Abstract
Reprogrammed metabolism is a key feature of cancer cells. The pyruvate kinase M2 (PKM2) isoform, which is commonly upregulated in many human cancers, has been recently shown to play a crucial role in metabolism reprogramming, gene transcription and cell cycle progression. In this Cell Science at a glance article and accompanying poster, we provide a brief overview of recent advances in understanding the mechanisms underlying the regulation of PKM2 expression, enzymatic activity, metabolic functions and subcellular location. We highlight the instrumental role of the non-metabolic functions of PKM2 in tumorigenesis and evaluate the potential to target PKM2 for cancer treatment.
KEY WORDS: PKM2, Pyruvate kinase M2, Metabolism reprogramming, Protein kinase, Gene transcription
Introduction
Pyruvate kinase (PK) regulates the final rate-limiting step of glycolysis by catalyzing the transfer of a phosphate group from phosphoenolpyruvate (PEP) to ADP to produce pyruvate and ATP (Altenberg and Greulich, 2004; Majumder et al., 2004). PKM1, PKM2 (encoded by PKM), PKL and PKR (encoded by PKLR) are the four pyruvate kinase isoforms. PKL and PKR are expressed in the liver and erythrocytes, respectively, whereas PKM1 and PKM2 are expressed in different types of cells and tissues. Alternative splicing of PKM pre-mRNA by heterogeneous nuclear ribonucleoproteins (hnRNPs) A1 and A2 and polypyrimidine-tract binding (PTB) protein splicing factors results in the generation of PKM2 by the inclusion of exon 10 and the exclusion of exon 9, which is specific to PKM1 (David et al., 2010; Noguchi et al., 1987). PKM1 is highly expressed in normal tissues, whereas PKM2 expression is also detected in normal tissues, including those from lung, liver, colon, thyroid, kidney and bladder (Bluemlein et al., 2011; Yang and Lu, 2013b). Analyses of 16 tumor types using the cancer genome atlas RNA-Seq and exon array datasets has revealed that an isoform switch from PKM1 to PKM2 occurs in glioblastomas (Desai et al., 2014). Despite a lack of isoform switches in other tumor types, PKM2 expression has been found to be increased in all cancer types examined (Bluemlein et al., 2011; Desai et al., 2014), and replacement of PKM2 with PKM1 has been found to inhibit aerobic glycolysis and tumor growth (Christofk et al., 2008a; Gumińska et al., 1988; Mellati et al., 1992). These findings point to a crucial role for expression of PKM2 in tumor growth.
In addition to its well-known role in glycolysis, PKM2 has also been reported to be involved in other cellular functions. PKM2 has been shown to be the cytosolic receptor for thyroid hormone (Kato et al., 1989). Importantly, PKM2 has recently been found to translocate into the nucleus upon mitogenic and oncogenic stimulation (Lv et al., 2013; Yang et al., 2012c). In the nucleus, PKM2 functions as a transcriptional co-activator and a protein kinase that phosphorylates histones, highlighting the crucial role of PKM2 in the epigenetic regulation of gene transcription that is important for the G1-S phase transition and the Warburg effect (which states that most cancer cells produce energy by a high level of glycolysis followed by lactic acid fermentation) (Yang et al., 2012b; Yang et al., 2011). In addition to the crucial role of PKM2 in G1-S phase, it phosphorylates important cell cycle regulators, such as the spindle checkpoint protein Bub3, to regulate chromatid segregation and the mitotic checkpoint during mitosis, and myosin light chain 2 (MLC2, encoded by MYL2) to initiate cytokinesis, leading to enhanced and governed tumor cell proliferation (Jiang et al., 2014a).
In this Cell Science at a Glance article and the accompanying poster, we briefly review recent findings regarding the metabolic and non-metabolic functions of PKM2 and the mechanisms that regulate PKM2 expression, its glycolytic enzymatic activity and subcellular location.
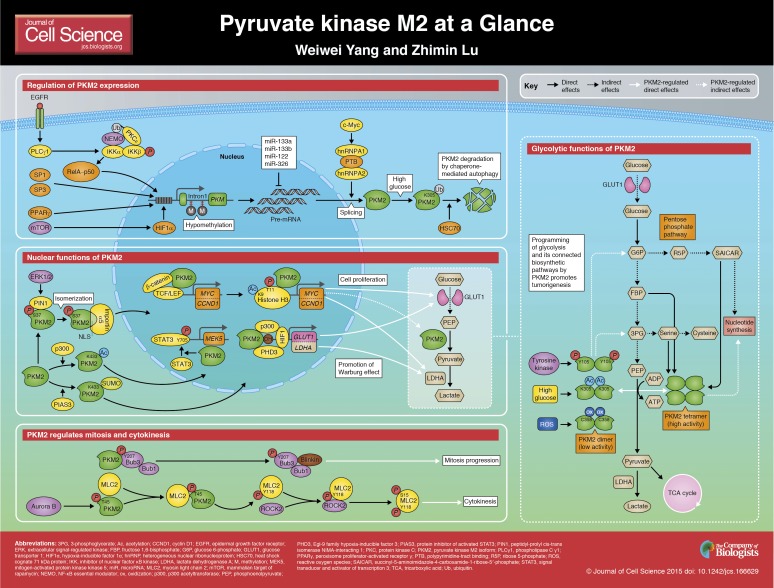
Regulation of PKM2
Regulation of PKM2 expression
The expression of PKM2 is regulated at multiple levels through regulation of DNA methylation, transcription factors, pre-mRNA splicing of PKM, PKM2-specific microRNAs (miRNAs) and post-translational modifications of the PKM2 protein (see poster).
Analyses of The Cancer Genome Atlas DNA methylation data have revealed that elevated PKM2 expression correlates well with a hypomethylation status of intron 1 of the PKM gene in multiple cancer types, suggesting that epigenetic regulation by DNA methylation is an important mechanism in controlling PKM transcription in tumors (Desai et al., 2014).
Several transcriptional factors have been reported to regulate the activity of the PKM promoter, which contains five putative binding sites for SP1 and SP3. Both SP1 and SP3 interact with the nuclear factor (NF)-YA transcriptional factor (see poster). Indeed, overexpression of SP1 or SP3 and NF-YA synergistically stimulates the distal promoter activity of the PKM gene (Discher et al., 1998; Yamada et al., 2000). Phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) activation, which can be induced by insulin stimulation, has also been shown to increase PKM2 expression through hypoxia-inducible factor 1α (HIF1α)-regulated transcription of the PKM gene (Iqbal et al., 2013; Sun et al., 2011). Peroxisome proliferator-activated receptor γ (PPARγ), a nuclear hormone receptor, can also specifically and transcriptionally regulate PKM2 expression. Activation of AKT in PTEN-deficient fatty livers results in the binding of PPARγ to PPAR response elements (PPRE) in the promoter region of both PKM and the hexokinase-2 (HK2) gene, and contributes to liver steatosis, hypertrophy and hyperplasia (Panasyuk et al., 2012). These results suggest that activation of the PI3K–AKT–mTOR pathway, coupled with other activated signaling modulators, regulates PKM2 expression in a manner that depends on the signaling context and is tissue specific. In response to epidermal growth factor receptor (EGFR) activation, which occurs in many types of human cancers, PKM transcription is upregulated by a signaling cascade that includes EGFR, phospholipase C γ1 (PLCγ1), protein kinase C ε (PKCε), and NF-κB. Activation of EGFR results in the activation of PLCγ1 and the subsequent production of diacylglycerol; this in turn activates PKCε, which is then monoubiquitylated by the E3 ligase RINCK1 (also known as TRIM41) at K321, allowing it to interact with a ubiquitin-binding motif located in the zinc finger region of NF-κB essential modulator (NEMO; also known as IKKγ). This interaction recruits the cytosolic IκB kinase (IKK) complex, which is composed of NEMO, IKKα and IKKβ, to the plasma membrane, where PKCε phosphorylates IKKβ at S177 and activates IKKβ. Activated IKKβ phosphorylates inhibitor of nuclear factor κB (IκB) and abrogates its repressive effect on RelA (the p65 subunit of NF-κB), thereby allowing it to translocate to the nucleus where it directly binds to the PKM promoter and enhances PKM2 expression, resulting in the Warburg effect and tumorigenesis (Yang et al., 2012a) (see poster).
PKM2 expression can also be regulated at the level of transcribed PKM pre-mRNA by splicing factors. Heterogeneous nuclear ribonucleoproteins (hnRNPs), including PTB (also known as hnRNP1), hnRNPA1 and hnRNPA2, are upregulated by the oncogenic transcription factor c-Myc and subsequently bind to splicing signals that flank PKM exon 9, repressing the inclusion of exon 9 and thus promoting an enhanced expression of the PKM2 isoform (David et al., 2010; Sun et al., 2011).
miRNAs, noncoding RNAs that bind to specific target mRNAs and promote their degradation and/or hinder their translation, provide another means for regulating PKM2 mRNA. Both miR-133a and miR-133b target the PKM transcript, and these miRNAs have been found to be significantly reduced in tongue squamous cell carcinoma cells, resulting in PKM2 overexpression (Wong et al., 2008). miR-122, which is highly expressed in normal liver tissue, is reduced in hepatocellular carcinoma and directly targets PKM2 mRNA (Liu et al., 2014), whereas miR-326 regulates PKM2 expression in human glioma (Kefas et al., 2010), suggesting that different miRNAs are involved in the tissue-specific regulation of PKM2 expression. In addition, the mRNAs encoding the splicing factors PTB, hnRNPA1 and hnRNPA2 are targeted by miR-124, miR-137 and miR-340. Consequently, these miRNAs mediate a switch in expression of the PKM gene from the PKM2 isoform to PKM1, which results in a reduced glycolysis rate and promotes the glucose flux into oxidative phosphorylation, consequently leading to impaired cancer cell growth (Sun et al., 2012).
Finally, PKM2 protein levels are also regulated at the level of post-translational modifications. For instance, acetylation of PKM2 at K305 promotes its degradation under high-glucose concentrations. Acetylated PKM2 interacts with heat shock protein HSC70 (also known as HSPA8), which leads to lysosomal-dependent degradation of PKM2 by chaperone-mediated autophagy (see poster). Accordingly, expression of an acetylation-mimetic PKM2 K305Q mutant, which undergoes degradation at high concentrations of glucose, results in the accumulation of glycolytic intermediates upstream of PKM2 for biosynthesis, which promotes cell proliferation and tumorigenesis (Lv et al., 2011).
Regulation of the enzymatic activity and metabolic functions of PKM2
PKM2 catalyzes the last step of glycolysis, and its activity can be regulated by glycolytic intermediates. Fructose 1,6-bisphosphate (FBP), an allosteric activator of PKM2, binds to PKM2 and promotes its tetramerization. Tetrameric PKM2 is more active than dimeric PKM2, and the conversion between these two forms is dynamically regulated (Dombrauckas et al., 2005) (see poster). Binding of PKM2 to phosphorylated tyrosine releases FBP and disrupts tetrameric PKM2 into the PKM2 dimer (Christofk et al., 2008b). Human PKM2 mutants with heterozygous missense mutations H391Y and K422R, which have been identified in cells from Bloom syndrome patients, heterooligomerize with the wild-type PKM2 and reduce the overall activity of PKM2, resulting in an increased accumulation of glycolytic intermediates and NADPH, cell proliferation, polyploidy and tumor growth (Gupta et al., 2010; Iqbal et al., 2014a; Iqbal et al., 2014b). JMJD5, a dioxygenase containing a Jumonji C domain, interacts with the region of PKM2 at the intersubunit interface, which impedes PKM2 tetramerization and blocks pyruvate kinase activity (Wang et al., 2014a). Decreased PKM2 pyruvate kinase activity has been shown to result in PEP-dependent histidine phosphorylation and activation of phosphoglycerate mutase (PGAM1), as well as in reduced levels of PEP-dependent ATP production; this metabolic switch might provide an alternate glycolytic pathway that decouples ATP from PEP-mediated phosphotransfer, thereby allowing for the high rate of glycolysis to support the anabolic metabolism (Vander Heiden et al., 2010b). Furthermore, in primary mouse embryonic fibroblasts in which PKM2 was deleted, PKM1 expression is upregulated and impairs nucleotide production and the ability of cells to synthesize DNA and progress the cell cycle, suggesting that an appropriate level of PKM2 expression is important for normal cell proliferation (Lunt et al., 2015).
Serine is another allosteric activator of PKM2 (Chaneton et al., 2012) (see poster). It binds to and activates PKM2 in a manner similar to but independent of FBP. When serine is abundant, PKM2 is fully active, enabling maximal use of glucose through glycolysis. When serine is limited, however, PKM2 activity is immediately curtailed, resulting in rapid diversion of glucose-derived carbon to serine biosynthesis and thus compensating for the serine shortfall (Chaneton et al., 2012). Succinyl-5-aminoimidazole-4-carboxamide-1-ribose-5′-phosphate (SAICAR), an intermediate of the de novo purine nucleotide synthesis pathway, also regulates PKM2 activity allosterically and independently of FBP. Cellular SAICAR concentration increases upon glucose starvation, which stimulates PKM2 activity to enhance glucose and glutamine consumption rates, and promotes cancer cell survival (Keller et al., 2012). Thus, allosteric regulation of PKM2 might allow cancer cells to coordinate different metabolic pathways to support cancer cell growth in the often nutrient-limited tumor microenvironment.
PKM2 activity can also be regulated by post-translational modifications (see poster). Fibroblast growth factor receptor type 1 (FGFR1) phosphorylates PKM2 at Y105, which disrupts the binding of FBP to PKM2 (Hitosugi et al., 2009) (see poster). This phosphorylation, which promotes the tetramer-to-dimer conversion of PKM2, inhibits its pyruvate kinase activity and enhances its protein kinase activity towards STAT3 phosphorylation (Gao et al., 2013). Expression of the PKM2 Y105F mutant impairs cell proliferation and tumorigenesis (Hitosugi et al., 2009). In addition, acute increase in intracellular concentrations of reactive oxygen species (ROS) induced by H2O2, diamide (a thiol-oxidizing compound) or hypoxia inhibits PKM2 activity through oxidation of C358 of PKM2, leading to diversion of glucose flux into the pentose phosphate pathway to generate a reducing potential for the detoxification of ROS and tumor growth (Anastasiou et al., 2011). Similarly, reduction of PKM2 activity by ROS-induced PKM2 oxidation in response to insulin stimulation has also been reported (Iqbal et al., 2013).
Regulation of the subcellular localization of PKM2
As a glycolytic enzyme, PKM2 predominantly localizes in the cytosol. However, PKM2 translocates into the nucleus to promote cell proliferation (see poster). Our group has demonstrated a crucial mechanism underlying the nuclear translocation of PKM2. Upon EGFR activation, activated extracellular signal-regulated kinase 1 and 2 (ERK1/2) binds to the region that is encoded by exon 10 of PKM2 through a docking groove in ERK1/2, resulting in phosphorylation of S37 of PKM2, but not PKM1. Phosphorylated PKM2 then recruits peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (PIN1), which specifically catalyzes the cis-trans isomerization of peptide bonds between phosphorylated serine or threonine residues and proline residues (Lu and Hunter, 2014). This isomerization of PKM2 exposes its nuclear localization sequence (NLS) and promotes its binding to importin α5, which facilitates its nuclear translocation (Yang et al., 2012c). In addition, sumoylation of PKM2 mediated by the SUMO-E3 ligase PIAS3, and acetylation of PKM2 at K433 mediated by the p300 acetyltransferase (also known as EP300) prevent the binding of FBP to PKM2, thereby enhancing its nuclear translocation (Lv et al., 2013; Spoden et al., 2009).
Non-metabolic functions of PKM2
In addition to its glycolytic function, PKM2 possesses non-metabolic functions that are instrumental for gene expression and cell cycle progression. Our group has reported that nuclear PKM2 interacts with c-Src-phosphorylated β-catenin Y333 and enhances the transactivation activity of β-catenin, highlighting the importance of PKM2 as a transcriptional co-activator (Yang et al., 2011) (see poster). Importantly, β-catenin-associated PKM2 directly binds to and phosphorylates histone H3 at T11. This phosphorylation, which uses PEP as a phosphate donor, is required for the dissociation of histone deacetylase 3 (HDAC3) from the promoters of the β-catenin target genes CCND1 (encoding cyclin D1) and MYC, and for the subsequent acetylation of histone H3 at lysine 9. PKM2-dependent histone H3 phosphorylation is essential for EGFR-mediated gene expression, cell proliferation and tumorigenesis (Lu, 2012b; Yang et al., 2012b). PKM2 phosphorylates not only threonine residues but also tyrosine residues, as demonstrated by its ability to phosphorylate STAT3 at Y705. This phosphorylation enhances the transcriptional activity of STAT3 and STAT3-dependent MEK5 (also known as MAP2K5) transcription (Gao et al., 2012). These findings reveal a new function for PKM2 as a dual-specificity protein kinase that directly regulates gene expression. The role of PKM2 as a pleiotropic protein kinase has been demonstrated by protein microarray experiments, which found that more than 100 human proteins, mostly protein kinases, are phosphorylated by PKM2. In particular, PKM2 phosphorylates and activates ERK1/2, and the sustained ERK1/2 activation mediated by PKM2 is instrumental for cell proliferation (Keller et al., 2014). PKM2 has also been shown to interact with Oct4 (also known as POU5F1) upon treatment with dichloroacetate, a pyruvate dehydrogenase kinase inhibitor that promotes mitochondrial oxidative phosphorylation (Morfouace et al., 2014). This interaction is responsible for the decrease in the transcriptional activity of Oct4 and thus induces the differentiation of glioma spheroids. This study has also shown that PKM2 depletion enhances both apoptosis and differentiation of glioma spheroids, suggesting that PKM2 maintains glioma stemness and promotes gliomagenesis through Oct4 (Morfouace et al., 2014).
PKM2 regulates not only G1-S phase transition by controlling cyclin D1 expression but also regulates mitosis (see poster). We recently demonstrated that PKM2, but not PKM1, binds to the spindle checkpoint protein Bub3 during mitosis and phosphorylates Bub3 at Y207. This phosphorylation leads to the recruitment of the Bub3–Bub1 complex to the outer kinetochore protein Blinkin (also known as CASC5), which acts as a receptor for Bub proteins; this regulation exerts a precise control over the kinetochore–spindle attachment and the mitotic (spindle assembly) checkpoint, and, subsequently, accurate chromosome segregation and proliferation of tumor cells (Jiang et al., 2014a).
PKM2 is also involved in cytokinesis, the final stage of cell division, during which the two daughter cells separate completely (see poster). During cytokinesis, Aurora B phosphorylates PKM2 at T45, which is required for the localization of PKM2 and its interaction with MLC2 in the contractile ring region of mitotic cells; this leads to PKM2-mediated phosphorylation of MLC2 at Y118. This phosphorylation primes the binding of Rho-associated protein kinase 2 (ROCK2) to MLC2 and subsequent ROCK2-dependent MLC2 S15 phosphorylation. PKM2-mediated MLC2 phosphorylation, which is greatly enhanced by EGFR variant III, K-Ras G12V and B-Raf V600E mutant expression, thus plays a pivotal role in completion of cytokinesis, cell proliferation and tumor development. These findings underscore the instrumental function of PKM2 in oncogenic signaling, regulating the fidelity of chromosome segregation, cell cycle progression, cytokinesis and tumorigenesis (Jiang et al., 2014b).
Integrated metabolic and non-metabolic functions of PKM2
PKM2 can also regulate cell metabolism through its non-metabolic functions (Lu, 2012b; Yang and Lu, 2013a; Yang and Lu, 2013b). Upon activation of EGFR and platelet-derived growth factor receptor (PDGFR), PKM2 translocates into the nucleus where it activates β-catenin to induce c-Myc expression. Upregulated c-Myc, in turn, induces the upregulation of GLUT1 and lactate dehydrogenase A (LDHA) and, in a positive-feedback loop, PTB-dependent PKM2 expression (Lu, 2012a; Yang et al., 2011; Yang et al., 2012c) (see poster). Upregulation of these glycolysis genes enhances glucose consumption and lactate production, and subsequently promotes tumorigenesis. In addition, nuclear PKM2 can also regulate HIF1α activity to reprogram cancer metabolism. PKM2 interacts directly with HIF1α, which requires prolyl hydroxylase 3 (PHD3)-mediated PKM2 hydroxylation. Interaction of HIF1α with PKM2 promotes the expression of HIF1α target genes by enhancing the binding of HIF1α to hypoxia response elements and the recruitment of p300 to these sites. Enhanced HIF1α activity increases the transcription of a variety of glycolytic genes and thereby promotes glycolysis of cancer cells (Luo et al., 2011). These findings underscore the importance of the integrated metabolic and non-metabolic functions of PKM2 in tumorigenesis.
PKM2 as a molecular target for cancer treatment
The specific role of PKM2 in cancer makes PKM2 an attractive therapeutic target for cancer treatment. However, the complexity of PKM2 regulation points to several potential strategies to target PKM2, and, accordingly, approaches to inhibit as well as to activate PKM2 have been pursued.
Because enhanced glycolysis and high expression of PKM2 have been observed in many types of cancer cells, it is reasonable to assume that repressing PKM2 activity will inhibit glycolysis and thereby reduce proliferation of tumor cells. The recently identified protein kinase activity of PKM2 and the instrumental role of this activity in regulating the Warburg effect and cell cycle progression of tumor cells (Jiang et al., 2014a; Yang and Lu, 2013b) have provided the molecular basis for the inhibition of growth and survival of tumor cells that has been observed previously with specific PKM2 inhibitors (Spoden et al., 2008; Vander Heiden et al., 2010a). Furthermore, a Phase II clinical trial with a peptidic inhibitor of PKM2 has been initiated to evaluate the potential of PKM2 as a therapeutic target in patients with refractory metastatic renal cell carcinoma, and encouraging results have been obtained (Porporato et al., 2011). In addition, shikonin and its enantiomeric isomer alkannin have been shown to be able to inhibit more than 50% of the cellular PKM2 without affecting the activities of PKM1 and PKL, which harbor the same FBP-binding site as PKM2 (Chen et al., 2011). Shikonin treatment inhibits glucose consumption and lactate release, and so induces tumor cell death.
In contrast, there is also evidence that supports the notion that the activation of PKM2 might be beneficial for the inhibition of tumor growth. Tyrosine phosphorylation or acetylation of PKM2, which reduces PKM2 activity or downregulates PKM2 expression, promotes tumor cell growth (Hitosugi et al., 2009; Lv et al., 2011). High-throughput screening of a chemical compound library has identified some PKM2 activators that effectively promote PKM2 tetramerization and inhibit lung cancer cell proliferation in different models (Anastasiou et al., 2012; Xu et al., 2014). However, other potent and selective PKM2 activators that have a different mode of binding to the PKM2 protein do not affect cancer cell glycolysis and are not effective in suppressing cancer cell growth in vitro (Guo et al., 2013). These results suggest that activation of PKM2 per se is not the key to regulating cell growth. Given that PKM2 translocates into the nucleus and regulates gene expression, likely as a monomer (Yang and Lu, 2013a; Yang and Lu, 2013b), the tetramerization of PKM2, which is induced by some activators, or their binding to PKM2, might disturb the nuclear translocation of PKM2 and/or cellular activities that are regulated by PKM2 protein kinase activity. Further studies are therefore warranted to understand the distinct regulatory effects of different PKM2 activators on cells.
It is also worth noting that PKM2 expression levels vary among different types of cancer cells (Desai et al., 2014), suggesting that the role of PKM2 in tumorigenesis depends on the signaling context. For instance, recent reports showed that PKM2 deficiency in mice did not block Brca1-loss-driven mammary tumor formation but did inhibit cyclin D1 expression and leukemia initiation that is induced by the fusion proteins BCR-ABL and MLL-AF9 (Israelsen et al., 2013; Wang et al., 2014b). These findings suggest that PKM2 regulates tumor development in a signaling-context-dependent manner and underscore the requirement for a personalized cancer therapy that takes into account the specific PKM2 activities present.
Conclusions
Reprogrammed metabolism provides tumor cells with a special growth advantage compared with normal cells. PKM2, a key rate-limiting enzyme in glycolysis, plays a central role in this process. On the one hand, both expression and enzymatic activity of PKM2 are regulated at multiple levels, including transcription, pre-mRNA slicing, miRNA-regulated mRNA stability, protein stability, post-translational modifications and allosteric regulation. These regulations rewire the metabolic pathways, thereby shunting the glycolytic intermediates to the different branches of glycolysis to support the synthesis of biomass. On the other hand, the newly characterized non-metabolic functions of PKM2 demonstrate its ability to epigenetically control gene transcription and to promote cell cycle progression, cytokinesis and feedback-regulated metabolism. Integration of the metabolic and non-metabolic functions of PKM2 promotes the proliferation of tumor cells. Further deciphering the cellular functions of PKM2 might lead to successful cancer therapy.
Supplementary Material
Acknowledgements
We thank Michael Worley and the Department of Scientific Publications at MD Anderson Cancer Center for critical reading of the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Our work was supported by the National Science Foundation of China [grant numbers 31471324, 31422034 to W.Y.]; National Cancer Institute [grant numbers 2R01CA109035 to Z.L., 1R0CA169603 to Z.L., and CA16672 to the Cancer Center Support Grant]; a research grant from the Cancer Prevention and Research Institute of Texas (CPRIT) [grant number RP130389 to Z.L.]; a James S. McDonnell Foundation 21st Century Science Initiative in Brain Cancer Research Award [grant number 220020318 to Z.L.]; a Odyssey Fellowship from The University of Texas MD Anderson Cancer Center (to W.Y.); the Harold C. and Mary L. Daily Endowment Fellowship (W.Y.); the Lupe C. Garcia Fellowship in Cancer Research (to W.Y.); and the Thomas H. and Mayme P. Scott Fellowship in Cancer Research (W.Y). Deposited in PMC for release after 12 months.
Cell science at a glance
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.166629/-/DC1.
References
- Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. doi: 10.1016/j.ygeno.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS. et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A. et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 2012;8:839–847. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluemlein K, Grüning NM, Feichtinger RG, Lehrach H, Kofler B, Ralser M. No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget. 2011;2:393–400. doi: 10.18632/oncotarget.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaneton B, Hillmann P, Zheng L, Martin AC, Maddocks OD, Chokkathukalam A, Coyle JE, Jankevics A, Holding FP, Vousden KH. et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491:458–462. doi: 10.1038/nature11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene. 2011;30:4297–4306. doi: 10.1038/onc.2011.137. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008a;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008b;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–368. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S, Ding M, Wang B, Lu Z, Zhao Q, Shaw K, Yung WK, Weinstein JN, Tan M, Yao J. Tissue-specific isoform switch and DNA hypomethylation of the pyruvate kinase PKM gene in human cancers. Oncotarget. 2014;5:8202–8210. doi: 10.18632/oncotarget.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DJ, Bishopric NH, Wu X, Peterson CA, Webster KA. Hypoxia regulates beta-enolase and pyruvate kinase-M promoters by modulating Sp1/Sp3 binding to a conserved GC element. J. Biol. Chem. 1998;273:26087–26093. doi: 10.1074/jbc.273.40.26087. [DOI] [PubMed] [Google Scholar]
- Dombrauckas JD, Santarsiero BD, Mesecar AD. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry. 2005;44:9417–9429. doi: 10.1021/bi0474923. [DOI] [PubMed] [Google Scholar]
- Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol. Cell. 2012;45:598–609. doi: 10.1016/j.molcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wang H, Yang JJ, Chen J, Jie J, Li L, Zhang Y, Liu ZR. Reciprocal regulation of protein kinase and pyruvate kinase activities of pyruvate kinase M2 by growth signals. J. Biol. Chem. 2013;288:15971–15979. doi: 10.1074/jbc.M112.448753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumińska M, Stachurska MB, Ignacak J. Pyruvate kinase isoenzymes in chromatin extracts of Ehrlich ascites tumour, Morris hepatoma 7777 and normal mouse and rat livers. Biochim. Biophys. Acta. 1988;966:207–213. doi: 10.1016/0304--4165(88)90113--4. [DOI] [PubMed] [Google Scholar]
- Guo C, Linton A, Jalaie M, Kephart S, Ornelas M, Pairish M, Greasley S, Richardson P, Maegley K, Hickey M. et al. Discovery of 2-((1H-benzo[d]imidazol-1-yl)methyl)-4H-pyrido[1,2-a]pyrimidin-4-ones as novel PKM2 activators. Bioorg. Med. Chem. Lett. 2013;23:3358–3363. doi: 10.1016/j.bmcl.2013.03.090. [DOI] [PubMed] [Google Scholar]
- Gupta V, Kalaiarasan P, Faheem M, Singh N, Iqbal MA, Bamezai RN. Dominant negative mutations affect oligomerization of human pyruvate kinase M2 isozyme and promote cellular growth and polyploidy. J. Biol. Chem. 2010;285:16864–16873. doi: 10.1074/jbc.M109.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ. et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci. Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal MA, Siddiqui FA, Gupta V, Chattopadhyay S, Gopinath P, Kumar B, Manvati S, Chaman N, Bamezai RN. Insulin enhances metabolic capacities of cancer cells by dual regulation of glycolytic enzyme pyruvate kinase M2. Mol. Cancer. 2013;12:72. doi: 10.1186/1476-4598-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal MA, Gupta V, Gopinath P, Mazurek S, Bamezai RN. Pyruvate kinase M2 and cancer: an updated assessment. FEBS Lett. 2014a;588:2685–2692. doi: 10.1016/j.febslet.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Iqbal MA, Siddiqui FA, Chaman N, Gupta V, Kumar B, Gopinath P, Bamezai RN. Missense mutations in pyruvate kinase M2 promote cancer metabolism, oxidative endurance, anchorage independence, and tumor growth in a dominant negative manner. J. Biol. Chem. 2014b;289:8098–8105. doi: 10.1074/jbc.M113.515742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW. et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155:397–409. doi: 10.1016/j.cell.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Li X, Yang W, Hawke DH, Zheng Y, Xia Y, Aldape K, Wei C, Guo F, Chen Y. et al. PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Mol. Cell. 2014a;53:75–87. doi: 10.1016/j.molcel.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wang Y, Wang T, Hawke DH, Zheng Y, Li X, Zhou Q, Majumder S, Bi E, Liu DX. et al. PKM2 phosphorylates MLC2 and regulates cytokinesis of tumour cells. Nat. Commun. 2014b;5:5566. doi: 10.1038/ncomms6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Fukuda T, Parkison C, McPhie P, Cheng SY. Cytosolic thyroid hormone-binding protein is a monomer of pyruvate kinase. Proc. Natl. Acad. Sci. USA. 1989;86:7861–7865. doi: 10.1073/pnas.86.20.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefas B, Comeau L, Erdle N, Montgomery E, Amos S, Purow B. Pyruvate kinase M2 is a target of the tumor-suppressive microRNA-326 and regulates the survival of glioma cells. Neuro-oncol. 2010;12:1102–1112. doi: 10.1093/neuonc/noq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Tan IS, Lee YS. SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Science. 2012;338:1069–1072. doi: 10.1126/science.1224409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Doctor ZM, Dwyer ZW, Lee YS. SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells. Mol. Cell. 2014;53:700–709. doi: 10.1016/j.molcel.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AM, Xu Z, Shek FH, Wong KF, Lee NP, Poon RT, Chen J, Luk JM. miR-122 targets pyruvate kinase M2 and affects metabolism of hepatocellular carcinoma. PLoS ONE. 2014;9:e86872. doi: 10.1371/journal.pone.0086872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z. Nonmetabolic functions of pyruvate kinase isoform M2 in controlling cell cycle progression and tumorigenesis. Chin. J. Cancer. 2012a;31:5–7. doi: 10.5732/cjc.011.10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z. PKM2 functions as a histone kinase. Cell Cycle. 2012b;11:4101–4102. doi: 10.4161/cc.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Hunter T. Prolyl isomerase Pin1 in cancer. Cell Res. 2014;24:1033–1049. doi: 10.1038/cr.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt SY, Muralidhar V, Hosios AM, Israelsen WJ, Gui DY, Newhouse L, Ogrodzinski M, Hecht V, Xu K, Acevedo PN. et al. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol. Cell. 2015;57:95–107. doi: 10.1016/j.molcel.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L, Li D, Zhao D, Lin R, Chu Y, Zhang H, Zha Z, Liu Y, Li Z, Xu Y. et al. Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol. Cell. 2011;42:719–730. doi: 10.1016/j.molcel.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L, Xu YP, Zhao D, Li FL, Wang W, Sasaki N, Jiang Y, Zhou X, Li TT, Guan KL. et al. Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization. Mol. Cell. 2013;52:340–352. doi: 10.1016/j.molcel.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR. et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- Mellati AA, Yücel M, Altinörs N, Gündüz U. Regulation of M2-type pyruvate kinase from human meningioma by allosteric effectors fructose 1,6 diphosphate and L-alanine. Cancer Biochem. Biophys. 1992;13:33–41. [PubMed] [Google Scholar]
- Morfouace M, Lalier L, Oliver L, Cheray M, Pecqueur C, Cartron PF, Vallette FM. Control of glioma cell death and differentiation by PKM2-Oct4 interaction. Cell Death Dis. 2014;5:e1036. doi: 10.1038/cddis.2013.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Yamada K, Inoue H, Matsuda T, Tanaka T. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J. Biol. Chem. 1987;262:14366–14371. [PubMed] [Google Scholar]
- Panasyuk G, Espeillac C, Chauvin C, Pradelli LA, Horie Y, Suzuki A, Annicotte JS, Fajas L, Foretz M, Verdeguer F. et al. PPARγ contributes to PKM2 and HK2 expression in fatty liver. Nat. Commun. 2012;3:672. doi: 10.1038/ncomms1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front. Pharmacol. 2011;2:49. doi: 10.3389/fphar.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoden GA, Mazurek S, Morandell D, Bacher N, Ausserlechner MJ, Jansen-Durr P, Eigenbrodt E, Zwerschke W. Isotype-specific inhibitors of the glycolytic key regulator pyruvate kinase subtype M2 moderately decelerate tumor cell proliferation. Int. J. Cancer. 2008;123:312–321. doi: 10.1002/ijc.23512. [DOI] [PubMed] [Google Scholar]
- Spoden GA, Morandell D, Ehehalt D, Fiedler M, Jansen-Dürr P, Hermann M, Zwerschke W. The SUMO-E3 ligase PIAS3 targets pyruvate kinase M2. J. Cell. Biochem. 2009;107:293–302. doi: 10.1002/jcb.22125. [DOI] [PubMed] [Google Scholar]
- Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, Wang Y, Jing Y, Yang H, Chen R. et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc. Natl. Acad. Sci. USA. 2011;108:4129–4134. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhao X, Zhou Y, Hu Y. miR-124, miR-137 and miR-340 regulate colorectal cancer growth via inhibition of the Warburg effect. Oncol. Rep. 2012;28:1346–1352. doi: 10.3892/or.2012.1958. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Christofk HR, Schuman E, Subtelny AO, Sharfi H, Harlow EE, Xian J, Cantley LC. Identification of small molecule inhibitors of pyruvate kinase M2. Biochem. Pharmacol. 2010a;79:1118–1124. doi: 10.1016/j.bcp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM. et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010b;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Hsieh YJ, Cheng WC, Lin CP, Lin YS, Yang SF, Chen CC, Izumiya Y, Yu JS, Kung HJ. et al. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1α-mediated glucose metabolism. Proc. Natl. Acad. Sci. USA. 2014a;111:279–284. doi: 10.1073/pnas.1311249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Israelsen WJ, Lee D, Yu VW, Jeanson NT, Clish CB, Cantley LC, Vander Heiden MG, Scadden DT. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014b;158:1309–1323. doi: 10.1016/j.cell.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TS, Liu XB, Chung-Wai Ho A, Po-Wing Yuen A, Wai-Man Ng R, Ignace Wei W. Identification of pyruvate kinase type M2 as potential oncoprotein in squamous cell carcinoma of tongue through microRNA profiling. Int. J. Cancer. 2008;123:251–257. doi: 10.1002/ijc.23583. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu XH, Saunders M, Pearce S, Foulks JM, Parnell KM, Clifford A, Nix RN, Bullough J, Hendrickson TF. et al. Discovery of 3-(trifluoromethyl)-1H-pyrazole-5-carboxamide activators of the M2 isoform of pyruvate kinase (PKM2). Bioorg. Med. Chem. Lett. 2014;24:515–519. doi: 10.1016/j.bmcl.2013.12.028. [DOI] [PubMed] [Google Scholar]
- Yamada K, Tanaka T, Miyamoto K, Noguchi T. Sp family members and nuclear factor-Y cooperatively stimulate transcription from the rat pyruvate kinase M gene distal promoter region via their direct interactions. J. Biol. Chem. 2000;275:18129–18137. doi: 10.1074/jbc.M001543200. [DOI] [PubMed] [Google Scholar]
- Yang W, Lu Z. Nuclear PKM2 regulates the Warburg effect. Cell Cycle. 2013a;12:3343–3347. doi: 10.4161/cc.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Lu Z. Regulation and function of pyruvate kinase M2 in cancer. Cancer Lett. 2013b;339:153–158. doi: 10.1016/j.canlet.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, Gao X, Aldape K, Lu Z. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Xia Y, Cao Y, Zheng Y, Bu W, Zhang L, You MJ, Koh MY, Cote G, Aldape K. et al. EGFR-induced and PKCε monoubiquitylation-dependent NF-κB activation upregulates PKM2 expression and promotes tumorigenesis. Mol. Cell. 2012a;48:771–784. doi: 10.1016/j.molcel.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, Aldape K, Hunter T, Alfred Yung WK, Lu Z. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012b;150:685–696. doi: 10.1016/j.cell.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, Lyssiotis CA, Aldape K, Cantley LC, Lu Z. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat. Cell Biol. 2012c;14:1295–1304. doi: 10.1038/ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.