Abstract
Malignant glioma is a relentless burden to both patients and clinicians, and calls for innovation to overcome the limitations in current management. Glioma therapy using viruses has been investigated to accentuate the nature of a virus, killing a host tumor cell during its replication. As virus mediated approaches progress with promising therapeutic advantages, combination therapy with chemotherapy and oncolytic viruses has emerged as a more synergistic and possibly efficacious therapy. Here, we will review malignant glioma as well as prior experience with oncolytic viruses, chemotherapy and combination of the two, examining how the combination can be optimized in the future.
Malignant glioma (MG) is well established as one of the more challenging and deadly diseases in medicine. Glial derived malignancies include anaplastic astrocytoma and glioblastoma multiforme [GBM], WHO grades III & IV, respectively. Together they represent the most common type of primary malignant brain tumors in adults, representing almost 70% of the 22,500 new primary brain tumors in the USA each year [1]. Despite being the focus of intense clinical and translational research, the prognosis for malignant glioma has not changed significantly in the past several decades.
The current standard of care consists of surgery with the goal of establishing the diagnosis and achieving gross total resection when possible, followed by radiotherapy and chemotherapy with temozolomide (TMZ) [2]. Radiation therapy was established in the 1970s and extends survival from 6 to 12 months. TMZ has a clear benefit in the management of GBM, extending survival by approximately 2 months when added to surgery and radiation [3]. It continues to be limited, however, by serious hematological side effects and the prevalence of resistance. Both TMZ and radiation are limited by off-target toxicity, though this has improved with current dosing and technology, respectively. In the end, these three modalities still result in a rather dismal outcome for MG, almost uniformly fatal with an average survival of 14 months [3].
While standard therapy may be nearing its peak efficacy, the addition of new agents and modalities is crucial [4]. Novel therapies are likely to focus on a few key aspects: local delivery of therapeutic doses, optimization of the tumor's immunologic milieu, stimulation of an antitumor immune response and enhanced tumor cell death. Chemotherapeutic advances may provide a window of opportunity for another promising treatment modality: oncolytic viruses.
Oncolytic viral (OV) therapy has been pursued in cancer therapy since it was discovered roughly a century ago, when it was noted that viral illnesses induced remission in patients with leukemia and lymphoma [5,6]. OV therapy has long been an appealing adjuvant approach as it utilizes a live, obligate intra-cellular organism that exploits host machinery and genetic changes unique to cancer cells. OV vectors are able to infect cancer cells specifically, replicate and lyse the cells [7–18]. The resulting viral progeny are then able to spread within the tumor and propagate the effect. In addition to direct tumor killing, viruses are immunogenic, with the potential to stimulate a long-term host response to the tumor. OVs have progressed to clinical trials based on their favorable safety profile and antitumor activity in preclinical studies.
Enthusiasm for the advancement of OV therapy stems from its potential to efficiently kill cancer cells in an immunostimulatory manner. OVs effect multifaceted tumor cytotoxicity. Viral infection and replication induce cellular stress that causes cell lysis [19,20]. OVs are also known to both infect tumor endothelium and inhibit tumor angiogenesis, depleting the tumor's supply of oxygen and nutrients, which results in additional cell death [19,21]. Regardless of mode, cell death as a result of OV therapy is immunogenic via the release of large amounts of tumor associated antigens and other danger signals [19,22–26]. It is this potential for long-term antitumor immunity that continues to encourage new approaches with the hope of achieving clinical efficacy in OV therapy.
Combination therapy with OV and chemotherapy is of intense interest as the effects of chemotherapy optimize the tumor environment for viruses to infect and lyse cells in an immunogenic fashion [27–32]. The two modalities also have a synergistic effect in causing progressive DNA damage and overwhelming cell repair mechanisms. The following is a review of the types of oncolytic viruses and their respective histories in the treatment of glial malignances as well as prior attempts at combination with chemotherapy.
Oncolytic viruses
The most challenging of malignancies continues to be glioma due to its unique features, namely the blood– brain barrier (BBB) and a locally immunosuppressive environment. In spite of these limitations, OVs are able to infect and kill glioma, with evidence that they may enhance the efficacy of current standard therapy [33].
Oncolytic viruses consist of both naturally occurring and genetically modified viral vectors with specificity for and propensity to kill tumor cells [7–18,34]. OVs have emerged as favorable options for glioma therapy because they have tumor and/or neurotropism, and trigger multifaceted responses including oncolysis and oncolysis-mediated stimulation of antitumor immunity, with minimal toxicity. These OVs have been studied and optimized with positive in vitro and in vivo results, advancing to clinical trials [6,10,17–18,35–47].
For current OV mediated glioma therapy, OVs are deployed intratumorally [7–8,48–53]. While intravenous (systemic) injections are able to achieve tumor infection via a non-invasive route, intratumoral injections are more favorable because most OVs are not able to pass the BBB when systemically injected, maximum dose of OVs can be directly delivered into tumor, and direct tumor injection can minimize systemic immune attack against OVs.
The physical and genetic manipulations of viruses have made them more efficient in targeting glioma while limiting toxicity. Altering the surface of viruses with ligands for specific tumor receptors has improved tumor specificity and distribution [10,54]. Genetic modifications of OVs have decreased neurovirulence by making OVs conditionally replicative, only able to replicate in rapidly dividing cancer cells that harbor specific genetic mutations. This prevents the virus from damaging normal neurons and glia while lysing cancer cells and spreading throughout the tumor. Below is a review of viruses that have demonstrated antitumor capabilities in glioma, and are the subject of ongoing research.
Herpes simplex virus type 1 (HSV-1)
HSV-1 is an enveloped, dsDNA virus, a human pathogen causing both active, acute infection and latent reactivation. It has inherent neurotropism and is capable of infecting and replicating in both mitotic and quiescent cells. A large genome (>150 kb) allows for extensive genetic manipulation. HSV-1 can cause encephalitis but is controllable with antiviral agents such as acyclovir via thymidine kinase.
Modified, avirulent HSV-1 vectors have been developed specifically for oncolytic glioma therapy [7–9,55]. The y34.5 gene induces phosphorylation of eIF-2α, which restores protein synthesis in infected cells. Mutation of y34.5 limits infectivity to tumor cells, where altered cell signaling pathways such as Ras lead to continued viral and host protein synthesis. UL39 encodes the large subunit of viral ribonucleotide reductase, limiting replication to proliferating cells. G207 is an HSV-1 vector genetically optimized via mutation of both copies of y34.5, decreasing neurovirulence and inactivation of UL39 via insertion of the lacZ gene [8,55]. Another HSV-1 mutant strain that is more tumor specific, 1716, has deletions of both copies of the gene RL1, which encodes y34.5 [7]. The third significant HSV-1 vector is G47delta [9]. This virus has a deletion of the α47 gene and overlapping US11 promoter region, reversing inhibition of a transporter associated with antigen presentation and increasing MHC-I expression in infected cells [9]. Continued efforts to improve HSV-1 vectors are aimed at genetic manipulation of tumor entry, tumor-specific gene expression, efficient replication and genes for prodrug conversion [10,11].
HSV-1 and its related vectors have shown antitumor efficacy in both in vitro and in vivo studies, providing the basis for clinical trials [6,10,35]. Rampling et al. conducted two Phase I clinical trials with the vector 1716 in patients with recurrent malignant glioma [7,48]. In the first study, 9 patients were treated with intratumoral injection of 105 plaque forming units (PFU). Four patients survived 14–24 months after treatment and no adverse effects, including encephalitis, were attributed to the treatment. The next study involved 12 patients receiving intratumoral injections of 105 PFU of 1716 [7]. Patients underwent tumor resection 4–9 days later. Examination of tumor tissue was indicative of efficient replication, showing viral DNA in excess of the injection dose in two patients, viral DNA by PCR at the tumor site in ten patients and viral DNA at distant sites in four patients.
In another Phase I trial, Markert et al. treated malignant glioma patients with G207. This trial recruited 21 patients for an escalating dose from 106 PFU at a single inoculation site to 3 × 109 PFU at five different sites [8]. Four out of 21 patients were alive at a mean of 12.8 months after inoculation with no incidence of encephalitis. Four patients underwent resection and two of the tumors were positive for both HSV-1 and lacZ sequences by PCR. A follow-up Phase Ib trial after these results evaluated the safety of multiple injections, including into surrounding brain tissue [49]. Patients received two injections, which were intratumoral followed by resection, and into the tissue surrounding the resection cavity. Again, no cases of encephalitis were found and PCR confirmed infection. Median survival was 6.6 months from initial treatment.
Unfortunately, clinical efficacy of HSV-1 type OVs has not matched the expectations formed based on pre-clinical studies [48,56]. Although the efforts to eliminate neurotoxicity have been successful, they have left the viral vectors at a disadvantage in terms of infectivity, cytotoxicity and replicative ability [57]. Replication is especially hampered by y34.5 deletions, with new efforts to selectively activate this gene via tumor-specific promoters [57]. Additionally, armed HSV-1 vectors have been introduced to attempt to enhance anti-tumor immunity [9]. For example, viruses have been engineered with immunostimulatory genes, such as IL-12, IL-18 and soluble B7–1 [9]. Prior exposure to HSV-1 is fairly prevalent, with an uncertain effect on immune clearance.
Adenovirus
Adenovirus (Ad) was first isolated in attempts to develop cell lines from tonsils and adenoids by Rowe et al. [58]. It is a nonenveloped dsDNA virus, highly immunogenic but with mild human pathogenicity. It was identified as harboring cytopathic effects on human epithelial cells, infecting them via coxsackie and adenovirus receptor (CAR) and causing self-limited respiratory illness. The knob of the fiber protein in the virus' capsid recognizes CAR on human epithelial cells. Due to the high infectivity and efficient lysis of host cells, Ad has been modified to infect various cancers including glioma, which do not express CAR. Modification of the knob domain is the most frequently used, and a switch to RGD enables the virus to bind to integrins on the surface and infect glioma [10]. Nearly all of the Ad vectors to date have a variation that expresses RGD [10,54].
There are several serotypes of Ad; however, Ad sero-type 5 (Ad5) is commonly employed in the literature as it can infect a wide range of cell types [59]. Genetic modification has produced tumor specific, conditionally replicative Ad vectors. The E1 genes of Ad are essential for viral replication, leading to cancer cell lysis. E1A binds to Rb and initiates signaling causing non-dividing cells to enter into the synthesis phase. E1B binds p53, decreasing pro-apoptotic signaling and improving viral replication. Replication of vectors with these deletions is limited to cells lacking normal protein as is frequently the case in glioma [10,11]. MG is heterogenous, though, and does not have ubiquitous deletions of p53 or Rb, therefore sensitivity to different vectors is variable [10,60–61]. E1B is deleted in ONYX-015 and decreases the replicative ability of the vector [10]. Ad-delta24 is a versatile vector based on a 24 bp deletion in the E1A gene [11]. The Ad-delta24 vector has also been modified with several transgenes and RGD [10]. Ad-survivin is a vector with E1 gene expression under control of the survivin promoter. Survivin is an anti-apoptotic protein over-expressed in glioma and associated with poor prognosis [62].
A number of researchers have also developed hybrid Ad vectors to overcome the transient infection ability and immunogenic nature (Ad2 and Ad5) of the Ad vectors by combining various elements from different vectors (Retroviral, AdenoAssociated virus and EBV vectors) [63]. These hybrid Ad vectors include pseudotype vector (Ad5/3-RGD) for CAR independent infection of cells and Ad/Retroviral hybrid vector that exhibits high infectivity and gene integration capability [64,65].
Ad vectors have shown both antitumor efficacy and immunostimulatory effects in preclinical studies [36–40]. Mice with glioma, when injected with delta24-RGD were found to have increased intratumoral cytokine and chemokine production as well as increased infiltration of macrophages, CD4+ and CD8+ tumor-specific lymphocytes [36,38]. These studies provide the basis for prior and ongoing clinical trials with Ad vectors. Chiocca et al. used ONYX-015 in a Phase I dose-escalation study utilizing groups of 6 patients with recurrent malignant glioma [50]. Virus was injected into multiple sites within the cavity after tumor resection. Injections were well-tolerated without significant toxicity [50]. Median time to progression and survival were 46 days and 6.2 months, respectively. These results have led to multiple clinical trials with Ad in glioma [66,67–75].
The use of Ad in MG offers possibilities for future improvement. The intratumoral replication of conditional vectors is not yet adequate for widespread tumor distribution and lysis [76]. Due to the high prevalence of prior exposure and existing immunity to this common virus, Ad is likely best utilized when protected from the immune response. Chemotherapeutics and cell-mediated delivery are two options that merit more study with adenovirus [76,77].
Poliovirus
Poliovirus (Polio) is strictly a human pathogen, a single stranded RNA enterovirus with a protein capsid. The virus causes a self-limited illness in the vast majority of cases. A small percentage of patients develop polio-myelitis, but this devastating complication is now rare due to vaccination practices. The virus enters cells via binding CD-155, expressed on a wide variety of cells including motor neurons and solid tumors including GBM [12]. Due to its tropism, polio has recently garnered attention as a potential oncolytic virotherapy for patients with GBM.
Modifications of wild type polio have produced vectors uniquely suited for trial in glioma [10,12]. Vectors are capable of recognizing and infecting cancer cells via the poliovirus receptor Necl-5 (CD155), an oncofetal cell adhesion molecule widely expressed in GBM [12]. Genetic mutations in the internal ribosome entry sequences limit replication to cancer cells while limiting toxicity to surrounding neurons [10,12]. PVS-RIPO, the primary vector used in studies, has the genome of the live attenuated poliovirus serotype I and contains the internal ribosomal entry site (IRES) of the human rhinovirus type 2 [78].
Preclinical studies have demonstrated that this engineered vector is lethal to glioma cells in culture, and in vivo shows antitumor activity that generates an immunological cell death that recruits the immune system to attack the tumor [78–80]. It does not have dangerous side effects such as neuropathy or poliomyelitis and the virus does not convert back to the virulent wild-type poliovirus [78,80–81]. The ideal safety profile and immunological response generated by poliovirus led to an ongoing Phase I clinical trial. Recurrent GBM patients receive intratumoral infusion of PVS-RIPO via convection-enhanced delivery, and results are yet to be published [80,82].
Paramyxovirus
Newcastle disease virus
Newcastle disease virus (NDV) is an enveloped, linear negative sense avian RNA virus that is rarely pathogenic in humans but only causes mild symptoms. It has limited replication in humans but is very immunogenic, the virulence mediated by the variant of viral fusion protein [13]. NDV has lytic and nonlytic strains, with the lytic strain being used more frequently in cancer therapy. In addition to its oncolytic activity, NDV stimulates peripheral mononuclear cells to produce TNF-α and improves the cytolytic effect of TNF-α on cancer cells [6,83]. NDV utilizes autophagy, the vesicular sequestration of cytoplasmic compartments, to enhance tumor cell infection. Upon infection, NDV stimulates autophagy, which increases viral replication via the inhibition of apoptosis and type I IFN production and signaling [84,85]. NDV is most effective in tumors that overexpress anti-apoptotic factors, which provides the virus with more time to replicate.
The primary NDV strains used in glioma are live-attenuated, replication competent viruses. MTH-68/H is mesogenic (moderate infectivity) with good potential tumor infiltration and infection but a higher likelihood of offsite toxicity [14]. HUJ is lentigenic (low infectivity), causing minimal or no host disease and persisting in tumors. In preclinical studies, NDV was cytotoxic to glioma cell lines in vitro and also induced tumor regression in vivo [41,42]. Furthermore, it has been shown to be safe in malignant glioma in two clinical trials. Csatary et al. conducted a Phase I trial in four patients with recurrent GBM [51]. They used MTH-68/H and reported survival of 5–9 years. A Phase I/II trial using intravenous administration of 1010 IU/injection of NDV-HUJ in recurrent GBM showed safety in all 11 patients. The patients were shown to have anti-NDV hemagglutinin antibodies 1–4 weeks post injection, and were seropositive for virus. However, virus was only found in the tumor of one patient [52].
The major limitations of NDV are limited replication and cytolytic activity in lentigenic strains, but unfortunately neurotoxicity precludes the use of more virulent forms in patients [10]. Additionally, NDV is inherently sensitive to type I IFN, which induces the antiviral immune response and clearance of the virus. NDV is also highly prevalent, and prior exposure and sensitization could reduce the therapeutic efficacy of the virus administration.
Measles
Measles is an enveloped, nonsegmented, negative sense RNA virus that is commonly pathogenic in humans, mostly causing a self-limited respiratory illness with rare instances of subacute sclerosing panencephalitis. It has emerged more recently than most other viruses as a therapeutic option in malignant glioma. Measles virus has a specific cell surface receptor, CD46, that is upregulated in many glioma cell lines and allows the virus to target glioma [10].
One study found measles viruses to be effective against glioma cell lines and orthotopic xenografts in mice and the effect was augmented by radiotherapy [43]. Also, Allen et al. recently found measles virus to have antitumor activity against glioma stem cells (GSCs), both in vitro and in vivo [15]. In preclinical studies, measles virus has proven to be safe, with no evidence of neurotoxicity in a primate model [86]. The preclinical efficacy of measles virus against glioma sparked a Phase I trial in 2006, but no results have been published to date [87]. A concern facing therapeutic measles virus administration is the presence of antibodies in as much as 90% of the population, due to infection and current vaccination practices [86].
Pox viruses
Vaccinia
Vaccinia is an enveloped, lytic dsDNA virus with a linear genome and large transgene capacity (28 kb) that is nonpathogenic in humans. The wild-type virus does not have specific tropism but is able to infect a variety of cell types. Vaccinia is relatively safe and easily controlled with cidofovir or vaccinia immunoglobulin [88].
The currently used Vaccinia vectors all have one common feature, a double deletion of the tk gene [14,89,90]. vvDD is vaccinia growth factor deficient, acting in concert with tk deletion to limit viral infection to tumor cells [16]. JX-594 is a vector expressing GMCSF to enhance the induced immune response [16]. The GL-ONC1 vector expresses β-galactosidase and β-glucuronidase, each of which may play a role in stimulation of the host immune response [16]. Vaccinia has shown oncolytic activity against GBM cell lines, and has resulted in tumor regression and prolonged survival in mice [44,45]. Vaccinia's efficacy and use are limited by three factors: its immunogenicity results in clearance by the immune system, a significant rate of pre-existing immunity and replication cannot be regulated or enhanced via transcriptional targeting.
Myxoma
Myxoma is an enveloped, dsDNA virus endemic to lagomorphs, a non-human pathogen with almost no potential for pre-existing immunity. The lack of human pathogenicity confers a low likelihood of inducing an antiviral immune response as well. It has a large genome with vast therapeutic transgenic potential [91]. A unique feature of myxoma is that it down-regulates MHC I and CD4 expression, thereby decreasing the T-cell response promoting NK cell lysis of glioma cells [92].
Myxoma virus has been tested in combination with rapamycin and TMZ in vitro and in a murine glioma model grown from patient-derived GSCs [46]. Even TMZ-resistant cells showed susceptibility to myxoma infection and killing, and all treatment groups experienced a survival benefit. Not only does myxoma infection inhibit tumor growth, but it may also affect genes in the PI3K-Akt-mTOR signaling pathway, which could result in a synergistic therapeutic effect when combined with chemotherapeutic agents [93]. Studies are not all positive, however, as Zemp et al. demonstrated that myxoma failed to sustain replication or show significant antitumor efficacy in vivo [91].
Parvovirus
Parvovirus is a small, nonenveloped, single stranded DNA virus that has minimal pathogenicity in humans. It has a small genome that limits its potential for gene therapy. Paglino et al. found recently that it may have an advantage in the glioma microenvironment as it did not induce an IFN response [94]. Further, the survival and replication of parvovirus were unaffected by IFNs of the typical antiviral immune response. Parvovirus only replicates in rapidly dividing cells, which provides tumor specificity as well.
In mouse models, parvovirus has not only proven safe but has also demonstrated antitumor activity [17]. Its efficiency in replication and oncolytic activity is improved using LuIII, a specific capsid protein. Grekova et al. showed the induction of an immune response and protection from future glioma challenge after treatment with parvovirus [95]. Parvovirus is currently being studied in a Phase I/IIa clinical trial in recurrent GBM [53]. Two groups of nine patients each will receive either intravenous or intratumoral parvovirus, followed by resection and viral injection into the cavity. No results have been published from this study to date.
Reovirus
Reovirus (respiratory enteric orphan) is a double stranded RNA virus isolated from gastrointestinal and respiratory tract in humans. It does not cause any disease in adult humans and therefore is an attractive candidate for oncolytic virotherapy. Reovirus is a natural oncolytic dependent on activated Ras and its downstream effectors for tumor lysis activity, which also spares normal cells [18,34]. Although Ras signaling in cancer cells affects several events of the Reovirus replication cycle, the infectivity of Reovirus is a determining factor of oncolytic potential. Specifically the Ras/RalGEF/p38 axis regulates the infectivity of Reovirus, as the expression of dominant negative RalA or a p38 inhibitor renders the cancer cells (Ras activated cells which are permissive) nonpermissive to Reovirus infection [34,96].
Reovirus infection leads to oncolysis in 20 out of 24 different patient-derived GBM cell lines [18]. Further, intratumoral injection of Reovirus resulted in significant improvement in survival in U251 and U87 GBM models [18]. The main concerns raised by this study were the risk of encephalitis with live virus and incomplete tumor regression in some of the cell lines. Yang et al., determined the safety and efficacy of replication competent Reovirus in immunocompetent glioma models including nonhuman primates. The authors found minimal toxicity in non-human primates after injection of high doses of Reovirus [97]. The same group conducted a dose escalation study and found reovirus to be well tolerated in recurrent malignant glioma patients [47].
Limitations
Overall, the efficacy of OVs in glioma is limited by three main factors: insufficient replication/cell lysis, clearance by the innate immune system and lack of a sustained antitumor immune response. Among these limitations, the human immune system is the common thread and most significant hurdle. Upon viral infection, an immune response is triggered, led by the production and release of type I IFNs. IFN's downstream effects result in cell cycle arrest, anti-angiogenic signals, induction of apoptosis, inhibition of protein synthesis and activation of the immune response [6]. Attenuated, tumor specific viruses have a disadvantage in this environment, which limits replication, cell lysis and spread. The genetic enhancements so effective in terms of tropism and limiting toxicity also limit the potency of OVs. The glioma environment is also specifically limiting to the antitumor immune response, one of the more desirable effects of OV therapy. The prevalence of myeloid derived suppressor cells (MDSCs) and Tregs creates an immunosuppressive environment. Tumor and viral antigens released are more likely to induce anergy than a beneficial adaptive immune response.
The hypoxic tumor microenvironment characteristic of MG also limits the replicative ability of viruses, most markedly Ad vectors [98]. To circumvent this Ad vectors have been developed that rely on hypoxia-induced promoter activity for conditional replication [99]. Interestingly, hypoxia may enhance replication of HSV-type vectors via a GADD34-dependent pathway [100]. The mediator of tumor hypoxia, aberrant vasculature, can be a significant limitation of OV therapy as well. Insufficient vascular supply limits tumor delivery of systemically administered virus.
Oncolytic viruses remain an enticing option in glioma therapy because their limitations are modifiable. The combination of OV with current standard of care chemotherapy, as well as exploring new agents, is of recent interest in maximizing the benefits of both for a synergistic approach. Chemotherapeutic agents and their various mechanisms of action offer a unique opportunity to augment the effect of OVs. The mechanisms are, in fact, complementary to the limitations present in OVs and combination therapy may be the key to optimizing both agents.
Combination therapy: oncolytic viruses & chemotherapy
While chemotherapy is a mainstay of current therapy for MG, its greatest utility going forward may be in combination with OVs. Chemotherapeutic drugs, despite their shortcomings in monotherapy, address the barriers to effective OV therapy. Many chemotherapeutics induce cellular and DNA damage that synergize with the effect of OVs, arresting the cell cycle at multiple points and promoting apoptosis [27]. In this regard, tumor cell death via chemotherapy alone may be less desirable as it often occurs via apoptosis, which can be immunologically silent [101,102]. Other drugs have a distinct effect on the tumor microenvironment, enhancing OV infection and its sequelae. Chemotherapeutic agents at least temporarily suppress innate immunity, which provides a window of opportunity for viral infection, replication and spread [28,29].
In addition to suppressing the innate immune response, chemotherapy depletes Tregs and MDSCs, preventing their recognition of viral and tumor antigens and allowing infiltration of immune effectors. Subsequently, the released tumor and viral antigens are presented to populations of cells capable of adaptive immunity [28–31]. Immune system stimulation by OV infection is further potentiated via induction of inflammatory cytokines and increased tumor immunogenicity after chemotherapy [103]. Tumors treated with chemotherapy are also inherently more sensitive to cytotoxic T lymphocytes via increased expression of M6P receptors on their surface [104]. The cumulative outcome of this combination therapy is enhanced tumor killing with the potential to induce a lasting immune response.
Here we present the agents that have been studied in combination with OV therapy in glioma to date, including agents currently used in MG as well as novel agents and those previously found to be ineffective or toxic. Chemotherapeutic agents in general augment OVs via enhanced cell death, optimization of the tumor and immune environment, or in some cases both. TMZ has a well-established albeit limited benefit as part of standard glioma therapy, but may be the most versatile chemotherapeutic in terms of combination therapy with OVs.
Previous studies
Enhanced cytotoxicity
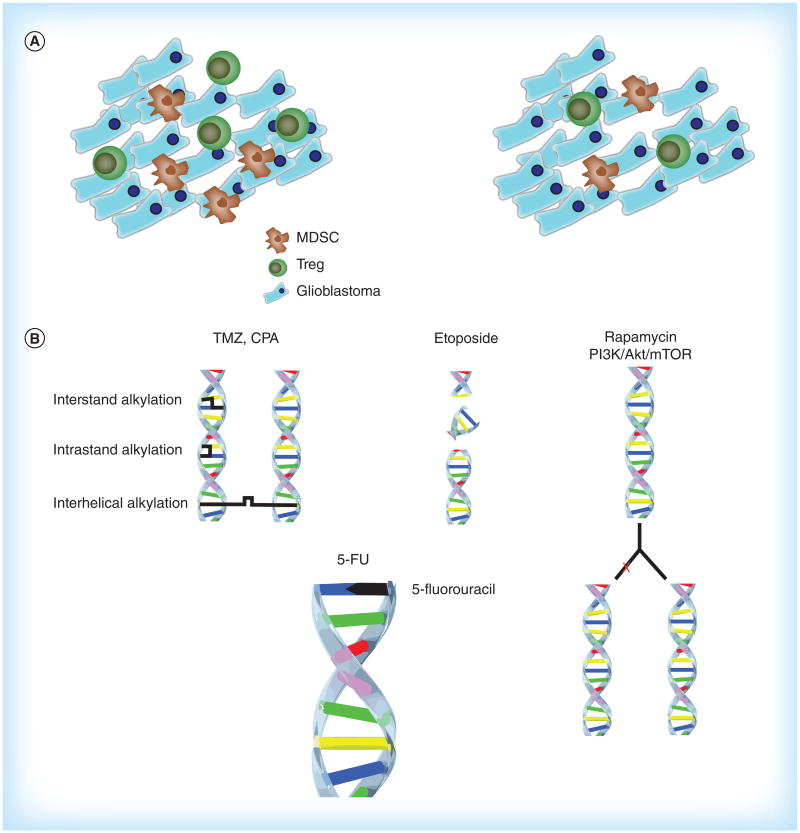
Chemotherapeutic drugs that induce synergistic killing include TMZ, cyclophosphamide (CPA), rapamycin, inhibitors of the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR pathway, cisplatin, etoposide and 5-fluorouracil (5-FU) (Figure 1B). These drugs have myriad mechanisms of altering the behavior of tumor cells, causing cell cycle arrest and/or apoptosis. The mechanisms include DNA alkylation/cross-linking (TMZ, CPA, cisplatin, 5-FU), inhibition of repair of dsDNA breaks (etoposide) and base pair substitution (5-FU) [27,30,32,93]. Glioma utilizes DNA repair mechanisms as a means to escape chemotherapy-mediated cell death. OVs can either activate (HSV) or inhibit (Ad) DNA repair mechanisms to enhance their own replication and cytotoxicity [105]. OVs and chemotherapy collectively overwhelm DNA repair mechanisms, promoting cytotoxicity [106]. The additive stress on the tumor cell either induces apoptosis or leads to viral cell lysis.
Figure 1. Immunological and genetic effects of chemotherapy.
(A) Effects of the chemotherapeutic agents temozolomide, cyclophosphamide, cisplatin and 5-FU on the local tumor environment. Depletion of Tregs and MDSCs allows infiltration of CD4+ and CD8+ effector T cells. (B) Cytotoxic effects of chemotherapeutic agents. TMZ and CPA induce intra- and interstrand DNA cross-links. Etoposide allows for accumulation of double stranded DNA breaks. Rapamycin and other inhibitors of the PI3K/Akt/mTOR pathway decrease DNA replication and cell proliferation. 5-FU inhibits thymidylate synthase and uracil is incorporated into DNA instead of thymidine, inhibiting DNA synthesis.
5-FU: 5-Fluorouracil; CPA: Cyclophosphamide; MDSC: Myeloid derived suppressor cells; mTOR: Mammalian target of rapamycin; PI3K: Phosphatidylinositol 3-kinase; TMZ: Temozolomide.
The PI3K/Akt/mTOR pathway may be mutated in up to 80% of malignant gliomas [107]. These genes are responsible for improved proliferative capacity and survival and include the tumor suppressor PTEN. Akt1 is a serine/threonine-specific protein kinase inhibitor of apoptosis. Small molecule inhibitors of this pathway have been developed including GDC-0941 (PI3K), triciribine (Akt), BEZ235 (mTOR/PI3K) and LY294002 (PI3K). Rapamycin inhibits the mTOR pathway by binding to FKBP12 [108]. Inhibitors of the PI3K/Akt/mTOR pathway used alone have a beneficial effect in glioma therapy by inducing autophagy, restricting tumor growth and improving survival [107]. Combination therapy with these agents enhances tumor cell cytotoxicity [109].
Immunomodulation
TMZ, CPA, rapamycin, cisplatin and 5-FU have beneficial effects on the tumor microenvironment as well, making it more conducive to viral infection, replication, and the induction of an immune response (Figure 1A) [30–31,110–111]. TMZ has been shown to suppress innate immunity and deplete Tregs and MDSCs [28]. TMZ and cisplatin not only deplete Tregs, they decrease the ratio of Tregs to CD4+ T cells and decrease the function of Tregs [30–31,112]. In tumor cells TMZ combined with Ad also induces autophagy, improving immunogenic cell death via cell surface presentation of calreticulin and the release of ATP, and HMGB1 [22,29]. Release of these substances stimulates an inflammatory response and recruits immune effectors.
CPA also selectively reduces Tregs as well as phagocytic cells (NK, macrophages/microglia) in the tumor environment [29]. While it has significant toxicity in therapeutic doses, lower doses can enhance the effect of OV (HSV) therapy as measured by viral replication and animal survival [32,113–114]. This is achieved by decreasing the expression of NK cell effectors IFN-γ, granzyme B and perforin [113,115]. NK cell killing of infected tumor cells is inhibited allowing increased viral replication and spread. Based on the results of animal studies, CPA is being used in clinical trials in multiple myeloma and warrants consideration in glioma [113,115].
Rapamycin is a macrolide immunosuppressant that limits the activation and amplification of B and T cells by limiting their response to IL-2. Rapamycin also decreases infiltration of macrophages/microglia, activates Akt and decreases type I IFN production, increasing the infectivity of OVs [46]. Rapamycin's collective immunosuppressive effects allow increased viral replication [110,116]. 5-FU depletes MDSCs in addition to its cytotoxic effects [111].
A unique approach to this concept going forward is the consideration of drugs not classically considered chemotherapy, in the sense that they do not directly kill tumor cells. Histone deacetylase inhibitors (HDIs) such as valproic acid have garnered some recent attention for their effect on the immune system as it processes viral infections. HDIs decrease expression of IFN-γ and NK cell recruitment and killing of virally infected cells, creating an environment that favors viral infection and propagation [113]. Clodronic acid is another drug in this class that has improved the tumor infectivity of OVs [113]. Importantly, these drugs do not appear to increase the toxicity of OV therapy. These results are not yet definitive, however, as others have found that treatment with HDIs can increase the expression of NK ligands on tumor cells infected with OVs and promote clearance [117].
Armed OVs represent another modification aimed at capitalizing on the optimized tumor environment created by chemotherapeutic agents that deplete Tregs and MDSCs. The use of armed OVs in combination with chemotherapy has drawn recent attention in enhancing the tumor cytotoxic effect and immune response [118]. Many of the vectors currently used have easily modifiable genomes with large transgene capacity [9,88,91]. OVs expressing enzymes, antigens and/or cytokines may have particular utility in combination with chemotherapy. Genes previously studied with OV and found to increase their efficacy via induction of cell death include TRAIL, FasL, Ki-67 and others [119,120]. Expressible cytokines under investigation include IL-2, I IL-4, IL-18, IL-24, TNF-α and GM-CSF [119]. IL-12 is of particular interest because it promotes the cytotoxic activity of NK cells and CD8+ T lymphocytes [121]. Additional downstream effects of IL-12 are to increase IFN-γ release, inhibit angiogenesis and reduce the regulatory T-cell population in the tumor microenvironment [121]. This concept has proven effective in vivo using Ad modified to express chemo inducible TNF-α. Combination with TMZ led to increased apoptosis and survival in mice with U87 tumors [122].
Preclinical results
The concepts of enhanced tumor cell killing and immunologic optimization show promise in recent preclinical testing, supporting further effort to elucidate the ideal regimen and translate it to the clinical setting. Studies to date have largely focused on HSV and Ad, the two viruses used most frequently in glioma up to this point. The efficacy of HSV is impacted positively by TMZ, etoposide and PI3K/Akt/mTOR inhibitors [27,33,109]. In these studies, combination therapy has proven superior to OV therapy alone in regard to glioma cell killing in vitro, viral replication, tumor regression and survival in animal models [27,33,109]. TMZ improves viral infectivity and distribution, while etoposide and PI3K/Akt/mTOR inhibitors augment cytotoxicity. Etoposide does not appear to increase viral replication, but combination therapy is able to target and kill a large population of cells. The virus is most effective before G1 with etoposide targeting cells in the G2/M phase [27].
Ad has a similarly positive interaction with TMZ and cisplatin, both separately and administered together. Both increase glioma cell apoptosis and autophagy, while in vivo combination with TMZ decreases angiogenesis, reduces tumor size and provides a survival benefit [30,123]. Holzmuller et al. found complete tumor regression in 33% of combination treated animals [30]. Cisplatin has yet to be studied in vivo, but it warrants further exploration. Ad expressing cytosine deaminase improves tumor cytotoxicity and survival by producing active 5-FU locally in the tumor, which augments oncolysis and necrosis [124].
Other viruses benefit from combination therapy too, as shown with vaccinia and myxoma. Vaccinia vector JX-594 with rapamycin increases viral replication and survival in mice compared with virus alone [89]. The combination also shows efficacy in vitro, including cultures of brain tumor initiating cells (BTICs), believed to be an important population for resistance and recurrence [125]. Myxoma, when combined with 5-FU, causes tumor stability and in some instances regression. Tumors treated with this regimen were noted to have decreased expression of PI3K, Akt and mTOR [93]. Treatment had a cytostatic effect, allowing for prolonged oncolytic activity.
The current studies point out that combination therapy is additive and often synergistic, but has not realized its full potential. Many questions remain regarding the ideal regimen that will maximize the therapeutic effect. The timing of chemotherapy doses in combination therapy appears to be a critical detail [77]. Administration of an appropriate agent prior to OV suppresses the immune response allowing infection and continued replication, however, no consensus exists on when this effect peaks or for how long to continue chemo-therapy [32]. The subsequent reconstitution of adaptive immunity is of interest both to capitalize on tumor cell death and antigen presentation, and in the possibility of repeat OV dosing requiring suppression of the immune response to virus [114]. On the other hand, OVs can sensitize tumors to chemotherapy, highlighting the need for further study to maximize the combined benefit [126]. Recent studies have attempted to better characterize the ideal regimen to enhance OV infection and generate long-lasting immunity [27,29,32,114]. It bears further study, as even cytostatic chemotherapy appears to enhance OV therapy based on these results.
Additional benefits of combination therapy Clinical studies
One of the most important limitations in chemo-therapy is toxicity, and combination therapy offers an avenue for improvement. In addition to TMZ, some of the agents above are either used in refractory glioma (etoposide), or have been tried in the past but were limited due to toxicity (CPA, cisplatin) [27]. Side effects are often a significant barrier to therapy, ranging from patient discomfort (nausea, vomiting, fatigue) to life-threatening (neutropenia) [28]. Combination therapy may permit lower doses that are better tolerated by patients because of the modification of therapeutic goals [27,124,127]. As opposed to relying fully on chemo-therapy to kill tumor cells and induce an immunologic response, combination therapy is given to modify the tumor environment and synergistically attack the tumor. Metronomic dosing (low, repetitive) is a promising technique used with TMZ and CPA [29,31]. Liikanen et al. utilized this strategy in refractory cancer patients, treating them with oncolytic Ad and low-dose TMZ (100 mg/day) and CPA (50 mg/day) [29]. Treated patients were noted to have cytokine release, viral replication, increased autophagy and circulating specific antibodies. They achieved a survival benefit despite the use of doses of chemotherapy previously considered subtherapeutic [27,29].
Perhaps just as important as reducing the total dose is reducing the systemically active dose, the portion responsible for side effects. Off-target toxicity can be reduced using prodrugs with tumor-specific OVs transfected with drug-activating genes. Active drug is only present at the tumor actively infected with virus, at a therapeutic local concentration and healthy tissues are not exposed to the drug [128]. This was studied using HSV vectors with genes that convert CPA into its active metabolites, and in Ad that activates 5-FU as described above [128,129]. Given the plethora of viruses and drugs available currently, this seems to offer many options going forward.
Persistent limitations
While the enhanced cytotoxicity of combination therapy is beneficial, the immunologic response continues to be the most avidly pursued as it is believed to be the gateway to sustained antitumor efficacy. The immuno-logic effects of chemotherapy are likely of more utility in achieving a long-term response. Several chemotherapeutic agents are already known to suppress Tregs, MDSCs and innate immunity mediated by type I IFN/NK cells long enough for viral replication, lysis and spread throughout the tumor. At least temporary breaches in innate immunity permit viral infection and replication, leading to the release of tumor and viral antigens capable of stimulating an immune response. The caveat to this is that the adaptive immune system needs to remain intact or capable of recovery to then recognize the released viral and tumor antigens to generate long-lasting immunity.
A recently explored complication is that chemotherapy permitting viral infection can indefinitely, and perhaps permanently, suppress the response to released antigens and induction of antitumor immunity [103,119]. Dying tumor cells release tumor associated and viral antigens that are recognized by antigen-presenting cells (APCs) and stimulate expansion of a responsive T cell population. Stimulated T cells begin rapidly dividing to amplify the response while becoming susceptible to the effects of chemotherapy in the process. Alkylating agents, including TMZ and CPA, in particular may inhibit the ability of the host T cells to replicate and mount an effective and sustained immune response to the tumor [103]. This is a crucial area for future study considering the ultimate goal of stimulating an immune response in an inherently suppressive environment.
Conclusion & future perspective
OV therapy remains one of the more promising options in translational research in the treatment of malignant glioma. While clinical efficacy is not yet satisfactory, recent discovery continues to provide future hope. The future of oncolytic viral therapy is almost certainly headed the direction that all prior glioma therapeutics have – combination with others to target a highly evolved and aggressive menace. Viral vectors, while already highly specialized and efficient, will continue to be manipulated to maximize virulence and maintain tumor specificity. The main foci of interest going forward will likely be the continuation of efforts to provide the most favorable tumor microenvironment, enhance immunogenicity and arm the immune system to capitalize.
While regulating the local immune environment is advantageous, it must be done in a way that preserves the host's ability to respond to both virus and tumor. The challenges remaining in this arena are based on preventing premature viral clearance and depleting immunosuppressive cell populations at least temporarily while preserving the crucial adaptive response to both virus and tumor. Further study of the chemotherapeutic agents used to date will be necessary to determine those with a synergistic effect not causing long-term damage to the immune system, negating a crucial aspect of OV therapy [103]. TMZ, while providing a survival benefit to some, may be limiting future progress in regards to immunity. The immune response holds the promise of a prolonged antitumor response, hopefully translated into an elusive boost in survival.
Another important tenet of immunomodulation is the potential toxicity of OVs in an immunocompromised host. While decreased immune surveillance allows more efficient viral replication and delayed clearance, it makes the host more susceptible to off-target infection including encephalitis. Currently used OVs pose little threat in this regard, being therapeutically optimized via selective virulence. However, as new viruses and vectors emerge in oncolytic therapy their human pathogenicity must be considered in the context of induced immunosuppression, and how combination therapy might affect viral replication and toxicity [28,119].
Stimulating the immune system is a goal that can be attacked via methods already in study as well as with the trial of other chemotherapeutic drugs. Viral vectors expressing immunostimulatory cytokines can be regularly engineered and may become more prevalent with continued focus on inducing antitumor immunity [9,29]. Drugs with mainly antitumor activity, such as etoposide and cisplatin, will likely never be efficacious in monotherapy but have desirable effects in lysing tumor cells. The synergistic approach with OVs can help to expose large amounts of foreign antigen to the immune system and is a potential mechanism for overcoming immunosuppression, aberrant antigen processing and lack of costimulatory signals.
In balance with preserving the long-term capabilities of the immune system, adding the capability of repetitive, cyclic doses of OV may be of utility. Given that viruses have passed several clinical trials with satisfactory safety profiles, the justification is present to increase the cumulative dose [140]. This requires limitation of the immunity developed to the virus after each dose, with the perils of chemotherapeutic intervention previously discussed. Alternatives such as viral delivery with neural stem cells and possibly macrophages could be attractive options [130,131]. Combination of slightly different vectors on subsequent doses warrants consideration as well [132].
Biodistribution of virus within the tumor is another aspect that is yet to be maximized. Barriers that are only beginning to be addressed include physical barriers to infection like extra-cellular matrix, necrosis/calcification, tumor size, local hypoxia and insufficient vasculature [119,133]. Recent investigations have begun to characterize the extracellular matrix of malignant glioma. Matrix metalloproteinases are a developing target of interest to remove a barrier to efficient viral distribution and appear to be affected by some agents already in use [134].
Radiation therapy will continue to have a prominent role as well, and its role in augmenting OV therapy has already been investigated. In addition to using a vaccinia virus expressing anti-VEGF, Buckel et al. also found that OV therapy sensitized tumors to radiotherapy [135]. Markert et al. conducted a Phase I clinical trial using HSV G207 with radiation therapy, showing it to be safe while inducing radiographic response [56]. Opyrchal et al. also showed efficacy of radiovirotherapy using a measles virus [43]. Advani et al. showed that viral replication was increased and tumor regression improved in pre-irradiated glioma treated with vaccinia [45].
An important aspect of therapy going forward is to consider the contribution of GSCs as not only brain tumor initiating cells, but as mediators of chemoand radio-resistance [136–139]. By these mechanisms GSCs are believed to have a prominent role in recurrence and spread. This has particular relevance in OV therapy, as some vectors have been shown to have activity specifically against these cells [46,89].
Much possibility remains in the pursuit of more effective therapy for malignant glioma in general. Recent developments and creative approaches will continue to bring OV therapy closer to the realization of its vast potential. Combination with chemo-therapy is but one of these options and one of the more versatile given the large number of drugs and their diverse effector mechanisms.
Key terms.
Malignant glioma
Intracranial malignancies derived from the glial or supporting cells of the brain (astrocytes, microglia). WHO grades III (anaplastic astrocytoma) and IV (glioblastoma multiforme).
Chemotherapy
Class of cytotoxic drugs with specific activity against rapidly proliferating cells, such as cancer cells.
Oncolytic virus
Viral vectors, natural and engineered, capable of infecting tumor cells where they are able to replicate, lyse the cells and spread throughout the tumor.
Synergistic
When a combination of agents with complementary actions produce an effect greater than the expected sum.
Innate immunity
Early, nonspecific host response to pathogens characterized by the release of cytokines and recruitment/activation of effector cells (natural killer cells, macrophages, neutrophils).
Executive summary.
Background
Standard of care in malignant glioma is in need of improvement.
Oncolytic viruses
Oncolytic viruses continue to be a promising option in current and future therapy for malignant glioma.
Oncolytic viruses are safe in clinical practice but lack efficacy.
Combination therapy
Chemotherapeutic agents have great potential for synergistic antitumor activity in combination with oncolytic viruses.
Chemotherapy enhances tumor cytotoxicity in combination with oncolytic viruses.
Chemotherapeutic immunosuppression creates an optimal environment for viral infection and replication.
Future perspective
Continued research must focus on the immune response: optimal viral infection and replication with a strong antitumor adaptive response.
Acknowledgments
This work was supported by the National Cancer Institute (R01CA122930 and R01CA138587) and the National Institutes of Neurological Disorders and Health (U01NS069997, R01NS07388).
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Mcgirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez YP, Weatherbee JL, Wheelhouse RT, Ross AH. Glioblastoma multiforme therapy and mechanisms of resistance. Pharmaceuticals. 2013;6(12):1475–1506. doi: 10.3390/ph6121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 6.Dey M, Auffinger B, Lesniak MS, Ahmed AU. Antiglioma oncolytic virotherapy: unattainable goal or a success story in the making? Future Virol. 2013;8(7):675–693. doi: 10.2217/fvl.13.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papanastassiou V, Rampling R, Fraser M, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9(6):398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 8.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a Phase I trial. Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 9.Todo T. Oncolytic virus therapy using genetically engineered herpes simplex viruses. Front Biosci. 2008;13:2060–2064. doi: 10.2741/2823. [DOI] [PubMed] [Google Scholar]
- 10.Parker JN, Bauer DF, Cody JJ, Markert JM. Oncolytic viral therapy of malignant glioma. Neurotherapeutics. 2009;6(3):558–569. doi: 10.1016/j.nurt.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwalla PK, Barnard ZR, Curry WT., Jr Virally mediated immunotherapy for brain tumors. Neurosurg Clin N Am. 2010;21(1):167–179. doi: 10.1016/j.nec.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Merrill MK, Bernhardt G, Sampson JH, Wikstrand CJ, Bigner DD, Gromeier M. Poliovirus receptor CD155-targeted oncolysis of glioma. Neuro-oncology. 2004;6(3):208–217. doi: 10.1215/S1152851703000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinkovics JG, Horvath JC. Natural and genetically engineered viral agents for oncolysis and gene therapy of human cancers. Arch Immunol Ther Exp (Warsz) 2008;56(Suppl. 1):s3–s59. doi: 10.1007/s00005-008-0047-9. [DOI] [PubMed] [Google Scholar]
- 14.Zemp FJ, Corredor JC, Lun X, Muruve DA, Forsyth PA. Oncolytic viruses as experimental treatments for malignant gliomas: using a scourge to treat a devil. Cytokine Growth Factor Rev. 2010;21(2–3):103–117. doi: 10.1016/j.cytogfr.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Allen C, Opyrchal M, Aderca I, et al. Oncolytic measles virus strains have significant antitumor activity against glioma stem cells. Gene Ther. 2013;20(4):444–449. doi: 10.1038/gt.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lusky M, Erbs P, Foloppe J, Acres RB. Oncolytic vaccinia virus: a silver bullet? Expert Rev Vaccines. 2010;9(12):1353–1356. doi: 10.1586/erv.10.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paglino JC, Ozduman K, Van Den Pol AN. LuIII parvovirus selectively and efficiently targets, replicates in, and kills human glioma cells. J Virol. 2012;86(13):7280–7291. doi: 10.1128/JVI.00227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcox ME, Yang W, Senger D, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93(12):903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 19.Bartlett DL, Liu Z, Sathaiah M, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12(1):103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cary ZD, Willingham MC, Lyles DS. Oncolytic vesicular stomatitis virus induces apoptosis in U87 glioblastoma cells by a type II death receptor mechanism and induces cell death and tumor clearance in vivo. J Virol. 2011;85(12):5708–5717. doi: 10.1128/JVI.02393-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breitbach CJ, Paterson JM, Lemay CG, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15(9):1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- 22•.Guo ZS, Liu Z, Bartlett DL. Oncolytic Immunotherapy: Dying the Right Way is a Key to Eliciting Potent Antitumor Immunity. Front Oncol. 2014;4:74. doi: 10.3389/fonc.2014.00074. Highlights the importance of immunogenic forms of cell death in generating antitumor immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Workenhe ST, Mossman KL. Oncolytic virotherapy and immunogenic cancer cell death: sharpening the sword for improved cancer treatment strategies. Mol Ther. 2014;22(2):251–256. doi: 10.1038/mt.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14(8):559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 25.Aymeric L, Apetoh L, Ghiringhelli F, et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70(3):855–858. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 26.Guillerme JB, Boisgerault N, Roulois D, et al. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin Cancer Res. 2013;19(5):1147–1158. doi: 10.1158/1078-0432.CCR-12-2733. [DOI] [PubMed] [Google Scholar]
- 27.Cheema TA, Kanai R, Kim GW, et al. Enhanced antitumor efficacy of low-dose Etoposide with oncolytic herpes simplex virus in human glioblastoma stem cell xenografts. Clin Cancer Res. 2011;17(23):7383–7393. doi: 10.1158/1078-0432.CCR-11-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liikanen I, Ahtiainen L, Hirvinen ML, et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther. 2013;21(6):1212–1223. doi: 10.1038/mt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holzmuller R, Mantwill K, Haczek C, et al. YB-1 dependent virotherapy in combination with temozolomide as a multimodal therapy approach to eradicate malignant glioma. Int J Cancer. 2011;129(5):1265–1276. doi: 10.1002/ijc.25783. [DOI] [PubMed] [Google Scholar]
- 31.Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immun. 2009;58(10):1627–1634. doi: 10.1007/s00262-009-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young BA, Spencer JF, Ying B, Tollefson AE, Toth K, Wold WS. The role of cyclophosphamide in enhancing antitumor efficacy of an adenovirus oncolytic vector in subcutaneous Syrian hamster tumors. Cancer Gene Ther. 2013;20(9):521–530. doi: 10.1038/cgt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadjipanayis CG, Fellows-Mayle W, Deluca NA. Therapeutic efficacy of a herpes simplex virus with radiation or temozolomide for intracranial glioblastoma after convection-enhanced delivery. Mol Ther. 2008;16(11):1783–1788. doi: 10.1038/mt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong J, Mita MM. Activated ras signaling pathways and reovirus oncolysis: an update on the mechanism of preferential reovirus replication in cancer cells. Front Oncol. 2014;4:167. doi: 10.3389/fonc.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wollmann G, Ozduman K, Van Den Pol AN. Oncolytic virus therapy for glioblastoma multiforme: concepts and candidates. Cancer J. 2012;18(1):69–81. doi: 10.1097/PPO.0b013e31824671c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleijn A, Kloezeman J, Treffers-Westerlaken E, et al. The in vivo therapeutic efficacy of the oncolytic adenovirus Delta24-RGD is mediated by tumor-specific immunity. PLoS ONE. 2014;9(5):e97495. doi: 10.1371/journal.pone.0097495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakashima H, Chiocca EA. Switching a replication-defective adenoviral vector into a replication-competent, oncolytic adenovirus. J Virol. 2014;88(1):345–353. doi: 10.1128/JVI.02668-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang H, Clise-Dwyer K, Ruisaard KE, et al. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS ONE. 2014;9(5):e97407. doi: 10.1371/journal.pone.0097407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Razumov IA, Sviatchenko VA, Protopopova EV, et al. Oncolytic properties of some orthopoxviruses, adenoviruses and parvoviruses in human glioma cells. Vestn Ross Akad Med Nauk. 2013;12:4–8. [PubMed] [Google Scholar]
- 40.Li X, Mao Q, Wang D, Xia H. A novel Ad5/11 chimeric oncolytic adenovirus for improved glioma therapy. Int J Oncol. 2012;41(6):2159–2165. doi: 10.3892/ijo.2012.1674. [DOI] [PubMed] [Google Scholar]
- 41.Zulkifli MM, Ibrahim R, Ali AM, et al. Newcastle diseases virus strain V4UPM displayed oncolytic ability against experimental human malignant glioma. Neurol Res. 2009;31(1):3–10. doi: 10.1179/174313208X325218. [DOI] [PubMed] [Google Scholar]
- 42.Ali R, Alabsi AM, Ali AM, et al. Cytolytic effects and apoptosis induction of Newcastle disease virus strain AF2240 on anaplastic astrocytoma brain tumor cell line. Neurochem Res. 2011;36(11):2051–2062. doi: 10.1007/s11064-011-0529-8. [DOI] [PubMed] [Google Scholar]
- 43.Opyrchal M, Allen C, Iankov I, et al. Effective radiovirotherapy for malignant gliomas by using oncolytic measles virus strains encoding the sodium iodide symporter (MV-NIS) Hum Gene Ther. 2012;23(4):419–427. doi: 10.1089/hum.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duggal R, Geissinger U, Zhang Q, et al. Vaccinia virus expressing bone morphogenetic protein-4 in novel glioblastoma orthotopic models facilitates enhanced tumor regression and long-term survival. J Transl Med. 2013;11(1):155. doi: 10.1186/1479-5876-11-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Advani SJ, Buckel L, Chen NG, et al. Preferential replication of systemically delivered oncolytic vaccinia virus in focally irradiated glioma xenografts. Clin Cancer Res. 2012;18(9):2579–2590. doi: 10.1158/1078-0432.CCR-11-2394. [DOI] [PubMed] [Google Scholar]
- 46.Zemp FJ, Lun X, Mckenzie BA, et al. Treating brain tumor-initiating cells using a combination of myxoma virus and rapamycin. Neuro-oncology. 2013;15(7):904–920. doi: 10.1093/neuonc/not035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forsyth P, Roldan G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16(3):627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 48.Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 49.Markert JM, Liechty PG, Wang W, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17(1):199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 51.Csatary LK, Gosztonyi G, Szeberenyi J, et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neuro-oncol. 2004;67(1–2):83–93. doi: 10.1023/b:neon.0000021735.85511.05. [DOI] [PubMed] [Google Scholar]
- 52.Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006;13(1):221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 53.Geletneky K, Huesing J, Rommelaere J, et al. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer. 2012;12:99. doi: 10.1186/1471-2407-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang H, White EJ, Rios-Vicil CI, Xu J, Gomez-Manzano C, Fueyo J. Human adenovirus type 5 induces cell lysis through autophagy and autophagy-triggered caspase activity. J Virol. 2011;85(10):4720–4729. doi: 10.1128/JVI.02032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aghi M, Rabkin S, Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98(1):38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- 56.Markert JM, Razdan SN, Kuo HC, et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther. 2014;22(5):1048–1055. doi: 10.1038/mt.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanai R, Zaupa C, Sgubin D, et al. Effect of gamma34.5 deletions on oncolytic herpes simplex virus activity in brain tumors. J Virol. 2012;86(8):4420–4431. doi: 10.1128/JVI.00017-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huebner RJ, Rowe WP, Chanock RM. Newly recognized respiratory tract viruses. Annu Rev Microbiol. 1958;12:49–76. doi: 10.1146/annurev.mi.12.100158.000405. [DOI] [PubMed] [Google Scholar]
- 59.Alemany R, Balague C, Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18(7):723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 60.Roos WP, Batista LF, Naumann SC, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26(2):186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 61.Song Y, Zhang Q, Kutlu B, et al. Evolutionary etiology of high-grade astrocytomas. Proc Natl Acad Sci USA. 2013;110(44):17933–17938. doi: 10.1073/pnas.1317026110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lv S, Dai C, Liu Y, et al. The Impact of Survivin on Prognosis and Clinicopathology of Glioma Patients: A Systematic Meta-Analysis. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8823-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 63.Murphy SJ, Chong H, Bell S, Diaz RM, Vile RG. Novel integrating adenoviral/retroviral hybrid vector for gene therapy. Hum Gene Ther. 2002;13(6):745–760. doi: 10.1089/104303402317322302. [DOI] [PubMed] [Google Scholar]
- 64.Tyler MA, Ulasov IV, Borovjagin A, et al. Enhanced transduction of malignant glioma with a double targeted Ad5/3-RGD fiber-modified adenovirus. Mol Cancer Ther. 2006;5(9):2408–2416. doi: 10.1158/1535-7163.MCT-06-0187. [DOI] [PubMed] [Google Scholar]
- 65.Feng M, Jackson WH, Jr, Goldman CK, et al. Stable in vivo gene transduction via a novel adenoviral/retroviral chimeric vector. Nat Biotechnol. 1997;15(9):866–870. doi: 10.1038/nbt0997-866. [DOI] [PubMed] [Google Scholar]
- 66.ClinicalTrials.gov. NCT00870181 (2009).
- 67.ClinicalTrials.gov. NCT00031083 (2002).
- 68.ClinicalTrials.gov. NCT00805376 (2008).
- 69.ClinicalTrials.gov. NCT02197169 (2014).
- 70.ClinicalTrials.gov. NCT00751270 (2008).
- 71.ClinicalTrials.gov. NCT00589875 (2007).
- 72.ClinicalTrials.gov. NCT01582516 (2012).
- 73.ClinicalTrials.gov. NCT01956734 (2013).
- 74.ClinicalTrials.gov. NCT02026271 (2013).
- 75.ClinicalTrials.gov. NCT01811992 (2013).
- 76.Ahmed AU, Thaci B, Tobias AL, et al. A preclinical evaluation of neural stem cell-based cell carrier for targeted antiglioma oncolytic virotherapy. J Natl Cancer Inst. 2013;105(13):968–977. doi: 10.1093/jnci/djt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tobias AL, Thaci B, Auffinger B, et al. The timing of neural stem cell-based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cell Transl Med. 2013;2(9):655–666. doi: 10.5966/sctm.2013-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goetz C, Gromeier M. Preparing an oncolytic poliovirus recombinant for clinical application against glioblastoma multiforme. Cytokine Growth Factor Rev. 2010;21(2–3):197–203. doi: 10.1016/j.cytogfr.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci USA. 2000;97(12):6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown MC, Dobrikova EY, Dobrikov MI, et al. Oncolytic polio virotherapy of cancer. Cancer. 2014;120(21):3277–3286. doi: 10.1002/cncr.28862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dobrikova EY, Broadt T, Poiley-Nelson J, et al. Recombinant oncolytic poliovirus eliminates glioma in vivo without genetic adaptation to a pathogenic phenotype. Mol Ther. 2008;16(11):1865–1872. doi: 10.1038/mt.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.ClinicalTrials.gov. NCT01491893 (2011).
- 83.Lorence RM, Rood PA, Kelley KW. Newcastle disease virus as an antineoplastic agent: induction of tumor necrosis factor-alpha and augmentation of its cytotoxicity. J Natl Cancer Inst. 1988;80(16):1305–1312. doi: 10.1093/jnci/80.16.1305. [DOI] [PubMed] [Google Scholar]
- 84.Mustafa Z, Shamsuddin HS, Ideris A, et al. Viability reduction and Rac1 gene downregulation of heterogeneous ex-vivo glioma acute slice infected by the oncolytic Newcastle disease virus strain V4UPM. BioMed Res Int. 2013;2013:248507. doi: 10.1155/2013/248507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun Y, Yu S, Ding N, et al. Autophagy benefits the replication of Newcastle disease virus in chicken cells and tissues. J Virol. 2014;88(1):525–537. doi: 10.1128/JVI.01849-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Myers R, Harvey M, Kaufmann TJ, et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther. 2008;19(7):690–698. doi: 10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.ClinicalTrials.gov. NCT00390299 (2006).
- 88.Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24(52):7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 89.Lun X, Chan J, Zhou H, et al. Efficacy and safety/toxicity study of recombinant vaccinia virus JX-594 in two immunocompetent animal models of glioma. Mol Ther. 2010;18(11):1927–1936. doi: 10.1038/mt.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mccart JA, Ward JM, Lee J, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61(24):8751–8757. [PubMed] [Google Scholar]
- 91.Zemp FJ, Mckenzie BA, Lun X, et al. Resistance to oncolytic myxoma virus therapy in nf1(-/-)/trp53(-/-) syngeneic mouse glioma models is independent of anti-viral type-I interferon. PLoS ONE. 2013;8(6):e65801. doi: 10.1371/journal.pone.0065801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ogbomo H, Zemp FJ, Lun X, et al. Myxoma virus infection promotes NK lysis of malignant gliomas in vitro and in vivo. PLoS ONE. 2013;8(6):e66825. doi: 10.1371/journal.pone.0066825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang QS, Zhang M, Liang SJ, Lin HZ, Ji T, Li WP. Effects of myxoma virus on gliomas of rats models in vivo. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2012;26(2):105–107. [PubMed] [Google Scholar]
- 94.Paglino JC, Andres W, Van Den Pol AN. Autonomous parvoviruses neither stimulate nor are inhibited by the type I interferon response in human normal or cancer cells. J Virol. 2014;88(9):4932–4942. doi: 10.1128/JVI.03508-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grekova SP, Raykov Z, Zawatzky R, Rommelaere J, Koch U. Activation of a glioma-specific immune response by oncolytic parvovirus Minute Virus of Mice infection. Cancer Gene Ther. 2012;19(7):468–475. doi: 10.1038/cgt.2012.20. [DOI] [PubMed] [Google Scholar]
- 96.Norman KL, Hirasawa K, Yang AD, Shields MA, Lee PW. Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc Natl Acad Sci USA. 2004;101(30):11099–11104. doi: 10.1073/pnas.0404310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang WQ, Lun X, Palmer CA, et al. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: racine and nonhuman primates. Clin Cancer Res. 2004;10(24):8561–8576. doi: 10.1158/1078-0432.CCR-04-0940. [DOI] [PubMed] [Google Scholar]
- 98.Pipiya T, Sauthoff H, Huang YQ, et al. Hypoxia reduces adenoviral replication in cancer cells by downregulation of viral protein expression. Gene Ther. 2005;12(11):911–917. doi: 10.1038/sj.gt.3302459. [DOI] [PubMed] [Google Scholar]
- 99.Post DE, Devi NS, Li Z, et al. Cancer therapy with a replicating oncolytic adenovirus targeting the hypoxic microenvironment of tumors. Clin Cancer Res. 2004;10(24):8603–8612. doi: 10.1158/1078-0432.CCR-04-1432. [DOI] [PubMed] [Google Scholar]
- 100.Aghi MK, Liu TC, Rabkin S, Martuza RL. Hypoxia enhances the replication of oncolytic herpes simplex virus. Mol Ther. 2009;17(1):51–56. doi: 10.1038/mt.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 102.Tesniere A, Panaretakis T, Kepp O, et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15(1):3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- 103••.Litterman AJ, Zellmer DM, Grinnen KL, et al. Profound impairment of adaptive immune responses by alkylating chemotherapy. J Immunol. 2013;190(12):6259–6268. doi: 10.4049/jimmunol.1203539. Examines the responsiveness of the immune system after standard chemotherapy and consider the effect on the antitumor immune response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramakrishnan R, Assudani D, Nagaraj S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120(4):1111–1124. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105•.Jiang H, Alonso MM, Gomez-Manzano C, Piao Y, Fueyo J. Oncolytic viruses and DNA-repair machinery: overcoming chemoresistance of gliomas. Expert Rev Anticancer. 2006;6(11):1585–1592. doi: 10.1586/14737140.6.11.1585. Explores the molecular pathways affected by both oncolytic viruses and chemotherapy, and how they can act in a synergistic fashion. [DOI] [PubMed] [Google Scholar]
- 106.Kanai R, Rabkin SD, Yip S, et al. Oncolytic virus-mediated manipulation of DNA damage responses: synergy with chemotherapy in killing glioblastoma stem cells. J Natl Cancer Inst. 2012;104(1):42–55. doi: 10.1093/jnci/djr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koul D, Shen R, Kim YW, et al. Cellular and in vivo activity of a novel PI3K inhibitor, PX-866, against human glioblastoma. Neuro-oncology. 2010;12(6):559–569. doi: 10.1093/neuonc/nop058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iwamaru A, Kondo Y, Iwado E, et al. Silencing mammalian target of rapamycin signaling by small interfering RNA enhances rapamycin-induced autophagy in malignant glioma cells. Oncogene. 2007;26(13):1840–1851. doi: 10.1038/sj.onc.1209992. [DOI] [PubMed] [Google Scholar]
- 109.Kanai R, Wakimoto H, Martuza RL, Rabkin SD. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells. Clin Cancer Res. 2011;17(11):3686–3696. doi: 10.1158/1078-0432.CCR-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lun XQ, Jang JH, Tang N, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15(8):2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- 111.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 112.Roselli M, Cereda V, Di Bari MG, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology. 2013;2(10):e27025. doi: 10.4161/onci.27025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alvarez-Breckenridge CA, Yu J, Price R, et al. The histone deacetylase inhibitor valproic acid lessens NK cell action against oncolytic virus-infected glioblastoma cells by inhibition of STAT5/T-BET signaling and generation of gamma interferon. J Virol. 2012;86(8):4566–4577. doi: 10.1128/JVI.05545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114•.Peng KW, Myers R, Greenslade A, et al. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther. 2013;20(3):255–261. doi: 10.1038/gt.2012.31. Demonstrates a beneficial effect from combination therapy using clinically relevant dosing schedule. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fulci G, Breymann L, Gianni D, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103(34):12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lun X, Alain T, Zemp FJ, et al. Myxoma virus virotherapy for glioma in immunocompetent animal models: optimizing administration routes and synergy with rapamycin. Cancer Res. 2010;70(2):598–608. doi: 10.1158/0008-5472.CAN-09-1510. [DOI] [PubMed] [Google Scholar]
- 117.Zhang C, Wang Y, Zhou Z, Zhang J, Tian Z. Sodium butyrate upregulates expression of NKG2D ligand MICA/B in HeLa and HepG2 cell lines and increases their susceptibility to NK lysis. Cancer Immunol Immun. 2009;58(8):1275–1285. doi: 10.1007/s00262-008-0645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rainov NG, Heidecke V. Clinical development of experimental virus-mediated gene therapy for malignant glioma. Anticancer Agents Med Chem. 2011;11(8):739–747. doi: 10.2174/187152011797378724. [DOI] [PubMed] [Google Scholar]
- 119•.Nguyen A, Ho L, Wan Y. Chemotherapy and oncolytic virotherapy: advanced tactics in the war against cancer. Front Oncol. 2014;4:145. doi: 10.3389/fonc.2014.00145. Review of the molecular opportunities and potential drawbacks related to combination therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsamis KI, Alexiou GA, Vartholomatos E, Kyritsis AP. Combination treatment for glioblastoma cells with tumor necrosis factor-related apoptosis-inducing ligand and oncolytic adenovirus delta-24. Cancer Invest. 2013;31(9):630–638. doi: 10.3109/07357907.2013.849724. [DOI] [PubMed] [Google Scholar]
- 121.Passer BJ, Cheema T, Wu S, Wu CL, Rabkin SD, Martuza RL. Combination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer models. Cancer Gene Ther. 2013;20(1):17–24. doi: 10.1038/cgt.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamini B, Yu X, Gillespie GY, Kufe DW, Weichselbaum RR. Transcriptional targeting of adenovirally delivered tumor necrosis factor alpha by temozolomide in experimental glioblastoma. Cancer Res. 2004;64(18):6381–6384. doi: 10.1158/0008-5472.CAN-04-2117. [DOI] [PubMed] [Google Scholar]
- 123.Ulasov IV, Sonabend AM, Nandi S, Khramtsov A, Han Y, Lesniak MS. Combination of adenoviral virotherapy and temozolomide chemotherapy eradicates malignant glioma through autophagic and apoptotic cell death in vivo. Brit J Cancer. 2009;100(7):1154–1164. doi: 10.1038/sj.bjc.6604969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Conrad C, Miller CR, Ji Y, et al. Delta24-hyCD adenovirus suppresses glioma growth in vivo by combining oncolysis and chemosensitization. Cancer Gene Ther. 2005;12(3):284–294. doi: 10.1038/sj.cgt.7700750. [DOI] [PubMed] [Google Scholar]
- 125.Cheng L, Wu Q, Guryanova OA, et al. Elevated invasive potential of glioblastoma stem cells. Biochem Biophys Res Commun. 2011;406(4):643–648. doi: 10.1016/j.bbrc.2011.02.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gomez-Manzano C, Fueyo J. Oncolytic adenoviruses for the treatment of brain tumors. Curr Opin Mol Ther. 2010;12(5):530–537. [PubMed] [Google Scholar]
- 127.Kanai R, Wakimoto H, Cheema T, Rabkin SD. Oncolytic herpes simplex virus vectors and chemotherapy: are combinatorial strategies more effective for cancer? Future Oncol. 2010;6(4):619–634. doi: 10.2217/fon.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kasai K, Nakashima H, Liu F, et al. Toxicology and biodistribution studies for MGH2.1, an oncolytic virus that expresses two prodrug-activating genes, in combination with prodrugs. Mol Ther. 2013;2:e113. doi: 10.1038/mtna.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ichikawa T, Petros WP, Ludeman SM, et al. Intraneoplastic polymer-based delivery of cyclophosphamide for intratumoral bioconversion by a replicating oncolytic viral vector. Cancer Res. 2001;61(3):64–868. [PubMed] [Google Scholar]
- 130.Muthana M, Rodrigues S, Chen YY, et al. Macrophage delivery of an oncolytic virus abolishes tumor regrowth and metastasis after chemotherapy or irradiation. Cancer Res. 2013;73(2):490–495. doi: 10.1158/0008-5472.CAN-12-3056. [DOI] [PubMed] [Google Scholar]
- 131.Ahmed AU, Thaci B, Alexiades NG, et al. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol Ther. 2011;19(9):1714–1726. doi: 10.1038/mt.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang W, Fulci G, Wakimoto H, et al. Combination of oncolytic herpes simplex viruses armed with angiostatin and IL-12 enhances antitumor efficacy in human glioblastoma models. Neoplasia. 2013;15(6):591–599. doi: 10.1593/neo.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thaci B, Ulasov IV, Ahmed AU, Ferguson SD, Han Y, Lesniak MS. Anti-angiogenic therapy increases intratumoral adenovirus distribution by inducing collagen degradation. Gene Ther. 2013;20(3):318–327. doi: 10.1038/gt.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang W, Fulci G, Buhrman JS, et al. Bevacizumab with angiostatin-armed oHSV increases antiangiogenesis and decreases bevacizumab-induced invasion in U87 glioma. Mol Ther. 2012;20(1):37–45. doi: 10.1038/mt.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Buckel L, Advani SJ, Frentzen A, et al. Combination of fractionated irradiation with anti-VEGF expressing vaccinia virus therapy enhances tumor control by simultaneous radiosensitization of tumor associated endothelium. Int J Cancer. 2013;133(12):2989–2999. doi: 10.1002/ijc.28296. [DOI] [PubMed] [Google Scholar]
- 136.Auffinger B, Tobias AL, Han Y, et al. Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ. 2014;21(7):1119–1131. doi: 10.1038/cdd.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bao S, Wu Q, Mclendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 138.Beier D, Schulz JB, Beier CP. Chemoresistance of glioblastoma cancer stem cells – much more complex than expected. Mol Cancer. 2011;10:128. doi: 10.1186/1476-4598-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cheng L, Bao S, Rich JN. Potential therapeutic implications of cancer stem cells in glioblastoma. Biochem Pharmacol. 2010;80(5):654–665. doi: 10.1016/j.bcp.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.ClinicalTrials database. www.clinicaltrials.gov