Abstract
Ischemic stroke and Alzheimer’s disease (AD), despite being distinct disease entities, share numerous pathophysiological mechanisms such as those mediated by inflammation, immune exhaustion, and neurovascular unit compromise. An important shared mechanistic link is acute and chronic changes in protein kinase C (PKC) activity. PKC isoforms have widespread functions important for memory, blood-brain barrier maintenance, and injury repair that change as the body ages. Disease states accelerate PKC functional modifications. Mutated forms of PKC can contribute to neurodegeneration and cognitive decline. In some cases the PKC isoforms are still functional but are not successfully translocated to appropriate locations within the cell. The deficits in proper PKC translocation worsen stroke outcome and amyloid-β toxicity. Cross talk between the innate immune system and PKC pathways contribute to the vascular status within the aging brain. Unfortunately, comorbidities such as diabetes, obesity, and hypertension disrupt normal communication between the two systems. The focus of this review is to highlight what is known about PKC function, how isoforms of PKC change with age, and what additional alterations are consequences of stroke and AD. The goal is to highlight future therapeutic targets that can be applied to both the treatment and prevention of neurologic disease. Although the pathology of ischemic stroke and AD are different, the similarity in PKC responses warrants further investigation, especially as PKC-dependent events may serve as an important connection linking age-related brain injury.
Keywords: Alzheimer’s disease, blood-brain barrier, immune exhaustion, innate immunity, ischemic stroke, protein kinase C
INTRODUCTION
The most prominent clinical symptom of Alzheimer’s disease (AD) is progressive cognitive decline [1]. The characteristic loss of episodic memories is an area under focused investigation and heavily dependent on amyloid-β (Aβ) plaques and neurofibrillary tau tangles (NFTs) [2]. A promising field of study is the contribution of Protein Kinase C (PKC) to cognitive decline and how it changes with aging and during AD progression. PKC isoforms have been classified as “memory kinases” for the role they play in acquisition and modification of dendritic spines [3]. Recent findings have highlighted PKC dysfunction as a process of aging. Aβ contributes to accelerated PKC changes that lead to downregulation of AMPA receptors [4]. Overactivity of damaging PKC isoforms, α and δ, contributes to cognitive decline and dendritic shortening [5]. Neurite retraction from PKC activity has also been reported in neurons of the hippocampus [6]. Interestingly, selective pharmacologic activation of PKCε can improve synaptogenesis [7]. PKCγ also contributes to the preservation of synaptic plasticity [8]. Besides the role that PKC isoforms play in memory formation, they also have important functions as tau kinases [9]. In particular, age-related changes in PKC translocation have been linked to tau hyperphosphorylation and the phosphorylation of glycogen synthase kinase 3β (p-GSK3β) [10]. Restoration of the PKCε cytosol-to-cell membrane translocation and activity decrease both NFTs and Aβ deposition in transgenic animal models [11]. What has yet to be fully determined is which isoforms are protective with aging, at what time are they protective, and when should they be selectively targeted.
Ischemic stroke, another prominent age-related disease, is the leading cause of disability in the US [12]. The severity of ischemic stroke outcome is closely linked to the extent of blood-brain barrier (BBB) disruption. Several deleterious PKC isoforms are increased in the endothelial cells of the vasculature following ischemia [13]. PKC θ and ζ contribute to disruption of the tight junction proteins, claudin-5, occludin, and ZO1 [14]. The extent of BBB disruption is biphasic in that acute disruption is detrimental while some chronic disruption is required for recovery. Interestingly, extensive motor training following stroke increases neuroprotective isoforms of PKC in a time-dependent manner leading to decreased BBB permeability [15]. Likewise δ opioid agonists increase the translocation of the neuroprotective isoform PKCε from the cytosol to nuclear membrane following stroke, thus providing protection for neurons [16].
The complex interrelations between AD and ischemic stroke include and are dependent on immune exhaustion. Atherosclerosis, cardiovascular disease, and AD are made worse by the inflammatory cascade released during immune exhaustion [17]. The risk for immune exhaustion is magnified in both AD and stroke with comorbidities such as diabetes, obesity, and hypertension [18, 19]. PKC activity is intimately linked to the immune system through both the complement system and toll-like receptors [20, 21]. In this review, we highlight what is known about PKC isoforms in aging, stroke, and AD, discuss areas requiring further investigation in order to successfully advance toward PKC-activated treatment regimens, and evaluate the contribution of immune exhaustion to PKC activity modification.
BACKGROUND OF PKC IN THE CENTRAL NERVOUS SYSTEM
PKC isoforms are found throughout the body, but in the brain they regulate vesicle movement and synapse secretion [22]. The isoforms can be broadly grouped into three classes: conventional (α, β, γ), novel (δ, ε, η, θ), and atypical (ι, ζ, N1–N3). Conventional isoforms require diacylglycerol, Ca2+, and diphorbol ester for activation. Novel isoforms require only diacylglycerol, and atypical isoforms do not require co-factors. Common PKC isoforms within the brain include PKC α, β, δ, ε, γ, and ζ [3]. PKC isoforms are differentiated according to structure and function. PKCα has an organized linear configuration consisting of N-terminal pseudosubstrate domains, a kinase domain, targeting domains, and inhibitory regulatory domains [23]. PKCα provides biochemical and structural support for synaptic architecture through activation of protein synthesis and has been associated with memory capacity [24, 25]. PKCβ has a distinct active site with a Cα backbone surrounded by supportive side chains [26]. The active site plays important roles as a memory kinase that mediates cognition [27]. The characteristic features of PKCδ are a catalytic domain and a highly reactive regulatory domain, C1B, which interacts with diacylglycerol [28]. PKCδ plays important roles in the regulation of apoptosis [29]. PKCε has a catalytic domain and two C1 domains that help direct translocation from the plasma membrane to nuclear membrane [30]. PKCε contributes to recognition memory and wound healing [31, 32]. PKCγ has a flexible C1B domain that can be phosphorylated at serine 109 [33]. PKCγ plays a vital role in pain regulation and reward seeking behavior [34, 35]. PKCζ has a series of N-terminal PB1 domains that have important roles in cellular processes [36]. PKCζ contributes to memory consolidation and maintenance [37, 38].
PKC REGULATION
PKC isoforms can be upregulated or downregulated depending on which pathways are active [39]. Common regulators include ceramide, annexins, and ellagic acid [40–42]. In order for PKC isoforms to be activated, they must be externally phosphorylated at a threonine residue tightly coiled within the active site. Subsequently, PKC undergoes autophosphorylation to internalize its hydrophobic residues [43]. It is only at this point that the C2 domain can bind to the receptor for activated C-kinases (RACKs) [44]. RACKs play a vital role in transporting PKC isoforms from the cytosol to the membrane [45]. Each PKC isoform has a binding site for specific RACKs in order to facilitate the appropriate translocation destination [46]. Once at the membrane, A-kinase regulating-proteins (AKAPs) and heat shock proteins (HSPs) direct PKC isoforms into close proximity with substrates [41]. AKAP7α enhances the speed by which PKC can phosphorylate substrates as well as stabilizes PKC activity over time [47]. HSP90 maintains the phosphorylation state of PKC for extended periods increasing its efficiency [48]. PKC is cleaved by caspase 3, transported in association with heat shock protein 70 (HSP70), and degraded by the proteasome [48, 49]. An alternative pathway for PKC degradation involves the lysosomal system [50].
PKC AND AGING
Two predominant theories have been proposed to explain how PKC activity changes with age [51]. The first theory is that as aging occurs, PKC isoforms become dysfunctional resulting in a gradual downregulation of PKC isoforms over time [52, 53]. Epigenetic modification triggers PKC repression [54]. Repression of the PKC gene has been directly associated with neurodegeneration as well as impaired memory and learning [7, 55]. The second theory is that PKC isoforms are still viable but the translocation process is dysfunctional [52]. RACKs are downregulated with aging, which leads to decreased PKC stabilization at the membrane [56]. Age-related decreases in RACK1 may explain, at least in part, age-related decreases in memory function [57].
Both theories are most likely relevant to the process of neuroaging but depend heavily on isoform specific interactions. For example, age-related decreases in expression with age of PKC α and ε in the frontal cortex and hippocampus have been linked to poor spatial memory [58]. Dysfunctional PKCα can also lead to an increase in matrix metalloproteinases within the aged brain [59]. In contrast, PKCγ levels are maintained at a constant level in the aged hippocampus, but translocation of this isoform is impaired. Such deficits in PKCγ translocation leads to poor performance on cognitive tasks in aged-animal models [27]. Furthermore, age-related comorbidities confound the expression of various isoforms. PKC α and β are increased with diabetes leading to the enhanced formation of advanced glycosylated end products [60, 61]. PKC δ and β are increased with atherosclerosis and contribute to endothelial cell damage [62, 63]. PKCδ also contributes to aortic contraction and adipocyte apoptosis in obese individuals [64]. In addition, complement mediated immunity activates PKC isoforms and triggers neurodegeneration during aging [65]. PKCβ accelerates inflammatory vascular disruption contributing to immune exhaustion [66]. What has yet to be fully elucidated is how age and co-morbidities alter PKC dynamics in diseases such as stroke and AD.
PKC AND STROKE
Following ischemic stroke, several PKC isoforms are altered within the brain [67]. PKC isoforms α, β, δ, θ, and ζ have an initial spike during the onset of ischemia, but are quickly degraded within the penumbra at later time points [68]. PKC isoforms ε and η are acutely downregulated but may play a role in recovery at extended time points [14]. PKCα has been linked to increased risk for hemorrhagic transformation following ischemic stroke [69]. PKCδ contributes to a release of reactive oxygen species and apoptosis following ischemia [70, 71]. PKCδ likewise contributes to increased BBB permeability via activation of matrix metalloproteinase-9 and phosphorylation of occludin [72, 73]. PKCε is downregulated leaving neuronal mitochondria susceptible to injury [74]. PKCζ and PKCβ contribute to tight junction disruption within the BBB during hypoxia (Fig. 1) [14, 75]. Of significance, PKC mediated vasoconstriction is disrupted allowing an influx of inflammatory markers and cytokines into the cerebrovasculature [76]. Similarly PKC isoforms likewise inhibit BBB transport proteins leaving the brain permeable to inflammatory toxins [77] such that the P-glycoprotein efflux capability is eventually overwhelmed and the tissue succumbs to infarct [78].
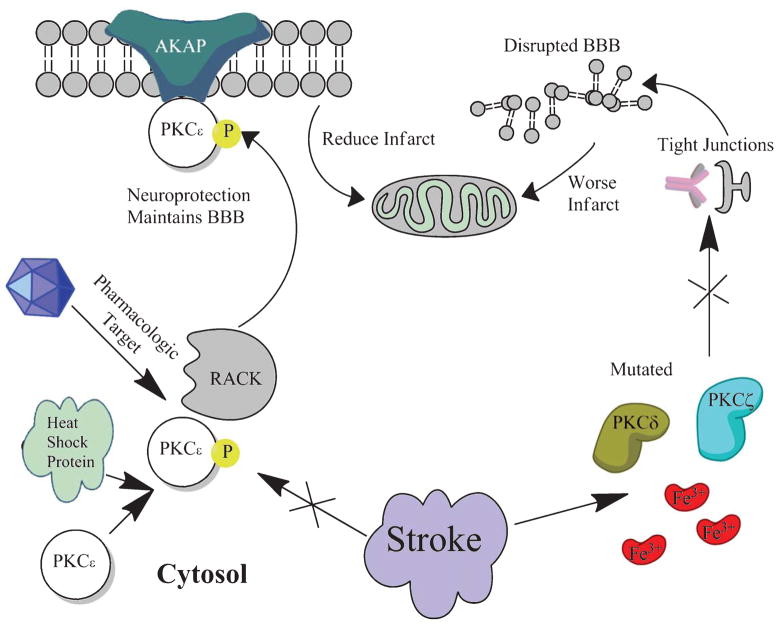
Fig. 1.
Following stroke, PKCδ and PKCζ become dysfunctional and are increased. The result is an increase in BBB disruption and worse ischemic infarct. If PKCε is targeted pharmacologically in order to enhance translocation to the membrane, the BBB is maintained and ischemic infarct is reduced.
PKC is initially activated by increased intracellular calcium and adenosine following ischemia, but a delayed induction is also seen due to changes in gene expression [68, 79]. PKCβ, in particular, quickly increases the RhoA/myosin-regulated light chain 2 pathway leading to increased brain edema following stroke [80]. Some isoforms are unable to translocate following ischemic injury and trigger intracellular pathways that contribute to neuronal death or injury [81]. One such response is activation of NADPH oxidase [82]. PKCζ triggers NADPH oxidase, which subsequently causes the release of superoxide. Superoxide changes the conformation of NMDA receptors predisposing the cell to excitotoxicity [83]. PKC activity also increases the permeability of chloride channels resulting in increased neuronal death following ischemia and triggers increased expression of nitric oxide synthase [84, 85]. If PKCε is increased, however, scavenging molecules that protect cells from reactive oxygen species are elevated [86]. The level of PKCε activity is inversely correlated with infarct volume [87]. PKCε exerts its protective effects through mitochondrial stabilization [88].
If the brain is reperfused by thrombolytics, PKCδ can contribute to injury expansion by triggering an influx of neutrophils and activating platelets within compromised vasculature [89]. PKC isoforms α, δ, ε, and ζ are intimately involved in toll-like receptor signaling linking PKC activity closely with the innate immune system [90]. Comorbidities can also exacerbate stroke outcome and injury. Hyperglycemia in diabetes primes PKCδ allowing for more extensive BBB disruption following stroke [91]. Obesity increases PKCζ, which predisposes the body to the development of the metabolic syndrome [92]. Hypertension can develop following obesity due to PKC specific activation of mitogen activated protein kinases. Such activation, leads to chronic vascular smooth muscle constriction in arteries [93]. Besides age itself, hypertension is the biggest risk factor for stroke [94]. Alternatively, ischemic preconditioning increases PKCε and decreases PKCδ, which has been shown to decrease infarct volume in animal models of stroke [95, 96]. PKCε is coupled with toll-like receptor 4 through MyD88. Toll-like receptor 4 exerts protective effects through downstream activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) [97]. PKC activity during stroke is ultimately time dependent and heavily mediated by vascular changes that are associated with comorbidities.
PKC AND AD
The process of memory formation and memory failure is an issue that has gained resurgence in the past few years with the increased prevalence of AD in the aging population [98]. PKC activity has recently been shown to be essential for memory formation and learning [3]. Memory in its basic form is dependent on synaptic remodeling, formation of dendritic spines, and mitochondria functionality [99, 100]. Isoforms of PKC are involved in multiple synaptic transmissions, including those involving glutamate, dopamine, acetylcholine, and serotonin [101–104]. The synaptic connections are intimately linked to cognitive processing and learning with different PKC isoforms being involved in distinct memory domains. PKCα is linked to the formation of aversive and high-impact memories, whereas PKCε is important in spatial memory formation and object recognition [25, 31]. Additionally, PKCζ is essential in maintenance and storage of long-term memory, and overexpression of this isoform has been shown to improve memory processes [105].
Stress-related dysfunction of PKC isoforms with age is linked to a progressive decline of memory and cognition with the potential for dementia and tau-related pathology [10]. In a transgenic PKCβ knockout model, animals did worse than controls on fear conditioning and cued learning [106], both tests detecting the neuroplasticity of the basolateral nucleus of the amygdala [107–109]. These data suggests that PKCβ is essential in normal amygdala synaptic plasticity, limbic driven memory, and learning. Transgenic animals with a PKCζ knockout have disrupted memory formation as well as poor memory recall [110]. In addition to dysfunction, downregulation of PKC isoforms is associated with AD, but not other types of dementia such as multi-infarct dementia and corticobasal degeneration [111]. PKC downregulation is also independent of other extraneous factors such as hydrocephalus and gender [112]. PKC downregulation may therefore be closely tied to the cognitive decline seen in AD [111]. A defect in PKC anchoring is associated with impairment of TNF-α production linking PKC dysfunction to immune senescence [113]. Moreover, the intracellular aggregation of hyperphosphorylated tau and extracellular amyloid accumulation are known to be detrimental to neurons and are suggested to be both directly and indirectly mediated by PKC [114] (Fig. 2).
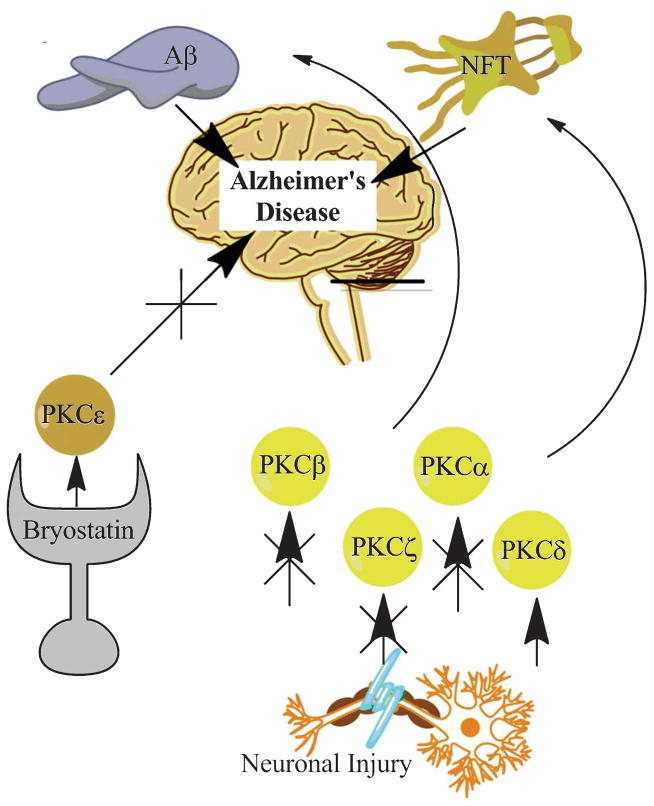
Fig. 2.
Neuronal injury causes dysregulation of PKC β, ζ, and α as well as an increase in PKCδ. These changes contribute to the development and progression of Aβ pathology and NFTs. Targeting PKCε with the pharmacologic agent Bryostatin may prove beneficial in protecting the brain against harmful PKC changes. By increasing PKCε, the progression of NFTs and Aβ pathology will be slowed.
PKCα is known to upregulate α-secretase, an enzyme important in non-pathogenic amyloid processing. Activation of α-secretase degrades amyloid-β protein precursor (AβPP), promotes the formation of soluble AβPP α (sAβPPα), and prevents Aβ accumulation. α-secretase is believed to be activated directly by PKCα and PKCε, and indirectly through the mitogen-activated protein kinase (MAPK) pathway [115]. Dysfunctional PKCα is deficient in activating α-secretase leading to disrupted AβPP processing and subsequent Aβ accumulation. It is important to note that sAβPPα, formed by the α-secretase cleavage of AβPP, also promotes translocation of PKCβ to the plasma membrane by RACK1 [116]. If PKCβ is not translocated, it can hyperphosphorylate tau and substantially contribute to AD pathology [117]. Intracellular PKC has recently been proposed as an AD biomarker because dysfunctional PKC translocation can be successfully detected in red blood cells thereby mimicking the activation state of PKC within the brain [118].
Another isoform, PKCε, when fully functional reduces Aβ accumulation. PKCε knockout mice display poor reward seeking behavior and have severe cognitive decline on memory tasks indicating the importance of this isoform [119]. PKCε induces the endothelin-converting enzyme to degrade Aβ40 and Aβ42 to small fragments [120], and facilitates the clearance of the Aβ fragments [115]. Aβ fragment clearance is associated with improved histological findings as well as potential neurological and cognitive benefits. In PKCε transgenic knock-in mice, the amyloid plaque burden is significantly reduced as well as a reduction in neuritic dystrophy, reactive astrocytosis, and other neurodegenerative changes [115]. This isoform acts through the MAPK dependent Ets-1 pathway. MAPK induces the formation of Ets protein complexes, and acts to promote the activation of endothelin-converting enzyme. Ets-1 also forms protein complexes that act as important transcription factors [121]. Further work is needed in order to determine the full extent that the PKC triggered Ets-1 pathway plays in AD pathophysiology.
Extracellular amyloid buildup can itself interfere with PKC function. Aβ is known to downregulate PKC activity [112, 122]. Aβ decreases PKC in a dose-dependent manner by binding to the PKC pseudosubstrate domain and inhibiting activation [123]. Aβ also disrupts cytosol to membrane translocation of PKCα and PKCε. The disrupted translocation prevents the clearance of Aβ [122]. Improving RACK1 translocation can drastically decrease the Aβ burden by allowing protective PKC isoforms to stimulate the degradation and reduction of Aβ. Novel PKC isoforms, including PKCδ and PKCθ, are heavily involved in mediating Aβ42 processing. Aβ42 triggers changes in phosphatidylinositol 3-kinase, phosphoinositol-dependent kinase, and Rac 1 that ultimately result in cell lysis and the release of reactive oxygen species [124]. It is not yet known if the increase in novel isoforms is strictly an age-dependent adjustment or an indication of accumulated neural injuries. What is known is that increased expression of these isoforms is detrimental to neurons within the brain and leads to an increase in vascular endothelial growth factor [125, 126].
Baseline levels of amyloid and tau within the brain are dependent on protein clearance and cellular metabolism. Imbalances in amyloid metabolism and tau regulation are believed to be critical to AD pathophysiology. Although the toxic effects of Aβ are widely known, the study into the evolutionary benefit of Aβ as an antioxidant is in its infancy [127]. PKC isoforms also serve as potent regulators of tau phosphorylation at serine 199–202 [9]. Importantly, PKCα regulates tau binding to tubulin within axons. If PKCα activity is dysfunctional, tau readily dissociates from tubulin leading to increased tau pathology [6]. Another well-known tau kinase, GSK3β, downregulates the neuroprotective isoform PKCε during AD [128].
INTERRELATING PKC, STROKE, AND AD
Common disease mechanisms link AD and stroke. Loss of synapses is common to both AD and stroke and in AD is most closely correlated with cognitive impairment [129, 130]. Hypoxia is also important for both AD and ischemic disease and increases with age, hypertension, diabetes, and congestive heart failure [131]. AD and ischemic stroke not surprisingly are both independent risk factors for one another [132]. Iron mediated inflammation can activate PKC pathways through glutamate activity in both diseases (Fig. 3) [133]. Toxic iron can be released by microhemorrhages, red blood cell breakdown in the peripheral vasculature, or contusions [134]. Iron contributes to inflammation in the caudate nucleus of AD brains [135]. Through PKC activation, iron enhances the toxicity of Aβ [136]. In stroke, iron overload contributes to peroxynitrate formation and the release of reactive oxygen species [137]. The similarities in injury response between the two diseases are the result of early immune suppression. The development of dementia and atherosclerosis takes a heavy burden on the body’s immune system with inevitable immune exhaustion over time [17]. The heightened state of inflammation and susceptibility to injury is likely due to an altered innate immune response seen in the elderly who are most at risk for these diseases. Toll-like receptors play important roles in neurogenesis and axonal growth in the adult brain, but have also been implicated in the pathology of both stroke and AD [138]. Toll-like receptor 4 contributes to microglia activation in a healthy brain [139]. Toll-like receptor 4 also activates PKCδ leading to neuronal apoptosis, which eliminates damaged cells [140]. It is therefore likely that immune exhaustion, characteristic of AD and ischemic stroke, has broad reaching implications for PKC activity and localization. Such detriments may in part account for functional deficits seen in both of those diseases. Future therapeutics should be targeting both a reconstitution of the immune system as well as directly modulating PKC activity.
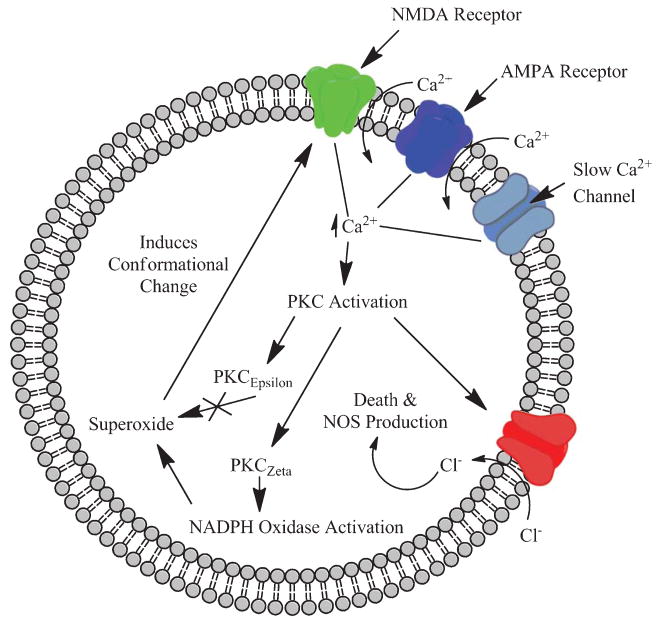
Fig. 3.
Glutamate activation of NMDA and AMPA receptors causes an increase in intracellular calcium. The calcium surge triggers an increase in PKCζ that subsequently leads to superoxide formation. PKC activation also contributes to the formation of nitric oxide synthase (NOS) and associated cell death. An increase in PKCε can mitigate the detrimental effects of oxidative stress and prevent conformational changes at the membrane.
Additionally, the neurovascular unit plays an important role in both AD and ischemic stroke. PKC remodeling of the neurovascular unit has been proposed as a mechanism by which blood-born products enter and accumulate within the brain [141]. Pericytes, astrocytes, and endothelial cells can become damaged during stroke onset and AD progression [142]. A key role of PKC is regulation of tight junction proteins. Tight junction complexes are altered with disease and the integrity of these complexes becomes compromised [143]. Abnormal vascular phenotypes may account for why PKC activity increases in at risk individuals. Vascular phenotypes more susceptible to injury can be driven into a pro-inflammatory state by obesity and diabetes [144]. A recent meta-analysis found that obesity and diabetes are independent risk factors for AD [145]. The important association of PKC changes in specific brain regions during disease and aging is a topic of ongoing investigation (Table 1). Markers such as cyclooxygenase 2 and interleukin 6 interact with PKC through toll-like receptors [21]. Modulation of toll-like receptor 4 acutely will likely decrease BBB disruption, help prevent immune exhaustion, and preserve the neurovascular unit. PKCε would likely be increased at later time points preserving neuronal function and slowing the decline seen in AD and stroke.
Table 1.
PKC isoform changes within the brain for aging, stroke, and AD organized by brain region
| PKC Isoforms | Brain region | Aging | Stroke | AD |
|---|---|---|---|---|
| PKCα | Hippocampus Vasculature | ↓ Hongpaisan et al. [7] | ↑ Ladage et al. [159] | ↓ Sozio et al. [160] |
| PKCβ | Cortex Hippocampus Vasculature | ↓ Shelton et al. [161] | ↑ Gerschutz et al. [162] | ↑ Srivastava et al. [80] |
| PKCδ | Cortex Hippocampus Vasculature | =Pascale et al. [163] | ↓ Bright et al., [164] | =Yi et al. [153] |
| PKCε | Hippocampus Vasculature | ↓ Hongpaisan et al. [7] | ↓ Bright et al. [165] | ↓ Yi et al. [153] |
| PKCζ | Cortex Hippocampus Vasculature | ↓ Galve-Roperh et al. [166] | ↑ Willis et al. [14] | ↓ Moore et al. [167] |
Melatonin administered post-stroke inhibits PKCδ in a rat model, effectively reducing aquaporin-1, brain edema, and infarct size [146]. Curcumin inhibits neuroinflammation by mitigating PKC induced toll-like receptor activation [147]. Our laboratory has shown that the PKC modulator, bryostatin-1, given post-MCAO increased PKCε in an aged-female rat model, improves survival, decreases infarct volume, and leads to an increase in salvageable tissue [148]. At low doses bryostatin activates PKC isoforms, but in excess it has an inhibitory effect. Histamine administration likewise increases PKCε and improves function after stroke [149]. Another approach is to deliver HSP90 or the PKCε specific RACK in order to facilitate enhanced translocation to the mitochondrial membrane, which was shown to reduce stroke infarct volume in a mouse model [74]. PKC mediated platelet aggregation can be inhibited by the phospholipase D inhibitor, FIPI. FIPI decreased the coagulability of platelets following middle cerebral artery occlusion [150]. Further work is required before PKC modulators are ready for clinical treatment. Meanwhile it will be necessary to determine when and where PKC activity is beneficial after stroke and at what time points PKC modification may prove detrimental.
Since PKC isoforms are closely connected to changes in amyloid and tau, PKC modulators are promising therapeutics warranting further investigation. PKC modulators are known to alter concentrations of hyperphosphorylated tau and Aβ. For instance, bryostatin-1, a potent modulator of classic and novel PKC isoforms, effectively reduces Aβ40 and Aβ42 plaques and improves behavioral outcomes [151, 152]. In addition, the effect of bryostatin-1 is significantly greater for transgenic AD mice compared to non-pathologic controls [152]. Bryostatin-1 is not solely dependent on functional PKC in that it directly activates α-secretase as well by increasing PKCε [153]. Low dose bryostatin-1 is currently being used in phase II clinical trials for the treatment of AD. Omega-3 polyunsaturated fatty acids reduce PKC mediated oxidative stress in a transgenic Aβ model [154]. Yessotoxin, a PKC activator, also decreases both hyperphosphorylated tau and Aβ accumulation [10]. It works by inhibiting the tau kinase, GSK3β [155]. GSK3β has an important association with PKC in that both contribute to tau hyperphosphorylation and eventually the development of neurofibrillary tangles in the diseased brain [156, 157]. Alternatively, (1H-indol-3-yl)-maleimide, a selective PKC inhibitor, can increase Aβ accumulation. Increased Aβ disrupts BBB transport and cellular metabolism contributing to rapid AD progression [158]. Many of the available compounds that target PKC have broad reaching endpoints that modulate several different isoforms. Future work will require the development of PKC isoform-specific compounds as well as increased use of transgenic models to tease out the exact role of PKC in AD pathology.
CONCLUSION
PKC isoforms have varied roles in normal and age-related physiology. Alterations in these isoforms contribute to the development of ischemic stroke and AD. Once ischemic stroke has occurred, altered PKC β, δ, and ζ contribute to BBB disruption and reperfusion injury. If PKCε is properly translocated, it can provide neuroprotection. Often, however, pre-existing comorbidities lead to disrupted PKC translocation and worse outcome following ischemic infarction. PKCε is also protective against memory decline in AD, but toxic Aβ contributes to epigenetic downregulation of PKC isoforms with time. Shared pathways between the two diseases such as iron mediated toxicity and immune suppression highlight important targets in injury development and progression. Although much work is yet to be done to increase our understanding about PKC activity in the brain, modulating PKC activity/translocation will enhance neuroprotective strategies for treating neurodegenerative diseases. Future studies are needed to investigate the time points at which PKC isoforms are neuroprotective, and furthermore when they switch to being detrimental.
Acknowledgments
We would like to thank West Virginia University School of Medicine for use of its facility. Funding for this project was provided by a WVU research funding and development grant and funding from the WVU Department of Neurosurgery.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=2418).
References
- 1.Cai HY, Holscher C, Yue XH, Zhang SX, Wang XH, Qiao F, Yang W, Qi JS. Lixisenatide rescues spatial memory and synaptic plasticity from amyloid beta protein-induced impairments in rats. Neuroscience. 2014;277C:6–13. doi: 10.1016/j.neuroscience.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Sutovsky S, Blaho A, Kollar B, Siarnik P, Csefalvay Z, Dragasek J, Turcani P. Clinical accuracy of the distinction between Alzheimer’s disease and frontotemporal lobar degeneration. Bratisl Lek Listy. 2014;115:161–167. doi: 10.4149/bll_2014_034. [DOI] [PubMed] [Google Scholar]
- 3.Sun MK, Alkon DL. The “memory kinases”: Roles of PKC isoforms in signal processing and memory formation. Prog Mol Biol Transl Sci. 2014;122:31–59. doi: 10.1016/B978-0-12-420170-5.00002-7. [DOI] [PubMed] [Google Scholar]
- 4.Liu SJ, Gasperini R, Foa L, Small DH. Amyloid-beta decreases cell-surface AMPA receptors by increasing intracellular calcium and phosphorylation of GluR2. J Alzheimers Dis. 2010;21:655–666. doi: 10.3233/JAD-2010-091654. [DOI] [PubMed] [Google Scholar]
- 5.Dickstein DL, Weaver CM, Luebke JI, Hof PR. Dendritic spine changes associated with normal aging. Neuroscience. 2013;251:21–32. doi: 10.1016/j.neuroscience.2012.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korulu S, Yildiz-Unal A, Yuksel M, Karabay A. Protein kinase C activation causes neurite retraction via cyclinD1 and p60-katanin increase in rat hippocampal neurons. Eur J Neurosci. 2013;37:1610–1619. doi: 10.1111/ejn.12185. [DOI] [PubMed] [Google Scholar]
- 7.Hongpaisan J, Xu C, Sen A, Nelson TJ, Alkon DL. PKC activation during training restores mushroom spine synapses and memory in the aged rat. Neurobiol Dis. 2013;55:44–62. doi: 10.1016/j.nbd.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Menard C, Bastianetto S, Quirion R. Neuroprotective effects of resveratrol and epigallocatechin gallate polyphenols are mediated by the activation of protein kinase C gamma. Front Cell Neurosci. 2013;7:281. doi: 10.3389/fncel.2013.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Montigny A, Elhiri I, Allyson J, Cyr M, Massicotte G. NMDA reduces Tau phosphorylation in rat hippocampal slices by targeting NR2A receptors, GSK3beta, and PKC activities. Neural Plast. 2013;2013:261593. doi: 10.1155/2013/261593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso E, Vale C, Vieytes MR, Botana LM. Translocation of PKC by yessotoxin in an in vitro model of Alzheimer’s disease with improvement of tau and beta-amyloid pathology. ACS Chem Neurosci. 2013;4:1062–1070. doi: 10.1021/cn400018y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun MK, Alkon DL. Activation of protein kinase C isozymes for the treatment of dementias. Adv Pharmacol. 2012;64:273–302. doi: 10.1016/B978-0-12-394816-8.00008-8. [DOI] [PubMed] [Google Scholar]
- 12.Magkou D, Tziomalos K. Antidiabetic treatment, stroke severity and outcome. World J Diabetes. 2014;5:84–88. doi: 10.4239/wjd.v5.i2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu YL, Gao L, Shi L, Li J, Liu WH, Du YH. Effect of electroacupuncture intervention on expression of vascular PKC in the ischemic cerebral tissue in rats with cerebral infarction. Zhen Ci Yan Jiu. 2012;37:218–223. [PubMed] [Google Scholar]
- 14.Willis CL, Meske DS, Davis TP. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cereb Blood Flow Metab. 2010;30:1847–1859. doi: 10.1038/jcbfm.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider A, Rogalewski A, Wafzig O, Kirsch F, Gretz N, Kruger C, Diederich K, Pitzer C, Laage R, Plaas C, Vogt G, Minnerup J, Schabitz WR. Forced arm use is superior to voluntary training for motor recovery and brain plasticity after cortical ischemia in rats. Exp Transl Stroke Med. 2014;6:3. doi: 10.1186/2040-7378-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Shah K, Wang H, Karamyan VT, Abbruscato TJ. Characterization of neuroprotective effects of biphalin, an opioid receptor agonist, in a model of focal brain ischemia. J Pharmacol Exp Ther. 2011;339:499–508. doi: 10.1124/jpet.111.184127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brod SA. Unregulated inflammation shortens human functional longevity. Inflamm Res. 2000;49:561–570. doi: 10.1007/s000110050632. [DOI] [PubMed] [Google Scholar]
- 18.Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, Benatti S, Gibellini L, Cotichini R, Stazi MA, Trenti T, Franceschi C, Cossarizza A. Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflammaging”. Eur J Immunol. 2014;44:1552–1562. doi: 10.1002/eji.201343921. [DOI] [PubMed] [Google Scholar]
- 19.Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Mol Metab. 2013;2:356–363. doi: 10.1016/j.molmet.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang XS, Liu MY, Zhang HM, Xue BZ, Shi H, Liu DX. Protein kinase C-delta mediates sepsis-induced activation of complement 5a and urokinase-type plasminogen activator signaling in macrophages. Inflamm Res. 2014;63:581–589. doi: 10.1007/s00011-014-0729-1. [DOI] [PubMed] [Google Scholar]
- 21.Mesquita RF, Paul MA, Valmaseda A, Francois A, Jabr R, Anjum S, Marber MS, Budhram-Mahadeo V, Heads RJ. Protein kinase Cepsilon-calcineurin cosignaling downstream of toll-like receptor 4 downregulates fibrosis and induces wound healing gene expression in cardiac myofibroblasts. Mol Cell Biol. 2014;34:574–594. doi: 10.1128/MCB.01098-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu SZ, Bullock L, Shan CJ, Cornelius K, Rajanna B. PKC isoforms were reduced by lead in the developing rat brain. Int J Dev Neurosci. 2005;23:53–64. doi: 10.1016/j.ijdevneu.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Ziemba BP, Li J, Landgraf KE, Knight JD, Voth GA, Falke JJ. Single-molecule studies reveal a hidden key step in the activation mechanism of membrane-bound protein kinase C-alpha. Biochemistry. 2014;53:1697–1713. doi: 10.1021/bi4016082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takigami S, Sunada H, Lukowiak K, Kuzirian AM, Alkon DL, Sakakibara M. Protein kinase C mediates memory consolidation of taste avoidance conditioning in Lymnaea stagnalis. Neurobiol Learn Mem. 2014;111:9–18. doi: 10.1016/j.nlm.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 25.de Quervain DJ, Kolassa IT, Ackermann S, Aerni A, Boesiger P, Demougin P, Elbert T, Ertl V, Gschwind L, Hadziselimovic N, Hanser E, Heck A, Hieber P, Huynh KD, Klarhofer M, Luechinger R, Rasch B, Scheffler K, Spalek K, Stippich C, Vogler C, Vukojevic V, Stetak A, Papas-sotiropoulos A. PKCalpha is genetically linked to memory capacity in healthy subjects and to risk for post-traumatic stress disorder in genocide survivors. Proc Natl Acad Sci U S A. 2012;109:8746–8751. doi: 10.1073/pnas.1200857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vijayakumar B, Velmurugan D. Designing of Protein Kinase C beta-II Inhibitors against Diabetic complications: Structure Based Drug Design, Induced Fit docking and analysis of active site conformational changes. Bioinformation. 2012;8:568–573. doi: 10.6026/97320630008568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, You L, Sunyer B, Patil S, Hoger H, Pollak A, Stork O, Lubec G. Hippocampal protein kinase C family members in spatial memory retrieval in the mouse. Behav Brain Res. 2014;258:202–207. doi: 10.1016/j.bbr.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 28.Shanmugasundararaj S, Das J, Sandberg WS, Zhou X, Wang D, Messing RO, Bruzik KS, Stehle T, Miller KW. Structural and functional characterization of an anesthetic binding site in the second cysteine-rich domain of protein kinase Cdelta*. Biophys J. 2012;103:2331–2340. doi: 10.1016/j.bpj.2012.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YA, Kim MY, Jung YS. Glutathione depletion by L-buthionine-S,R-sulfoximine induces apoptosis of cardiomyocytes through activation of PKC-delta. Biomol Ther (Seoul) 2013;21:358–363. doi: 10.4062/biomolther.2013.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheeseman KL, Ueyama T, Michaud TM, Kashiwagi K, Wang D, Flax LA, Shirai Y, Loegering DJ, Saito N, Lennartz MR. Targeting of protein kinase C-epsilon during Fcgamma receptor-dependent phagocytosis requires the epsilonC1B domain and phospholipase C-gamma1. Mol Biol Cell. 2006;17:799–813. doi: 10.1091/mbc.E04-12-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zisopoulou S, Asimaki O, Leondaritis G, Vasilaki A, Sakellaridis N, Pitsikas N, Mangoura D. PKC-epsilon activation is required for recognition memory in the rat. Behav Brain Res. 2013;253:280–289. doi: 10.1016/j.bbr.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Zhang M, Sun GY, Liu YP, Ran WZ, Peng L, Guan CX. Calcitonin gene-related peptide promotes the wound healing of human bronchial epithelial cells via PKC and MAPK pathways. Regul Pept. 2013;184:22–29. doi: 10.1016/j.regpep.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Lauer J, Banerjee D, Shanks D, Dai H, Gong YX, Prakash O, Takemoto D. NMR structure/function relationships of peptides corresponding to the C1B1 Region of PKC gamma. Protein Pept Lett. 2010;17:1–10. doi: 10.2174/092986610789909485. [DOI] [PubMed] [Google Scholar]
- 34.Sanna MD, Quattrone A, Ghelardini C, Galeotti N. PKC-mediated HuD-GAP43 pathway activation in a mouse model of antiretroviral painful neuropathy. Pharmacol Res. 2014;81C:44–53. doi: 10.1016/j.phrs.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt HD, Schassburger RL, Guercio LA, Pierce RC. Stimulation of mGluR5 in the accumbens shell promotes cocaine seeking by activating PKC gamma. J Neurosci. 2013;33:14160–14169. doi: 10.1523/JNEUROSCI.2284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren J, Wang J, Wang Z, Wu J. Structural and biochemical insights into the homotypic PB1-PB1 complex between PKCzeta and p62. Sci China Life Sci. 2014;57:69–80. doi: 10.1007/s11427-013-4592-z. [DOI] [PubMed] [Google Scholar]
- 37.Furini CR, Myskiw JC, Benetti F, Izquierdo I. New frontiers in the study of memory mechanisms. Rev Bras Psiquiatr. 2013;35:173–177. doi: 10.1590/1516-4446-2012-1046. [DOI] [PubMed] [Google Scholar]
- 38.Kwapis JL, Helmstetter FJ. Does PKM(zeta) maintain memory? Brain Res Bull. 2014;105:36–45. doi: 10.1016/j.brainresbull.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solstad T, Bjorgo E, Koehler CJ, Strozynski M, Torgersen KM, Tasken K, Thiede B. Quantitative proteome analysis of detergent-resistant membranes identifies the differential regulation of protein kinase C isoforms in apoptotic T cells. Proteomics. 2010;10:2758–2768. doi: 10.1002/pmic.201000164. [DOI] [PubMed] [Google Scholar]
- 40.Tanabe F, Nakajima T, Ito M. The thiol proteinase inhibitor E-64-d ameliorates amyloid-beta-induced reduction of sAPPalpha secretion by reversing ceramide-induced protein kinase C down-regulation in SH-SY5Y neuroblastoma cells. Biochem Biophys Res Commun. 2013;441:256–261. doi: 10.1016/j.bbrc.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 41.Hoque M, Rentero C, Cairns R, Tebar F, Enrich C, Grewal T. Annexins – Scaffolds modulating PKC localization and signaling. Cell Signal. 2014;26:1213–1225. doi: 10.1016/j.cellsig.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Mishra S, Vinayak M. Ellagic acid inhibits PKC signaling by improving antioxidant defense system in murine T cell lymphoma. Mol Biol Rep. 2014;41:4187–4197. doi: 10.1007/s11033-014-3289-0. [DOI] [PubMed] [Google Scholar]
- 43.Kim HR, Gallant C, Morgan KG. Regulation of PKC autophosphorylation by calponin in contractile vascular smooth muscle tissue. Biomed Res Int. 2013;2013:358643. doi: 10.1155/2013/358643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farah CA, Sossin WS. The role of C2 domains in PKC signaling. Adv Exp Med Biol. 2012;740:663–683. doi: 10.1007/978-94-007-2888-2_29. [DOI] [PubMed] [Google Scholar]
- 45.Miller LD, Lee KC, Mochly-Rosen D, Cartwright CA. RACK1 regulates Src-mediated Sam68 and p190RhoGAP signaling. Oncogene. 2004;23:5682–5686. doi: 10.1038/sj.onc.1207735. [DOI] [PubMed] [Google Scholar]
- 46.Liron T, Chen LE, Khaner H, Vallentin A, Mochly-Rosen D. Rational design of a selective antagonist of epsilon protein kinase C derived from the selective allosteric agonist, pseudo-RACK peptide. J Mol Cell Cardiol. 2007;42:835–841. doi: 10.1016/j.yjmcc.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenwald EC, Redden JM, Dodge-Kafka KL, Saucerman JJ. Scaffold state switching amplifies, accelerates, and insulates protein kinase C signaling. J Biol Chem. 2014;289:2353–2360. doi: 10.1074/jbc.M113.497941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lum MA, Balaburski GM, Murphy ME, Black AR, Black JD. Heat shock proteins regulate activation-induced proteasomal degradation of the mature phosphorylated form of protein kinase C. J Biol Chem. 2013;288:27112–27127. doi: 10.1074/jbc.M112.437095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui ZG, Piao JL, Kondo T, Ogawa R, Tsuneyama K, Zhao QL, Feril LB, Jr, Inadera H. Molecular mechanisms of hyperthermia-induced apoptosis enhanced by docosa-hexaenoic acid: Implication for cancer therapy. Chem Biol Interact. 2014;215:46–53. doi: 10.1016/j.cbi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Cone AC, Cavin G, Ambrosi C, Hakozaki H, Wu-Zhang AX, Kunkel MT, Newton AC, Sosinsky GE. Protein kinase Cdelta-mediated phosphorylation of connexin43 gap junction channels causes movement within gap junctions followed by vesicle internalization and protein degradation. J Biol Chem. 2014;289:8781–8798. doi: 10.1074/jbc.M113.533265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner RC, Lucke-Wold B, Tan Z, Rosen CL, Huber JD. Modulation of protein kinase C isoforms: A potential therapeutic for ischemic stroke? In: Lakatos V, Somogyi B, editors. Ischemic Stroke: Symptoms, Prevention and Recovery (Neuroscience Research Progress) Nova Science Publishers Inc; Hauppauge, NY: 2012. pp. 171–190. [Google Scholar]
- 52.Pascale A, Amadio M, Govoni S, Battaini F. The aging brain, a key target for the future: The protein kinase C involvement. Pharmacol Res. 2007;55:560–569. doi: 10.1016/j.phrs.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Poulose SM, Bielinski DF, Carrihill-Knoll K, Rabin BM, Shukitt-Hale B. Exposure to 16O-particle radiation causes aging-like decrements in rats through increased oxidative stress, inflammation and loss of autophagy. Radiat Res. 2011;176:761–769. doi: 10.1667/rr2605.1. [DOI] [PubMed] [Google Scholar]
- 54.Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKCepsilon gene repression in rat hearts. Circ Res. 2010;107:365–373. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang S, Huang S, Gaertig MA, Li XJ, Li S. Age-dependent decrease in chaperone activity impairs MANF expression, leading to Purkinje cell degeneration in inducible SCA17 mice. Neuron. 2014;81:349–365. doi: 10.1016/j.neuron.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Battaini F, Pascale A, Lucchi L, Pasinetti GM, Govoni S. Protein kinase C anchoring deficit in postmortem brains of Alzheimer’s disease patients. Exp Neurol. 1999;159:559–564. doi: 10.1006/exnr.1999.7151. [DOI] [PubMed] [Google Scholar]
- 57.Liu W, Dou F, Feng J, Yan Z. RACK1 is involved in beta-amyloid impairment of muscarinic regulation of GABAergic transmission. Neurobiol Aging. 2011;32:1818–1826. doi: 10.1016/j.neurobiolaging.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perovic M, Tesic V, Mladenovic Djordjevic A, Smiljanic K, Loncarevic-Vasiljkovic N, Ruzdijic S, Kanazir S. BDNF transcripts, proBDNF and proNGF, in the cortex and hippocampus throughout the life span of the rat. Age (Dordr) 2013;35:2057–2070. doi: 10.1007/s11357-012-9495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin CC, Hsieh HL, Shih RH, Chi PL, Cheng SE, Chen JC, Yang CM. NADPH oxidase 2-derived reactive oxygen species signal contributes to bradykinin-induced matrix metalloproteinase-9 expression and cell migration in brain astrocytes. Cell Commun Signal. 2012;10:35. doi: 10.1186/1478-811X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vetri F, Chavez R, Xu HL, Paisansathan C, Pelligrino DA. Complex modulation of the expression of PKC isoforms in the rat brain during chronic type 1 diabetes mellitus. Brain Res. 2013;1490:202–209. doi: 10.1016/j.brainres.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang L, Huang D, Shen D, Zhang C, Ma Y, Babcock SA, Chen B, Ren J. Inhibition of protein kinase C betaII isoform ameliorates methylglyoxal advanced glycation endproduct-induced cardiomyocyte contractile dysfunction. Life Sci. 2014;94:83–91. doi: 10.1016/j.lfs.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 62.Klymenko K, Novokhatska T, Kizub I, Parshikov A, Dosenko V, Soloviev A. PKC-delta isozyme gene silencing restores vascular function in diabetic rat. J Basic Clin Physiol Pharmacol. 2014;26:1–9. doi: 10.1515/jbcpp-2013-0147. [DOI] [PubMed] [Google Scholar]
- 63.Fan Y, Li J, Zhang YQ, Jiang LH, Zhang YN, Yan CQ. Protein kinase C delta mediated cytotoxicity of 6-Hydroxydopamine via sustained extracellular signal-regulated kinase 1/2 activation in PC12 cells. Neurol Res. 2014;36:53–64. doi: 10.1179/1743132813Y.0000000267. [DOI] [PubMed] [Google Scholar]
- 64.Liu L, Liu J, Gao Y, Yu X, Dou D, Huang Y. Protein kinase Cdelta contributes to phenylephrine-mediated contraction in the aortae of high fat diet-induced obese mice. Biochem Biophys Res Commun. 2014;446:1179–1183. doi: 10.1016/j.bbrc.2014.03.065. [DOI] [PubMed] [Google Scholar]
- 65.Yang P, Baciu P, Parker Kerrigan B, Etheridge M, Sung E, Toimil BA, Berchuck JE, Jaffe GJ. Retinal pigment epithelial cell death by the alternative complement cascade: Role of membrane regulatory proteins, calcium, PKC and oxidative stress. Invest Ophthalmol Vis Sci. 2014;55:3012–3021. doi: 10.1167/iovs.13-13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kong L, Shen X, Lin L, Leitges M, Rosario R, Zou YS, Yan SF. PKCbeta promotes vascular inflammation and acceleration of atherosclerosis in diabetic ApoE null mice. Arterioscler Thromb Vasc Biol. 2013;33:1779–1787. doi: 10.1161/ATVBAHA.112.301113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gebremedhin D, Gopalakrishnan S, Harder DR. Endogenous events modulating myogenic regulation of cerebrovascular function. Curr Vasc Pharmacol. 2013;11:1–11. doi: 10.2174/15701611113116660153. [DOI] [PubMed] [Google Scholar]
- 68.Bright R, Mochly-Rosen D. The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke. 2005;36:2781–2790. doi: 10.1161/01.STR.0000189996.71237.f7. [DOI] [PubMed] [Google Scholar]
- 69.Cui GY, Gao XM, Qi SH, Gillani A, Gao L, Shen X, Zhang YD. The action of thrombin in intracerebral hemorrhage induced brain damage is mediated via PKCalpha/PKCdelta signaling. Brain Res. 2011;1398:86–93. doi: 10.1016/j.brainres.2010.11.095. [DOI] [PubMed] [Google Scholar]
- 70.Jeong C, Shin T. Immunohistochemical localization of protein kinase C (PKC) beta I in the pig retina during postnatal development. Acta Histochem. 2012;114:18–23. doi: 10.1016/j.acthis.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Rex EB, Rankin ML, Yang Y, Lu Q, Gerfen CR, Jose PA, Sibley DR. Identification of RanBP 9/10 as interacting partners for protein kinase C (PKC) gamma/delta and the D1 dopamine receptor: Regulation of PKC-mediated receptor phosphorylation. Mol Pharmacol. 2010;78:69–80. doi: 10.1124/mol.110.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang HH, Hsieh HL, Wu CY, Yang CM. Oxidized low-density lipoprotein-induced matrix metalloproteinase-9 expression via PKC-delta/p42/p44 MAPK/Elk-1 cascade in brain astrocytes. Neurotox Res. 2010;17:50–65. doi: 10.1007/s12640-009-9077-2. [DOI] [PubMed] [Google Scholar]
- 73.Angelow S, Zeni P, Hohn B, Galla HJ. Phorbol ester induced short- and long-term permeabilization of the blood-CSF barrier in vitro. Brain Res. 2005;1063:168–179. doi: 10.1016/j.brainres.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 74.Sun X, Budas GR, Xu L, Barreto GE, Mochly-Rosen D, Giffard RG. Selective activation of protein kinase C in mitochondria is neuroprotective in vitro and reduces focal ischemic brain injury in mice. J Neurosci Res. 2013;91:799–807. doi: 10.1002/jnr.23186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim YA, Park SL, Kim MY, Lee SH, Baik EJ, Moon CH, Jung YS. Role of PKCbetaII and PKCdelta in blood-brain barrier permeability during aglycemic hypoxia. Neurosci Lett. 2010;468:254–258. doi: 10.1016/j.neulet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 76.Payne GW, Smeda JS. Cerebrovascular alterations in pressure and protein kinase C-mediated constriction in Dahl salt-sensitive rats. J Hypertens. 2002;20:1355–1363. doi: 10.1097/00004872-200207000-00022. [DOI] [PubMed] [Google Scholar]
- 77.Zhou F, Lee AC, Krafczyk K, Zhu L, Murray M. Protein kinase C regulates the internalization and function of the human organic anion transporting polypeptide 1A2. Br J Pharmacol. 2011;162:1380–1388. doi: 10.1111/j.1476-5381.2010.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ji BS, Cen J, He L, Liu M, Liu YQ, Liu L. Modulation of P-glycoprotein in rat brain microvessel endothelial cells under oxygen glucose deprivation. J Pharm Pharmacol. 2013;65:1508–1517. doi: 10.1111/jphp.12122. [DOI] [PubMed] [Google Scholar]
- 79.Yagami T, Yamamoto Y, Koma H. The role of secretory phospholipase A2 in the central nervous system and neurological diseases. Mol Neurobiol. 2014;49:863–876. doi: 10.1007/s12035-013-8565-9. [DOI] [PubMed] [Google Scholar]
- 80.Srivastava K, Shao B, Bayraktutan U. PKC-beta exacerbates in vitro brain barrier damage in hyperglycemic settings via regulation of RhoA/Rho-kinase/MLC2 pathway. J Cereb Blood Flow Metab. 2013;33:1928–1936. doi: 10.1038/jcbfm.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mizutani K, Sonoda S, Wakita H, Katoh Y, Shimpo K. Functional recovery and alterations in the expression and localization of protein kinase C following voluntary exercise in rat with cerebral infarction. Neurol Sci. 2014;35:53–59. doi: 10.1007/s10072-013-1477-7. [DOI] [PubMed] [Google Scholar]
- 82.Shao B, Bayraktutan U. Hyperglycaemia promotes cerebral barrier dysfunction through activation of protein kinase C-beta. Diabetes Obes Metab. 2013;15:993–999. doi: 10.1111/dom.12120. [DOI] [PubMed] [Google Scholar]
- 83.Brennan-Minnella AM, Shen Y, El-Benna J, Swanson RA. Phosphoinositide 3-kinase couples NMDA receptors to superoxide release in excitotoxic neuronal death. Cell Death Dis. 2013;4:e580. doi: 10.1038/cddis.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang YP, Zhang H, Duan DD. Chloride channels in stroke. Acta Pharmacol Sin. 2013;34:17–23. doi: 10.1038/aps.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Katakam PV, Snipes JA, Steed MM, Busija DW. Insulin-induced generation of reactive oxygen species and uncoupling of nitric oxide synthase underlie the cerebrovascular insulin resistance in obese rats. J Cereb Blood Flow Metab. 2012;32:792–804. doi: 10.1038/jcbfm.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gundimeda U, McNeill TH, Elhiani AA, Schiffman JE, Hinton DR, Gopalakrishna R. Green tea polyphenols precondition against cell death induced by oxygen-glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase Cepsilon. J Biol Chem. 2012;287:34694–34708. doi: 10.1074/jbc.M112.356899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng S, Li D, Li Y, Yang X, Han S, Li J. Insight into hypoxic preconditioning and ischemic injury through determination of nPKCepsilon-interacting proteins in mouse brain. Neurochem Int. 2013;63:69–79. doi: 10.1016/j.neuint.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 88.Ye Z, Guo Q, Wang N, Xia P, Yuan Y, Wang E. Delayed neuroprotection induced by sevoflurane via opening mitochondrial ATP-sensitive potassium channels and p38 MAPK phosphorylation. Neurol Sci. 2012;33:239–249. doi: 10.1007/s10072-011-0665-6. [DOI] [PubMed] [Google Scholar]
- 89.Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, Lowell CA, Ferriero DM, Messing RO. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J Clin Invest. 2004;114:49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loegering DJ, Lennartz MR. Protein kinase C and toll-like receptor signaling. Enzyme Res. 2011;2011:537821. doi: 10.4061/2011/537821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farese RV, Sajan MP. Metabolic functions of atypical protein kinase C: “good” and “bad” as defined by nutritional status. Am J Physiol Endocrinol Metab. 2010;298:E385–E394. doi: 10.1152/ajpendo.00608.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70:1537–1547. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howard VJ, Woolson RF, Egan BM, Nicholas JS, Adams RJ, Howard G, Lackland DT. Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens. 2010;4:32–41. doi: 10.1016/j.jash.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao X, Zhang H, Takahashi T, Hsieh J, Liao J, Steinberg GK, Zhao H. The Akt signaling pathway contributes to postconditioning’s protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem. 2008;105:943–955. doi: 10.1111/j.1471-4159.2008.05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yeung J, Apopa PL, Vesci J, Kenyon V, Rai G, Jadhav A, Simeonov A, Holman TR, Maloney DJ, Boutaud O, Holinstat M. Protein kinase C regulation of 12-lipoxygenase-mediated human platelet activation. Mol Pharmacol. 2012;81:420–430. doi: 10.1124/mol.111.075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Faisal A, Saurin A, Gregory B, Foxwell B, Parker PJ. The scaffold MyD88 acts to couple protein kinase Cepsilon to Toll-like receptors. J Biol Chem. 2008;283:18591–18600. doi: 10.1074/jbc.M710330200. [DOI] [PubMed] [Google Scholar]
- 98.Simpkins JW, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Brain Res Rev. 2008;57:421–430. doi: 10.1016/j.brainresrev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 99.Simpkins JW, Yi KD, Yang SH, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Biochim Biophys Acta. 2010;1800:1113–1120. doi: 10.1016/j.bbagen.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amadoro G, Corsetti V, Florenzano F, Atlante A, Ciotti MT, Mongiardi MP, Bussani R, Nicolin V, Nori SL, Campanella M, Calissano P. AD-linked, toxic NH2 human tau affects the quality control of mitochondria in neurons. Neurobiol Dis. 2014;62:489–507. doi: 10.1016/j.nbd.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 101.Hasham MI, Pelech SL, Krieger C. Glutamate-mediated activation of protein kinase C in hippocampal neurons. Neurosci Lett. 1997;228:115–118. doi: 10.1016/s0304-3940(97)00382-0. [DOI] [PubMed] [Google Scholar]
- 102.Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vicente-Torres MA, Davila D, Bartolome MV, Carricondo F, Gil-Loyzaga P. Biochemical evidence for the presence of serotonin transporters in the rat cochlea. Hear Res. 2003;182:43–47. doi: 10.1016/s0378-5955(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 104.Chen XK, Wang LC, Zhou Y, Cai Q, Prakriya M, Duan KL, Sheng ZH, Lingle C, Zhou Z. Activation of GPCRs modulates quantal size in chromaffin cells through G(betagamma) and PKC. Nat Neurosci. 2005;8:1160–1168. doi: 10.1038/nn1529. [DOI] [PubMed] [Google Scholar]
- 105.Shema R, Hazvi S, Sacktor TC, Dudai Y. Boundary conditions for the maintenance of memory by PKMzeta in neocortex. Learn Mem. 2009;16:122–128. doi: 10.1101/lm.1183309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R, Leitges M, Sweatt JD. A role for the beta isoform of protein kinase C in fear conditioning. J Neurosci. 2000;20:5906–5914. doi: 10.1523/JNEUROSCI.20-16-05906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 108.Bordi F, Marcon C, Chiamulera C, Reggiani A. Effects of the metabotropic glutamate receptor antagonist MCPG on spatial and context-specific learning. Neuropharmacology. 1996;35:1557–1565. doi: 10.1016/s0028-3908(96)00101-3. [DOI] [PubMed] [Google Scholar]
- 109.Hargreaves EL, Cain DP. Hyperactivity, hyper-reactivity, and sensorimotor deficits induced by low doses of the N-methyl-D-aspartate non-competitive channel blocker MK801. Behav Brain Res. 1992;47:23–33. doi: 10.1016/s0166-4328(05)80249-9. [DOI] [PubMed] [Google Scholar]
- 110.Shema R, Haramati S, Ron S, Hazvi S, Chen A, Sacktor TC, Dudai Y. Enhancement of consolidated long-term memory by overexpression of protein kinase Mzeta in the neocortex. Science. 2011;331:1207–1210. doi: 10.1126/science.1200215. [DOI] [PubMed] [Google Scholar]
- 111.Govoni S, Bergamaschi S, Racchi M, Battaini F, Binetti G, Bianchetti A, Trabucchi M. Cytosol protein kinase C downregulation in fibroblasts from Alzheimer’s disease patients. Neurology. 1993;43:2581–2586. doi: 10.1212/wnl.43.12.2581. [DOI] [PubMed] [Google Scholar]
- 112.Masliah E, Cole GM, Hansen LA, Mallory M, Albright T, Terry RD, Saitoh T. Protein kinase C alteration is an early biochemical marker in Alzheimer’s disease. J Neurosci. 1991;11:2759–2767. doi: 10.1523/JNEUROSCI.11-09-02759.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Corsini E, Battaini F, Lucchi L, Marinovich M, Racchi M, Govoni S, Galli CL. A defective protein kinase C anchoring system underlying age-associated impairment in TNF-alpha production in rat macrophages. J Immunol. 1999;163:3468–3473. [PubMed] [Google Scholar]
- 114.McKee AC, Kosik KS, Kennedy MB, Kowall NW. Hippocampal neurons predisposed to neurofibrillary tangle formation are enriched in type II calcium/calmodulin-dependent protein kinase. J Neuropathol Exp Neurol. 1990;49:49–63. doi: 10.1097/00005072-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 115.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 116.Buoso E, Biundo F, Lanni C, Aiello S, Grossi S, Schettini G, Govoni S, Racchi M. Modulation of Rack-1/PKCbetaII signalling by soluble AbetaPPalpha in SH-SY5Y cells. Curr Alzheimer Res. 2013;10:697–705. doi: 10.2174/15672050113109990145. [DOI] [PubMed] [Google Scholar]
- 117.Gerschutz A, Heinsen H, Grunblatt E, Wagner AK, Bartl J, Meissner C, Fallgatter AJ, Al-Sarraj S, Troakes C, Ferrer I, Arzberger T, Deckert J, Riederer P, Fischer M, Tatschner T, Monoranu CM. Neuron-specific mitochondrial DNA deletion levels in sporadic Alzheimer’s disease. Curr Alzheimer Res. 2013;10:1041–1046. doi: 10.2174/15672050113106660166. [DOI] [PubMed] [Google Scholar]
- 118.de Barry J, Liegeois CM, Janoshazi A. Protein kinase C as a peripheral biomarker for Alzheimer’s disease. Exp Gerontol. 2010;45:64–69. doi: 10.1016/j.exger.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 119.Kumar S, Sieghart W, Morrow AL. Association of protein kinase C with GABA(A) receptors containing alpha1 and alpha4 subunits in the cerebral cortex: Selective effects of chronic ethanol consumption. J Neurochem. 2002;82:110–117. doi: 10.1046/j.1471-4159.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- 120.Pacheco-Quinto J, Eckman EA. Endothelin-converting enzymes degrade intracellular beta-amyloid produced within the endosomal/lysosomal pathway and autophagosomes. J Biol Chem. 2013;288:5606–5615. doi: 10.1074/jbc.M112.422964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sementchenko VI, Watson DK. Ets target genes: Past, present and future. Oncogene. 2000;19:6533–6548. doi: 10.1038/sj.onc.1204034. [DOI] [PubMed] [Google Scholar]
- 122.Pakaski M, Papp H, Rakonczay Z, Fakla I, Kasa P. Effects of acetylcholinesterase inhibitors on the metabolism of amyloid precursor protein in vitro. Neurobiology (Bp) 2001;9:55–57. doi: 10.1556/neurob.9.2001.1.11. [DOI] [PubMed] [Google Scholar]
- 123.Chauhan A, Chauhan VP, Brockerhoff H, Wisniewski HM. Action of amyloid beta-protein on protein kinase C activity. Life Sci. 1991;49:1555–1562. doi: 10.1016/0024-3205(91)90328-9. [DOI] [PubMed] [Google Scholar]
- 124.Manterola L, Hernando-Rodriguez M, Ruiz A, Apraiz A, Arrizabalaga O, Vellon L, Alberdi E, Cavaliere F, Lacerda HM, Jimenez S, Parada LA, Matute C, Zugaza JL. 1-42 beta-amyloid peptide requires PDK1/nPKC/Rac 1 pathway to induce neuronal death. Transl Psychiatry. 2013;3:e219. doi: 10.1038/tp.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rigor RR, Beard RS, Jr, Litovka OP, Yuan SY. Interleukin-1beta-induced barrier dysfunction is signaled through PKC-theta in human brain microvascular endothelium. Am J Physiol Cell Physiol. 2012;302:C1513–C1522. doi: 10.1152/ajpcell.00371.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee MC, Wei SC, Tsai-Wu JJ, Wu CH, Tsao PN. Novel PKC signaling is required for LPS-induced soluble Flt-1 expression in macrophages. J Leukoc Biol. 2008;84:835–841. doi: 10.1189/jlb.1007691. [DOI] [PubMed] [Google Scholar]
- 127.Luna S, Cameron DJ, Ethell DW. Amyloid-beta and APP deficiencies cause severe cerebrovascular defects: Important work for an old villain. PLoS One. 2013;8:e75052. doi: 10.1371/journal.pone.0075052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cai Z, Zhao Y, Zhao B. Roles of glycogen synthase kinase 3 in Alzheimer’s disease. Curr Alzheimer Res. 2012;9:864–879. doi: 10.2174/156720512802455386. [DOI] [PubMed] [Google Scholar]
- 129.Sun MK, Hongpaisan J, Nelson TJ, Alkon DL. Post-stroke neuronal rescue and synaptogenesis mediated in vivo by protein kinase C in adult brains. Proc Natl Acad Sci U S A. 2008;105:13620–13625. doi: 10.1073/pnas.0805952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hongpaisan J, Sun MK, Alkon DL. PKC epsilon activation prevents synaptic loss, Abeta elevation, and cognitive deficits in Alzheimer’s disease transgenic mice. J Neurosci. 2011;31:630–643. doi: 10.1523/JNEUROSCI.5209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun MK, Hongpaisan J, Alkon DL. Postischemic PKC activation rescues retrograde and anterograde long-term memory. Proc Natl Acad Sci U S A. 2009;106:14676–14680. doi: 10.1073/pnas.0907842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heikkinen R, Malm T, Heikkila J, Muona A, Tanila H, Koistinaho M, Koistinaho J. Susceptibility to focal and global brain ischemia of Alzheimer mice displaying abeta deposits: Effect of immunoglobulin. Aging Dis. 2014;5:76–87. doi: 10.14336/AD.2014.050076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pretorius E, Kell DB. Diagnostic morphology: Biophysical indicators for iron-driven inflammatory diseases. Integr Biol (Camb) 2014;6:486–510. doi: 10.1039/c4ib00025k. [DOI] [PubMed] [Google Scholar]
- 134.Yavuz BB, Cankurtaran M, Haznedaroglu IC, Halil M, Ulger Z, Altun B, Ariogul S. Iron deficiency can cause cognitive impairment in geriatric patients. J Nutr Health Aging. 2012;16:220–224. doi: 10.1007/s12603-011-0351-7. [DOI] [PubMed] [Google Scholar]
- 135.De Reuck JL, Deramecourt V, Auger F, Durieux N, Cordonnier C, Devos D, Defebvre L, Moreau C, Caparros-Lefebvre D, Leys D, Maurage CA, Pasquier F, Bordet R. Iron deposits in post-mortem brains of patients with neurodegenerative and cerebrovascular diseases: A semi-quantitative 7.0 T magnetic resonance imaging study. Eur J Neurol. 2014;21:1026–1031. doi: 10.1111/ene.12432. [DOI] [PubMed] [Google Scholar]
- 136.Bandyopadhyay S, Rogers JT. Alzheimer’s disease therapeutics targeted to the control of amyloid precursor protein translation: Maintenance of brain iron homeostasis. Biochem Pharmacol. 2014;88:486–494. doi: 10.1016/j.bcp.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gamez A, Carbonell T, Rama R. Does nitric oxide contribute to iron-dependent brain injury after experimental cerebral ischaemia? J Physiol Biochem. 2003;59:249–254. doi: 10.1007/BF03179881. [DOI] [PubMed] [Google Scholar]
- 138.Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem. 2010;114:13–27. doi: 10.1111/j.1471-4159.2010.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- 141.Elali A, Theriault P, Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci. 2014;15:6453–6474. doi: 10.3390/ijms15046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kelleher RJ, Soiza RL. Evidence of endothelial dysfunction in the development of Alzheimer’s disease: Is Alzheimer’s a vascular disorder? Am J Cardiovasc Dis. 2013;3:197–226. [PMC free article] [PubMed] [Google Scholar]
- 143.Kook SY, Seok Hong H, Moon M, Mook-Jung I. Disruption of blood-brain barrier in Alzheimer disease pathogenesis. Tissue Barriers. 2013;1:e23993. doi: 10.4161/tisb.23993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim E, Zhang J, Hong K, Benoit NE, Pathak AP. Vascular phenotyping of brain tumors using magnetic resonance microscopy (muMRI) J Cereb Blood Flow Metab. 2011;31:1623–1636. doi: 10.1038/jcbfm.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 146.Bhattacharya P, Pandey AK, Paul S, Patnaik R. Melatonin renders neuroprotection by protein kinase C mediated aquaporin-4 inhibition in animal model of focal cerebral ischemia. Life Sci. 2014;100:97–109. doi: 10.1016/j.lfs.2014.01.085. [DOI] [PubMed] [Google Scholar]
- 147.Yang Z, Zhao T, Zhang JH, Feng H. Curcumin inhibits microglia inflammation and confers neuroprotection in intracerebral hemorrhage. Immunol Lett. 2014;160:89–95. doi: 10.1016/j.imlet.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 148.Tan Z, Turner RC, Leon RL, Li X, Hongpaisan J, Zheng W, Logsdon AF, Naser ZJ, Alkon DL, Rosen CL, Huber JD. Bryostatin improves survival and reduces ischemic brain injury in aged rats after acute ischemic stroke. Stroke. 2013;44:3490–3497. doi: 10.1161/STROKEAHA.113.002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang XF, Hu WW, Yan HJ, Tan L, Gao JQ, Tian YY, Shi XJ, Hou WW, Li J, Shen Y, Chen Z. Modulation of astrocytic glutamine synthetase expression and cell viability by histamine in cultured cortical astrocytes exposed to OGD insults. Neurosci Lett. 2013;549:69–73. doi: 10.1016/j.neulet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 150.Elvers M, Grenegard M, Khoshjabinzadeh H, Munzer P, Borst O, Tian H, Di Paolo G, Lang F, Gawaz M, Lindahl TL, Falker K. A novel role for phospholipase D as an endogenous negative regulator of platelet sensitivity. Cell Signal. 2012;24:1743–1752. doi: 10.1016/j.cellsig.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 151.Sridhar J, Wei ZL, Nowak I, Lewin NE, Ayres JA, Pearce LV, Blumberg PM, Kozikowski AP. New bivalent PKC ligands linked by a carbon spacer: Enhancement in binding affinity. J Med Chem. 2003;46:4196–4204. doi: 10.1021/jm0302041. [DOI] [PubMed] [Google Scholar]
- 152.Etcheberrigaray R, Tan M, Dewachter I, Kuiperi C, Van der Auwera I, Wera S, Qiao L, Bank B, Nelson TJ, Kozikowski AP, Van Leuven F, Alkon DL. Therapeutic effects of PKC activators in Alzheimer’s disease transgenic mice. Proc Natl Acad Sci U S A. 2004;101:11141–11146. doi: 10.1073/pnas.0403921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yi P, Schrott L, Castor TP, Alexander JS. Bryostatin-1 vs. TPPB: Dose-dependent APP processing and PKC-alpha, -delta, and -epsilon isoform activation in SH-SY5Y neuronal cells. J Mol Neurosci. 2012;48:234–244. doi: 10.1007/s12031-012-9816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hammamieh R, Chakraborty N, Gautam A, Miller SA, Muhie S, Meyerhoff J, Jett M. Transcriptomic analysis of the effects of a fish oil enriched diet on murine brains. PLoS One. 2014;9:e90425. doi: 10.1371/journal.pone.0090425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fang X, Yu S, Tanyi JL, Lu Y, Woodgett JR, Mills GB. Convergence of multiple signaling cascades at glycogen synthase kinase 3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol Cell Biol. 2002;22:2099–2110. doi: 10.1128/MCB.22.7.2099-2110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribe E, Dalfo E, Avila J. Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathies. Curr Alzheimer Res. 2005;2:3–18. doi: 10.2174/1567205052772713. [DOI] [PubMed] [Google Scholar]
- 157.Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol. 1999;58:1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 158.Wang DS, Dickson DW, Malter JS. beta-Amyloid degradation and Alzheimer’s disease. J Biomed Biotechnol. 2006;2006:58406. doi: 10.1155/JBB/2006/58406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ladage D, Tilemann L, Ishikawa K, Correll RN, Kawase Y, Houser SR, Molkentin JD, Hajjar RJ. Inhibition of PKCalpha/beta with ruboxistaurin antagonizes heart failure in pigs after myocardial infarction injury. Circ Res. 2011;109:1396–1400. doi: 10.1161/CIRCRESAHA.111.255687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sozio P, Rapino M, Di Valerio V, Laserra S, Pacella S, Di Stefano A, Cataldi A. pPKCalpha-mediated effect on in vitro Abeta production in response to gamma secretase inhibitor LY411575 in rat CTXTNA2 astrocytes. J Biol Regul Homeost Agents. 2012;26:245–251. [PubMed] [Google Scholar]
- 161.Shelton RC, Sanders-Bush E, Manier DH, Lewis DA. Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience. 2009;158:1406–1415. doi: 10.1016/j.neuroscience.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gerschutz A, Heinsen H, Grunblatt E, Wagner AK, Bartl J, Meissner C, Fallgatter AJ, Al-Sarraj S, Troakes C, Ferrer I, Arzberger T, Deckert J, Riederer P, Fischer M, Tatschner T, Monoranu CM. Neuron-specific alterations in signal transduction pathways associated with Alzheimer’s disease. J Alzheimers Dis. 2014;40:135–142. doi: 10.3233/JAD-131280. [DOI] [PubMed] [Google Scholar]
- 163.Pascale A, Fortino I, Govoni S, Trabucchi M, Wetsel WC, Battaini F. Functional impairment in protein kinase C by RACK1 (receptor for activated C kinase 1) deficiency in aged rat brain cortex. J Neurochem. 1996;67:2471–2477. doi: 10.1046/j.1471-4159.1996.67062471.x. [DOI] [PubMed] [Google Scholar]
- 164.Bright R, Steinberg GK, Mochly-Rosen D. DeltaPKC mediates microcerebrovascular dysfunction in acute ischemia and in chronic hypertensive stress in vivo. Brain Res. 2007;1144:146–155. doi: 10.1016/j.brainres.2007.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bright R, Sun GH, Yenari MA, Steinberg GK, Mochly-Rosen D. epsilonPKC confers acute tolerance to cerebral ischemic reperfusion injury. Neurosci Lett. 2008;441:120–124. doi: 10.1016/j.neulet.2008.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Galve-Roperh I, Malpartida JM, Garcia-Barreno P, Haro A, Laviada ID. Levels and activity of brain protein kinase C alpha and zeta during the aging of the medfly. Mech Ageing Dev. 1996;92:21–29. doi: 10.1016/s0047-6374(96)01799-x. [DOI] [PubMed] [Google Scholar]
- 167.Moore P, White J, Christiansen V, Grammas P. Protein kinase C-zeta activity but not level is decreased in Alzheimer’s disease microvessels. Neurosci Lett. 1998;254:29–32. doi: 10.1016/s0304-3940(98)00653-3. [DOI] [PubMed] [Google Scholar]