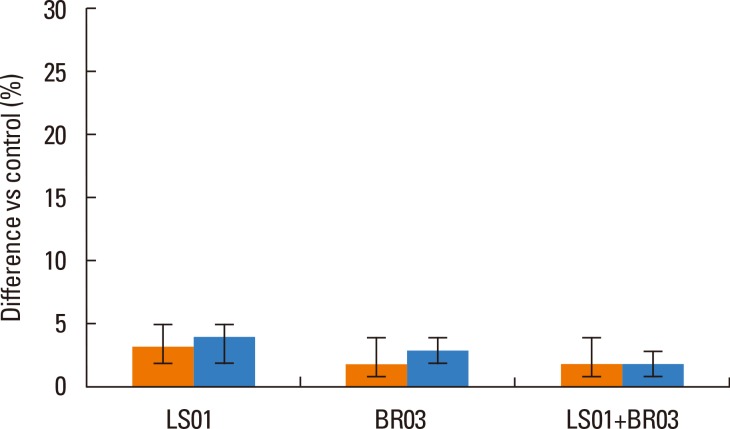
Results were reported as differences (%) vs control of the 3 different experiments in duplicate. IL-10 secretion by PBMCs after stimulation with probiotic strains (alone and in combination) was significantly higher than secretion by non-stimulated PBMCs (Fig. 1), in particular the combination of LS01 and BR03 led to a higher increase in IL-10 production than LS01 and BR03 used alone. Secretion of TGF-β was significantly lower after stimulation of PBMCs with probiotic strains (Fig. 2A), using both the mixture of strains and the strains alone. Otherwise, IL-13 and IL-17 amounts were significantly lower only when PBMCs were stimulated with LS01-BR03 co-culture compared to the amount of cytokines produced after stimulation with LS01 or BR03 alone (Fig. 2B and C). No differences in the secretion of IL-4 and IL-5 were observed in PBMCs stimulated with probiotic strains (alone and in combination) if compared to control (Fig. 3).
Fig. 1. Release of IL-10 by PBMCs. *P<0.05 vs control (non-stimulated cells). Data are means±SD of the 3 experiments.
Fig. 2. Release of TGF-β (A), IL-13 (B), and IL-17 (C) by PBMCs. *P<0.05 vs control (non-stimulated cells). Data are means±SD of the 3 experiments.
Fig. 3. Release of IL-4 (orange bars) and IL-5 (blue bars) by PBMCs. *P<0.05 vs control (non-stimulated cells). Data are means±SD of the 3 experiments.
DISCUSSION
One of the main characteristics of probiotic strains used in combination in the same product is their growth compatibility, as it is fundamental that each strain does not inhibit the growth or probiotic activity of each strains present in the mixture. In our study, L. salivarius LS01 and B. breve BR03 did not show growth incompatibility when mixed together in the same broth; as a consequence the 2 tested strains can be used in the same probiotic mixture without affecting their functionality each other in the human gut. Furthermore, this study showed that only LS01 and LS01-BR03 co-culture supernatant not neutralized to pH 6.5 was able to inhibit the growth of all the tested pathogens, and in particular, it inhibited the growth of E. coli and, slightly, the growth of S. aureus when the pH was neutralized to 6.5. As a consequence, the antimicrobial effect of LS01and LS01-BR03 supernatant against E. coli and S. aureus may be due not only to the low pH that had an inhibition activity on pathogens growth, but also to the production of bacteriocins that seemed to be specific for these microorganisms. Bacteriocins are oligopeptides, proteins, or protein complexes with antimicrobial activity against several bacterial strains, and in recent years, the production of this compounds by L. salivarius has been described.19 These molecules are produced on the surface of ribosomes in microbial cells and may attach to the target cell wall anywhere on the surface, as no specific receptors on the target cell wall apparently exist.20 Moreover, the production of a family of 2-component salivaricin P-like bacteriocin may be a common feature among intestinal L. salivarius strains.21 Furthermore, the ability of LS01 to modify the intestinal microbiota composition, leading to a significant reduction in S. aureus load has been already described by Drago et al.16 who highlighted the modulatory activity of the aforementioned strain in rebalancing the altered intestinal microbiota in patients affected by atopic dermatitis. The antibacterial effect of LS01 in this study is probably due to an altered intestinal ecosystem, leading to a significant decrease in S. aureus load. On the other hand, BR03 did not show antimicrobial activity, even if different studies underlined that several Bifidobacterium strains produce antibacterial substances, different from organic acids, which could have a strong killing activity against pathogenic bacteria.22 Furthermore, the antimicrobial effect of bifidobacteria seems to be strain-specific and not species-specific.23 As a consequence, the antimicrobial effect shown by LS01-BR03 supernatant when the pH was neutralized to 6.5 seems to be especially due to the production of bacteriocins by L. salivarius LS01. Regarding the immunomodulatory properties of the tested probiotic strains, we observed that LS01 and BR03 were able to induce massive secretion of IL-10 by human PBMCs of subjects with allergic asthma. Previous studies showed that the absence of IL-10 in knockout mice led to a harmful inflammatory state, indicating that inflammation is induced by an imbalanced local or systematic cytokine milieu.9 The massive stimulation of IL-10 secretion by PBMCs stimulated with probiotic strains has been already observed by Michalkiewicz et al.24 who highlighted the physiological role of lactic acid bacteria (LAB) in limiting the proinflammatory response, simultaneously improving the process of maintaining the state of tolerance to external antigens. Consequently, the ability of LS01 and BR03 to induce high levels of IL-10 is of great importance in maintaining the physiological profile of the immune response in mucosal lymphoid tissue. IL-10 is a pleiotropic cytokine that can exert either immunosuppressive or immunostimulatory effects on several cell types. This cytokine is a powerful inhibitor of proinflammatory cytokines, such as IL-4 and IL-5, and there is evidence that IL-10 mRNA and protein expression are reduced in alveolar macrophages from asthmatic subjects compared to those from non-asthmatic subjects.25 Furthermore, our analysis highlighted the synergic effect of LS01 and BR03 in stimulating the production of IL-10; indeed, the amount of IL-10 is higher when PBMCs were stimulated with LS01 and BR03 at the same time. Interestingly, the probiotic strains tested in this study were able to inhibit the secretion of proinflammatory cytokines, such as TGF-β, IL-13, and IL-17. TGF-β is one of the mediators involved in many aspects of persistent inflammation and tissue remodelling in the asthmatic lung, and it was suggested that excessive TGF-β secretion may lead to the dysregulation of regulatory T (TReg) cell activity and to the development of allergic diseases in human.26 IL-13, instead, has been shown to be associated with asthma severity, lung function, and airways hyper-responsiveness in asthmatic patients,27 and together with the novel proinflammatory cytokine IL-17, it should exert a combined effect for inflammatory reactions in patients with allergic asthma.27 Moreover, it has been demonstrated that the production of IL-13 by PBMCs from allergic patients stimulated with LAB was highly reduced, highlighting the strong immunomodulatory effect of probiotic strains.28 Therefore, the ability of LS01 and BR03 to down-regulate the secretion of TGF-β, IL-13, and IL-17 in asthmatic subjects may lead to the rebalancing of Th1/Th2 ratio and to the improvement of allergic symptoms. Finally, we did not find any differences in IL-4 and IL-5 production by PBMCs after probiotic stimulation. These results are quite different from those obtained from Pochard et al.28 who observed a significant decrease in the secretion of the proinflammatory cytokines IL-4 and IL-5 by PBMCs from allergic subjects stimulated with several Lactobacillus strains. In conclusion, L. salivarius LS01 and B. breve BR03 showed promising probiotic features, as these strains may act as anti-inflammatory mediators able to inhibit the Th2 cytokine profile that is increased in allergic diseases, and interestingly their combination in the same formulation seems to implement the immunomodulatory activity of each strain. Further studies should be carried out to assess the clinical impact of LS01 and BR03 in subjects affected by allergic asthma and to develop a cost-effective biotherapy able to improve the symptoms of allergic diseases.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Björkstén B. Environmental risk factors for atopy. Clin Rev Allergy Immunol. 1997;15:125–143. doi: 10.1007/BF02826583. [DOI] [PubMed] [Google Scholar]
- 2.Björkstén B. The environmental influence on childhood asthma. Allergy. 1999;54(Suppl 49):17–23. doi: 10.1111/j.1398-9995.1999.tb04383.x. [DOI] [PubMed] [Google Scholar]
- 3.Julge K, Vasar M, Björkstén B. The development of atopic sensitization in Estonian infants. Acta Paediatr. 1997;86:1188–1194. doi: 10.1111/j.1651-2227.1997.tb14842.x. [DOI] [PubMed] [Google Scholar]
- 4.Robinson DS, Kay AB. Role of Th1 and Th2 cells in human allergic disorders. Chem Immunol. 1996;63:187–203. [PubMed] [Google Scholar]
- 5.Isolauri E, Salminen S Nutrition, Allergy, Mucosal Immunology, and Intestinal Microbiota (NAMI) Research Group Report. Probiotics: use in allergic disorders: a Nutrition, Allergy, Mucosal Immunology, and Intestinal Microbiota (NAMI) Research Group Report. J Clin Gastroenterol. 2008;42(Suppl 2):S91–S96. doi: 10.1097/MCG.0b013e3181639a98. [DOI] [PubMed] [Google Scholar]
- 6.Gourbeyre P, Denery S, Bodinier M. Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. J Leukoc Biol. 2011;89:685–695. doi: 10.1189/jlb.1109753. [DOI] [PubMed] [Google Scholar]
- 7.Drago L, Toscano M, Pigatto PD. Probiotics: immunomodulatory properties in allergy and eczema. G Ital Dermatol Venereol. 2013;148:505–514. [PubMed] [Google Scholar]
- 8.Food and Agriculture Organization of the United Nations (IT); World Health Organization (CH) Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO Working Group on drafiting guidelines for the evaluation of probiotics in food. Rome: Food and Agriculture Organization of the United Nations; 2002. [Google Scholar]
- 9.Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, Rizzardini G, et al. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol. 2012;46(Suppl):S33–S40. doi: 10.1097/MCG.0b013e31826a8468. [DOI] [PubMed] [Google Scholar]
- 10.Mueller C, Macpherson AJ. Layers of mutualism with commensal bacteria protect us from intestinal inflammation. Gut. 2006;55:276–284. doi: 10.1136/gut.2004.054098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramakrishna BS. Probiotic-induced changes in the intestinal epithelium: implications in gastrointestinal disease. Trop Gastroenterol. 2009;30:76–85. [PubMed] [Google Scholar]
- 12.Pessi T, Sütas Y, Hurme M, Isolauri E. Interleukin-10 generation in atopic children following oral Lactobacillus rhamnosus GG. Clin Exp Allergy. 2000;30:1804–1808. doi: 10.1046/j.1365-2222.2000.00948.x. [DOI] [PubMed] [Google Scholar]
- 13.Kilpi T, Kero J, Jokinen J, Syrjänen R, Takala AK, Hovi T, et al. Common respiratory infections early in life may reduce the risk of atopic dermatitis. Clin Infect Dis. 2002;34:620–626. doi: 10.1086/338783. [DOI] [PubMed] [Google Scholar]
- 14.Drago L, Nicola L, Iemoli E, Banfi G, De Vecchi E. Strain-dependent release of cytokines modulated by Lactobacillus salivarius human isolates in an in vitro model. BMC Res Notes. 2010;3:44. doi: 10.1186/1756-0500-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gore C, Custovic A, Tannock GW, Munro K, Kerry G, Johnson K, et al. Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: randomized controlled trial with follow-up until age 3 years. Clin Exp Allergy. 2012;42:112–122. doi: 10.1111/j.1365-2222.2011.03885.x. [DOI] [PubMed] [Google Scholar]
- 16.Drago L, Toscano M, De Vecchi E, Piconi S, Iemoli E. Changing of fecal flora and clinical effect of L. salivarius LS01 in adults with atopic dermatitis. J Clin Gastroenterol. 2012;46(Suppl):S56–S63. doi: 10.1097/MCG.0b013e318265ef38. [DOI] [PubMed] [Google Scholar]
- 17.Michałkiewicz J, Stachowski J, Barth C, Patzer J, Dzierzanowska D, Madaliński K. Effect of Pseudomonas aeruginosa exotoxin A on IFN-gamma synthesis: expression of costimulatory molecules on monocytes and activity of NK cells. Immunol Lett. 1999;69:359–366. doi: 10.1016/s0165-2478(99)00121-2. [DOI] [PubMed] [Google Scholar]
- 18.Socha P, Michałkiewicz J, Stachowski J, Pawłowska J, Jankowska I, Barth C, et al. Deficiency of the expression of CD45RA isoform of CD45 common leukocyte antigen in CD4+ T lymphocytes in children with infantile cholestasis. Immunol Lett. 2001;75:179–184. doi: 10.1016/s0165-2478(00)00305-9. [DOI] [PubMed] [Google Scholar]
- 19.Robredo B, Torres C. Bacteriocin production by Lactobacillus salivarius of animal origin. J Clin Microbiol. 2000;38:3908–3909. doi: 10.1128/jcm.38.10.3908-3909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleveland J, Montville TJ, Nes IF, Chikindas ML. Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol. 2001;71:1–20. doi: 10.1016/s0168-1605(01)00560-8. [DOI] [PubMed] [Google Scholar]
- 21.Barrett E, Hayes M, O'Connor P, Gardiner G, Fitzgerald GF, Stanton C, et al. Salivaricin P, one of a family of two-component antilisterial bacteriocins produced by intestinal isolates of Lactobacillus salivarius. Appl Environ Microbiol. 2007;73:3719–3723. doi: 10.1128/AEM.00666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makras L, De Vuyst L. The in vitro inhibition of Gram-negative pathogenic bacteria by bifidobacteria is caused by the production of organic acids. Int Dairy J. 2006;16:1049–1057. [Google Scholar]
- 23.Ibrahim SA, Dharmavaram SR, Seo CW, Shahbazi G. Antimicrobial activity of Bifidobacterium longum (NCFB 2259) as influenced by spices. Int J Food Safety. 2005;2:6–8. [Google Scholar]
- 24.Michalkiewicz J, Krotkiewski M, Gackowska L, Wyszomirska-Golda M, Helmin-Basa A, Dzierźanowska D, et al. Immunomodulatory effects of lactic acid bacteria on human peripheral blood mononuclear cells. Microb Ecol Health Dis. 2003;15:185–192. [Google Scholar]
- 25.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97:1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt-Weber CB, Blaser K. The role of TGF-beta in allergic inflammation. Immunol Allergy Clin North Am. 2006;26:233–244. vi–vii. doi: 10.1016/j.iac.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui DS, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125:177–183. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pochard P, Gosset P, Grangette C, Andre C, Tonnel AB, Pestel J, et al. Lactic acid bacteria inhibit TH2 cytokine production by mononuclear cells from allergic patients. J Allergy Clin Immunol. 2002;110:617–623. doi: 10.1067/mai.2002.128528. [DOI] [PubMed] [Google Scholar]