Abstract
One new alkaloid, 4-geissoschizine N-oxide methyl ether (1), was isolated from the EtOH extract of the hook-bearing branch of Uncaria rhynchophylla, together with 10 known alkaloids, 3-epi-geissoschizine methyl ether (2) isolated from U. rhynchophylla for the first time, geissoschizine methyl ether (3), 4-hirsuteine N-oxide (4), hirsuteine (5), hirsutine (6), 3α-dihydro-cadambine (7), 3β-isodihydro-cadambine (8), cadambine (9), strictosamide (10), and akuammigine (11). The structures were elucidated by spectroscopic methods including UV, ESI-QTOF MS, NMR, and circular dichroism experiments. Neuroprotective effects of 1–9 were investigated against 3 mM glutamate-induced HT22 cell death. The activity assay showed that 2, 3, 5, and 6 exhibited potent neuroprotective effects against glutamate-induced HT22 cell death. However, only weak neuroprotective activities were observed for 1, 4, 7, 8, and 9.
Keywords: Uncaria rhynchophylla, indole alkaloids, neuroprotection, glutamate, HT22 cells
1. Introduction
Some unique tetracyclic or pentacyclic indole alkaloids, such as hirsutine, and hirsuteine and geissoschizine methyl ether, are the primary constituents of Uncaria sp. responsible for a diversity of effects, including lowering blood pressure, vasodilatation, sedation, and protection against ischemia-induced neuronal damage [1–3]. The hook-bearing branch of Uncaria rhynchophylla (Miq.) Miq. ex Havil. is used as a traditional Chinese medicine in China for the treatment of hypertension, headache, and stroke [4]. In our previous studies, 15 oxindole and indole alkaloids were isolated from the herb, some of which exhibited potent inhibitory activity on lipopolysaccharide-induced nitric oxide (NO) release in microglia [5–7]. The present study describes the isolation and structure elucidation of one new compound 1, together with 10 known compounds 2–11, and the neuroprotection of 1–9 against 3 mM glutamate-induced HT22 cell death.
2. Results and discussion
The EtOH extract of the hook-bearing branch of U. rhynchophylla was prepared by percolation. Eleven indole alkaloids were isolated from the CHCl3-soluble fraction. The structures of 1–4 were elucidated by 1D and 2D NMR, UV, MS, and circular dichroism (CD) techniques, and 5–11 were identified by comparing their 1H NMR, UV, MS, and CD data with reported values [8–10]. Compound 1 was confirmed as a new alkaloid, 4-geissoschizine N-oxide methyl ether (1), together with the following 10 known alkaloids 3-epi-geissoschizine methyl ether (2) isolated from U. rhynchophylla for the first time [11], geissoschizine methyl ether (3), 4-hirsuteine N-oxide (4), hirsuteine (5), hirsutine (6), 3α-dihydro-cadambine (7), 3β-isodihydro-cadambine (8), cadambine (9), strictosamide (10), and akuammigine (11). The neuroprotection of 1–9 against glutamate-induced HT22 cell death was assayed for the first time. The neuroprotective activities in the HT22 cell assay of 2, 3, 5, and 6 were confirmed. However, 1, 4, 7, 8, and 9 showed weak activities at the maximum concentration (100 μM).
Compound 1 was isolated as a hygroscopic yellow powder. The molecular formula was determined to be C22H26N2O4 from the [M + H]+ quasi molecular ion peak at m/z 383.1974 in the ESI-QTOF-MS. This implies that it has 11 degrees of unsaturation. 1H NMR spectrum gave a group of o-substituted benzene ring proton signals (δH 7.22, 1H, d, J = 7.2 Hz; δH 6.92, 1H, d, J = 7.2 Hz; δH 6.88, 1H, td, J = 7.2, 1.0 Hz; and δH 6.78, 1H, td, J = 7.2, 1.0 Hz), which were in connection with four aromatic carbon signals (δC 117.6, 111.6, 121.2, and 118.7) in HSQC spectrum, and a proton connected to the nitrogen atom (δH 9.80, 1H, br s). 13C NMR (100MHz, CDCl3) spectrum gave two aromatic quaternary carbon signals (δC 130.2 and 104.8). These data indicated that 1 possesses an indole structure [12]. Moreover, the resonances for the carbons and protons in the 1H and 13C NMR as well as HSQC spectra included four methylenes (δH 3.32, 3.56, δC 61.9; δH 2.74, 3.28, δC 18.0; δH 2.13, 2.41, δC 32.6; and δH 3.85, 4.74, δC 76.3), one methine (δH 4.03, δC 32.4), a methane connected to N atom or O atom (δH 4.27; δC 72.2), two methoxyl groups (δH 3.71, 3H, s, δC 61.7; δH 3.59, 3H, s, δC 51.2), an sp2 methine (δH 7.22, 1H, s; δC 159.5) that shouldbe directly attached to oxygen, an sp2 methine (δH 5.74, 1H, q, J = 6.8 Hz; δC 129.3), a methyl group (δH 1.58, 3H, dd, J = 6.8, 2.0 Hz; δC 13.3), an sp2 quaternary (δC 110.8), and two carbonyl groups (δC 130.2 and 167.6). HMBC spectrum gave some correlations that were determined between H-6 and C-2, C-3, C-5, and C-7, between H-3 and C-2 and C-7, between H-21 and C-3, C-15, C-19, and C-20, and between H-15 and C-20, respectively. Its NMR data (Table 1) suggested that its tetracyclic framework was similar to that of geissoschizine methyl ether [13], except for those of C-3, C-5, and C-21 as well as related protons. The downfield shifts of C-3 (δC 72.2, + 13.5 ppm), C-5 (δC 61.9, + 10.3 ppm), and C-21 (δC 76.3, + 11.8 ppm), relative to the corresponding signals of geissoschizine methyl ether, revealed the formation of an N-4 oxide. In addition, the constitutions and locations of two chain substituents being attached to the cyclic framework separately at C-15 and C-20 were the same to those of geissoschizine methyl ether, as evidenced by the substantial 2D NMR spectrum. The absolute configuration of 1 was determined from the CD spectrum and 2D NOESY correlation. As a tetracyclic indole alkaloid, its 3α-orientation was established according to a positive Cotton effect at 270 nm [14,15], as evidenced by the substantial NOE correlation between H-3 and aromatic H-9. The strong NOE correlations between α-H-3 at δH 4.27 and α-H-14 and between α-H-14 and H-15 indicated that both α-H-14 and H-15 were cofacial with α-H-3. Therefore, 1 possesses 3S, 15S absolute configurations, which are consistent with geissoschizine methyl ether. Meanwhile, the NOE correlations between H-15 and H-18 suggested that C-20 connected with E-ethylidene side chain. The geometry of the trans C-16–C-17 double bond was confirmed on the basis of the olefinic proton in a downfield shift relative to the corresponding signals of the cis compounds. Compound 1 was determined to be 4-geissoschizine N-oxide methyl ether.
Table 1.
1H and 13C NMR spectral data of 1 and 2.
| 1
|
2
|
|||
|---|---|---|---|---|
| No. | δHa (J in Hz) | δCb mult. | δHa (J in Hz) | δCb mult. |
| 2 | 130.2, qC | 134.6, qC | ||
| 3 | 4.27, d (10.6) | 72.2, CH | 3.65, d (10.2) | 55.8, CH |
| 5 | 3.56, dd, β (11.0, 5.5) 3.32, ddd, α (11.0, 4.8) |
61.9, CH2 | 3.08–3.12, m, β 2.75–2.83, m, α |
52.6, CH2 |
| 6 | 3.28, o, α 2.74, br d, β (15.0) |
18.0, CH2 | 2.98–3.01, m, α 2.75–2.83, m, β |
20.8, CH2 |
| 7 | 104.8, qC | 106.8, qC | ||
| 8 | 126.0, qC | 126.3, qC | ||
| 9 | 7.22, d (7.2) | 117.6, CH | 7.46, d (7.8) | 118.3, CH |
| 10 | 6.78, td (7.2, 1.0) | 118.7, CH | 7.07, t (7.2) | 118.7, CH |
| 11 | 6.88, td (7.2, 1.0) | 121.2, CH | 7.12, td (7.2, 1.0) | 121.1, CH |
| 12 | 6.92, d (7.2) | 111.6, CH | 6.92, d (7.8) | 111.3, CH |
| 13 | 137.0, qC | 137.0, qC | ||
| 14 | 2.41, br d, β (13.4) 2.13, ddd, α (13.4, 12.0, 7.2) |
32.6, CH2 | 2.35, dd, β (25.2, 12.6) 1.94, d, α (10.8) |
34.9, CH2 |
| 15 | 4.03, d (7.3) | 32.4, CH | 3.77, d (7.3) | 32.2, CH |
| 16 | 110.8, qC | 110.8, qC | ||
| 17 | 7.22, s | 159.5, CH | 7.35, s | 159.1, CH |
| 18 | 1.58, dd (6.8, 2.0) | 13.3, CH3 | 1.54, d (6.6) | 12.8, CH3 |
| 19 | 5.74, q (6.8) | 129.3, CH | 5.43–5.49, m | 123.2, CH |
| 20 | 129.8, qC | 134.8, qC | ||
| 21 | 4.74, d, β (12.7) 3.85, d, α (12.8) |
76.3, CH2 | 3.52, d, β (12.0) 3.26, d, α (9.2) |
64.5, CH2 |
| 22 | 167.6, qC | 168.6, qC | ||
| 17-OCH3 | 3.71, s | 61.7, CH3 | 3.72, s | 61.7, CH3 |
| 22-OCH3 | 3.59, s | 51.2, CH3 | 3.84, s | 51.4, CH3 |
| NH | 9.80, br s | 10.02, br s | ||
Note: o, overlapped.
Measured separately at 400 MHz in CDCl3.
Measured separately at 100 MHz in CDCl3.
Glutamate is a mammalian central nervous system neurotransmitter in normal neuronal cells. However, high concentrations of glutamate produce oxidative stress, resulting in neurodegeneration such as in stroke, trauma, Alzheimer’s disease, and Parkinson’s disease [16–18]. HT22 cells, an immortalized mouse hippocampal cell line, have been widely used in recent years as an in vitro model for studying the mechanism of glutamate-induced neurotoxicity [19,20]. Neuroprotective effects of 1–9 against 3 mM glutamate-induced HT22 cell death were investigated for the first time (Figures 1–4). The activity assay showed that 2, 3, 5, and 6 exhibited potent neuroprotective effects against glutamate-induced HT22 cell death. However, weak neuroprotective activities were observed for 1, 4, 7, 8, and 9. In a parallel experiment, 2-(1-adamantyl)-4-methylestrone (ZYC-26), an estrogen-like compound, significantly possesses a neuroprotective against glutamate-induced cell death [21]. Compounds 1 and 4 are N-4-oxide derivative of geissoschizine methyl ether and hirsuteine that are major bioactive alkaloids of Uncaria sp., respectively. Geissoschizine methyl ether and hirsuteine potently inhibited glutamate-induced HT22 cell death. These results suggested that N-4-oxide formation caused the loss of the inhibitory activity of indole alkaloids.
Figure 1.
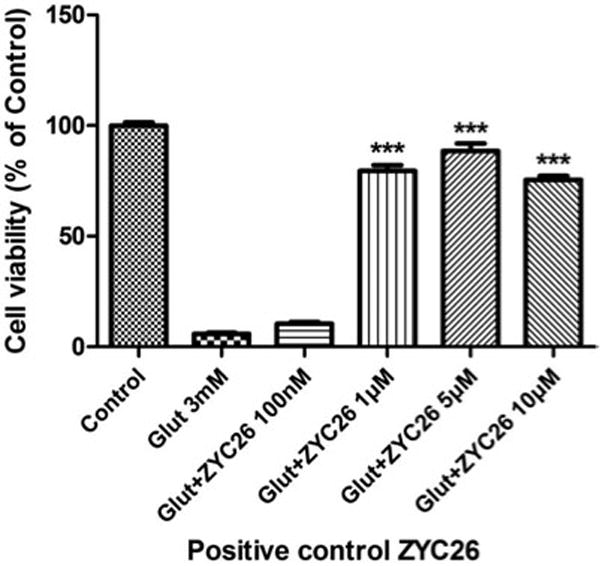
Effects of ZYC-26 against 3 mM glutamate-induced HT22 cell death.
Figure 4.
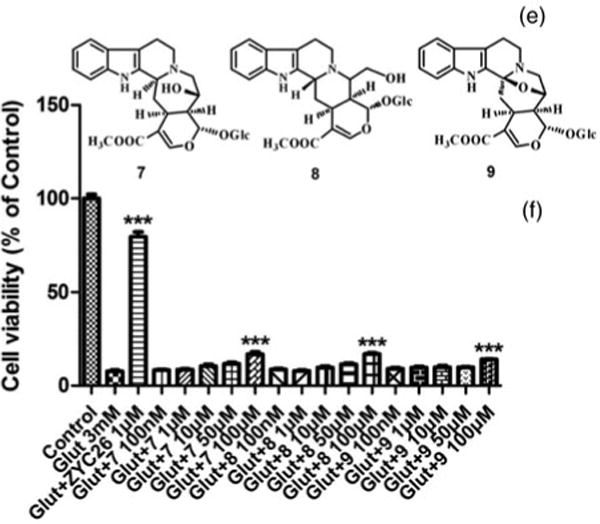
Structures of 7, 8, and 9 (E), and their effects against 3 mM glutamate-induced HT22 cell death (F). (1) HT22 cell treated simultaneously with 3 mM glutamate. (2) Cell viability was determined by calcein AM assay after 24 h exposure to the various samples. (3) All data were normalized to percentage survival of control. (4) Data are represented as mean ± SEM for n = 8. ***p < 0.001 versus glutamate-treated group alone.
3. Experimental
3.1 General experimental procedures
UV spectra were obtained using a Shimadzu UV-2201 spectrophotometer (Shimadzu Co. Ltd, Kyoto, Japan). IR spectra were measured on a Bruker IFS-55 infrared spectrometer (Bruker, Karlsruhe, Germany). CD spectra were recorded on a Bio-Logic Modular Optical System 450 (MOS-450; Bio-Logic, Grenoble, France). NMR spectra were measured on a Bruker ARX-600 and Bruker AVANCE III 400 MHz spectrometer (Bruker, Flländen, Switzerland), and chemical shifts are given in ppm downfield relative to tetramethylsilane as the internal standard. All compounds were dissolved in CDCl3. Quadrupole time-of-flight tandem mass spectrometry (Xevo G2 Qtof; Waters MS Technologies, Manchester, UK) was used to acquire both the exact molecular weight and the product ion spectra of any novel compounds. Parameters for analysis were set by using full-scan positive ion mode with spectra acquired over a mass range from m/z 100 to 1000. The ESI source was set to the following conditions: cone gas (N2) flow rate, 50 L h−1; desolution gas (N2) flow rate, 800 L h−1; source temperature, 100°C; desolution temperature, 400°C; and capillary voltage, 2.0 kV. Macroporous resin AB-8 was obtained from Nankai University Chemical Factory (Tianjin, China); Sephadex LH-20 was from GE Healthcare (Uppsala, Sweden); ODS was obtained from YMC Co., Ltd (Kyoto, Japan); and Silica gel GF254 for thin-layer chromatography was from Qingdao Ocean Chemical Co. (Qingdao, China). Other chemical reagents were of analytical or HPLC grade obtained from Shandong Yuwang Industrial Co., Ltd (Yucheng, China). Double-distilled water from Jinzhou Scientific Instrument Co., Ltd (Tianjin, China) was used in this study.
3.2 Plant material
The hook-bearing branches of U. rhynchophylla were collected in October 2011 in Shaming City, Fujian Province, China. A voucher specimen (No. 20111225) is maintained in the Department of Traditional Chinese Medicines, Shenyang Pharmaceutical University. Species identification was confirmed by Professor Zerong Jiang, Shenyang Pharmaceutical University.
3.3 Extraction and isolation
The air-dried hook-bearing branches (2.5 kg) of U. rhynchophylla were percolated with 75% aqueous EtOH (251). The EtOH extract (670g) was partitioned between n-hexane (3 × 41) and 5% aqueous EtOH (11), and the aqueous phase was further extracted with CHCl3 (3 × 41). The dried CHCl3-soluble fraction was subjected to Si gel column chromatograph (10 × 150 cm, 200–300 mesh; Qingdao Ocean Chemical Co.) eluting with a CHCl3–MeOH gradient. One gram of the fraction eluted with CHCl3–MeOH (20:1) (total 17.3g) was passed through a Sephadex LH-20 column (3.5 cm × 60 cm; GE Healthcare) and eluted with CHCl3−MeOH (50:50), and the eluates were grouped on the basis of TLC analysis into four major fractions (F1–F4). Fraction F3 (1.3 g) was further separated by open column chromatography using an MDS-5 reversed-phase packing (3.5 × 30cm, 200–300 mesh; Bejing Medicine Technology Center, Bejing, China) and eluting with a gradient of MeOH−H2O (50:50 to 100:0) to yield four major fractions (F3-1, F3-2, F3-3, and F3-4). Fraction F3-3 (0.9 g) was further subjected to ODS open column chromatography (3.0 cm cm × 30 cm, 50mm; YMC Co., Ltd) eluting with MeOH−H2O (50:50), and finally, preparative HPLC (Shim-pack PRC-ODS, 2.0 cm × 30 cm, Shimadzu Co. Ltd) was carried out using MeOH−H2O (40:60) plus 0.04% Et2NH as an eluent at a flow rate of 2 ml/min to afford 1 (32 mg, tR 11.7 min) and 2 (6 mg, tR 29.8 min). Fraction F2 (1.4 g) was subjected to ODS open column chromatography (3.0 × 30 cm) eluting with MeOH−H2O (60:40) and, finally, to preparative HPLC using MeOH−H2O (65:35) plus 0.04% Et2NH as an eluent at a flow rate of 12 ml/min to give 3 (24 mg, tR 28.1 min), 4 (7 mg, tR 14.3 min), 5 (15 mg, tR 29.1 min), and 6 (36.4 mg, tR 32.6 min).
One gram of the fraction eluted with CHCl3−MeOH (10:1) (total 2.9g) was subjected to MDS-5 open column chromatography (3.5 cm × 40 cm) eluting with a gradient of MeOH−H2O (50:50 to 100:0). The fraction eluted with MeOH−H2O (70:30) was purified for further two times using MDS-5 open column chromatography to give 7 (17.5 mg, tR 15.5 min), 8 (6 mg, tR 8.2 min), 9 (17.7 mg, tR 9.6 min), 10 (9.6 mg, tR 11.3 min), and 11 (6mg, tR 30.2 min).
3.3.1 4-Geissoschizine N-oxide methyl ether (1)
Yellow amorphous powder; (c = 1.0, MeOH); UV (MeOH) λmax: 221 nm. IR (KBr) νmax (cm−1): 2800, 2730. CD (MeOH): Δε205nm +0.879, Δε218nm −1.182, Δε235nm +0.515, Δε250nm +0.303 and Δε270nm +0.364; for 1H and 13C NMR spectral data, see Table 1; ESI-QTOF-MS: m/z 383.1974 [M + H]+ (calcd for C22H27N2O4, 383.1971).
3.4 Cell culture
HT-22 cells were cultured in Dulbecco’s minimum essential medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Atlanta Biological, Lawrenceville, GA, USA) at 37°C in an atmosphere containing 5% CO2 and 95% air [20]. HT-22 cells were obtained from David Schubert (Salk Institute, San Diego, CA, USA).
3.5 Cell viability assay
Cell viability was determined in HT-22 cells (gift from Dr David Schuber, Salk Institute) by the calcein AM viability assay [20]. In brief, HT-22 cells were seeded 24 h before the initiation of the experiment at a density of 5000 cells per well in 96-well plates and then treated with various test sample solutions with 3mM glutamate which induced 50–75% cell death for 24 h. After exposure to various treatment paradigms, cells were rinsed with PBS, and cell viability was measured using the membrane-permeant calcein-AM dye (Molecular Probes, Eugene, OR, USA). Calcein-AM is a fluorogenic esterase substrate that is hydrolyzed to a fluorescent product in cells having esterase activity and intact membranes. Cells were incubated in a solution of 1μM calcein-AM in PBS at 37°C in dark. Twenty minutes later, fluorescence was determined using a Bio-Tek FL600 microplate reader (Winooski, VT, USA) with an excitation/emission filter set of 485/530 nm. The results, obtained in relative fluorescent units, are expressed as the percentage of untreated control values.
Figure 2.
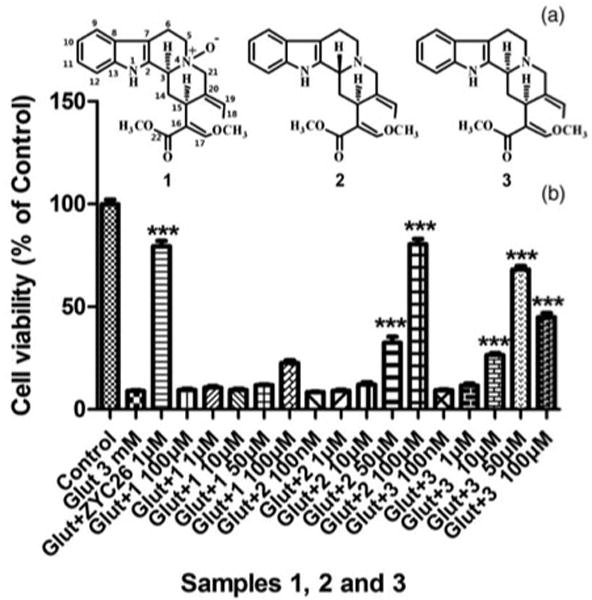
Structures of 1, 2, and 3 (A), and their effects against 3 mM glutamate-induced HT22 cell death (B).
Figure 3.
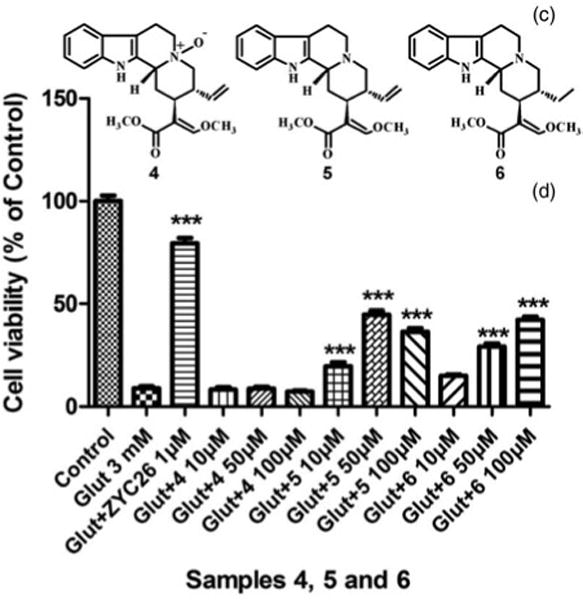
Structures of 4, 5, and 6 (C), and their effects against 3 mM glutamate-induced HT22 cell death (D).
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No. 81173544), the Distinguished Professor Foundation of Liaoning Province of China of 2011, and the Specialized Research Fund for the Doctoral Program of Higher Education of China (20112134120010).
References
- 1.Yuzurihara M, Ikarashi Y, Goto K, Sakakibara I, Hayakawa T, Sasaki H. Eur J Pharmacol. 2002;444:183. doi: 10.1016/s0014-2999(02)01623-0. [DOI] [PubMed] [Google Scholar]
- 2.Sakakibara I, Terabayashi S, Kubo M, Higuchi M, Komatsu Y, Okada M. Phytomedicine. 1999;6:163. doi: 10.1016/S0944-7113(99)80004-X. [DOI] [PubMed] [Google Scholar]
- 3.Shimada Y, Goto H, Itoh T, Sakakibara I, Kubo M, Sasaki H, Terasawa K. J Pharm Pharmacol. 1999;51:715. doi: 10.1211/0022357991772853. [DOI] [PubMed] [Google Scholar]
- 4.The Health Bureau of Guangxi Autonomous Region Revolutionary Committee. Guangxi Materia Medica Collection. Vol. 1. Guangxi People’s Press; Kunming: 1974. p. 886. [Google Scholar]
- 5.Yuan D, Ma B, Wu CF, Yang JY, Zhang LJ, Liu SK, Wu LJ. J Nat Prod. 2008;71:1271. doi: 10.1021/np8000305. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Yuan D, Ma B, Xie YY, Yin J, Kano Y. Chin J Med Chem. 2006;16:369. [Google Scholar]
- 7.Ma B, Wu CF, Yang JY, Wang R, Kano Y, Yuan D. Helv Chim Acta. 2009;92:1575. [Google Scholar]
- 8.Xin WB, Yu XG, Wang TZ. Chin Tradit Herb Drugs. 2009;40:204. [Google Scholar]
- 9.Lounasmaa M, Kan SK. Tetrahedron. 1980;36:1607. [Google Scholar]
- 10.Handbook of Analytical Chemistry. Vol. 7. Chemical Industry Press; Beijing: 1999. p. 901. [Google Scholar]
- 11.Matsuo H, Okamoto R, Zaima K, Hirasawa Y, Ismail IS, Lajis NH, Morita H. Bioorg Med Chem. 2011;19:4075. doi: 10.1016/j.bmc.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Aimi N, Yamanaka E, Shinma N, Fujiu M, Kurita J, Sakai S, Haginiwa J. Chem Pharm Bull. 1977;25:2067. [Google Scholar]
- 13.Takayama H, Watanabe T, Seki H, Aimi N, Sakai S. Tetrahedron Lett. 1992;33:6831. [Google Scholar]
- 14.Brown RT, Blackstock WP. Tetrahedron Lett. 1972;30:3063. [Google Scholar]
- 15.Kohda H, Namera A, Koyama A, Yamazaki K, Tani T. Chem Pharm Bull. 1996;44:352. [Google Scholar]
- 16.Coyle JT, Puttfarcken P. Science. 1993;262:689. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 17.Blandini F, Porter RH, Greenamyre JT. Mol Neurobiol. 1996;12:73. doi: 10.1007/BF02740748. [DOI] [PubMed] [Google Scholar]
- 18.Cherubinia A, Ruggieroa C, Polidorib MC, Mecoccia P. Free Rad Biol Med. 2005;39:841. [Google Scholar]
- 19.Tan S, Wood M, Maher P. J Neurochem. 1998;71:95. doi: 10.1046/j.1471-4159.1998.71010095.x. [DOI] [PubMed] [Google Scholar]
- 20.Yi KD, Chung J, Pang P, Simpkins JW. J Neurosci. 2005;25:7191. doi: 10.1523/JNEUROSCI.1328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpkins JW, Yang SH, Liu R, Perez E, Cai ZY, Covey DF, Green PS. Stroke. 2013 doi: 10.1161/01.STR.0000143734.59507.88. [DOI] [PubMed] [Google Scholar]


