Abstract
The direct, regioselective, and stereoselective arylation of activated alkynes with aryl iodides using a nickel catalyst and manganese reductant is described. The reaction conditions are mild (40 °C in MeOH, no acid or base) and an intermediate organomanganese reagent is unlikely. Functional groups tolerated include halides and pseudohalides, free and protected anilines, and a benzyl alcohol. Other activated alkynes including an amide and a ketone also reacted to form arylated products in good yields.
Keywords: reductive, arylation, alkyne, nickel, catalyzed
Tri-substituted alkenoates are important building blocks for the synthesis of natural products,1 and thus a variety of methods have been developed for their synthesis. These methods include olefination,2 cross-coupling,3, 4 Heck reactions of alkenes,5 reductive Heck reactions of alkynes,6 C-H activation,7 and the addition of organometallic reagents across alkynes.8, 9, 10 Of these approaches, the Heck reaction and the addition of organometallic reagents across alkynes are the most well developed. Reductive arylation combines attractive elements of these two approaches, the use of organic halides and addition across simple alkynes, but a simple β-selective reaction is unknown.
The reductive arylation of alkynes is less well developed than addition reactions to alkenes. Although palladium-catalyzed reactions tend to give products of multiple alkyne insertions11 and favor α-addition of alkynones and alkynoates,12 broadly useful approaches to vinyl arenes and heterocycles have been developed.6 Cheng has reported nickel-catalyzed annulations of alkynes to form lactones13 and quinolines,14 including a single example of β-selective arylation.15 Although it was unclear if stereochemical control and high chemical yield would be possible without a cyclization event, our own studies on reductive-Heck like reactions prompted us to investigate this process.16
After some initial exploration of reactivity, we found that methyl octynoate could be coupled with iodobenzene in the presence of a nickel(II) source, a bidentate amine ligand, and manganese reductant. While reasonable yields could be obtained in a variety of solvents, the best results were obtained in methanol. In contrast to Cheng’s work, we found nitrogen donor ligands, especially phenanthroline (L1, entry 1), to be superior to dppe (L2, entry 8). Control reactions demonstrated that nickel and ligand were both necessary for the reaction (Table 1, entries 2 and 3).
Table 1.
Reductive arylation of methyl octynoate with iodobenzenea

| |||
|---|---|---|---|
| entry | deviation from entry 1 | yieldb | E/Z ratioc |
| 1 | none | 92 | 8:1 |
| 2 | no nickel | 6 | 5:1 |
| 3 | no phenanthroline (L1) | 0 | NA |
| 4 | 5 mol% NiCl2•2H2O | 95 | 13:1 |
| 5 | 5 mol% L1 | 72 | 14:1 |
| 6 | no Mn0 powder | 0 | NA |
| 7 | 1 mol% catalyst | 56 | 10:1 |
| 8 | dppe (L2) instead of L1 | 1 | NA |
Standard reaction conditions: 1a (0.50 mmol), 2a (0.50 mmol), 2 mL methanol, heated in a reaction block at 40 °C for 20-24 h.
Yield reported is corrected GC yield of combined isomers with respect to decane.
E/Z ratio determined by GC analysis.
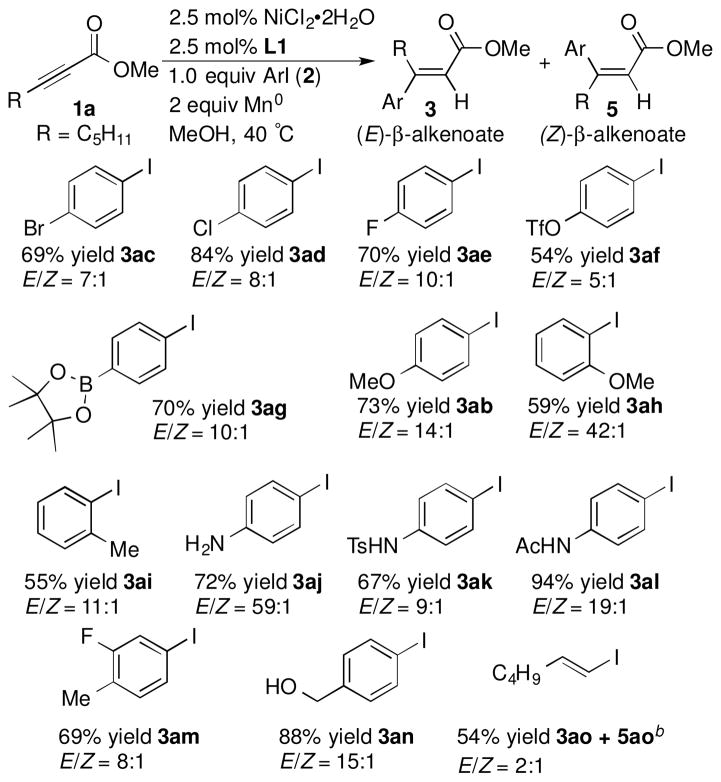
A variety of activated alkynes participated in the arylation reaction and provided primarily the products of syn addition across the alkyne, yielding the (E)-isomer 3, as well as minor amounts of the (Z)-isomer (Scheme 1). The exception was a reaction run with ethyl phenylpropiolate which formed α-aryl alkenoate 4ca and only a trace amount of the β-arylation product. In addition, product 4ca was found to be a methyl ester instead of an ethyl ester due to competitive transesterification. Transesterification was not observed in reactions with a tert-butyl ester and a dimethyl amide (see products 3ba and 3db).
Scheme 1.
Alkyne scopea
a Reactions conducted as in Table 1, footnote a. Yields reported are of isolated yields of E olefin (major isomer). E/Z and α/β ratios determined by GC analysis. Ar = Ph unless noted. b Alkynoate used was ethyl 3-phenylpropiolate. c Reaction was run at 60 °C.
The chemistry tolerated aryl iodides with a wide range of functional groups (Scheme 2). The selective functionalization of the carbon-iodine bond over other carbon-halogen/pseudohalogen/boron bonds was observed, providing products that could be further functionalized using a variety of methods. Aryl iodides with a variety of other functional groups, including anilines and a benzyl alcohol, as well as a vinyl iodide also provided trisubstituted alkenoates 3aj, 3ak, 3al, 3an and 3ao in good yields.
Scheme 2.
Aryl halide scopea
a Reactions conducted as in Table 1, footnote a. Yields reported are of isolated yields of E olefin 3 (major isomer). E/Z ratio determined by GC analysis. b Yield reported is of combined E and Z isomers.
Both sterics and electronics of the aryl iodide affected the selectivity of the arylation. Aryl iodides with ortho-substitution resulted in better selectivity for the (E) product (Scheme 2, 3ab vs. 3ah), as did reactions with electron-rich aryl iodides (Scheme 2, 3ab and 3aj vs. 3aa in Scheme 1). In contrast, π-withdrawing substituents on aryl iodides provided significant amounts of the α-addition products in addition to the major (E)-β-aryl alkenoate (Scheme 3).
Scheme 3.
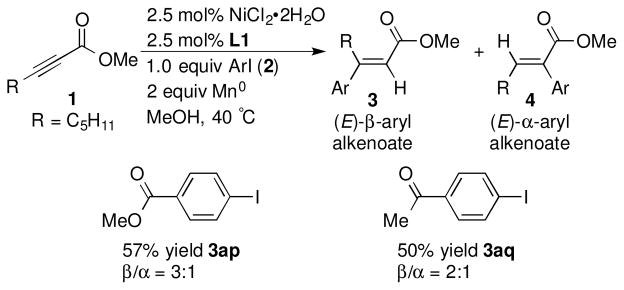
Aryl iodides that yielded α-arylation product 4a
a Reactions conducted as in Table 1, footnote a. Yields reported are of isolated yields. β/α ratio determined by GC analysis.
For some of the low-yielding substrates, byproducts including biaryl, hydrodehalogenation, and alkyne-dimer were observed. Specifically, with 2-iodotrifluorotoluene, there was an equal amount of hydrodehalogenated, hydroarylated, and bisarylated products, as well as a small amount of alkyne dimer observed. For ortho-iodotoluene, there was a significant amount of iodoarene remaining, and some bisarylated product. Efforts to minimize bisarylated product using protic additives were not successful.
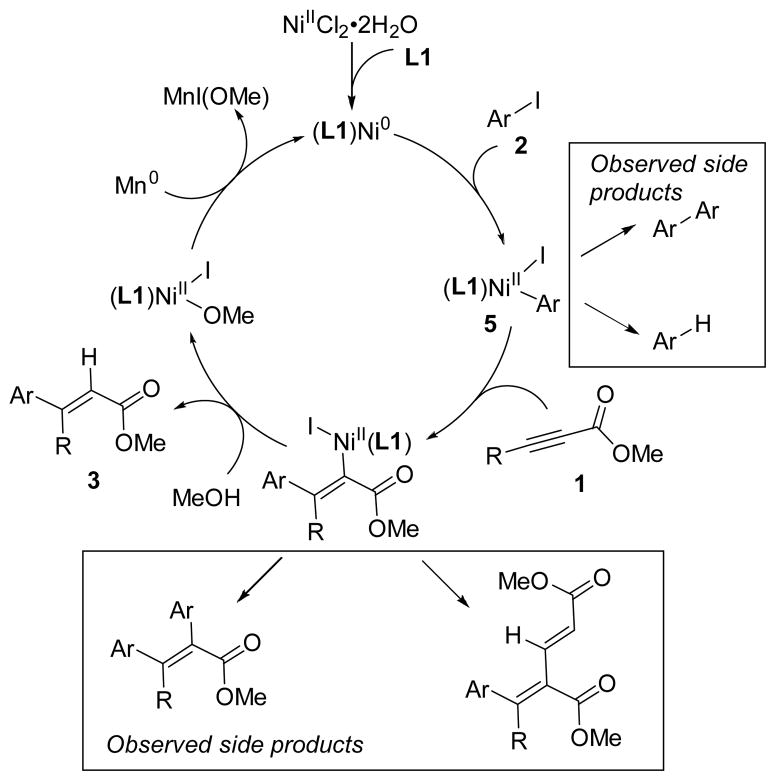
Mechanistic experiments support a reductive Heck mechanism, as suggested by Cheng (Scheme 4).13,14 An organomanganese intermediate is not likely because 1) acidic funtional groups are tolerated (benzyl alcohol, primary aniline), 2) the reaction is run in an alcohol solvent, and 3) stoichiometric reactions without added manganese produce product with similar yield and selectivity (Table 2). Additionally, the synthesis of arylmanganese reagents requires the use of additives (indium) or highly activated manganese.17 We also confirmed that the α-vinyl proton in the product is derived from the methanol solvent by running the reaction in deuterated methanol (see SI for details).
Scheme 4.
Proposed catalytic cycle
Table 2.
Stoichiometric studies of an arylnickel(II) complexa

| ||||
|---|---|---|---|---|
| entry | equiv ArI | equiv Mn0 | equiv alkyne | yieldb |
| 1c | Catalytic reaction with NiCl2•2H2O and L1 | 78 | ||
| 2 | 0 | 0 | 1.0 | 83 |
| 3 | 0 | 2 | 1.0 | 68 |
| 4 | 0 | 0 | 50 | 62 |
| 5 | 0 | 2 | 50 | 81 |
| 6c | 40 | 80 | 40 | 63 |
Reactions conducted as in Table 1, footnote a.
Yield reported is corrected GC yield calculated with respect to an internal standard.
ArI remaining after 24 h.
Further evidence in support of a reductive Heck mechanism is the stoichiometric reaction of (phenanthroline)NiII(aryl)I complex 6 with an alkynoate to form the expected addition product 3ar (Table 2). Arylnickel 6 was synthesized in analogy to our published method18 and characterized by 1H NMR and elemental analysis. Stoichiometric reactions of 6 with alkynoate 1a formed expected product 3ar under a variety of conditions in less than one hour (entries 2–5). Intermediate reduction of 6 is not required to form product because reactions run both with and without added Mn0 provided similar yields of 3ar (entries 2–5). Finally, 6 was a competent precatalyst, with similar intial rates to a reaction with a pre-formed catalyst (entries 1 and 6), although the final yield with 6 was slightly lower.
A proposed catalytic cycle, accounting for both product and byproduct formation, is shown in Scheme 4. The nickel(II) pre-catalyst is reduced to nickel(0) before sequential oxidative addition and migratory insertion. Protolysis liberates the product, completing the catalytic cycle.
In conclusion, a simple, nickel-catalyzed method for the synthesis of β-aryl alkenoates from alkynoates and aryl iodides has been developed. This method has broad functional-group compatibility, does not use a large excess of either reagent, and reactions can be set up on the benchtop with unpurified methanol.
Supplementary Material
Acknowledgments
This work was supported by the NIH (R01 GM097243). We are grateful for summer research support from the University of Rochester (REK and AKO Research and Innovation Grant) and the NSF REU program (REK and AKO, CHE-1156340 and CHE-0849892). We thank Adam Lee (University of Rochester) for the synthesis of aryliodides and Jill Caputo (University of Rochester) for the synthesis of NiCl2(dme).
Footnotes
Detailed experimental procedures, data on optimization and deuteration experiments, product characterization, and copies of NMR spectra are available online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Selected examples from recent syntheses of biologically active target molecules: Sasmal S, et al. Bioorg Med Chem Lett. 2012;22:3163. doi: 10.1016/j.bmcl.2012.03.049.Sashidhara KV, et al. Bioorg Med Chem Lett. 2012;22:3926. doi: 10.1016/j.bmcl.2012.04.100.Sridhar Reddy M, Thirupathi N, Babu MH. Eur J Org Chem. 2012;29:5803.Cushman M, et al. J Med Chem. 1998;41:2076. doi: 10.1021/jm9800595.Chen Z, et al. WO2011/68821 A1 2011Kumar TB, et al. Bioorg Med Chem Lett. 2012;22:5639. doi: 10.1016/j.bmcl.2012.06.100.
- 2.Wittig G. Chem Berichte. 1954;87:1318. [Google Scholar]; Maryanoff BE, Reitz AB. Chem Rev. 1989;89:863. [Google Scholar]; Schlosser M, Christmann KF. Angew Chem Int Ed. 1966;5:126. [Google Scholar]; Takeda T, editor. Modern Carbonyl Olefination. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2004. [Google Scholar]
- 3.With Ar-B, Simard-Mercier J, Jiang JL, Ho ML, Flynn AB, Ogilvie WW. J Org Chem. 2008;73:5899. doi: 10.1021/jo8008325.With Ar-Mg: Nishikado H, Nakatsuji H, Ueno K, Nagase R, Tanabe Y. Synlett. 2010:2087.Nakatsuji H, Ueno K, Misaka T, Tanabe Y. Org Lett. 2008;10:2131. doi: 10.1021/ol800480d.With Ar-Sn: Farina V, Krishnan B, Marshall DR, Roth GP. J Org Chem. 1993;58:5434.Rossi R, Bellina F, Carpita A, Mazzarella F. Tetrahedron. 1996;52:4095.With ArMgBr: Normant JF, Alexakis A. Synthesis. 1981:841.With R2CuLi: Corey EJ, Katzenellenbogen JA. J Am Chem Soc. 1969;91:1851.
- 4.Recent review of the palladium-catalyzed elementometallation of alkynes: Hydroboration: Lee J, Kwon J, Yun J. Chem Commun. 2008:733–734. doi: 10.1039/b716697d.Hydrostannylation: Piers E, Chong JM, Morton HE. Tetrahedron. 1989;45:363.Thibonnet J, Launay V, Abarbri M, Duchene A, Parrain J. Tetrahedron Lett. 1998;39:4277.
- 5.Gourtler C, Buchwald SL. Chem Eur J. 1999;5:3107. [Google Scholar]; Littke AF, Fu GC. J Am Chem Soc. 2001;123:6989. doi: 10.1021/ja010988c. [DOI] [PubMed] [Google Scholar]; Sustmann R, Hopp P, Holl P. Tetrahedron Lett. 1989;30:689. [Google Scholar]; Jeffery T. J Chem Soc, Chem Commun. 1984:1287. [Google Scholar]
- 6.Chinchilla R, Nájera C. Chem Rev. 2014;114:1783. doi: 10.1021/cr400133p. [DOI] [PubMed] [Google Scholar]
- 7.Reddy MC, Jeganmohan M. Chem Commun. 2013;49:481. doi: 10.1039/c2cc37758f. [DOI] [PubMed] [Google Scholar]
- 8.Using boron: Pd-catalyzed: Oh CH, Jung HH, Kim KS, Kim N. Angew Chem Int Ed. 2003;42:805. doi: 10.1002/anie.200390214.Gupta AK, Kim KS, Oh CH. Synlett. 2005:457.Zeng H, Hua R. J Org Chem. 2008;73:558. doi: 10.1021/jo7020554.Bush AG, Jiang JL, Rayne PR, Ogilvie WW. Tetrahedron. 2009;65:8502.Cu-catalyzed: Yamamoto Y, Kirai N, Harada Y. Chem Commun. 2008:2010. doi: 10.1039/b802231c.Co-catalyzed: Lin P-S, Jeganmohan M, Cheng C. Chem Eur J. 2008;14:11296. doi: 10.1002/chem.200801858.Nickel-catalyzed: Shirakawa E, Takahashi G, Tsuchimoto T, Kawakami Y. Chem Commun. 2001:2688. doi: 10.1039/b207185a.
- 9.Using Zn: Co-catalyzed: Murakami H, Yorimitsu H, Oshima K. Org Lett. 2009;11:2373. doi: 10.1021/ol900883j.
- 10.Cu-catalyzed with TMS-X additive: Mueller AJ, Jennings MP. Org Lett. 2007;9:5327. doi: 10.1021/ol702546w.Mueller AJ, Jennings MP. Org Lett. 2010;12:2750. doi: 10.1021/ol100854j.
- 11.Tao W, Silverberg LJ, Rheingold AL, Heck RF. Organometallics. 1989;8:2550. [Google Scholar]
- 12.Cacchi S, Fabrizi G, Marinelli F, Moro L, Pace P. Tetrahedron. 1996;52:10225. [Google Scholar]; Ahlquist M, Fabrizi G, Cacchi S, Norrby P-O. J Am Chem Soc. 2006;128:12785. doi: 10.1021/ja061543x. [DOI] [PubMed] [Google Scholar]; Cacchi S, Fabrizi G, Goggiamani A, Persiani D. Org Lett. 2008;10:1597. doi: 10.1021/ol800266e. [DOI] [PubMed] [Google Scholar]
- 13.Rayabarapu DK, Cheng C-H. J Am Chem Soc. 2002;124:5630. doi: 10.1021/ja017390p. [DOI] [PubMed] [Google Scholar]
- 14.Korivi RP, Cheng C-H. J Org Chem. 2006;71:7079. doi: 10.1021/jo060800d. [DOI] [PubMed] [Google Scholar]
- 15.Reference 13 contains one example in the context of a mechanistic study, but no yield was provided.
- 16.Shrestha R, Weix DJ. Org Lett. 2011;13:2766. doi: 10.1021/ol200881v. [DOI] [PubMed] [Google Scholar]; Shrestha R, Dorn SCM, Weix DJ. J Am Chem Soc. 2013;135:751. doi: 10.1021/ja309176h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng Z, Knochel P. Org Lett. 2011;13:3198. doi: 10.1021/ol201109g. [DOI] [PubMed] [Google Scholar]; Cahiez G, Duplais C, Buendia J. Chem Rev. 2009;109:1434. doi: 10.1021/cr800341a. [DOI] [PubMed] [Google Scholar]
- 18.Biswas S, Weix DJ. J Am Chem Soc. 2013;135:16192. doi: 10.1021/ja407589e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.