Introduction
Metastasis is the main cause of treatment failure and death for cancer patients. The involvement of lymphatic system with cancer has long been recognized as an important indicator of cancer aggressiveness. Lymph node status is one of the key parameters used for determining stage of disease progression and it is a powerful predictor of patient survival (Edge, 2010). Patients with lymph node metastases are also more likely to present with disease recurrence (Rosen, 2008). However, the causal link between lymphatic dissemination and the negative outcome is not understood and how exactly the lymphatic system contributes to cancer progression from localized to systemic, disseminated disease remains a critical open question. Although the number of publications on the topic of cancer lymphatics has been growing steadily over the past decade, there is still a lot to be learned. This review highlights advances in our understanding of the mechanisms by which lymphatic vessels, and in particular lymphatic endothelium, impact metastasis.
Tumor lymphangiogenesis
Upon identification of VEGF-C and VEGF-D as lymphangiogenesis factors(Jeltsch et al., 1997; Joukov et al., 1996; Joukov et al., 1997), we and others have reported more than a decade ago that induction of lymphangiogenesis by the tumor facilitates metastatic spread (Mandriota et al., 2001; Skobe et al., 2001; Stacker et al., 2001). Since then, work from many laboratories has recapitulated these findings in numerous animal models and further showed that inhibition of lymphangiogenesis by blockade of VEGF-C or its receptor VEGFR-3, prevents lymph node metastases without significantly affecting primary tumor growth (Brakenhielm et al., 2007; Burton et al., 2008; Chen et al., 2005; He et al., 2005; Kawakami et al., 2005; Krishnan et al., 2003; Lin et al., 2005; Mandriota et al., 2001; Mattila et al., 2002; Skobe et al., 2001; Yanai et al., 2001). VEGF-C also facilitates metastatic spread to distant sites and, conversely, blocking VEGF-C or VEGFR-3 inhibits distant metastases in majority of experimental models (Brakenhielm et al., 2007; Burton et al., 2008; Chen et al., 2005; Krishnan et al., 2003; Lin et al., 2005; Roberts et al., 2006). In agreement with the preclinical data, numerous clinical studies reaffirmed the negative correlation between VEGF-C, lymphangiogenesis and patient outcome (Alitalo and Carmeliet, 2002; Ding et al., 2007; Furudoi et al., 2002; Miyazaki et al., 2008; Mohammed et al., 2007; Pepper et al., 2003; Swartz and Skobe, 2001; Tsutsumi et al., 2005). VEGF-C and VEGF-D are most specific and best studied lymphangiogenesis factors, however, tumor lymphangiogenesis can be mediated also by several pleiotropic factors, including PDGF-BB, IGFs, FGF2, HGF, Ang2, adrenomedulin and IL-7 (Zheng et al., 2014).
Lymphangiogenesis associated with the primary tumor is thought to increase metastasis by increasing the probability for tumor cells to enter into the lymphatic vessels. Large numbers of newly generated lymphatics create more opportunities for tumor cell exit and close proximity of tumor cells to LECs could make more tumor cells respond to LEC-derived chemokines and be mobilized into the lymphatics. Furthermore, gene-profiling data of tumor-activated and quiescent lymphatic endothelium showed significantly different expression profile, suggesting that tumor cells may interact differently with the pre-existing and with the newly formed lymphatics (Clasper et al., 2008). The nature and significance of that cross-talk, however, remain to be elucidated. Importantly, while tumor lymphangiogenesis profoundly increases metastatic spread, it is not an obligatory step for metastasis. Controversy on this topic stems from the assumption that if angiogenesis is required for tumor growth, by inference, lymphangiogenesis must be a requirement for metastasis. However, paradigms established for tumor angiogenesis cannot be extrapolated on lymphangiogenesis, since function of lymphatics and blood vessels in tumors is very different despite the fact that the endothelial biology of these two vascular systems is shared on many levels.
Interestingly, lymphangiogenesis in the sentinel lymph nodes has been shown to precede lymph node metastasis in several studies(Dadras et al., 2005; Harrell et al., 2007; Hirakawa et al., 2007; Hirakawa et al., 2005; Ruddell et al., 2008; Van den Eynden et al., 2006; Van den Eynden et al., 2007). Lymph node lymphangiogenesis is a component of the normal host immune response (Angeli et al., 2006; Kim et al., 2012; Randolph et al., 2005), which in the tumor setting is thought to enhance metastasis by creating a pre-metastatic niche. Because selective inhibition of lymph node lymphangiogenesis is difficult to achieve, this concept is derived mainly from correlative studies and more work is needed to elucidate exact mechanisms and roles of LN lymphangiogenesis in cancer spread. Lymphangiogenesis has also been documented within metastases in the sentinel and more distal lymph nodes (Kerjaschki et al., 2011). Furthermore, this study indicated that tumor cell invasion into the intrametastatic lymphatic vessels and formation of tumor emboli is necessary for metastatic dissemination into more distal lymph nodes (Kerjaschki et al., 2011).
Mechanisms of lymph node metastasis
Many important questions about lymph node metastasis remain unresolved to date. Lymph nodes are usually first sites of detectable metastases, which could be due to the preference of tumor cells to enter into the lymphatic vessels. It is not known however, whether such preference exists and whether tumor cell rate of entry into the lymphatic and blood vessels is different. Alternatively, early metastasis in the lymph nodes could be a result of survival or growth advantage within the lymph node microenvironment. Another key unresolved question is to which extent lymph node metastases directly contribute to the formation of distant metastases. While these issues have been frequently debated, there is no data to clearly support or oppose any of the aforementioned concepts.
Over decades, lymphatics were portrayed as passive participants in metastasis and regarded mainly as a transportation system. Recent studies, however, indicate that tumor cells are guided into the lymphatic vessels by chemokines produced by lymphatic endothelium (Ben-Baruch, 2008; Das and Skobe, 2008). CCL21 is constitutively expressed by the lymphatic vessels (Gunn et al., 1998; Kerjaschki et al., 2004; Podgrabinska et al., 2002; Shields et al., 2007), immobilized by binding to heparin sulfates and forms steep gradients within the perilymphatic interstitium (Haessler et al., 2011; Schumann et al., 2010; Weber et al., 2013). These gradients induce directed migration of dendritic cells towards lymphatics from a distance of up to 90 microns (Weber et al., 2013), suggesting that melanoma and breast cancer cells expressing CCR7 receptor (Houshmand and Zlotnik, 2003; Muller et al., 2001) could also be guided into the vessels by such haptotactic chemokine gradients. Overexpression of CCR7 in melanoma has indeed been shown to facilitate lymph node metastasis in a mouse model (Wiley et al., 2001) and clinical studies have confirmed the correlation between CCR7 expression and lymph node metastasis (Cabioglu et al., 2005; Ishigami et al., 2007; Mashino et al., 2002). CXCL12 is another chemokine that has been shown to facilitate lymph node metastasis of CXCR4+ tumor cells (Hirakawa et al., 2009; Muller et al., 2001; Uchida et al., 2007). CXCL12 is upregulated on lymphatic vessels in the primary tumor and it has been shown to promote recruitment of CXCR4+/CD133+ melanoma cells into the proximity of lymphatic endothelium. However, direct evidence for its role in directing cells into the lymphatic capillaries is lacking. Several studies suggested that macrophage mannose receptor I (MR) and CLEVER-1 may be important mediators of cancer cell adhesion to lymphatic endothelium (Irjala et al., 2003; Irjala et al., 2001). MR and CLEVER-1 expression has been detected on tumor lymphatic vessels and it was associated with increased lymph node metastases (Irjala et al., 2003). There is no evidence, however, that adhesive interactions with LECs are indeed required for tumor cell entry into the lymphatics and the mechanisms of tumor cells intravasation into the lymphatic vessels remain elusive. Conventional wisdom implies that tumor cells will be delivered into the sentinel lymph nodes with the flow of lymph once they are inside the lymphatic lumen, and this has indeed been demonstrated for tumor cell transport within large, collecting lymphatic vessels (Dadiani et al., 2006; Hayashi et al., 2007). In lymphatic capillaries, however, dendritic cells have been shown to crawl along the luminal side of LECs towards lymph node in the direction of flow (Pflicke and Sixt, 2009; Tal et al., 2011), opening the possibility that tumor cells could employ similar mechanisms.
Subcapsular sinus (SCS) of the LN, which is lined by LECs, is the first site of lymph node metastasis (Carr, 1983; Carr et al., 1985; Dadiani et al., 2006; Das et al., 2013; Dewar et al., 2004). Dilation of SCS, which starts at the junction with the afferent lymphatic vessel, precedes arrival of tumor cells (Das et al., 2013) and may be a prerequisite for allowing the entry of tumor cells into the SCS. Indeed, in the absence of the primary tumor, when injected directly into the lymphatic system, osteosarcoma and melanoma cells arrest at the junction of the afferent lymphatic vessel and the LN (Hayashi et al., 2007). Scanning Electron Microscopy (SEM) analysis revealed that SCS is divided vertically and horizontally into smaller compartments, resulting in passages 5–15 micron wide (Das et al., 2013; Jia et al., 2012; Ohtani and Ohtani, 2008). Since the diameter of a single circulating tumor cell is at least 15 micron (Vona et al., 2000), it has been concluded that the small dimensions of the sinus prevent passive flow of tumor emboli into the SCS (Das et al., 2013). Chemokine CCL1 produced by the SCS LECs facilitates tumor cell entry into the open SCS as well as subsequent migration across the floor of the sinus into the LN cortex. Conversely, blocking CCR8, which is expressed in a large subset of melanomas, led to the arrest of tumor cells at the junction of the afferent lymphatic vessel and the LN (Das et al., 2013). These studies demonstrate that LECs of the LN SCS represent a barrier for entry of tumor cells into the lymph node and identify novel function for CCL1-CCR8 in controlling the egress of tumor cells from the afferent lymphatics (Figure 1). From the sentinel lymph node, metastatic cells advance into the subsequent LNs by the mechanisms that are not well understood. Chemokine CXCL12 could play a role in LN exit since it is upregulated on LN lymphatics when metastases are present (Kim et al., 2010). Ductal mammary carcinomas were shown to invade in bulk into the LN lymphatics through large openings in the lymphatic vessel’s walls (Kerjaschki et al., 2011; Yamaguchi et al., 2005). Large gaps in LECs were induced by the tumor-derived arachidonic acid metabolite 12S-HETE (Kerjaschki et al., 2011).
Figure 1. Model for tumor cell entry into the lymph node.
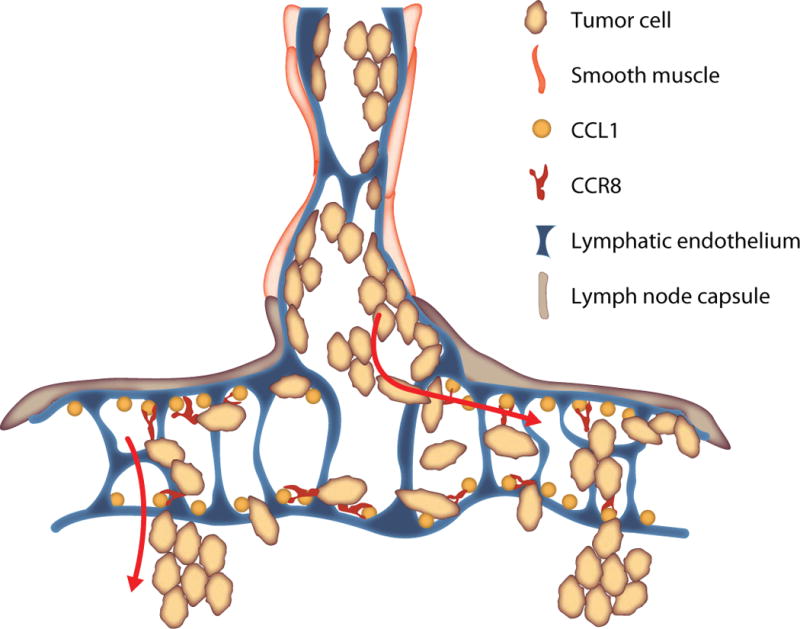
Prior to the arrival of tumor cells, subcapsular sinus (SCS) dilates starting at the orifice of the afferent lymphatic vessel. Tumor emboli arriving from the afferent lymph first arrest at the junction of the afferent lymphatic vessel and the subcapsular sinus. From here, tumor cells expressing CCR8 migrate laterally into the subcapsular sinuses, guided by the CCL1 chemokine which is presented on the surface of SCS LECs. Single cells and small cell clusters may move with the flow of lymph laterally into the sinus. Within the SCS, tumor cells attach to the floor and the roof of the sinus, where they continue to proliferate. Colonization of the SCS is a first step of lymph node metastasis and it is a result of concurrent migration and growth of tumor cells within the sinus. Next step is tumor cell migration across the floor of the sinus into the LN cortex, process also guided by the CCL1 chemokine presented by SCS LECs.
Lymphatics and distant metastasis
From the therapeutic perspective, it is critically important to understand what role lymphatics play in the formation and progression of distant metastases. Thus far, experimental evidence demonstrated that in most cases inhibiting lymph node metastases diminished incidence of lung metastases (Brakenhielm et al., 2007; Burton et al., 2008; Chen et al., 2005; Krishnan et al., 2003; Lin et al., 2005; Roberts et al., 2006), indicating that inhibiting lymphatic dissemination could be a promising approach for preventing distant metastases in certain patients. Concurrent inhibition of lymphangiogenesis and angiogenesis by inhibition of VEGFR-3 and VEGFR-2, respectively, has been shown to effectively diminish lung metastases in the intervention treatment regimen (Matsui et al., 2008; Roberts et al., 2006), suggesting that such combination approach could attenuate metastatic disease also in certain patients with established lung metastases. While it is clear that lymphatics contribute to the early stages of metastases serving as a route for dissemination from the primary tumor to the regional lymph nodes and possibly for the subsequent spread to distant sites, it is less well understood what role lymphatics in distant organs play for already disseminated disease.
In some patients, metastatic disease in the lung is characterized by extensive involvement of lung lymphatics with cancer (Acikgoz et al., 2006; Bruce et al., 1996; Goldsmith et al., 1967; Janower and Blennerhassett, 1971; Thomas and Lenox, 2008; Tomashefski and Dail, 2008). This type of metastasis is referred to as pulmonary lymphangitic carcinomatosis and it is most commonly observed in patients with breast, lung, gastric, pancreatic and prostate cancer (Thurlbeck, 1979; Tomashefski and Dail, 2008). Strikingly, most of these patients die within several months of diagnosis (Bruce et al., 1996; Thomas and Lenox, 2008; Tomashefski and Dail, 2008; Yang and Lin, 1972). How frequent this type of metastasis is in the patient population, however, is a subject of a debate. Studies reported the incidence of lymphangitic spread to be as low as 6%(Harold, 1952; Minor, 1950; Yang and Lin, 1972) and as high as 56%(Fichera and Hagerstrand, 1965). Because a hallmark of lymphangitic spread is its diffuse presentation, it is very difficult to diagnose in patients with current imaging techniques. For example, 50% of the cases of histologically proven pulmonary lymphangitic carcinomatosis present with normal radiographs (Amundson and Weiss, 1991; Fichera and Hagerstrand, 1965; Goldsmith et al., 1967; Janower and Blennerhassett, 1971; Thurlbeck, 1979; Trapnell, 1964). Because of these imaging limitations in patients and because histologic sampling of lung metastases even at autopsy, is not frequently performed, it is believed that the true incidence of lymphangitic spread is greatly underestimated (Tomashefski and Dail, 2008). Nevertheless, the evidence of lymphangitic carcinomatosis in a patient is invariably associated with extremely poor prognosis, indicating that pulmonary lymphatic vasculature facilitates rapid progression of metastatic disease.
Data from the spontaneous metastasis model in mouse, revealed that overexpression of VEGF-C in tumor cells induced lymphangiogenesis in the lung and changed pattern of metastases to pulmonary lymphangitic carcinomatosis (Das et al., 2010). Expansion of the pulmonary lymphatic network was accompanied with a dramatic increase in size of metastases, which were growing within the constraint of lymphatic vessel walls in the absence of angiogenesis. Together with the clinical observations, these experimental data demonstrate an unappreciated role of lymphatics in facilitating lung colonization. These data also suggest that lymphatic vasculature could be a niche which promotes survival and growth of metastases. Importantly, this opens the possibility that targeting lymphatics could be employed as a strategy for treatment of patients which already have disseminated disease, and not only for prevention of metastatic spread. One study demonstrated an association between CD133+ chemoresistant tumor cells and lymphatics at different metastatic sites (Kim et al., 2010), raising another intriguing possibility that lymphatics may modulate therapeutic response.
Immunoregulatory role of LECs in cancer
Several important functions have been attributed to LECs in the recent years which could influence cancer progression as well as directly impact immunotherapy approaches for cancer. LECs have emerged as important players in directing immune cell traffic from tissues into the lymphatic capillaries (Girard et al., 2012; Johnson and Jackson, 2008; Martin-Fontecha et al., 2009). Best studied chemokine made by LECs is CCL21, which binds to CCR7 on migratory DCs, certain macrophage subsets and T-cells and facilitates directed migration of these cells (Forster et al., 2008; Luther et al., 2000; Nagira et al., 1997; Saeki et al., 1999; Willimann et al., 1998). Importance of CCL21-CCR7 interaction in immunity is illustrated by the fact that mice lacking CCR7 ligands have drastically impaired DC and T cell homing to LNs and cannot mount adaptive immune responses (Forster et al., 2008). LECs express many other chemokines which can attract cells into the lymphatic capillaries (Card et al., 2014), but their exact role in controlling leukocyte traffic is yet to be determined.
In the lymph node, LECs lining subcapsular sinuses direct CCR8+ cells into the LN cortex by presenting CCL1 chemokine to the cells arriving from the afferent lymph (Das et al., 2013). CCR8 has been shown to be important for migration of DCs from the skin into the lymph node (Qu et al., 2004), and since its ligand CCL1 is not made by peripheral lymphatics, it has been concluded that CCL1 made by LECs of SCS controls DC entry into the LN (Das et al., 2013; Jakubzick et al., 2006; Qu et al., 2004). Further evidence for the role of lymph node LECs in guiding and selecting cells for entry into the LN comes from the studies which showed that entry of DCs into the LN occurs preferably across the LECs of afferent SCS, whereas T-cells arriving from afferent lymph preferentially enter via medullary sinuses (Braun et al., 2011). Underlying mechanisms governing this pattern of migration remain to be determined.
LECs of medullary sinuses regulate egress of T-cells from the LN by sphingosine-1-phosphate (S1P). Downregulation of S1P receptor 1 (S1P1) on antigen-activated naïve T cells promotes retention of differentiating T cells in the LN, whereas its upregulation makes cells responsive to the ligand and triggers migration into the cortical sinuses (Schwab and Cyster, 2007) where fluid flow promotes movement of T cells into efferent lymphatic vessels (Grigorova et al., 2009).
In addition to regulating cell traffic, growing body of evidence shows that LECs can directly modulate activity of immune cells and promote tolerance (Girard et al., 2012; Lukacs-Kornek et al., 2011; Norder et al., 2012; Podgrabinska et al., 2009). As DCs enter into and crawl along the lymphatic capillaries, they directly interact with LECs. Under inflammatory conditions, binding of Mac-1 on DCs to ICAM-1 on LECs leads to inhibition of DC maturation and suppresses the ability of DCs to activate T cells (Podgrabinska et al., 2009). Another study demonstrated that supernatant from IFNγ-activated LECs also impaired the ability of DCs to induce allogeneic CD4+ T cell proliferation (Lukacs-Kornek et al., 2011). Thus, LECs can regulate T-cell responses by limiting expansion of T-cells in the LNs (Lukacs-Kornek et al., 2011; Norder et al., 2012; Podgrabinska et al., 2009).
LECs express MHC class I (Cohen et al., 2010; Lund et al., 2012; Nichols et al., 2007) and II (Malhotra et al., 2012; Tewalt et al., 2012a) molecules, and can directly induce T cell tolerance as well as suppress T cell activation by expressing several immunoregulatory factors. For example, activated LECs secrete TGF-β, indoleamine-2,3-dioxygenase (IDO) and nitric oxide (NO), all of which are strongly immunosuppressive (Lukacs-Kornek et al., 2011; Malhotra et al., 2012; Norder et al., 2012; Podgrabinska et al., 2002). Furthermore, LECs can modulate T-cell function and induce peripheral tolerance through direct presentation of antigens to T-cells (Cohen et al., 2010; Gardner et al., 2008; Lee et al., 2007; Magnusson et al., 2008; Nichols et al., 2007). In a mouse model, LECs expressed melanoma antigen, tyrosinase epitope Tyr369, and induced tolerance of Tyr369-specific CD8+ T cells (Cohen et al., 2010; Fletcher et al., 2010). Since tyrosinase epitope is a major target for melanoma immunotherapy, these findings suggested that LEC-induced tolerance could have direct impact on the clinical efficacy of anti-melanoma immunotherapies. Interestingly, VEGF-C was shown to promote immune tolerance in B16 F10 murine melanoma which expressed OVA as a foreign antigen; VEGF-C promoted local deletion of OVA-specific CD8(+) T cells and protected tumors against pre-existing antitumor immunity (Lund et al., 2012).
LECs induce tolerance due to high levels of expression of the inhibitory ligand PD-L1 and absence of co-stimulatory molecules on the surface of LECs (Malhotra et al., 2012; Norder et al., 2012; Tewalt et al., 2012a; Tewalt et al., 2012b). Lack of co-stimulation leads to upregulation of programmed cell death 1 (PD-1) receptor expression on CD8 T cells and ultimately antigen-specific deletion of CD8+ T cells (Tewalt et al., 2012a). PD-1 is an important negative regulator of T-cell function and a marker of T-cell exhaustion associated with immunosuppression in cancer. PD-1 has emerged as an important target in immunotherapy: inhibition of PD-1 and PD-L1 potently increases anti-tumor CD8+ T-cells effector response and has shown very promising results in cancer patients (Brahmer et al., 2012; Topalian et al., 2012). In addition to PD-L1, LECs were most recently shown to upregulate CTLA-4 in T-cells, another important marker of T-cell exhaustion and a key target for cancer immunotherapy. Together, these findings that multiple inhibitory receptors are expressed at high levels by LECs (Tewalt et al., 2012a) point to an important role of LECs in cancer immunosuppression and indicate that further insight into the mechanisms by which LECs mediate T-cell exhaustion offers potential for discovery of novel therapeutic targets for cancer immunotherapy.
Concluding remarks
Slowly but steadily, perspectives on the role of lymphatics in cancer have been changing. Traditionally viewed only as a transportation system, it has now become clear that lymphatics perform many functions which could profoundly affect cancer progression. Recent discoveries that LECs can modulate adaptive immune responses put lymphatics in the spotlight as a new player in cancer immunoediting. Proper functioning of LECs in controlling immune cell traffic could be important for immunosurveillance during early stages of tumor development and promote host protection against cancer. On the contrary, the ability of LECs to promote immunosuppression could facilitate immune escape and therefore promote tumor initiation, progression and dissemination. In the dynamic interplay with tumor cells and immune cells, LECs may help orchestrate protection against cancer and tumor elimination, but they may also be exploited to facilitate tumor progression. An example for this is lymphangiogenesis, which is anti-inflammatory and an intrinsic component of the immune response, yet in the cancer setting lymphangiogenesis potently augments metastatic spread. Based on the preclinical data showing that blockade of VEGF-C or VEGFR-3 inhibits lymphangiogenesis and metastasis, two humanized blocking antibodies have entered clinical trials in the past year. In view of the more recent data showing that lymphangiogenesis and VEGF-C not only increase regional spread, but also facilitate late steps of metastasis and immunosuppression, it is reasonable to assume that blocking VEGF-C and its receptors could benefit patients with early as well as late stages of cancer as an adjuvant therapy. Importantly, careful design of preclinical studies and clinical trials will be essential for evaluating the potential of targeting lymphatics in cancer. Testing these inhibitors in a broad patient population without attempting to define a subset of patients which are most likely to respond to this type of therapy will inevitably lead to failure. Since the therapeutic potential of targeting lymphatics is just beginning to be explored, we need to maximize the odds for seeing a positive response by assuring that such therapeutics are tested in the most relevant setting.
Acknowledgments
We thank Melody Swartz for helpful discussions and we apologize to the authors whose work we have not cited because of the article length restrictions. Research in our laboratory is currently supported by the grant from NIH/NCI R01 CA172637, by the Department of Defense grant W81XWH-12-1-0483, Susan G. Komen grant KG 110970, and by the grant from Breast Cancer Alliance.
Abbreviations
- CCL
CC Chemokine ligand
- CCR
CC Chemokine receptor
- CXCR
CXC chemokine receptor
- DC
Dendritic cell
- IL
Interleukin
- LEC
Lymphatic endothelial cells
- LN
Lymph node
- MR
Mannose receptor
- SCS
Subcapsular sinus
- SEM
Scanning Electron Microscopy
- TNF-α
Tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acikgoz G, Kim SM, Houseni M, Cermik TF, Intenzo CM, Alavi A. Pulmonary lymphangitic carcinomatosis (PLC): spectrum of FDG-PET findings. Clin Nucl Med. 2006;31:673–678. doi: 10.1097/01.rlu.0000242210.99022.fd. [DOI] [PubMed] [Google Scholar]
- Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- Amundson DE, Weiss PJ. Hypoxemia in malignant carcinoid syndrome: a case attributed to occult lymphangitic metastatic involvement. Mayo Clin Proc. 1991;66:1178–1180. doi: 10.1016/s0025-6196(12)65802-9. [DOI] [PubMed] [Google Scholar]
- Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25:345–356. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakenhielm E, Burton JB, Johnson M, Chavarria N, Morizono K, Chen I, Alitalo K, Wu L. Modulating metastasis by a lymphangiogenic switch in prostate cancer. Int J Cancer. 2007;121:2153–2161. doi: 10.1002/ijc.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bolter J, Munk A, Forster R. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol. 2011;12:879–887. doi: 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- Bruce DM, Heys SD, Eremin O. Lymphangitis carcinomatosa: a literature review. J R Coll Surg Edinb. 1996;41:7–13. [PubMed] [Google Scholar]
- Burton JB, Priceman SJ, Sung JL, Brakenhielm E, An DS, Pytowski B, Alitalo K, Wu L. Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res. 2008;68:7828–7837. doi: 10.1158/0008-5472.CAN-08-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabioglu N, Yazici MS, Arun B, Broglio KR, Hortobagyi GN, Price JE, Sahin A. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11:5686–5693. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- Card CM, Yu SS, Swartz MA. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J Clin Invest. 2014;124:943–952. doi: 10.1172/JCI73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr I. Lymphatic metastasis. Cancer Metastasis Rev. 1983;2:307–317. doi: 10.1007/BF00048483. [DOI] [PubMed] [Google Scholar]
- Carr I, Levy M, Orr K, Bruni J. Lymph node metastasis and cell movement: ultrastructural studies on the rat 13762 mammary carcinoma and Walker carcinoma. Clin Exp Metastasis. 1985;3:125–139. doi: 10.1007/BF01758961. [DOI] [PubMed] [Google Scholar]
- Chen Z, Varney ML, Backora MW, Cowan K, Solheim JC, Talmadge JE, Singh RK. Down-regulation of vascular endothelial cell growth factor-C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res. 2005;65:9004–9011. doi: 10.1158/0008-5472.CAN-05-0885. [DOI] [PubMed] [Google Scholar]
- Clasper S, Royston D, Baban D, Cao Y, Ewers S, Butz S, Vestweber D, Jackson DG. A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Res. 2008;68:7293–7303. doi: 10.1158/0008-5472.CAN-07-6506. [DOI] [PubMed] [Google Scholar]
- Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, Farr AG, Tung KS, Engelhard VH. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010;207:681–688. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadiani M, Kalchenko V, Yosepovich A, Margalit R, Hassid Y, Degani H, Seger D. Real-time imaging of lymphogenic metastasis in orthotopic human breast cancer. Cancer Research. 2006;66:8037–8041. doi: 10.1158/0008-5472.CAN-06-0728. [DOI] [PubMed] [Google Scholar]
- Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, Detmar M. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18:1232–1242. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- Das S, Ladell DS, Podgrabinska S, Ponomarev V, Nagi C, Fallon JT, Skobe M. Vascular endothelial growth factor-C induces lymphangitic carcinomatosis, an extremely aggressive form of lung metastases. Cancer Res. 2010;70:1814–1824. doi: 10.1158/0008-5472.CAN-09-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, Gordon R, Nagi CS, Wang Y, Entenberg D, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J Exp Med. 2013;210:1509–1528. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Skobe M. Lymphatic vessel activation in cancer. Ann N Y Acad Sci. 2008;1131:235–241. doi: 10.1196/annals.1413.021. [DOI] [PubMed] [Google Scholar]
- Dewar DJ, Newell B, Green MA, Topping AP, Powell BW, Cook MG. The microanatomic location of metastatic melanoma in sentinel lymph nodes predicts nonsentinel lymph node involvement. J Clin Oncol. 2004;22:3345–3349. doi: 10.1200/JCO.2004.12.177. [DOI] [PubMed] [Google Scholar]
- Ding S, Li C, Lin S, Han Y, Yang Y, Zhang Y, Li L, Zhou L, Kumar S. Distinct roles of VEGF-A and VEGF-C in tumour metastasis of gastric carcinoma. Oncol Rep. 2007;17:369–375. [PubMed] [Google Scholar]
- Edge SB, editor. AJCC Cancer Staging Manual. 7. Springer; 2010. [Google Scholar]
- Fichera G, Hagerstrand I. The small lymph vessels of the lungs in lymphangiosis carcinomatosa. Acta Pathol Microbiol Scand. 1965;65:505–513. doi: 10.1111/apm.1965.65.4.505. [DOI] [PubMed] [Google Scholar]
- Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, Collier AR, Boyd RL, Turley SJ. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207:689–697. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- Furudoi A, Tanaka S, Haruma K, Kitadai Y, Yoshihara M, Chayama K, Shimamoto F. Clinical significance of vascular endothelial growth factor C expression and angiogenesis at the deepest invasive site of advanced colorectal carcinoma. Oncology. 2002;62:157–166. doi: 10.1159/000048262. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, Anderson MS. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- Goldsmith HS, Bailey HD, Callahan EL, Beattie EJ., Jr Pulmonary lymphangitic metastases from breast carcinoma. Arch Surg. 1967;94:483–488. doi: 10.1001/archsurg.1967.01330100047007. [DOI] [PubMed] [Google Scholar]
- Grigorova IL, Schwab SR, Phan TG, Pham TH, Okada T, Cyster JG. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat Immunol. 2009;10:58–65. doi: 10.1038/ni.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haessler U, Pisano M, Wu M, Swartz MA. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. Proc Natl Acad Sci U S A. 2011;108:5614–5619. doi: 10.1073/pnas.1014920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold JT. Lymphangitis carcinomatosa of the lungs. Q J Med. 1952;21:353–360. [PubMed] [Google Scholar]
- Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Jiang P, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Research. 2007;67:8223–8228. doi: 10.1158/0008-5472.CAN-07-1237. [DOI] [PubMed] [Google Scholar]
- He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–4746. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109:1010–1017. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa S, Detmar M, Kerjaschki D, Nagamatsu S, Matsuo K, Tanemura A, Kamata N, Higashikawa K, Okazaki H, Kameda K, et al. Nodal lymphangiogenesis and metastasis: Role of tumor-induced lymphatic vessel activation in extramammary Paget’s disease. Am J Pathol. 2009;175:2235–2248. doi: 10.2353/ajpath.2009.090420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houshmand P, Zlotnik A. Therapeutic applications in the chemokine superfamily. Curr Opin Chem Biol. 2003;7:457–460. doi: 10.1016/s1367-5931(03)00086-3. [DOI] [PubMed] [Google Scholar]
- Irjala H, Alanen K, Grenman R, Heikkila P, Joensuu H, Jalkanen S. Mannose receptor (MR) and common lymphatic endothelial and vascular endothelial receptor (CLEVER)-1 direct the binding of cancer cells to the lymph vessel endothelium. Cancer Res. 2003;63:4671–4676. [PubMed] [Google Scholar]
- Irjala H, Johansson EL, Grenman R, Alanen K, Salmi M, Jalkanen S. Mannose receptor is a novel ligand for L-selectin and mediates lymphocyte binding to lymphatic endothelium. J Exp Med. 2001;194:1033–1042. doi: 10.1084/jem.194.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigami S, Natsugoe S, Nakajo A, Tokuda K, Uenosono Y, Arigami T, Matsumoto M, Okumura H, Hokita S, Aikou T. Prognostic value of CCR7 expression in gastric cancer. Hepatogastroenterology. 2007;54:1025–1028. [PubMed] [Google Scholar]
- Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006;176:3578–3584. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- Janower ML, Blennerhassett JB. Lymphangitic spread of metastatic cancer to the lung. A radiologic-pathologic classification. Radiology. 1971;101:267–273. doi: 10.1148/101.2.267. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- Jia L, Xie Z, Zheng J, Liu L, He Y, Liu F. Morphological studies of lymphatic labyrinths in the rat mesenteric lymph node. Anat Rec (Hoboken) 2012;295:1291–1301. doi: 10.1002/ar.22509. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Jackson DG. Cell traffic and the lymphatic endothelium. Ann N Y Acad Sci. 2008;1131:119–133. doi: 10.1196/annals.1413.011. [DOI] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M, Yanai Y, Hata F, Hirata K. Vascular endothelial growth factor C promotes lymph node metastasis in a rectal cancer orthotopic model. Surg Today. 2005;35:131–138. doi: 10.1007/s00595-004-2896-0. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D, Bago-Horvath Z, Rudas M, Sexl V, Schneckenleithner C, Wolbank S, Bartel G, Krieger S, Kalt R, Hantusch B, et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J Clin Invest. 2011;121:2000–2012. doi: 10.1172/JCI44751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- Kim H, Kataru RP, Koh GY. Regulation and implications of inflammatory lymphangiogenesis. Trends Immunol. 2012;33:350–356. doi: 10.1016/j.it.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Kim M, Koh YJ, Kim KE, Koh BI, Nam DH, Alitalo K, Kim I, Koh GY. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res. 2010;70:10411–10421. doi: 10.1158/0008-5472.CAN-10-2591. [DOI] [PubMed] [Google Scholar]
- Krishnan J, Kirkin V, Steffen A, Hegen M, Weih D, Tomarev S, Wilting J, Sleeman JP. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713–722. [PubMed] [Google Scholar]
- Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, Tu GH, Koprivnikar K, VanRoey MJ, He Y, et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–6909. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier AR, Turley SJ. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 2011;12:1096–1104. doi: 10.1038/ni.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell reports. 2012;1:191–199. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson FC, Liblau RS, von Boehmer H, Pittet MJ, Lee JW, Turley SJ, Khazaie K. Direct presentation of antigen by lymph node stromal cells protects against CD8 T-cell-mediated intestinal autoimmunity. Gastroenterology. 2008;134:1028–1037. doi: 10.1053/j.gastro.2008.01.070. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, Gonzalez SF, Elpek KG, Chang SK, Knoblich K, Hemler ME, et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol. 2012;13:499–510. doi: 10.1038/ni.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fontecha A, Lanzavecchia A, Sallusto F. Dendritic cell migration to peripheral lymph nodes. Handbook of experimental pharmacology. 2009:31–49. doi: 10.1007/978-3-540-71029-5_2. [DOI] [PubMed] [Google Scholar]
- Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–2941. [PubMed] [Google Scholar]
- Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14:5459–5465. doi: 10.1158/1078-0432.CCR-07-5270. [DOI] [PubMed] [Google Scholar]
- Mattila MM, Ruohola JK, Karpanen T, Jackson DG, Alitalo K, Harkonen PL. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int J Cancer. 2002;98:946–951. doi: 10.1002/ijc.10283. [DOI] [PubMed] [Google Scholar]
- Minor GR. A clinical and radiologic study of metastatic pulmonary neoplasms. J Thorac Surg. 1950;20:34–42. [PubMed] [Google Scholar]
- Miyazaki T, Okada N, Ishibashi K, Ogata K, Ohsawa T, Ishiguro T, Nakada H, Yokoyama M, Matsuki M, Kato H, et al. Clinical significance of plasma level of vascular endothelial growth factor-C in patients with colorectal cancer. Jpn J Clin Oncol. 2008;38:839–843. doi: 10.1093/jjco/hyn106. [DOI] [PubMed] [Google Scholar]
- Mohammed RA, Green A, El-Shikh S, Paish EC, Ellis IO, Martin SG. Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br J Cancer. 2007;96:1092–1100. doi: 10.1038/sj.bjc.6603678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Nagira M, Imai T, Hieshima K, Kusuda J, Ridanpaa M, Takagi S, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine secondary lymphoid-tissue chemokine that is a potent chemoattractant for lymphocytes and mapped to chromosome 9p13. J Biol Chem. 1997;272:19518–19524. doi: 10.1074/jbc.272.31.19518. [DOI] [PubMed] [Google Scholar]
- Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- Norder M, Gutierrez MG, Zicari S, Cervi E, Caruso A, Guzman CA. Lymph node-derived lymphatic endothelial cells express functional costimulatory molecules and impair dendritic cell-induced allogenic T-cell proliferation. FASEB J. 2012;26:2835–2846. doi: 10.1096/fj.12-205278. [DOI] [PubMed] [Google Scholar]
- Ohtani O, Ohtani Y. Structure and function of rat lymph nodes. Arch Histol Cytol. 2008;71:69–76. doi: 10.1679/aohc.71.69. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Tille JC, Nisato R, Skobe M. Lymphangiogenesis and tumor metastasis. Cell Tissue Res. 2003;314:167–177. doi: 10.1007/s00441-003-0748-7. [DOI] [PubMed] [Google Scholar]
- Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. 2009;206:2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A. 2002;99:16069–16074. doi: 10.1073/pnas.242401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, Skobe M. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol. 2009;183:1767–1779. doi: 10.4049/jimmunol.0802167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, Pytowski B, Skobe M. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- Rosen PP. Rosen’s Breast Pathology. 3. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Ruddell A, Kelly-Spratt KS, Furuya M, Parghi SS, Kemp CJ. p19/Arf and p53 suppress sentinel lymph node lymphangiogenesis and carcinoma metastasis. Oncogene. 2008;27:3145–3155. doi: 10.1038/sj.onc.1210973. [DOI] [PubMed] [Google Scholar]
- Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- Schumann K, Lammermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, Schuler G, Forster R, Lutz MB, Sorokin L, Sixt M. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- Shields JD, Emmett MS, Dunn DB, Joory KD, Sage LM, Rigby H, Mortimer PS, Orlando A, Levick JR, Bates DO. Chemokine-mediated migration of melanoma cells towards lymphatics–a mechanism contributing to metastasis. Oncogene. 2007;26:2997–3005. doi: 10.1038/sj.onc.1210114. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- Swartz MA, Skobe M. Lymphatic function, lymphangiogenesis, and cancer metastasis. Microsc Res Tech. 2001;55:92–99. doi: 10.1002/jemt.1160. [DOI] [PubMed] [Google Scholar]
- Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, Ng LG, Angeli V, Shakhar G. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med. 2011;208:2141–2153. doi: 10.1084/jem.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewalt EF, Cohen JN, Rouhani SJ, Engelhard VH. Lymphatic endothelial cells – key players in regulation of tolerance and immunity. Frontiers in immunology. 2012a;3:305. doi: 10.3389/fimmu.2012.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, Fahl SP, Conaway MR, Bender TP, Tung KS, Vella AT, et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood. 2012b;120:4772–4782. doi: 10.1182/blood-2012-04-427013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Lenox R. Pulmonary lymphangitic carcinomatosis as a primary manifestation of colon cancer in a young adult. CMAJ. 2008;179:338–340. doi: 10.1503/cmaj.080142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlbeck WM. In: Neoplasia of the pulmonary vascular bed In Pulmonary vascular disease. Moser KM, editor. New York: Marcel Dekker; 1979. [Google Scholar]
- Tomashefski JF, Dail DH. Dail and Hammar’s pulmonary pathology. 3. New York: Springer; 2008. [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell DH. Radiological Appearances of Lymphangitis Carcinomatosa of the Lung. Thorax. 1964;19:251–260. doi: 10.1136/thx.19.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S, Kuwano H, Shimura T, Morinaga N, Mochiki E, Asao T. Vascular endothelial growth factor C (VEGF-C) expression in pT2 gastric cancer. Hepatogastroenterology. 2005;52:629–632. [PubMed] [Google Scholar]
- Uchida D, Onoue T, Tomizuka Y, Begum NM, Miwa Y, Yoshida H, Sato M. Involvement of an autocrine stromal cell derived factor-1/CXCR4 system on the distant metastasis of human oral squamous cell carcinoma. Mol Cancer Res. 2007;5:685–694. doi: 10.1158/1541-7786.MCR-06-0368. [DOI] [PubMed] [Google Scholar]
- Van den Eynden GG, Van der Auwera I, Van Laere SJ, Huygelen V, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA. Induction of lymphangiogenesis in and around axillary lymph node metastases of patients with breast cancer. Br J Cancer. 2006;95:1362–1366. doi: 10.1038/sj.bjc.6603443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eynden GG, Vandenberghe MK, van Dam PJ, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA. Increased sentinel lymph node lymphangiogenesis is associated with nonsentinel axillary lymph node involvement in breast cancer patients with a positive sentinel node. Clin Cancer Res. 2007;13:5391–5397. doi: 10.1158/1078-0432.CCR-07-1230. [DOI] [PubMed] [Google Scholar]
- Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schutze K, Capron F, Franco D, Pazzagli M, Vekemans M, et al. Isolation by size of epithelial tumor cells : a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, Luther SA, Bollenbach T, Sixt M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- Willimann K, Legler DF, Loetscher M, Roos RS, Delgado MB, Clark-Lewis I, Baggiolini M, Moser B. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur J Immunol. 1998;28:2025–2034. doi: 10.1002/(SICI)1521-4141(199806)28:06<2025::AID-IMMU2025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Yanai Y, Furuhata T, Kimura Y, Yamaguchi K, Yasoshima T, Mitaka T, Mochizuki Y, Hirata K. Vascular endothelial growth factor C promotes human gastric carcinoma lymph node metastasis in mice. J Exp Clin Cancer Res. 2001;20:419–428. [PubMed] [Google Scholar]
- Yang SP, Lin CC. Lymphangitic carcinomatosis of the lungs. The clinical significance of its roentgenologic classification. Chest. 1972;62:179–187. doi: 10.1378/chest.62.2.179. [DOI] [PubMed] [Google Scholar]
- Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest. 2014;124:878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]


