Abstract
Depression and cigarette smoking co-occur at high rates. However, the etiological mechanisms that contribute to this relationship remain unclear. Anhedonia and associated impairments in reward learning are key features of depression, which also have been linked to the onset and maintenance of cigarette smoking. However, few studies have investigated differences in anhedonia and reward learning among depressed smokers and depressed nonsmokers. The goal of this study was to examine putative differences in anhedonia and reward learning in depressed smokers (n = 36) and depressed nonsmokers (n = 44). To this end, participants completed self-report measures of anhedonia and behavioral activation (BAS reward responsiveness scores) and as well as a probabilistic reward task rooted in signal detection theory, which measures reward learning (Pizzagalli, Jahn, & O’Shea, 2005). When considering self-report measures, depressed smokers reported higher trait anhedonia and reduced BAS reward responsiveness scores compared to depressed nonsmokers. In contrast to self-report measures, nicotine-satiated depressed smokers demonstrated greater acquisition of reward-based learning compared to depressed nonsmokers as indexed by the probabilistic reward task. Findings may point to a potential mechanism underlying the frequent co-occurrence of smoking and depression. These results highlight the importance of continued investigation of the role of anhedonia and reward system functioning in the co-occurrence of depression and nicotine abuse. Results also may support the use of treatments targeting reward learning (e.g., behavioral activation) to enhance smoking cessation among individuals with depression.
Keywords: depression, smoking, anhedonia, reward learning, Veteran
Cigarette smoking (via its introduction of many chronic medical conditions such as cardiovascular diseases, respiratory diseases, and cancer) is the leading cause of premature death in the United States and constitutes the single most preventable cause of morbidity and mortality worldwide (Centers for Disease Control and Prevention, 2002). Smoking is responsible for enormous health and economic burdens, and is linked to over 440,000 deaths per year in the United States (Centers for Disease Control and Prevention, 2008) and approximately 5 million premature deaths annually worldwide (Warren, 2002). Epidemiological studies indicate that smoking rates are disproportionately high among samples with mental illness (Breslau, 1995), particularly those with depression (Breslau, Novak, & Kessler, 2004; Grant, Hasin, Chou, Stinson, & Dawson, 2004; Lasser et al., 2000). In fact, studies have shown that individuals with major depressive disorder (MDD) are approximately twice as likely to report smoking, compared to individuals without mental illness (35–45% versus 23%, respectively; Lasser et al., 2000).
The association between smoking and depression appears to be bidirectional in nature. Many studies have demonstrated that smoking individuals (compared with nonsmokers) are approximately 2–3 times as likely to be currently depressed (Grant et al., 2004; John, Meyer, Rumpf, & Hapke, 2004) and these individuals are also at increased risk for future depression (Brown, Lewinsohn, Seeley, & Wagner, 1996). For example, Breslau and colleagues (1991) reported that 39% of smokers with moderate nicotine dependence met criteria for MDD, compared to 10% of non-dependent smokers (Breslau, Kilbey, & Andreski, 1991). Similarly, rates of depression are higher among smokers (irrespective of dependence status) than nonsmokers (Morrell & Cohen, 2006). Specifically, smokers report higher levels of depressive symptoms (Anda et al., 1990) and experience more frequent depressive episodes (Glassman, 1993) compared to nonsmokers.
Despite the frequent and costly co-occurrence of smoking and depression, the etiological mechanisms that contribute to this relationship remain unknown (Danaei et al., 2009; Mokdad, Marks, Stroup, & Gerberding, 2004; Tsuang, Francis, Minor, Thomas, & Stone, 2012). MDD is a heterogeneous clinical condition marked by both elevations in negative affect and deficits in positive affect (i.e., anhedonia; Brown, Chorpita, & Barlow, 1998). Several studies have demonstrated a relationship between self-reported anhedonia, defined as loss of pleasure or reduced positive emotional reactivity to pleasurable stimuli, and smoking behavior (Carton, Jouvent, & Widlocher, 1994). Specifically, anhedonia has been associated with increased urge and craving to smoke, as well as poor smoking cessation outcomes (Ameringer & Leventhal, 2010; Cook, Spring, McChargue, & Doran, 2010; Leventhal, Ramsey, Brown, LaChance, & Kahler, 2008; Leventhal, Waters, Kahler, Ray, & Sussman, 2009). Thus, preliminary evidence suggests that anhedonia may play an important role in the association between these two conditions.
Dysfunction in the brain’s reward system is thought to contribute to reduced hedonic capacity in depression (Dillon et al., 2009; Pizzagalli, Jahn, & O’Shea, 2005). For example, individuals with MDD show weakened responses in striatal regions (caudate, putamen, nucleus accumbens) to rewards and reward-predicting cues (Pizzagalli et al., 2009). In addition, hypoactivity in these regions has been associated with anhedonia in depression and related disorders (Elman et al., 2009; Keedwell, Andrew, Williams, Brammer, & Phillips, 2005). A crucial element of reward system functioning is the capacity to acquire reward-based learning (i.e., the ability to modify behavior in response to positive reinforcement and to learn associations among neutral stimuli and unconditioned rewards). Recent studies suggest that impairments in the ability to adjust behavior as a function of reinforcement may be an important mechanism underlying the experience of anhedonia in mood disorders (Pizzagalli, Goetz, Ostacher, Iosifescu, & Perlis, 2008; Pizzagalli, Iosifescu, Hallett, Ratner, & Fava, 2008).
Phasic signaling in midbrain dopamine neurons has been implicated in reward learning processes. Specifically, dopamine bursts have been linked to both the receipt of unpredicted rewards in early learning phases and the presence of reward-predicting cues in later learning phases (Holroyd & Coles, 2002). These dopamine bursts are thought to signal the anterior cingulate cortex and striatal regions to integrate reward-based learning and implement approach-related behaviors. Consistent with this, interruptions in dopamine transmission (e.g., through administration of single low doses of a dopamine agonist hypothesized to reduce dopamine transmission via autoreceptor activation) weakened reinforcement learning in both humans (Pizzagalli, Evins, et al., 2008) and rodents (Der-Avakian, D’Souza, Pizzagalli, & Markou, 2013). Based on this model, MDD and anhedonia have been associated with decreased dopamine signaling in striatal and midbrain reward regions (Bressan & Crippa, 2005; Forbes, 2009; Kumar et al., 2008). Significantly, cigarette smoking has been shown to increase transient dopamine release in these regions (i.e., ventral striatum, predominantly in the left ventral caudate/nucleus accumbens and left ventral putamen; Brody et al., 2004). Withdrawal from nicotine has also been shown to dampen dopamine signaling in the nucleus accumbens, creating a hypodopaminergic state that is reversed by acute nicotine re-exposure (Zhang, Dong, Doyon, & Dani, 2012).
In line with these neurobiological findings suggesting that smoking modulates dopaminergic activity in the brain’s reward system, preclinical studies have shown that nicotine improves behavioral indices of reward-based learning (Barr, Pizzagalli, Culhane, Goff, & Evins, 2008; Blakey, 2005; Chaudhri et al., 2006; Kenny & Markou, 2006). In rodents, acute nicotine administration increases sensitivity to non-drug reward, while nicotine withdrawal diminishes reactivity to environmental incentives (Epping-Jordan, Watkins, Koob, & Markou, 1998; Kenny & Markou, 2006). Similarly, in humans, Barr and colleagues (2008) found that a single dose of nicotine increased reinforcement learning for non-drug cues among nonsmokers (Barr et al., 2008). Smoking also has been shown to promote positive emotional responding to mood induction stimuli among smokers with high levels of anhedonia (Cook, Spring, & McChargue, 2007). Consistent with these findings, a recent longitudinal study found that baseline levels of depressive symptoms were associated with diminished responding to alternative reinforcers over time, which led to subsequent increases in smoking onset and rate (Audrain-McGovern, Rodriguez, Rodgers, & Cuevas, 2011). Collectively, these studies suggest that depressed individuals with higher levels of anhedonia might utilize nicotine to ameliorate alterations in dopamine signaling associated with deficits in reward learning and responsiveness, ultimately leading to higher rates of smoking and nicotine dependence in this group.
Based on this literature, our goal was to examine putative differences in measures of anhedonia and reward-based learning among depressed smokers and nonsmokers. To this end, we used self-report measures of anhedonia and an established probabilistic reward-based learning task that yields a behavioral measure of reward responsiveness (Pizzagalli et al., 2005). We hypothesized that, relative to depressed nonsmokers, depressed smokers would report higher baseline levels of anhedonia. Furthermore, we predicted that depressed smokers, who were allowed to smoke to satiation immediately before the probabilistic reward task, would demonstrate increased reward-based learning relative to depressed nonsmokers during the course of the task. Results may provide important information about potential behavioral and neurocognitive mechanisms of action underlying the common co-occurrence of smoking and depression.
Material and Methods
Inclusion/Exclusion Criteria
The primary inclusion criterion was a current diagnosis of unipolar depression, which included diagnoses of either MDD or dysthymia (93.8% met criteria for MDD and 11.3% met for dysthymia). Additional inclusion criteria consisted of age over 18 years and ability to read and write English. Exclusionary criteria included: (a) bipolar disorder diagnosis; (b) current psychotic-spectrum diagnosis; (c) current suicidal or homicidal intent and/or plan; (d) unstable psychiatric symptoms (e.g., psychiatric hospitalization within the last two months) and/or symptoms that interfered with study procedures; and (e) limited mental competency and/or inability to provide informed, written consent.
Diagnostic Assessment
The Structured Clinical Interview for DSM-IV Axis I Disorders – Clinician Version (SCID-CV; First, Spitzer, Gibbon, & Williams, 1997) was used to determine current and lifetime Axis I diagnoses. Interviews were conducted by a trained doctoral level psychologist.
Self-Report Measures
Psychiatric symptoms
Beck Depression Inventory-II (BDI-II)
The BDI-II (Beck, Steer, & Brown, 1996) is a 21-item scale that is a widely used measure of depression. Each item is rated on a 4-point Likert scale, ranging from 0–3. Total scores on this measure range from 0–63, with higher scores indexing more severe depressive symptoms. The BDI-II has demonstrated excellent internal consistency, validity, and test-retest reliability (Beck, Steer, Ball, & Ranieri, 1996; Dozois, Dobson, & Ahnberg, 1998). In this study, the BDI-II total score was used as an index of total depression severity; items 4 and 12 of the BDI-II were used to assess loss of pleasure and loss of interest, respectively.
Mood and Anxiety Symptom Questionnaire (MASQ)
The MASQ (Watson, Weber, et al., 1995) Anhedonic Depression (AD) subscale was used to assess anhedonia. The 22-items on this subscale index loss of interest and reduced positive affect. Each item is rated on a Likert scale from 1 to 5. Scores for the MASQ AD subscale range from 22–110, with higher scores indicating greater levels of anhedonia. The MASQ-AD subscale has demonstrated good reliability and validity (e.g., Watson, Clark, et al., 1995).
Behavioral Inhibition/Behavioral Activation Scale
The BIS/BAS (Carver & White, 1994) is a 20-item scale that was administered to assess behavioral activation and reward responsivity. Each item is rated on a 4-point Likert scale, ranging from 1 to 4. The scale produces a BIS subscale score as well as a total BAS and three BAS subscale scores (i.e., Reward Responsiveness, Drive, and Fun Seeking). In this study, the 13-item BAS total and 5-item Reward Responsiveness scores were examined. Scores on each scale range from 13–52 and 5–20, respectively, with higher scores indexing greater symptom severity. The BIS/BAS has demonstrated excellent reliability and validity in clinical samples (Campbell-Sills, Liverant, & Brown, 2004).
Smoking behavior
Fagerstrom Test of Nicotine Dependence (FTND)
The FTND (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) is a 6-item widely used measure of nicotine dependence. Two of the six items are rated on a 0–3 scale, while four items are rated either “0” or “1.” Higher scores on this measure indicate greater levels of nicotine dependence. The FTND evidences good internal consistency and has demonstrated convergent validity with smoking behavior and biochemical measures (Heatherton et al., 1991).
Smoking Behavior Questionnaire (SBQ)
The SBQ is a scale developed for use in the current study. The measure consists of a combination of yes/no and open ended questions assessing current smoking behaviors (e.g., average number of cigarettes smoked per day, duration of smoking behavior).
Biochemical Verification
The EC50 Micro 4 Smokerlyzer (Bedfont Scientific, LTD) was used to measure carbon monoxide (CO) in expired rapid lung-breath. CO concentration was assessed in parts per million (ppm). The Alcomate Prestige AL6000 (AK Solutions, Palisades Park, NJ) was used to measure blood alcohol level. A breathalyzer reading of < 0.005 g/l was required to establish alcohol free status for the study visit and allow for participation.
Computerized Task Assessing Reward Learning
A previously validated signal detection task designed to assess modulation of behavior in response to rewards was used (i.e., reward learning; Pizzagalli et al., 2005). Prior to beginning, participants are informed that the goal of the task is to win as much money as possible. The task consists of two blocks of 100 trials. Each trial follows an identical sequence: a) presentation of a fixation point; b) appearance of a cartoon face without a mouth; c) presentation of either a short mouth (11.5 mm) or a long mouth (13 mm) for 100 ms-; and d) reappearance of the mouthless face, which remains on the screen until a response is made. Participants are asked to identify which mouth was presented (i.e., short or long) via button press. Unbeknownst to the participants, an asymmetrical reinforcer ratio (3:1 rich/lean) is used for correct identifications. Specifically, correct identification of the rich stimulus is associated with three times more positive feedback (“Correct! You won 5 cents”) than the lean stimulus (30 vs. 10 reward feedback). Designation of the short versus long mouth as the rich stimulus is counterbalanced across participants.
Three primary outcome variables are derived from the signal detection task: response bias (RB), discriminability, and reaction time (RT; see Pizzagalli et al., 2005). RB, the main variable of interest, reflects the preference for the more-frequently rewarded (rich) stimulus (i.e., an index of reward responsiveness). RB is calculated as:
Change in RB across blocks (ΔRB) has been identified as a primary index of reward learning during the task (e.g., Santesso et al., 2008). Prior findings with this task in MDD and bipolar samples have found reduced RB relative to non-psychiatric groups and have demonstrated inverse relationships between RB and anhedonia (Pizzagalli, Goetz, et al., 2008; Pizzagalli et al., 2009). Discriminability is an index of participants’ ability to differentiate between the two different mouth stimuli (i.e., a measure of task difficulty), and is calculated as follows:
RT is the time elapsed in milliseconds from the reappearance of the mouthless face and the participant’s response.
According to published scoring procedures (Pizzagalli et al., 2005), data were screened to identify outliers both within blocks and across participants. The following criteria were used to determine outlying data, which were subsequently removed from analyses: Within each block, RTs < 150 ms or > 2500 ms were used to determine individual outlier trials. The following criteria were used to determine outlier task administrations for individual participants: < 80% of valid trials within a Block; < 25 rich reward/block; > 30 outlier trials for any Block; and < 60% accuracy for each Block. Using these procedures, 24 (30%) participants were identified as task outliers (15 outliers were identified in the depressed nonsmoker group and 9 outliers were identified in the depressed smoker group), and their data were excluded. Outlier data for this study are consistent with rates from other investigations of this task in mood disorder and Veteran psychiatric samples (Ahnallen et al., 2012; Pizzagalli, Iosifescu, et al., 2008).
Study Design and Procedure
During a preliminary telephone screening interview, participants were provided with a description of the study and engaged in a brief assessment to determine possible inclusion/exclusion criteria. All participants provided written informed consent prior to study participation. The study visit consisted of three primary components: (a) diagnostic assessment using the SCID-CV, (b) completion of self-report questionnaires, and (c) completion of the computerized task. The SCID-CV was administered at the beginning of the study day to determine diagnostic eligibility. Participants then completed questionnaires assessing psychiatric symptoms. Next, lunch was provided. Participants who smoked were allowed to smoke ad lib during circumscribed periods throughout the study visit. All smokers took a standardized smoke break following lunch during which they were asked to smoke to satiation. Self-report measures assessing smoking were completed following this smoke break and immediately before initiation of the computerized reward learning task. This research was conducted in compliance with the Institutional Review Board of a large VA Healthcare System.
Statistical Analysis
Participants were classified as nonsmokers if they reported smoking zero cigarettes per day. Individuals reporting one or more cigarettes/day were classified as smokers. Independent samples t-tests and chi square analyses were used to examine group differences on socio-demographic, psychiatric, and medication use variables as well as self-report measures of anhedonia and reward responsiveness (i.e., BDI-II total score and loss of pleasure and loss of interest items, BISBAS – Reward Responsiveness subscale; BISBAS – BAS, MASQ – AD subscale). Mixed model repeated measures analysis of variance (ANOVA) was used to examine group differences on outcome measures for the task. Two separate 2 (Smoking status: smoker, nonsmoker) × 2 (Block: 1, 2) analyses were conducted to examine RB and discriminability. For RT, the repeated measure of Stimulus (Rich, Lean) was added. Reward learning was operationalized as change in RB from block 1 to 2 (ΔRB = RB Block 2 − RB Block 1). Analyses were performed using SPSS statistical software version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Participant Characteristics
Eighty Veterans were recruited from a large VA Healthcare System in the Northeastern United States. Consistent with other Veteran samples, the majority of the sample was male (87.5%). Participants reported an average age of 51.2 years (SD = 11.19, range = 25 – 80) and had completed an average of 13.7 (SD = 1.86) years of education. The sample was primarily composed of individuals who identified as White/Caucasian (78.8%), with smaller numbers of Black/African-American (12.5%), Latina/o (3.8%), Asian/Pacific Islander (2.5%), and other/multiracial (2.5%). The majority of the sample (65.0%) reported taking an antidepressant medication at the time of enrollment (SSRI: n = 31, SNRI: n = 6, NDRI: n = 16, tricyclics: n = 4, tetracyclics: n = 13). A total of 36 participants (45.0%) were classified as current smokers. On average, depressed smokers reported smoking 14.56 (SD = 7.28) cigarettes per day for a period of 28.77 (SD = 13.71) years. Smoking participants reported a moderate level of nicotine dependence per the FTND (M = 4.83, SD = 2.38).
Depressed smokers and depressed nonsmokers differed in terms of gender, marital status, education, and expired-air CO levels (Table 1). Specifically, depressed smokers were more likely to be male, χ2 (1) = 5.66, p = .02, less likely to be married, χ2 (1) = 11.32, p = .05, evidenced higher expired-air CO levels, t(77) = 9.49, p < .001, and reported fewer years of education, t(77) = −2.62, p = .01. Additionally, depressed smokers had lower rates of anti-depressant medication use, χ2 (1) = 9.09, p = .003; however, smokers and nonsmokers did not differ with respect to type (ps > .15) or number (t[50] = −.25, p = .80) of currently used antidepressant medications.
Table 1.
Demographic, Psychiatric, and Medical Characteristics of the Participant Sample as a Function of Smoking Status
| Characteristic | Smokers (n = 36)
|
Nonsmokers (n = 44)
|
p-value | ||
|---|---|---|---|---|---|
| Mean (SD) | n (%) | Mean (SD) | n (%) | ||
| Age (years) | 50.33 (10.13) | 51.86 (12.06) | 0.55 | ||
| Education (years) | 13.11 (1.50) | 14.18 (2.01) | 0.01 | ||
| Gender | |||||
| Male | 35 (97.2) | 35 (79.5) | |||
| Female | 1 (2.8) | 9 (20.5) | 0.02 | ||
| Ethnicity | |||||
| White/Caucasian | 27 (75.0) | 36 (81.8) | |||
| Black/African-American | 7 (19.4) | 3 (6.8) | |||
| Latina/o | 1 (2.8) | 2 (4.5) | |||
| Asian/Pacific Islander | 1 (2.8) | 1 (2.8) | |||
| Other/Multiracial | 0 (0.0) | 2 (4.5) | 0.35 | ||
| Marital status | |||||
| Never married | 13 (36.1) | 10 (22.7) | |||
| Married | 2 (5.6) | 13 (29.5) | |||
| Divorced/separated | 18 (50.0) | 19 (43.2) | |||
| Widowed | 3 (3.8) | 2 (4.5) | 0.05 | ||
| MDD diagnosisa | 34 (94.4) | 41 (93.2) | 0.82 | ||
| Dysthymic disorder diagnosisa | 4 (11.1) | 5 (11.4) | 0.97 | ||
| BDI-II score | 31.28 (9.21) | 29.02 (9.59) | 0.29 | ||
| CO level (ppm) | 10.81 (6.52) | 1.35 (.53) | < .001 | ||
| Anti-depressant medication use | 17 (47.2) | 35 (79.5) | 0.003 | ||
| Beta blocker use | 0 (0.0) | 4 (9.1) | 0.06 | ||
| Benzodiazepine use | 4 (11.1) | 7 (15.9) | 0.54 | ||
Note. N = 80. Bold indicates p < .05. CO = carbon monoxide; BDI-II = Beck Depression Inventory-II (Beck, Steer, & Brown, 1996); MDD = major depressive disorder; ppm = parts per million.
Assessed with the SCID-I for the DSM-IV – Clinician Version (First et al., 1997).
Measures of Anhedonia and Reward Responsiveness
Consistent with hypotheses, relative to depressed nonsmokers, depressed smokers, reported greater loss of interest (BDI-II item 12), t(78) = 2.03, p = .045, increased anhedonia (MASQ-AD), t(77) = 2.01, p = .048, and lower reward responsiveness (BIS/BAS Reward Responsiveness subscale), t(77) = −2.31, p = .024 (see Table 2). Cohen’s d values for all significant mean differences ranged from small to medium (.46 – .52; Table 2).
Table 2.
Differences between Depressed Smokers and Depressed Nonsmokers on Self-report Measures of Depression, Anhedonia, and Reward Responsiveness
| Self report measure | Smokers (n = 36)
|
Nonsmokers (n = 44)
|
t(df) | p-value | d | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| BDI-II total score | 31.28 | 9.21 | 29.02 | 9.59 | 1.07(78) | .290 | .24 |
| BDI-II – Loss of pleasure item | 1.86 | .72 | 1.77 | .74 | .54(78) | .594 | .12 |
| BDI-II – Loss of interest item | 2.22 | .83 | 1.84 | .83 | 2.03(78) | .045 | .46 |
| BIS/BAS – Reward Responsiveness Subscale | 14.78 | 2.88 | 16.19 | 2.54 | −2.31(77) | .024 | −.52 |
| BIS/BAS – BAS | 33.64 | 6.87 | 36.90 | 8.08 | −1.89(74) | .063 | −.44 |
| MASQ – AD subscale | 89.56 | 9.32 | 85.12 | 10.16 | 2.01(77) | .048 | .46 |
Note. Bold indicates p < .05. AD = Anhedonic Depression; BAS = total Behavioral Activation Scale; BDI-II = Beck Depression Inventory – II (Beck, Steer, & Brown, 1996); BIS/BAS = Behavioral Inhibition and Activation Scales (Carver & White, 1994); MASQ = Mood and Anxiety Symptom Questionnaire (Watson, Weber, et al., 1995); SD = standard deviation.
Probabilistic Reward Task
Response Bias
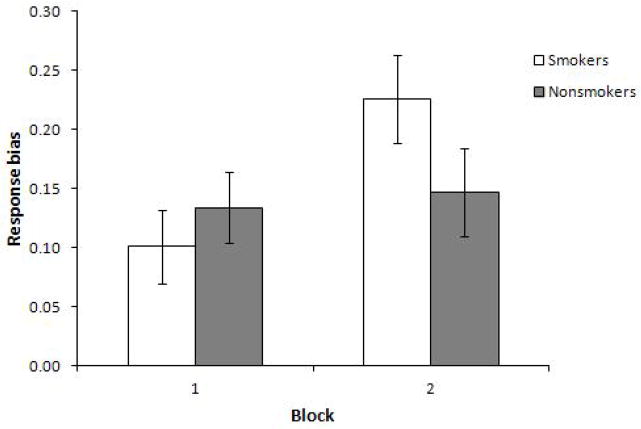
The ANOVA revealed a significant main effect for Block, F(1, 53) = 9.88, p = .003, η2 = .16, due to overall increased RB from Block 1 (M = 0.12, SD = .16) to Block 2 (M = 0.19, SD =.20). This effect was qualified by a significant interaction for Smoking status x Block, F(1, 53) = 6.54, p = .013, η2 = .11, indicating that the groups significantly differed in reward learning (i.e., ΔRB). Paired-samples t-tests indicated that RB significantly increased from Block 1 to 2 in the depressed smoker group (M = 0.10, SD = .16 vs. M = 0.23, SD = .20; t(26) = 4.12, p = .001), but not in the depressed nonsmoker group (M = 0.13, SD = .17 vs. M = 0.15, SD = .19; t(26) = −.41, p = .69; Figure 1). Independent samples t-tests were used to examine mean differences within individual blocks. Results did not show a significant difference between the depressed smoker and depressed nonsmoker groups in RB within Block 1 or Block 2.
Figure 1.
Estimated marginal means for Response Bias across Blocks 1 and 2 in the probabilistic reward task (significant main effect for Block and significant interaction effect for Block x Smoking status).
Discriminability
The Smoking status x Block ANOVA revealed a main effect for Block, F(1, 53) = 5.82, p = .02, η2 = .10, due to overall increases in discriminability from Block 1 (M = 0.50, SD = 0.03) to Block 2 (M = 0.56, SD = 0.04). All other effects were not significant, suggesting that smoking status did not affect discriminability.
RT
The Smoking status x Block x Stimulus ANOVA demonstrated a main effect for Block, F(1, 53) = 10.54, p = .002, η2 = .17, due to the expected faster RT in Block 2 (M = 571.32 ms, SD = 27.79) versus Block 1 (M = 629.61ms, SD = 30.59). There was a significant main effect for Stimulus, F(1, 53) = 21.16, p = .001, η2 = .29, with anticipated faster RT to the rich (M = 583.18 ms, SD = 27.15) versus lean (M = 617.84 ms, SD =28.94) stimulus. There was also a significant Block x Stimulus interaction, F(1, 53) = 8.53, p = .005, η2 = .14, indicating that RT decreased to a greater extent from Block 1 to 2 in response to the rich (M = 621.11 ms, SD = 231.57 vs. M = 545.80 ms, SD = 186.22) versus lean stimulus (M = 638.74 ms, SD = 223.48 vs. M = 597.60 ms, SD =228.11). All other effects were not significant, suggesting no modulations of smoking status on reaction time across blocks.
Analyses Controlling for Demographic and Medication Differences in the Smoker and Nonsmoker Groups
Although no significant differences were found between the depressed smoker and nonsmoker groups with respect to type or number of antidepressant medications used, additional t-tests and Pearson correlations were conducted to examine relationships between use of each type of antidepressant medication (SSRI, SNRI, NDRI, tricyclic, and tetracyclic) and number of antidepressant medications and task outcomes (Block 1 RB, Block 2 RB, and ΔRB). Significant associations were found between use of SNRI medication and Block 2 RB, t(53) = 2.48, p = .016, (No SNRI use: M = 0.17, SD = 0.19; SNRI use: M = 0.45, SD =0.10), as well as use of SNRI medication and ΔRB, t(53) = 2.34, p = .023 (No SNRI use: M = 0.06, SD = 0.16; SNRI use: M = 0.28, SD = 0.12). No other significant associations were found between type or number of antidepressant medications and RB.
A follow-up hierarchical regression analysis was conducted to determine if the relationship between smoking status and ΔRB remained after controlling for SNRI medication use as well as other demographic differences between depressed smokers and depressed nonsmokers. In this analysis, years of education, marital status, gender, and SNRI use were entered on Step 1 of the model and smoking status was entered on Step 2. Step 1 was not significant, F(4, 53) = 1.73, p = .16, R2 = .12. Step 2 contributed significantly to the model (Δ R2 = .14), F(5, 53) = 3.41, p = .01, R2 = .26, with smoking status (β = .42, t = 3.01, p = .004) significantly predicting ΔRB after controlling for SNRI use, years of education, gender, and marital status (Table 4).
Table 4.
Hierarchical Regression Analysis Predicting Change in Response Bias from Block 1 to 2 (ΔRB) Controlling for Group Difference Variables
| Predictor variables | R2 | t | β | p |
|---|---|---|---|---|
| Step 1 | .12 | |||
| Gendera | −1.01 | −.01 | .92 | |
| Education (years) | .96 | .14 | .34 | |
| SNRI useb | 2.33 | .31 | .02 | |
| Marital statusc | −.40 | −.06 | .69 | |
| Step 2 | .26 | |||
| Gender | .48 | .06 | .64 | |
| Education (years) | 2.08 | .29 | .04 | |
| SNRI use | 2.29 | .29 | .03 | |
| Marital status | .12 | .02 | .91 | |
| Smoking statusd | 3.01 | .42 | .004 |
Note: β = standardized beta weight. SNRI = serotonin–norepinephrine reuptake inhibitor.
Discussion
The current investigation examined putative differences in self-report and behavioral (Pizzagalli et al., 2005) measures of anhedonia and reward responsiveness between depressed smokers and nonsmokers. Consistent with hypotheses, depressed smokers reported higher baseline levels of trait anhedonia and reduced reward responsiveness compared with depressed nonsmokers. Specifically, depressed smokers endorsed greater loss of interest on the BDI-II, elevated anhedonia on the MASQ-AD, and diminished reward responsiveness on the BIS/BAS. These findings are in line with existing literature, which has shown positive associations between anhedonia and smoking behavior (i.e., increased craving and urge to smoke, poor response to smoking cessation treatments; Ameringer & Leventhal, 2010; Cook et al., 2010; Leventhal et al., 2008; Leventhal et al., 2009).
With respect to reward-based learning, depressed smokers (who were allowed to smoke to satiation prior to the reward learning task) evidenced stronger preference for the more frequently rewarded stimulus (i.e., significantly increased ΔRB). This suggests that nicotine-satiated smokers with depression demonstrated more robust acquisition of reward-based learning during the task as compared to depressed nonsmokers. These findings are consistent with preclinical and human studies indicating that nicotine acutely enhances reward learning and sensitivity to non-drug rewards (Barr et al., 2008; Epping-Jordan et al., 1998; Kenny & Markou, 2006). However, study results extend this literature to highlight the effects of nicotine on reward learning among individuals with clinical depression. Study findings offer support for nicotine’s role in enhancing reward learning among depressed individuals, suggesting a potential mechanism of action underlying the common co-occurrence of depression and smoking behavior (Breslau et al., 2004; Grant et al., 2004).
This mechanism may involve a complex interaction of neuroanatomical and biochemical pathways implicated in the brain’s reward system (Buhler et al., 2010; Forbes, 2009; Holroyd & Coles, 2002). More specifically, depression and anhedonia have been linked to hypoactivation in key structures of the reward pathway, including the caudate, nucleus accumbens, and putamen, during both the anticipatory/motivational and consummatory/hedonic phases of reward learning (Gard, Kring, Gard, Horan, & Green, 2007; Pizzagalli et al., 2009). Similarly, abstinent smokers demonstrate dysfunction in the brain’s motivational system during the anticipatory phase of reward learning, in particular decreased striatal activity in anticipation of non-drug reinforcers (Buhler et al., 2010; Peters et al., 2011). However, acute nicotine exposure appears to ameliorate this hypoactivation among dependent smokers, with data showing increased activation after nicotine administration in the dorsal striatum during anticipatory reward responding and in the medial prefrontal cortex associated with sensitivity to reward (Rose et al., 2013).
Collectively, these findings may point to the possibility that depressed individuals with higher levels of trait anhedonia utilize nicotine to enhance reward learning processes, leading to higher rates of smoking. Of note, significant differences were not found between depressed smokers and depressed nonsmokers in Block 1 RB in the current investigation. This finding may suggest that depressed smokers do not show baseline deficits in reward learning as compared to depressed nonsmokers. However, the presence of premorbid deficits in reward learning among depressed individuals who later become nicotine dependent is difficult to evaluate with the current study design and sample (i.e., participation of depressed smokers who self-administered nicotine to satiation prior to task completion). Studies that assess reward learning prior to initiation of smoking behavior and/or during smoking withdrawal support the presence of preexisting reward learning impairments, which are ameliorated by subsequent nicotine administration. For example, longitudinal research has shown that baseline levels of depression are associated with decreased responsivity to alternative reinforcers over time, leading to later elevations in the onset and frequency of cigarette smoking (Audrain-McGovern et al., 2011). In addition, Pergadia, Der-Avakian, Pizzagalli, and colleagues recently found that, in both human smokers and nicotine-treated rats tested with analogous versions of the Probabilistic Reward Task, 24-hour withdrawal from nicotine was associated with blunted reward responsiveness. Of note, abstinence-induced deficits in reward responsiveness were greatest in participants with past MDD. Moreover, among nicotine-treated rats, acute nicotine re-exposure long after withdrawal potentiated reward responsiveness (Der-Akavian et al., 2012; Pergadia et al., 2012). When combined with these findings, study results may point to the possibility that depressed individuals with higher levels of trait anhedonia utilize nicotine to ameliorate preexisting reward learning deficits associated with hypoactivity and altered dopaminergic signaling in midbrain reward regions, thus leading to higher rates of smoking. Results from the current investigation also may support the use of cognitive-behavioral treatments that aim to increase reward learning (e.g., behavioral activation) to improve smoking cessation outcomes among individuals with depression.
Although the present investigation may have important implications for understanding and treating the co-occurrence of smoking and depression, several limitations should be noted. First, this study did not have a nonpsychiatric smoker and/or nonsmoker group. Thus, we cannot determine whether smoking before the task administration normalized the blunted reward learning previously described in MDD (Pizzagalli, Iosifescu, et al., 2008). Second, this study utilized a naturalistic design, allowing depressed smokers to smoke to satiation as opposed to using an experimental manipulation of a controlled dose of nicotine. Thus, variation in the amount of nicotine consumed was not controlled for. Third, this study did not include a baseline nicotine deprivation condition, which would have allowed for examination of intra-individual differences in reward learning between nicotine withdrawal and acute nicotine re-exposure states among depressed smokers (e.g., Zhang et al., 2012). In addition, this study did not assess prior smoking history, and therefore possible inclusion of former smokers (i.e., quitters, categorized as current nonsmokers in the present study) may have influenced study results. Finally, similar to other Veteran samples, the current sample was primarily composed of men. Therefore, results should be interpreted cautiously, specifically when generalizing findings to women.
Despite these limitations, results may suggest a potential mechanism of action underlying the frequent co-occurrence of depression and smoking (Danaei et al., 2009; Lasser et al., 2000; Mokdad et al., 2004). Findings also suggest the importance of incorporating psychotherapeutic treatments that target reward learning (e.g., behavioral activation) to enhance smoking cessation outcomes among individuals with depression. More generally, findings highlight the need for continued investigation of the role of anhedonia and associated reward learning deficits in the onset and maintenance of smoking behavior among depressed individuals. Additional studies, particularly using longitudinal designs, are needed to better explicate the causal relationships among depression, anhedonia, smoking, and reward learning. Furthermore, future studies with depressed samples would benefit from examining effects of nicotine on the brain’s reward system during both anticipatory and consummatory reward processing, as well as effects on reward learning of nicotine withdrawal and subsequent acute nicotine re-exposure. Additional research in this area is particularly important given recent neuroimaging findings suggesting that attenuation of reward responsivity may be most acute during nicotine withdrawal among smokers (Sweitzer et al., 2013).
Table 3.
Performance Variables from the Reward-based Learning Task for Depressed Smokers and Depressed Nonsmokers.
| Variable | Smokers (n = 27)
|
Nonsmokers (n = 28)
|
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Response Bias | ||||
| RB Block 1 | .10 | .155 | .13 | .165 |
| RB Block 2 | .23 | .203 | .15 | .186 |
| RB Total Task | .16 | .163 | .14 | .158 |
| Discriminability | ||||
| Discriminability Block 1 | .47 | .234 | .52 | .210 |
| Discriminability Block 2 | .55 | .289 | .57 | .276 |
| Discriminability Total Task | .51 | .243 | .55 | .224 |
| RT (ms) | ||||
| RT Block 1 (Rich stimulus only) | 606.0 | 223.84 | 635.7 | 241.99 |
| RT Block 1 (Lean stimulus only) | 623.6 | 222.48 | 653.4 | 227.53 |
| RT Block 1 (Rich + Lean) | 614.8 | 221.66 | 644.4 | 231.64 |
| RT Block 2 (Rich stimulus only) | 531.0 | 185.12 | 560.1 | 189.54 |
| RT Block 2 (Lean stimulus only) | 576.0 | 221.14 | 618.4 | 236.78 |
| RT Block 2 (Rich + Lean) | 553.5 | 200.7 | 589.1 | 211.12 |
| RT Total (Rich stimulus only) | 568.4 | 195.34 | 597.5 | 205.57 |
| RT Total (Lean stimulus only) | 599.9 | 211.05 | 635.6 | 218.23 |
| RT Total Task (Rich + Lean) | 584.1 | 201.81 | 616.4 | 209.96 |
Note. RB = response bias; RT = reaction time.
Acknowledgments
The authors would like to acknowledge Kimberly Arditte and Daniel Lee for their assistance with recruitment and data collection for this study. This investigation was supported by a VA Career Development Award, Department of Veterans Affairs, awarded to the first author, Dr. Gabrielle Liverant. The study sponsor had no role in study design or implementation as well as manuscript preparation. Dr. Pizzagalli was partially supported by NIMH (R01MH68376).
Footnotes
Disclosure Statement
Dr. Pizzagalli has received consulting fees from ANT North America Inc. (Advanced Neuro Technology), AstraZeneca, Ono Pharma USA, Pfizer, Servier, and Shire. Maurizio Fava has the following lifetime disclosures: Research Support: Abbot Laboratories; Alkermes, Inc.; Aspect Medical Systems; AstraZeneca; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Clinical Trials Solutions, LLC; Clintara, LLC; Covance; Covidien; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; Ganeden Biotech, Inc.; GlaxoSmithKline; Icon Clinical Research; i3 Innovus/Ingenix; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Novartis AG; Organon Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; Pharmavite® LLC; Photothera; Roche Pharmaceuticals; RCT Logic, LLC; Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Synthelabo; Wyeth-Ayerst Laboratories. Advisory/Consulting: Abbott Laboratories; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Bayer AG; Best Practice Project Management, Inc.; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; BrainCells Inc; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Clinical Trials Solutions, LLC; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; i3 Innovus/Ingenis; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG; Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Otsuka Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite® LLC.; PharmoRx Therapeutics; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; RCT Logic, LLC; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex Pharmaceuticals, Inc.; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Vanda Pharmaceuticals, Inc. Speaking/Publishing: Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource, Corp.; Wyeth-Ayerst Laboratories. Equity Holdings: Compellis. Royalty/patent, other income: Patent for Sequential Parallel Comparison Design (SPCD) and patent application for a combination of azapirones and bupropion in Major Depressive Disorder (MDD), copyright royalties for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), and SAFER. Patent for research and licensing of SPCD with RCT Logic; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte.Ltd. For the remaining authors, no conflicts of interest were declared.
References
- Ahnallen CG, Liverant GI, Gregor KL, Kamholz BW, Levitt JJ, Gulliver SB, Kaplan GB. The relationship between reward-based learning and nicotine dependence in smokers with schizophrenia. Psychiatry Research. 2012;196:9–14. doi: 10.1016/j.psychres.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameringer KJ, Leventhal AM. Applying the tripartite model of anxiety and depression to cigarette smoking: An integrative review. Nicotine and Tobacco Reseacrh. 2010;12:1183–1194. doi: 10.1093/ntr/ntq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking: A national perspective. Journal of the American Medical Association. 1990;264:1541–1545. doi: 10.1001/jama.1990.03450120053028. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Rodgers K, Cuevas J. Declining alternative reinforcers link depression to young adult smoking. Addiction. 2011;106(1):178–187. doi: 10.1111/j.1360-0443.2010.03113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: Implications for development of dependence. Biological Psychiatry. 2008;63:1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Vol. 1. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Blakey J. Formal learning theory and nicotine dependence: Response to Balfour. Nicotine and Tobacco Research. 2005;7:481–482. doi: 10.1080/14622200500124800. author reply 483–484. [DOI] [PubMed] [Google Scholar]
- Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behavioral Genetics. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Archives of General Psychiatry. 1991;48:1069–1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Daily smoking and the subsequent onset of psychiatric disorders. Psychological Medicine. 2004;34:323–333. doi: 10.1017/S0033291703008869. [DOI] [PubMed] [Google Scholar]
- Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour--review of data from preclinical research. Acta Psychiatrica Scandinavica. 2005;111(Suppl s427):14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Mandelkern MA. Smoking-induced ventral striatum dopamine release. American Journal of Psychiatry. 2004;161:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lewinsohn PM, Seeley JR, Wagner EF. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1602–1610. doi: 10.1097/00004583-199612000-00011. [DOI] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology. 1998;107:179–192. doi: 10.1037/0021-843X.107.2.179. [DOI] [PubMed] [Google Scholar]
- Buhler M, Vollstadt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, Smolka MN. Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biological Psychiatry. 2010;67:745–752. doi: 10.1016/j.biopsych.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Liverant GI, Brown TA. Psychometric evaluation of the behavioral inhibition/behavioral activation scales in a large sample of outpatients with anxiety and mood disorders. Psychological Assessment. 2004;16:244–254. doi: 10.1037/1040-3590.16.3.244. [DOI] [PubMed] [Google Scholar]
- Carton S, Jouvent R, Widlocher D. Nicotine dependence and motives for smoking in depression. Journal of Substance Abuse. 1994;6:67–76. doi: 10.1016/S0899-3289(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67:319–333. doi: 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- Centers for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and economic costs --- United States, 1995–1999. Morbidity and Mortality Weekly Report. 2002;51(14):300–303. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses --- United States, 2000–2004. Morbidity and Mortality Weekly Report. 2008;57(45):1226–1228. [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology. 2007;192:87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: A brief report. Nicotine and Tobacco Reseacrh. 2010;12:978–982. doi: 10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Medicine. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, D’Souza MS, Pizzagalli DA, Markou A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Translational Psychiatry. 2013;3:e297. doi: 10.1038/tp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Pergadia ML, D’Souza MS, Madden PAF, Heath AC, Shiffman S, Markou A. Impaired reward responsiveness during nicotine withdrawal in rats and humans assessed in a translational behavioral procedure. Neuropsychopharmacology. 2012;38(Suppl 1):S381. doi: 10.1038/npp.2012.221. [DOI] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozois DJ, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychological Assessment. 1998;10:83–89. doi: 10.1037/1040-3590.10.2.83. [DOI] [Google Scholar]
- Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biological Psychiatry. 2009;66:1083–1090. doi: 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Forbes EE. Where’s the fun in that? Broadening the focus on reward function in depression. Biological Psychiatry. 2009;66:199–200. doi: 10.1016/j.biopsych.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93(1–3):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH. Cigarette smoking: Implications for psychiatric illness. American Journal of Psychiatry. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Hapke U. Smoking, nicotine dependence and psychiatric comorbidity: A population-based study including smoking cessation after three years. Drug and Alcohol Dependence. 2004;76:287–295. doi: 10.1016/j.drugalcdep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131(Pt 8):2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine and Tobacco Research. 2008;10:507–517. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine and Tobacco Research. 2009;11:1047–1054. doi: 10.1093/ntr/ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Journal of the American Medical Association. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Morrell HR, Cohen LM. Cigarette smoking, anxiety, and depression. Journal of Psychopathology and Behavioral Assessment. 2006;28:283–297. doi: 10.1007/s10862-005-9011-8. [DOI] [Google Scholar]
- Pergadia ML, Der-Avakian A, D’Souza MS, Madden PAF, Heath AC, Shiffman S, Pizzagalli DA. Cross-species effects of nicotine abstinence on reward responsiveness: Moving towards consilience. Poster session presented at the 14th annual meeting of Society for Research on Nicotine and Tobacco Europe; Helsinki, Finland. 2012. Aug, [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Consortium I. Lower ventral striatal activation during reward anticipation in adolescent smokers. American Journal of Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, Culhane M. Single dose of a dopamine agonist impairs reinforcement learning in humans: Behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 2008;196:221–232. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biological Psychiatry. 2008;64:162–168. doi: 10.1016/j.biopsych.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. Journal of Psychiatric Research. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biological Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose EJ, Ross TJ, Salmeron BJ, Lee M, Shakleya DM, Huestis MA, Stein EA. Acute nicotine differentially impacts anticipatory valence- and magnitude-related striatal activity. Biological Psychiatry. 2013;73:280–288. doi: 10.1016/j.biopsych.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Dillon DG, Birk JL, Holmes AJ, Goetz E, Bogdan R, Pizzagalli DA. Individual differences in reinforcement learning: Behavioral, electrophysiological, and neuroimaging correlates. Neuroimage. 2008;42:807–816. doi: 10.1016/j.neuroimage.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Geier CF, Joel DL, McGurrin P, Denlinger R, Forbes EE, Donny EC. Dissociated effects of anticipating smoking versus monetary reward in the caudate as a function of abstinence. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.11.013. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Francis T, Minor K, Thomas A, Stone WS. Genetics of smoking and depression. Human Genetics. 2012;131:905–915. doi: 10.1007/s00439-012-1170-6. [DOI] [PubMed] [Google Scholar]
- Warren CW. The Global Youth Tobacco Survey Collaborative Group. Tobbaco use among youth: A cross country comparison. Tobacco Control. 2002;11:252–270. doi: 10.1136/tc.11.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995;104:15–25. doi: 10.1037/0021-843X.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037/0021-843X.104.1.3. [DOI] [PubMed] [Google Scholar]
- Zhang L, Dong Y, Doyon WM, Dani JA. Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biological Psychiatry. 2012;71:184–191. doi: 10.1016/j.biopsych.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]