Abstract
Objectives
To characterize adverse drug events (ADEs) occurring within the high-risk 45-day period post-hospitalization in older adults.
Design
Clinical pharmacists reviewed the ambulatory records of 1000 consecutive discharges.
Setting
A large multispecialty group practice closely aligned with a Massachusetts-based health plan.
Participants
Hospitalized patients aged 65 years and older who were discharged to home.
Measurements
Possible drug-related incidents occurring during the 45-day period post-hospitalization were identified and presented to a pair of physician-reviewers who classified incidents as to whether an ADE was present, whether the event was preventable, and the severity of the event. Medications implicated in ADEs were further characterized according to their inclusion in the 2012 Beers Criteria for Potentially Inappropriate Medication Use in Older Adults.
Results
At least one ADE was identified during the 45-day period in 18.7% (187) of the 1000 discharges. Of the 242 ADEs identified, 35% (n=84) were deemed preventable, of which 32% (n=27) were characterized as serious, and 5% (n=4) as life threatening. Over half of all ADEs occurred within the first 14 days post-hospitalization. The percentage of ADEs in which Beers Criteria medications were implicated was 16.5% (n=40). Beers Criteria medications with both a high quality of evidence and strong strength of recommendation were implicated in 6.6% (n=16) of the ADEs.
Conclusion
ADEs are common and often preventable among older adults following hospital discharge, underscoring the need to address medication safety during this high-risk period in this vulnerable population. Beers Criteria medications played a small role in these events suggesting that efforts to improve the quality and safety of medication use during this critical transition period must extend beyond a singular focus on Beers criteria medications.
Keywords: adverse drug events, Beers medications, ambulatory care setting, older adults
INTRODUCTION
Adverse drug events (ADEs), especially those that may be preventable, pose a serious concern in older adults during the immediate post-hospitalization period. This is a time of high-risk for older patients, when multiple medication changes occur that can lead to confusion regarding medication management for both patients and providers.1–3 One study of older hospitalized patients found that 40% of all admission medications were discontinued by discharge, and 45% of all discharge medications were newly started during the hospitalization.4 It has been estimated that 12–17% of general medical patients experience ADEs after hospital discharge, of which a large percentage may be preventable.2,3
Multiple factors may contribute to suboptimal medication management following hospital discharge, including poor physician-patient communication and inadequate education regarding medication use,5 poor therapeutic monitoring,6,7 and incomplete or inaccurate information transfer between clinicians.8 During the immediate post-hospital discharge period, patients may receive medications from different prescribers, who may lack access to comprehensive reconciled medication information.9 In addition, lack of prompt follow-up care following a hospitalization may exacerbate problems during this particularly high-risk period.
The purpose of our study was to characterize the frequency, preventability, and severity of ADEs occurring during the 45-day post-hospitalization period among older adults discharged from hospital to home. Medications implicated in ADEs were further characterized according to their inclusion in the 2012 Beers Criteria for Potentially Inappropriate Medication Use in Older Adults.10
METHODS
Study Setting and Population
Our study was conducted in the setting of a large multispecialty group practice closely aligned with a Massachusetts-based health plan. Hospital care is delivered by hospitalists employed by the medical group. The group practice uses an electronic health record system. The study population was derived from the health plan’s senior plan membership, the majority of whom are cared for by the multispecialty group.
We studied 1000 consecutive hospital discharges between August 26, 2010 and December 27, 2010 who met the following criteria: (1) age of patient 65 years or older at the time of discharge; (2) discharged from the primary inpatient facility serving the medical group for a non-psychiatric condition; (3) no plans to enroll in hospice upon discharge; and (4) discharged to the community (not a skilled nursing facility or long-term care setting). The observation period included days 1 – 45 following hospital discharge.
The project was approved by the institutional review board of the University of Massachusetts Medical School, and the institutional review board of the group practice and health plan.
Definitions and Classification of Events
Three trained clinical pharmacists performed comprehensive medical record reviews to identify possible drug-related incidents during the 45-day post-hospital discharge period. Comprehensive medical record reviews consisted of: (1) review of hospital discharge summaries and emergency department visits; (2) review of office visit notes; and (3) review of telephone encounters and communications that occurred between the patient and providers, and among providers. If a hospital admission or an emergency department visit occurred within the observation period of interest, notes were reviewed for evidence of a drug-related incident that may have led to that hospital admission or emergency department visit. However, any drug-related incidents that occurred during a hospitalization or an emergency department visit were not considered in the context of this study. A computer generated list with signals of possible adverse events was produced relating to each discharged patient, including elevated drug concentrations, abnormal laboratory data, administration of antidotes that might relate to an ADE, and ICD-9 codes suggesting potential adverse effects of drugs.11 The clinical pharmacists reviewers used these reports to focus and enhance their reviews in order to identify possible drug-related incidents.
Preparation of Event Summaries
An event summary was prepared whenever the clinical pharmacist identified a possible drug-related incident. The event summary incorporated data obtained from a comprehensive review including medical and medication history, physical examination findings, laboratory data, and providers’ assessments. Additionally, the event summary captured information to assess the probability of the adverse event being attributable to the drug. Such information included timing, severity, and resolution. Drug references and literature were also reviewed to estimate the incidence of ADEs associated with the use of specific drug therapies.12
Outcome Measures
An ADE was defined as an injury resulting from a drug, rather than an underlying disease. An ADE can be related to an error or an adverse drug reaction without an error.6,7,13–15 All possible drug-related incidents were presented by the clinical pharmacists (A.O.K. and J.L.D.) to pairs of physician-reviewers (J.G., J.T., L.H., and S.C.). These physician-reviewers independently classified incidents using a structured implicit review process, which has been used in numerous prior studies of ADEs in various clinical settings.6,7,14–18 Briefly, the review assessed whether an ADE occurred, the preventability and severity of the event, and the effects of the event on the patient. In determining whether an ADE had occurred, the physician-reviewers considered the sequential relation between the drug exposure and the event, as well as whether the event reflected a known effect of the drug.
ADEs were considered preventable if they were due to an error and were preventable by any means available.7 Preventability was categorized as preventable, probably preventable, probably not preventable, or definitely not preventable; results were collapsed into the categories of preventable (preventable and probably preventable) and non-preventable (probably not preventable and definitely not preventable). Severity of ADEs was categorized as less serious, serious, life-threatening, or fatal.7,14 ADEs categorized as less serious included rashes, bruising, and constipation. Examples of serious events included falls, dizziness with orthostasis, and gastrointestinal bleeding not requiring hospitalization. Examples of life-threatening events included acute kidney injury with hyperkalemia, and thromboembolic events. The effects of ADEs on patients were categorized as a laboratory abnormality requiring only a change in therapy, symptoms of less than one day in duration, symptoms of one day and longer in duration, disability, and death. The stages of pharmaceutical care during which an error leading to a preventable ADE occurred (prescribing, dispensing, patient adherence, and monitoring) were also characterized. For a single ADE, it may have been possible to identify errors at more than one stage of pharmaceutical care, or to identify more than one error within a single stage of care.
Medications implicated in ADEs were further characterized according to whether or not they were included in the 2012 Beers Criteria for Potentially Inappropriate Medication Use in Older Adults.10 The most recent revision of the criteria was developed by an interdisciplinary panel and employed an evidence-based approach to rate the strength of recommendations for each medication as well as the strength of the evidence deeming each medication potentially inappropriate. Fifty-three medications or medication classes encompass the 2012 Beers Criteria, and they are divided into three categories: potentially inappropriate medications to avoid in older patients; potentially inappropriate medications to avoid in older adults with certain diseases and syndromes that the drugs can exacerbate; and medications to be used with caution in older patients. For the purpose of the present study, Beers Criteria medications included those medications classified as potentially inappropriate medications and medications to be used with caution according to the 2012 Beers Criteria. Dosing, gender, age, route, and drug form as outlined in the Beers Criteria tables were considered when classifying medications. Additionally, we characterized medications implicated in ADEs limited to those Beers criteria medications labeled as having both a high quality of evidence and a strong strength of recommendation.
When the physician-reviewers disagreed on the classification of an ADE, its preventability or its severity, they met and reached consensus; consensus was reached in all instances where there was initial disagreement. All initial assessments of the physician-reviewers were compared and interrater reliability was calculated using the қ statistic. For judgment about the presence of an ADE, the қ was 0.63 (95% Confidence Interval [CI]: 0.53 to 0.72); for preventability, 0.62 (95% CI: 0.51 to 0.73); and for severity, 0.47 (95% CI: 0.34 to 0.60). A қ score between 0.6 and 0.8 reflects “substantial” agreement, and a score between 0.41–0.6 is considered “moderate”. 19 We calculated the relative risk of preventability for serious vs. less than serious events with 95% confidence intervals using the epitab function in version 11 of Stata® data analysis and statistical software (Stata Corporation 4905 Lakeway Drive College Station, Texas 77845 USA).
RESULTS
The 1000 consecutive discharges involved a total of 850 different patients; 731 had one discharge and 119 had more than one discharge. The mean (± standard deviation) age of patients at the time of discharge was 78.8 (± 7.1) years and 51.6% were women.
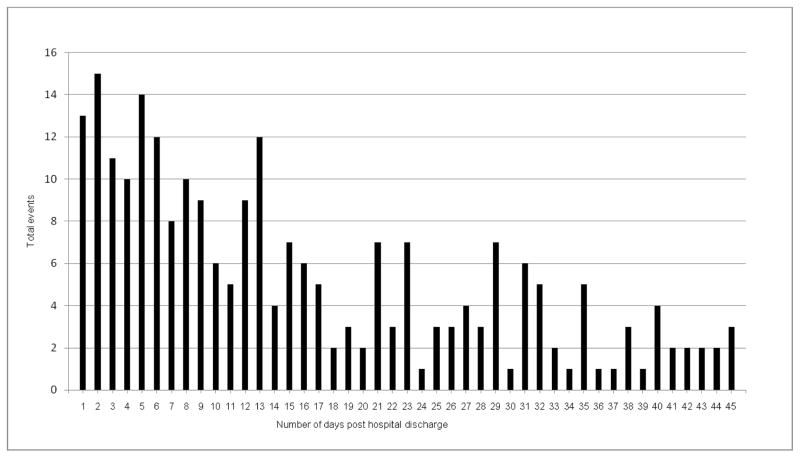
The clinical pharmacists identified 330 possible drug-related incidents occurring during the 1–45 day period post hospital discharge, of which 242 (73%) were deemed to be ADEs by the physician reviewers. There was at least one ADE identified in 18.7% (n=187) of hospital discharges. The number of discharges with more than one ADE was 44. Over half of ADEs occurred within 14 days post-hospital discharge (Figure 1).
Figure 1.
Number of days post discharge to first event
With regard to severity, 24% of ADEs were categorized as serious or life-threatening (Table 1). There were no fatal events. Abnormal laboratory results requiring only a change in therapy accounted for 18% of all ADEs. Nearly 60% (n=141) resulted in symptom duration lasting one or more days. Two events resulted in non-permanent disability and included a fall with an associated injury related to the use of cardiovascular and sedative/hypnotic medications, and a cardiovascular event related to the inappropriate discontinuation of drug therapy.
Table 1.
Characteristics of Adverse Drug Events Within 45-Day Period Post Hospitalization
| Overall (n=242) | Preventable (n=84) | |
|---|---|---|
| Category of severity, n (%) | ||
| Less Serious | 185 (76.4) | 53 (63.1) |
| Serious | 51 (21.1) | 27 (32.1) |
| Life-threatening | 6 (2.5) | 4 (4.8) |
| Effects of Adverse Drug Events, N (%) | ||
| Abnormal laboratory results without symptoms | 44 (18.2) | 12 (14.3) |
| Duration of symptoms, day(s) | ||
| < 1 | 55 (22.7) | 21 (25.0) |
| ≥ 1 | 141 (58.3) | 50 (59.5) |
| Non-permanent disability | 2 (0.8) | 1 (1.2) |
Of the 242 ADEs, 35% (n= 84) were considered to be preventable; of which 32% (n=27) were serious, and 5% (n=4) were life threatening (Table 2). More severe ADEs (serious and life threatening) were significantly more likely to be deemed preventable (relative risk = 1.9; 95% CI: 1.4–2.6).
Table 2.
Selected Examples of Preventable More Severe Adverse Drug Events
| A patient taking an angiotensin-converting–enzyme inhibitor (new medication started in hospital and continued at discharge), a beta-blocker (dose increased at discharge), a thiazide diuretic, and a loop diuretic developed hypotension (systolic blood pressure in the 50’s, as reported by a visiting nurse) required emergency transport and hospitalization. |
| A patient taking an opioid (started in hospital and continued at discharge) without an appropriate bowel regimen required evaluation in the emergency department for severe constipation. |
| A patient taking a sulfonylurea (glipizide) developed hypoglycemia (blood glucose in the 30’s) requiring emergency medical services. Patient’s HbA1C was below 7% and as low as 5.5% over the two years preceding the event; the patient was also taking the drug inappropriately with respect to meal times. |
Adverse Drug Events by Type and by Drug Class
Gastrointestinal events (e.g., diarrhea, constipation, abdominal pain) comprised the most common type of preventable ADEs (Table 3); other common preventable ADEs included cardiovascular and renal/electrolyte related events (hyperkalemia, hypokalemia, and renal insufficiency). Among preventable events, cardiovascular medications, diuretics, opioids, and anticoagulants/antiplatelets were most commonly involved (Table 4).
Table 3.
Frequency of Types of Adverse Drug Eventsa
| Core components of ADEs, n (%) | Overall n= 242 |
Preventable n= 84 |
|---|---|---|
| Cardiovascular | 54 (22.3) | 21 (25.0) |
| Renal/Electrolyte | 53 (21.9) | 24 (28.6) |
| Gastrointestinal tract | 43 (17.8) | 23 (27.4) |
| Hemorrhagic | 26 (10.7) | 7 (8.3) |
| Syncope/dizziness | 23 (9.5) | 11 (13.1) |
| Metabolic/endocrine | 18 (7.4) | 6 (7.1) |
| Neuropsychiatric | 18 (7.4) | 7 (8.3) |
| Fatigue | 15 (6.2) | 1 (1.2) |
| Dermatologic/allergic | 11 (4.5) | 0 (0) |
| Infection | 7 (2.9) | 2 (2.4) |
| Falls without injury | 6 (2.5) | 3 (3.6) |
| Respiratory tract | 2 (0.8) | 2 (2.4) |
| Hematologic | 3 (1.2) | 0 (0) |
| Ataxia/difficulty with gait | 1 (0.4) | 0 (0) |
| Anticholinergic | 2 (0.8) | 0 (0) |
| Fall with injury | 2 (0.8) | 2 (2.4) |
| Chills | 2 (0.8) | 1(1.2) |
Adverse drug events could manifest as more than one type.
Table 4.
Frequencies of Adverse Drug Events by Drug Classa
| Prescription Drug Class, n (%) | Overall n= 242 |
Preventable n= 84 |
|---|---|---|
| Cardiovascular | 86 (35.5) | 34 (40.5) |
| Diuretics | 49 (20.2) | 20 (23.8) |
| Opioids | 23 (9.5) | 14 (16.7) |
| Antibiotics/anti-infectives | 19 (7.9) | 2 (2.4) |
| Anticoagulants | 18 (7.4) | 5 (6.0) |
| Antiplatelets | 18 (7.4) | 4 (4.8) |
| Aspirin | 16 (6.6) | 5 (6.0) |
| Steroids | 13 (5.4) | 3 (3.6) |
| Antineoplastics | 12 (5.0) | 0 (0) |
| NSAIDs | 11 (4.5) | 7 (8.3) |
| Nutrients/supplements | 10 (4.1) | 4 (4.8) |
| Hypoglycemics | 8 (3.3) | 3 (3.6) |
| Gastrointestinal tract | 7 (2.9) | 3 (3.6) |
| Sedatives/hypnotics | 7 (2.9) | 4 (4.8) |
| Antidepressants | 5 (2.1) | 5 (6.0) |
| Antihyperlipidemics | 4 (1.7) | 0 (0) |
| Muscle relaxants | 4 (1.7) | 4 (4.8) |
| Antipsychotics | 4 (1.7) | 2 (2.4) |
| Other | 10 (4.1) | 3 (3.6) |
Drugs in more than one category were associated with some events. Frequencies in each column sum to greater than the total number of events.
Errors Associated with Adverse Drug Events
Among the 84 preventable ADEs, errors occurred most often at the prescribing (n=65 [54%]) and monitoring (n=44 [36%]) stages of pharmaceutical care. Errors accounting for preventable ADEs were less commonly identified at the dispensing (n=1 [1%]) and administration/adherence (n=11 [9%]) stages. Over 40% (n=34) of the preventable ADEs were associated with an error at two or more stages of pharmaceutical care.
Among the prescribing errors, the most common were wrong drug choice and wrong dose. Monitoring stage errors generally referred to inadequate laboratory monitoring of drug therapies or to a delayed response, or failure to respond to signs or symptoms of drug toxicity or laboratory evidence of toxicity.
Adverse Drug Events Associated with Beers Criteria
Of the 242 ADEs identified, 16.5% (n=40) involved one or more drugs included among Beers Criteria medications; specifically, 14.9% (n=36) had one or more “potentially inappropriate” drugs while 2.5% (n=6) had one or more “use with caution” drugs. Non-COX-selective NSAIDs were the most common types of Beers Criteria medications implicated in ADEs (n=7). Beers Criteria medications with both a high quality of evidence and strong strength of recommendation were implicated in 6.6% (n=16) of the ADEs.
DISCUSSION
Krumholz has recently described the post-hospital syndrome as a condition of generalized high-risk.20 Our findings serve to reinforce the importance of medication safety as a critically important issue during this period of high vulnerability for older patients. We determined that ADEs occurred frequently in older adults discharged to home during the 45-day period post-hospitalization, with an event identified in nearly one in five of all discharges. Over a third of ADEs were considered preventable and more severe events were more likely to be deemed preventable. Over half of ADEs happened within 14 days post-hospitalization and most errors associated with preventable events occurred at the prescribing and monitoring stages of pharmaceutical care. Other studies that have examined ADEs post-hospitalization have found that they occur commonly and are often preventable.2,3,21
Only a small proportion of the identified ADEs were related to medications found on the Beers list. While our study employed the most recently updated Beers Criteria, studies of ADEs conducted in other clinical settings have suggested that Beers list medications are associated with few adverse events.22–24 For example, an analysis of emergency hospitalizations for ADEs in older adults reported that only 6.6% of all hospitalizations were associated with Beers Criteria medications.22 Similarly, another study found that only 3.6% of emergency department visits for ADEs were associated with Beers Criteria medications.23 The somewhat higher percentage of ADEs associated with Beers criteria in our study may reflect the fact that ADEs that lead to an emergency department visit are generally severe, and Beers Criteria medications may lead to less severe events not requiring an emergency department visit or hospitalization.
The findings of this study can be directly applied to improve the quality of care for older patients discharged from the hospital. Our results suggest that errors in medication prescribing and monitoring are most commonly associated with the occurrence of adverse drug events during this high-risk post-hospitalization period. Enhanced monitoring of patients involving a team-based approach to care that includes the physician working together with other health disciplines, including nursing and pharmacy, seems an essential component of any initiative designed to reduce risk for older patients following hospital discharge.25–27 Clinical decision support incorporated into electronic health records that provides prompts for follow-up office visits, notification of medication changes that occurred during the hospitalization, and recommendations for laboratory monitoring may hold promise for reducing medication error rates and preventable adverse drug events in the post-hospital discharge period.28 Educational efforts relating to the optimal use of medications in older patients should target all health professionals providing care to older patients. In addition, enhanced surveillance and reporting systems for adverse drug events in the ambulatory setting, especially those relating to medication errors, are essential to inform the design of new system-based approaches to reducing risk of adverse drug events.
Our study benefitted from the availability of information contained in the electronic health records including prescription medications, laboratory results, and clinic notes and telephone communications. The clinical pharmacists performed a comprehensive medical record review using all of these sources of medical information. However, our study has some limitations. There is inherently a level of subjectivity in the process of classifying ADEs. Although the 2012 Beers Criteria were used in our study, this version of the criteria did not exist during the time period of the research. Our findings may have been somewhat different if the 2012 Beers Criteria had been available and widely publicized at that time. It is important to note that medications are only one facet of an analysis of medication safety. Errors of omission (i.e., missed opportunities to prescribe appropriate medications) and patient behaviors leading to ADEs are also important aspects of pharmaceutical care, but were not the focus of the present study.
In summary, we determined that ADEs are common among older adults during the immediate period post-hospitalization. Beers Criteria medications play only a small role in these events. Efforts to improve the quality and safety of medication use during this critical transition period for older adults are necessary. However, it is essential that these efforts extend beyond a singular focus on Beers criteria medications.
Acknowledgments
We thank Kevin Swearengin, PharmD, for his assistance with the data abstraction and Mary Ellen Stansky for the assistance with technical aspects of this study.
Funding source: This study was funded by grant R18 HS017203 from the Agency for Healthcare Research and Quality (AHRQ). Dr. Cutrona is supported by Award Number KL2RR031981 from the National Center for Research Resources (NCRR).
Footnotes
Authors presented this research abstract at the 2012 American College of Clinical Pharmacy Annual Meeting, 2013 Annual HMO Research Network Conference, and 2013 Annual Scientific Meeting of the American Geriatrics Society.
Author Contributions:
Abir O. Kanaan and Jennifer L. Donovan contributed to conception and design, acquisition of data, analysis and interpretation of data, as well as drafting and revising the article critically for important intellectual content.
Nerissa P. Duchin, Terry S. Field, Jennifer Tjia, Lawrence Garber, and Jerry H. Gurwitz contributed to conception and design, acquisition of data, analysis and interpretation of data, and revising the article critically for important intellectual content.
Sarah L. Cutrona, Shawn J. Gagne, Peggy Preusse, and Leslie R. Harrold contributed to acquisition of data, analysis and interpretation of data, and revised the article critically for important intellectual content.
All authors reviewed and approved the submitted version of the article.
Sponsor’s Role: The sponsor was not involved in the study design, methods, subject recruitment, data collections, analysis, or preparation of the paper. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Conflict of interest
Nerissa Duchin was supported by a Medical Student Training in Aging Research Award (MSTAR) from the American Federation for Aging Research (AFAR).
The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
References
- 1.Beers MH, Sliwkowski J, Brooks J. Compliance with medication orders among the elderly after hospital discharge. Hosp Formul. 1992;27:720–724. [PubMed] [Google Scholar]
- 2.Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170:345–349. [PMC free article] [PubMed] [Google Scholar]
- 3.Forster AJ, Murff HJ, Peterson JF, et al. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 4.Beers MH, Dang J, Hasegawa J, et al. Influence of hospitalization on drug therapy in the elderly. J Am Geriatr Soc. 1989;37:679–683. doi: 10.1111/j.1532-5415.1989.tb02227.x. [DOI] [PubMed] [Google Scholar]
- 5.Calkins DR, Davis RB, Reiley P, et al. Patient-physician communication at hospital discharge and patients’ understanding of the postdischarge treatment plan. Arch Intern Med. 1997;157:1026–1030. [PubMed] [Google Scholar]
- 6.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 7.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29–34. [PubMed] [Google Scholar]
- 8.van Walraven C, Seth R, Laupacis A. Dissemination of discharge summaries. Not reaching follow-up physicians. Can Fam Physician. 2002;48:737–742. [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51:549–555. doi: 10.1046/j.1532-5415.2003.51185.x. [DOI] [PubMed] [Google Scholar]
- 10.American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Field TS, Gurwitz JH, Harrold LR, et al. Strategies for detecting adverse drug events among older persons in the ambulatory setting. J Am Med Inform. 2004;11:492–498. doi: 10.1197/jamia.M1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micromedex® Healthcare Series [Internet database] Greenwood Village, CO: Thomson Healthcare; Available at http://www.micromedex.com. Updated periodically. [Google Scholar]
- 13.Leape LL, Bates DW, Cullen DJ, et al. Systems analysis of adverse drug events. JAMA. 1995;274:35–43. [PubMed] [Google Scholar]
- 14.Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventablity of adverse drug events in nursing homes. Am J Med. 2000;109:87–94. doi: 10.1016/s0002-9343(00)00451-4. [DOI] [PubMed] [Google Scholar]
- 15.Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation of physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267–270. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]
- 16.Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285:2114–2120. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- 17.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention on serious medication errors. JAMA. 1998;280:1311–1316. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 18.Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. JAMA. 1997;277:307–311. [PubMed] [Google Scholar]
- 19.Sackett DL, Haynes RB, Guyatt GH, et al. Clinical Epidemiology: A Basic Science for Clinical Medicine. 2. Boston, MA: Little Brown and Co; 1991. [Google Scholar]
- 20.Krumholz HM. Post-hospital syndrome-an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forster Aj, Murff HJ, Petereson JF, et al. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005;20:317–323. doi: 10.1111/j.1525-1497.2005.30390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budnitz DS, Lovegrove MC, Shehab N, et al. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 23.Budnitz DS, Shehab N, Kegler SR, et al. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147:755–765. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]
- 24.Page RL, 2nd, Ruscin JM. The risk of adverse drug events and hospital-related morbidity and mortality among older adults with potentially inappropriate medication use. Am J Geriatr Pharmacother. 2006;4:297–305. doi: 10.1016/j.amjopharm.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Makowsky MJ, Schindel TJ, Rosenthal M, et al. Collaboration between pharmacists, physicians and nurse practitioners: A qualitative investigation of working relationships in the inpatient medical setting. J Interprof Care. 2009;23:169–184. doi: 10.1080/13561820802602552. [DOI] [PubMed] [Google Scholar]
- 26.Jain M, Miller L, Belt D, et al. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15:235–239. doi: 10.1136/qshc.2005.016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isetts BJ, Brown LM, Schondelmeyer SW, et al. Quality assessment of a collaborative approach for decreasing drug-related morbidity and achieving therapeutic goals. Arch Intern Med. 2003;163:1813–1820. doi: 10.1001/archinte.163.15.1813. [DOI] [PubMed] [Google Scholar]
- 28.Field TS, Garber L, Gagne SJ, et al. Technological resources and personnel costs required to implement an automated alert system for ambulatory physicians when patients are discharged from hospitals to home. Inform Prim Care. 2012;20:87–93. doi: 10.14236/jhi.v20i2.29. [DOI] [PubMed] [Google Scholar]