Abstract
The molecular clock is intimately linked to metabolic regulation, and brown adipose tissue plays a key role in energy homeostasis. However, whether the cell-intrinsic clock machinery participates in brown adipocyte development is unknown. Here, we show that Bmal1 (also known as ARNTL), the essential clock transcription activator, inhibits brown adipogenesis to adversely affect brown fat formation and thermogenic capacity. Global ablation of Bmal1 in mice increases brown fat mass and cold tolerance, and adipocyte-selective inactivation of Bmal1 recapitulates these effects and demonstrates its cell-autonomous role in brown adipocyte formation. Further loss- and gain-of-function studies in mesenchymal precursors and committed brown progenitors reveal that Bmal1 inhibits brown adipocyte lineage commitment and terminal differentiation. Mechanistically, Bmal1 inhibits brown adipogenesis through direct transcriptional control of key components of the TGF-β pathway together with reciprocally altered BMP signaling; activation of TGF-β or blockade of BMP pathways suppresses enhanced differentiation in Bmal1-deficient brown adipocytes. Collectively, our study demonstrates a novel temporal regulatory mechanism in fine-tuning brown adipocyte lineage progression to affect brown fat formation and thermogenic regulation, which could be targeted therapeutically to combat obesity.
KEY WORDS: Brown adipocyte differentiation, Circadian rhythm, Adipogenesis, Obesity, TGF-β signaling pathway
INTRODUCTION
The molecular clock is an evolutionarily conserved timekeeping mechanism that entrains diverse biological processes to timing cues (Reppert and Weaver, 2002). Recently, it has been recognized that through temporal regulation of key metabolic pathways (Panda et al., 2002), the clock machinery ensures the adaptation of nutrient metabolism and energy homeostasis with circadian timing, and resident peripheral clocks in metabolic tissues are key components in this regulation (Bass and Takahashi, 2010; Green et al., 2008). However, whether the clock machinery functions in the metabolically active brown adipose tissue (BAT), a specialized thermogenic organ (Cannon and Nedergaard, 2004), is largely unknown.
In both the central clock – the suprachiasmatic nuclei (SCN) – and peripheral tissues, the ∼24-hour daily rhythm is driven by an intricate molecular feedback loop consisting of positive and negative regulators coupled with post-transcriptional control (Dibner et al., 2010). Brain and muscle Arnt like 1 (Bmal1, also known as ARNTL), a basic helix-loop-helix transcription factor, is an essential positive regulator of the core molecular clock loop (Reppert and Weaver, 2002). Bmal1 binds to conserved E-box elements (5′-CACGTG-3′) as a heterodimer with CLOCK (circadian locomotor output cycles kaput) and elicits transcription of the negative regulator period proteins (Per1, Per2 and Per3) and cryptochromes (Cry1 and Cry2). These factors in turn inhibit the transcriptional activity of Bmal1–CLOCK, which, coupled with temporally controlled proteasome-mediated degradation mechanisms, leads to rhythmic oscillation of the molecular clock and thus generates the daily rhythm. Interestingly, despite studies demonstrating that Bmal1 plays key roles in metabolic regulation (Cho et al., 2012; Rudic et al., 2004; Solt et al., 2012) and adipogenesis (Fontaine et al., 2003; Guo et al., 2012; Wang and Lazar, 2008), its function in brown adipocytes has not been studied.
BAT possesses a unique thermogenic ability mediated by the action of uncoupling protein-1 (UCP-1), an inner mitochondrial membrane proton channel that dissipates the chemical gradient derived from oxidative phosphorylation as heat instead of ATP generation (Cannon and Nedergaard, 2004). It has been increasingly recognized that, in addition to its crucial role in the maintenance of body temperature under physiological conditions and cold stress (Cannon and Nedergaard, 2004), the energy-dissipating capacity of BAT is an important regulatory component of whole-body energy balance (Saito et al., 2009; van Marken Lichtenbelt et al., 2009). Functional BAT is present in humans (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009) and its activity is inversely correlated with body mass index in obese patients (van Marken Lichtenbelt et al., 2009), suggesting that expanding or stimulating the thermogenic capacity of BAT might have therapeutic potential in treating obesity (Nedergaard and Cannon, 2010). Owing to its distinct function, the formation of mature brown adipocytes – brown adipogenesis – requires not only activation of the adipogenic cascade, but also the coordination of a thermogenic program and mitochondrial biogenesis (Peirce et al., 2014). Interestingly, recent lineage tracing studies of BAT embryonic development indicate that, although it originates from the mesoderm similar to the white adipose tissue (WAT), BAT diverges from a Myf5+ myogenic lineage under the instructive signal of Prdm16 (Seale et al., 2008), an early marker of brown adipocyte precursors.
We recently demonstrated that the essential core clock gene Bmal1 has a novel function in suppressing adipogenesis and WAT formation (Guo et al., 2012). By contrast, it potently promotes myogenic progression in skeletal muscle (Chatterjee et al., 2013). As BAT shares a common mesodermal origin with WAT and skeletal muscle, in the current study, we investigated whether Bmal1 participates in brown adipocyte development. Employing various genetic approaches in animal models and cellular systems, our study uncovers the adipocyte-intrinsic Bmal1 function in suppressing brown adipocyte lineage commitment and differentiation, which consequently affects brown fat thermogenic capacity.
RESULTS
Ablation of Bmal1 promotes brown fat formation and thermogenesis in vivo
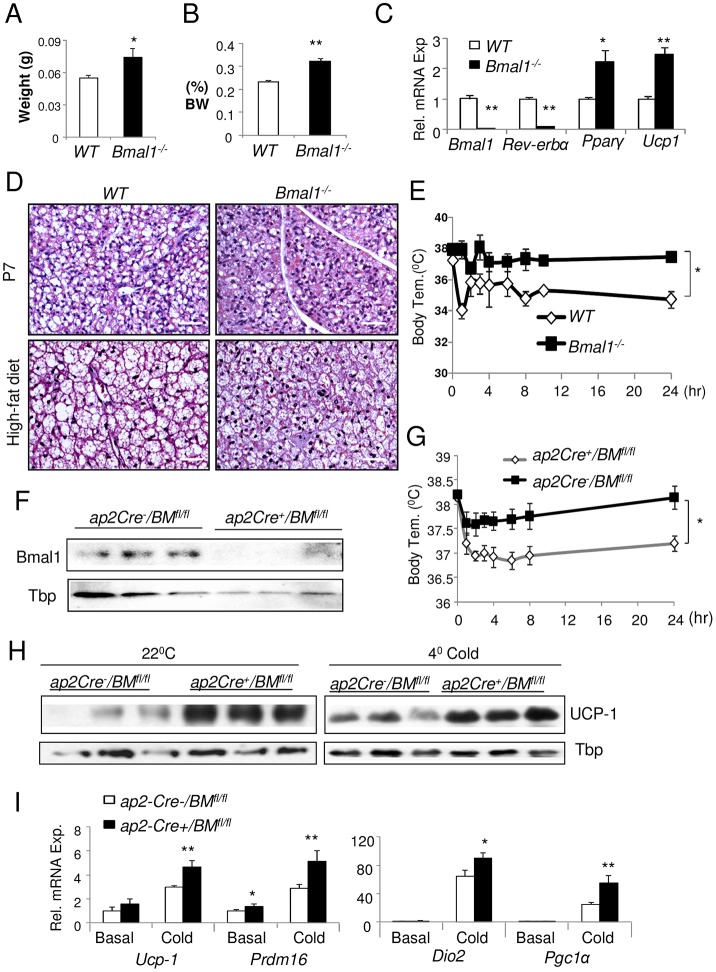
We first investigated the role of Bmal1 in BAT formation in the global Bmal1-null (Bmal1−/−) mouse model (Bunger et al., 2000). Ablation of Bmal1 leads to a ∼30% increase in the amount of BAT as compared to wild-type littermate controls, as assessed by fat pad weight (Fig. 1A) or its ratio to total body weight (Fig. 1B). In Bmal1−/− BAT, mRNA expression of Bmal1 and its direct target gene in the molecular clock, Rev-erbα (also known as Nr1d1), are nearly absent (Fig. 1C), as expected. Notably, the absence of Bmal1 leads to a marked 1.5–2-fold induction of Ucp-1, Cebpb and Pparg transcripts, indicating potentially increased thermogenesis and adipogenesis in BAT. In line with this finding, BAT from neonatal [postnatal day (P)5] Bmal1−/− mice or that from mice after 2 months of high-fat diet feeding exhibited stronger cytoplasmic staining typical of high mitochondrial density with less lipid accumulation as compared to wild type (Fig. 1D). Direct assessment of thermogenic response by using a cold-tolerance test revealed that lack of Bmal1 significantly improved the resistance to cold, as Bmal1−/− mice maintained higher core body temperature than the wild-type controls at 4°C (Fig. 1E).
Fig. 1.
Bmal1 ablation in mice enhances brown fat formation and function.(A,B) Effects of the loss of Bmal1 on BAT formation as analyzed by brown adipose tissue weight (A) and its ratio to body weight (BW) (B) in 8-week-old wild-type (WT) and Bmal1−/− mice (n = 6–8/group). (C–E) RT-qPCR analysis of BAT gene expression (C), representative hematoxylin and eosin staining of BAT histology (D) and 24 hour cold-tolerance test at 4°C (E) in wild-type and Bmal1−/− mice (n = 6/group). (F–I) The effect of adipocyte-specific Bmal1 ablation on BAT. (F) Immunoblot analysis of Bmal1 protein in ap2-Cre+/BMfl/fl and ap2-Cre−/BMfl/fl littermate control mice. (G) Cold-tolerance test at 4°C. Immunoblot analysis of UCP-1 (H), and RT-qPCR analysis of brown fat gene expression (I) at basal ambient temperature and under cold conditions (4°C) in ap2-Cre−/BMfl/fl and ap2-Cre+/BMfl/fl mice (n = 5–6). Tissue samples were collected the previous day at 09.00 prior to (basal) or after 24-hour cold challenge (4°C cold). All quantitative data show the mean±s.e.m. *P≤0.05; **P≤0.01 Bmal1−/− versus wild type [Student's t-test or one-way ANOVA (cold tolerance test)]. Scale bar: 50 µm.
To further test cell-autonomous functions of Bmal1 in brown fat, we selectively ablated Bmal1 in adipocytes by crossing Bmal1fl/fl mice (BMfl/fl) (Storch et al., 2007) with an adipocyte-specific deleter, the ap2-Cre (Ap2 is also known as Fabp4) transgenic mice (He et al., 2003). Mice with selective adipocyte Bmal1 deletion, ap2-Cre+/BMfl/fl, display near complete absence of Bmal1 protein in BAT (Fig. 1F). RT-qPCR analysis reveals an ∼80% reduction in Bmal1 mRNA in mature brown adipocytes, but not in preadipocytes, along with downregulation of the Bmal1 target genes Rev-erbα and Dbp (D-element binding protein, supplementary material Fig. S1A). Similarly, reduced Bmal1 expression is detected in white adipocytes (supplementary material Fig. S1B) in these mice, likely due to ap2-Cre activity in WAT (He et al., 2003). As indicated by intact Bmal1 immunostaining in suprachiasmatic nuclei of ap2-Cre+/BMfl/fl mice (supplementary material Fig. S1C), adipocyte-selective ablation of Bmal1 does not affect its protein expression in the central clock. Furthermore, the central clock function is preserved in these mice as compared to control ap2-Cre−/BMfl/fl littermates, because they display normal circadian behavioral rhythms as assessed by wheel-running actograms (supplementary material Fig. S1D). When subjected to 4°C challenge, ap2-Cre+/BMfl/fl mice exhibit enhanced tolerance to cold as compared to ap2-Cre−/BMfl/fl littermates (Fig. 2G). This similarly improved thermogenic response as seen in the Bmal1−/− mice suggests that this effect is likely attributable to the loss of Bmal1 function in brown adipocytes. In line with previous observations in Bmal1−/− mice (Guo et al., 2012), the body weight (supplementary material Fig. S2A) and brown adipose weight (supplementary material Fig. S2C) of ap2-Cre+/BMfl/fl mice are increased as compared to the controls without cold challenge. Interestingly, exposure to cold (4°C) for 8 hours induced a greater weight loss in ap2-Cre+/BMfl/fl mice (supplementary material Fig. S2B), suggesting higher thermogenic energy expenditure under this condition. The enhanced thermogenic ability of ap2-Cre+/BMfl/fl mice is accompanied by substantially elevated UCP-1 protein in BAT both at the basal state under ambient temperature and at 4°C (Fig. 1H). Gene expression analysis under these conditions revealed a significant induction of genes involved in the brown thermogenic program, including Prdm16, Dio2 (deiodinase 2) and Pgc1α (also known as Ppargc1a) in ap2-Cre+/BMfl/fl mice (Fig. 1I). Collectively, these findings indicate that Bmal1 inhibits brown fat formation and its thermogenic function.
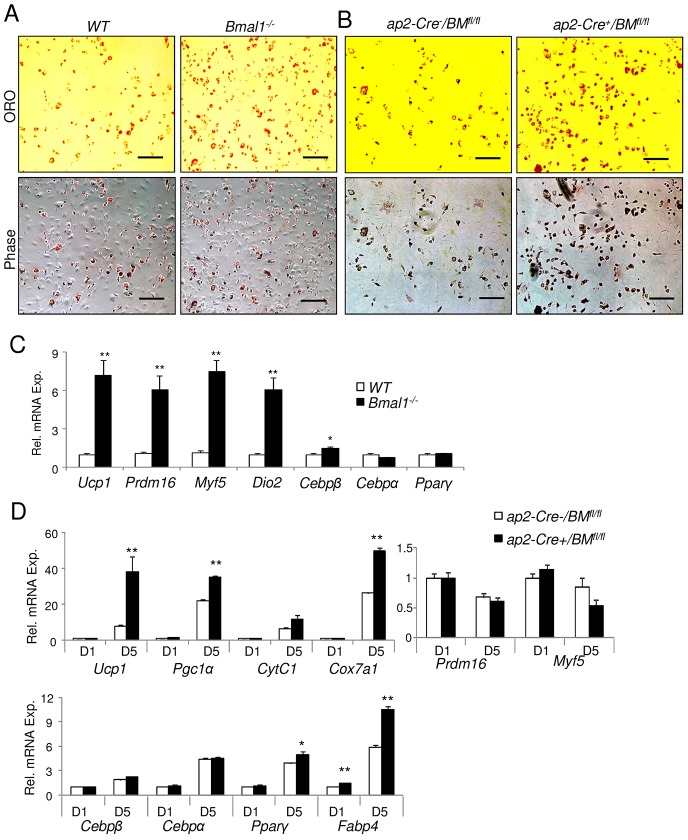
Fig. 2.
Bmal1-deficient primary brown preadipocytes display enhanced adipogenic differentiation.(A,B) Representative images of Oil Red O staining (ORO) and phase-contrast microscopy (Phase) of day-6-differentiated primary preadipocytes isolated from wild-type (WT) and Bmal1−/− mice (A) or ap2-Cre−/BMfl/fl and ap2-Cre+/BMfl/fl mice (B). Scale bars: 100 µm. (C) RT-qPCR analysis of brown adipogenic gene expression at day 5 of differentiation of wild-type and Bmal1−/− preadipocytes (n = 3). (D) RT-qPCR analysis of brown adipogenic gene expression at day 1 (D1) and day 5 (D5) of differentiation of ap2-Cre+/BMfl/fl versus ap2-Cre−/BMfl/fl preadipocytes (n = 3). Data show the mean±s.e.m. *P≤0.05; **P≤0.01 for Bmal1−/− versus wild type or for ap2-Cre+/BMfl/fl versus ap2-Cre−/BMfl/fl (Student's t-test).
The absence of Bmal1 promotes primary brown adipocyte differentiation
To directly test the function of Bmal1 in brown adipogenesis, we isolated brown primary preadipocytes from wild-type and Bmal1-null mice and induced differentiation using a brown-adipocyte-specific induction cocktail. Using Oil Red O staining of lipid accumulation to label differentiated brown adipocytes, preadipocytes devoid of Bmal1 were found to display enhanced differentiation to mature brown adipocytes as compared to wild-type preadipocytes (Fig. 2A). Furthermore, not only was the expression of mature brown adipocyte markers (Ucp-1, Dio2) markedly induced in Bmal1-null cells, the early brown lineage markers Prdm16 and Myf5 were robustly upregulated as well (Fig. 2C). Furthermore, similar to these findings, primary brown preadipocytes from ap2-Cre+/BMfl/fl mice displayed enhanced brown adipogenesis upon induction, as compared to ap2-Cre−/BMfl/fl control cells (Fig. 2B). This effect was accompanied by robust induction of brown-specific (Ucp-1) and adipogenic markers (Pparg, Fabp4), as well as mitochondrial genes [Pgc1α, CytC1 (also known as Cyc1), Cox7a1] at day 1 and day 5 through the differentiation timecourse (Fig. 2D). Interestingly, the early brown lineage markers Prdm16, Myf5 and Cebpb are not altered, likely owing to the nature of ap2-Cre-mediated Bmal1 deletion in these cells. Markedly elevated Ucp-1 expression along with induction of the brown adipogenic gene program is found in ap2-Cre+/BMfl/fl BAT as well (supplementary material Fig. S1E). Taken together, these findings indicate that Bmal1 inhibits brown fat formation and thermogenic function, and suggest that these effects could be mediated by its cell-autonomous action in suppressing brown adipogenesis.
Bmal1 inhibits brown adipocyte terminal differentiation and lineage specification
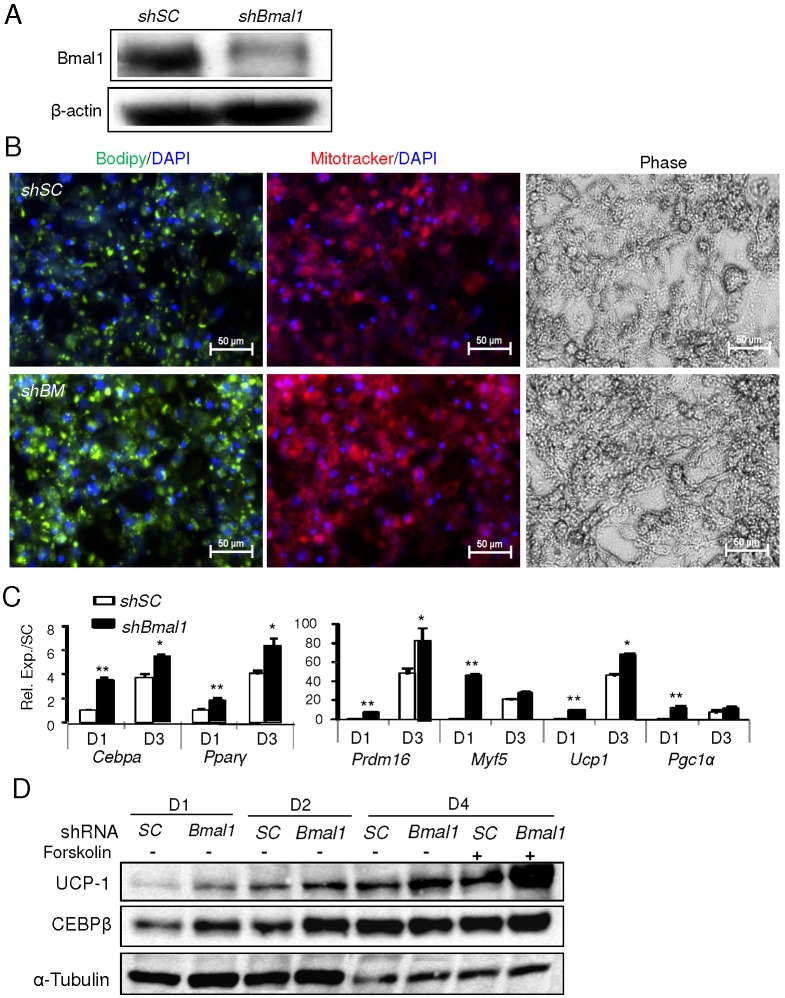
Brown adipocytes arise from mesenchymal precursors that undergo brown lineage commitment and subsequent terminal differentiation (Gesta et al., 2007). To directly test the cell-autonomous function of Bmal1 in these distinct stages of brown adipogenesis, we created stable cell lines with genetic gain or loss of function of Bmal1 in mesenchymal precursor cells, C3H10T1/2 (10T1/2), and brown preadipocytes, HIB1B. Analysis of Bmal1 protein expression reveals that it is more highly abundant in brown adipocyte cell lines than in white preadipocytes, 3T3-L1 cells, at levels comparable to those of primary myoblasts (Fig. 3A). Silencing of Bmal1 by a short hairpin RNA (shRNA) construct in 10T1/2 mesenchymal precursors reduced its protein level to ∼30% of that of scrambled control shRNA (shSC)-treated cells, similar to what we have reported previously (Guo et al., 2012). Under a specific brown adipogenic condition that induces 10T1/2 lineage determination and differentiation into mature brown adipocytes (Tseng et al., 2008), stable knockdown of Bmal1 resulted in an increase in the number of cells converting to mature brown adipocytes with rounded morphology and stronger lipid staining than that of the shSC-treated cells, as shown in Fig. 3B by BODIPY and phase-contrast images. Oil Red O staining in day 10 differentiated shSC- and shBmal1-treated cells further confirms these findings (supplementary material Fig. S3). Moreover, MitoTracker Red staining of functional mitochondria in live cells is substantially increased in Bmal1-deficient cells compared to that of the shSC-treated cells, indicating increased mitochondrial abundance associated with differentiation. Gene expression analysis at both early (day 1) and late (day 9) stages of differentiation demonstrated a substantially augmented brown adipogenic program with marked upregulation of the brown-adipocyte-specific marker Ucp-1, early brown progenitor genes, Myf5 and Prdm16, and adipogenic genes, Cebpb and Pparg (Fig. 3C).
Fig. 3.
Silencing of Bmal1 promotes C3H10T1/2 mesenchymal precursor differentiation to brown adipocytes. (A) Immunoblot analysis of Bmal1 protein in mesodermal lineage cell lines. (B) Representative images of BODIPY lipid staining, mitochondrial staining by Mitotracker and phase contrast microscopy of C3H10T1/2 cells with stable transfection of scrambled control shRNA (shSC) or Bmal1-specific shRNA (shBmal1) subjected to brown-adipocyte-specific differentiation conditions at day 9. (C) RT-qPCR analysis of brown adipogenic gene expression at day 1 (D1) and day 9 (D9) of differentiation in shSC- and shBmal1-treated cells (n = 3). Data show the mean±s.e.m. *P<0.05; **P<0.01 for shBmal1 versus shSC.
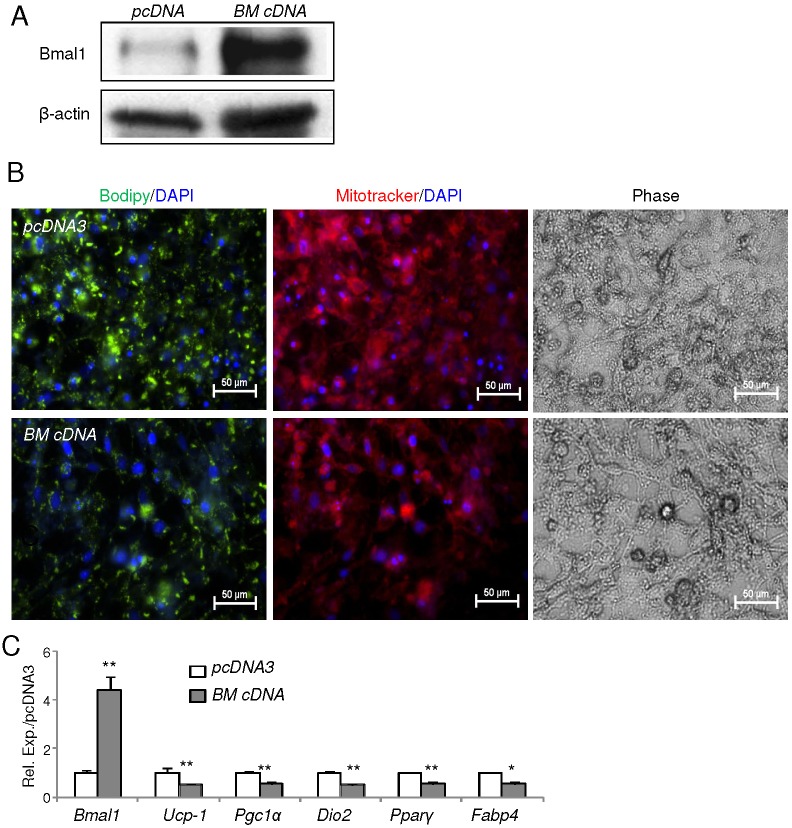
Next, we examined the role of Bmal1 in brown adipocyte terminal differentiation using the committed brown preadipocyte line HIB1B. Stable knockdown of Bmal1 (using shBmal1) in these cells largely depletes Bmal1 protein as compared to scrambled controls (Fig. 4A). Assessment of mature differentiation of these cells through lipid accumulation (BODIPY) and mitochondrial abundance (MitoTracker) reveals that Bmal1 depletion markedly enhances the formation of brown adipocytes (Fig. 4B). Analysis of brown-adipocyte-specific and adipogenic gene expression in the Bmal1-deficient cells further demonstrated that Bmal1 inhibits brown preadipocyte terminal differentiation (Fig. 4C). Moreover, UCP-1 and CEBP-β (also known as CEBPB) protein levels were elevated in Bmal1-depleted cells during the differentiation timecourse as compared to those of controls (Fig. 4D). Notably, UCP-1 also exhibited an augmented response to forskolin stimulation in day-4 terminally differentiated cells. As forskolin treatment mimics a cAMP-mediated cold-induced thermogenic response in brown adipocytes, this increased responsiveness of UCP-1 suggests a potentially augmented thermogenic response with Bmal1 depletion and is in line with the observed enhanced cold tolerance of Bmal1-null mice in vivo. In contrast, forced overexpression of Bmal1 in brown preadipocytes by a cDNA plasmid vector (BM cDNA), which results in high expression of Bmal1 protein and transcript (Fig. 5A,C) as compared to empty vector control (pcDNA3), leads to marked suppression of differentiation as demonstrated by lipid and mitochondrial staining (Fig. 5B). The significantly lower mRNA expression of brown adipogenic markers corroborates the inhibitory effect of Bmal1 overexpression on terminal differentiation of brown adipocytes (Fig. 5C). Taken together, these in vitro analyses further delineate the cell-intrinsic functions of Bmal1 in suppressing lineage commitment and terminal differentiation of brown adipocytes.
Fig. 4.
Silencing of Bmal1 promotes HIB1B brown preadipocyte terminal differentiation. (A) Immunoblot analysis of Bmal1 protein expression in HIB-1B cells with scrambled control shRNA (shSC) or stable shRNA-mediated Bmal1 knockdown (shBmal1). (B) The effect of stable Bmal1 knockdown on HIB-1B differentiation as shown by BODIPY and Mitotracker staining at day 3 of differentiation. (C) RT-qPCR analysis of brown adipocyte marker gene expression in shSC and shBmal1 cells (n = 3). Data show the mean±s.e.m. *P<0.05; **P<0.01 for shBmal1 versus shSC. (D) Immunoblot analysis of brown adipogenic markers during differentiation day 1–4 (D1–4) of shSC and shBmal1 cells, and their response to forskolin at day 4.
Fig. 5.
Forced expression of Bmal1 inhibits HIB1B brown preadipocyte terminal differentiation. (A) Immunoblot analysis of Bmal1 protein expression in HIB-1B cells with Bmal1 stable overexpression (BM cDNA) or empty vector control (pcDNA3). (B) The effect of Bmal1 overexpression on HIB-1B differentiation as shown by BODIPY and Mitotracker staining at day 3 of differentiation. (C) RT-qPCR analysis of brown adipocyte marker gene expression of day-3 differentiated HIB-1B cells expressing pcDNA3 or BM cDNA (n = 3). Data show the mean±s.e.m. *P<0.05; **P<0.01 (BM cDNA versus pcDNA3).
The TGF-β and BMP signaling cascade mediates Bmal1 function in brown adipogenesis
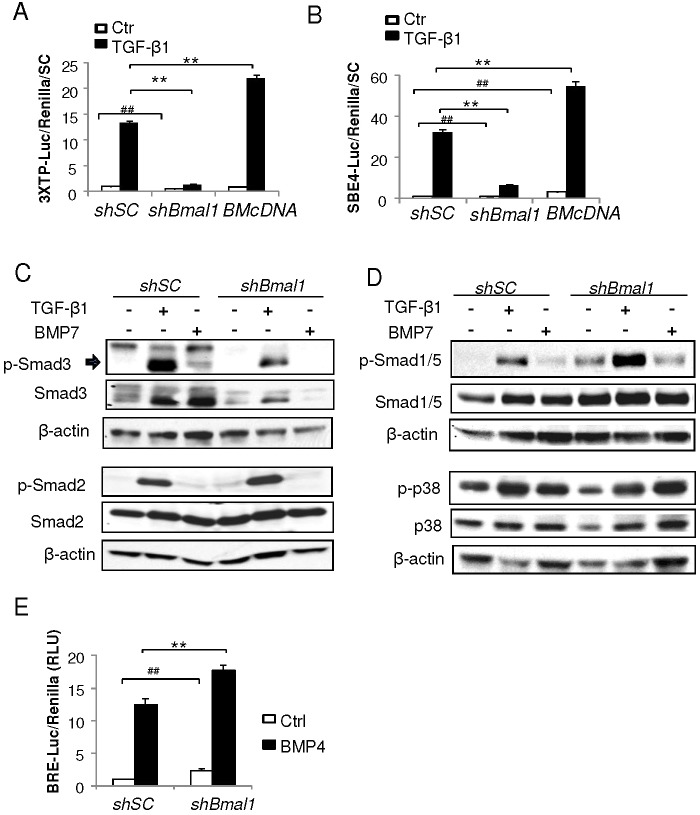
To investigate the underlying mechanisms responsible for Bmal1 inhibition of brown adipogenesis, we interrogated transcriptional targets of Bmal1 in Bmal1-knockdown 10T1/2 cells by gene expression profiling analysis, and found that the TGF-β and BMP cascade is among the top differentially regulated processes (supplementary material Table S1). As TGF-β and BMPs are key negative and positive signals controlling brown fat formation, respectively (Fournier et al., 2012; Koncarevic et al., 2012; Tseng et al., 2008; Yadav et al., 2011), we postulated that Bmal1 might alter the activities of these pathways to affect brown adipocyte differentiation. We thus determined TGF-β signaling activities in Bmal1 loss- and gain-of-function cells using the TGF-β-responsive luciferase reporters 3×TP-Luc (Wrana et al., 1992) and SBE4-Luc (Zhou et al., 1998). As demonstrated in Fig. 6A,B, whereas TGF-β treatment in normal control cells leads to robust 15-fold (3XTP-Luc) or 32-fold (SBE4-Luc) induction of reporter activities, depletion of Bmal1 profoundly compromised TGF-β signal transduction to ∼10% of that of controls. In contrast, forced expression of Bmal1 significantly increased TGF-β reporter activity by ∼67% under TGF-β-stimulated conditions. The TGF-β signaling defect in Bmal1-deficient cells was further corroborated by blunted phosphorylation of Smad3, the ultimate signal transducer of the TGF-β pathway (Massagué and Wotton, 2000), upon either TGF-β1 or BMP7 stimulation (Fig. 6C). Interestingly, the total amount of Smad3 protein was also reduced in Bmal1-knockdown cells as compared to controls, likely reflecting Bmal1 transcriptional regulation of Smad3 as suggested by microarray analysis (supplementary material Table S1). In contrast, Smad2 and its ligand-activated phosphorylation are not altered, suggesting potential specificity of Bmal1-regulated targets in the TGF-β signaling pathway. Surprisingly, Bmal1 deficiency also markedly increases the activity of the canonical BMP signaling pathway, as indicated by much higher Smad1 and Smad5 phosphorylation at basal and ligand-stimulated state as compared to shSC-treated cells (Fig. 6D). In contrast, the p38 MAP kinase (MAPK11–MAPK14) branch of the BMP signaling is not affected by loss of Bmal1 function. Additional assessment of BMP activity by using a well-characterized BMP-responsive luciferase reporter, BRE2-Luc (Korchynskyi and ten Dijke, 2002), further confirmed the robustly augmented BMP signaling under basal and BMP-stimulated conditions in Bmal1-depleted brown preadipocytes (Fig. 6E).
Fig. 6.
Bmal1 regulates the signaling activities of TGF-β and BMP cascades. (A,B) The effect of Bmal1 silencing and forced expression on TGF-β signaling as assessed by using the TGF-β-responsive luciferase reporters, 3×TP-Luc (A) and SBE4-Luc (B), under basal or TGF-β-stimulated conditions in C3H10T1/2 cells (n = 4). Ctr, control. ##P<0.01 under basal conditions; **P<0.01 under TGF-β-treated conditions (Student's t-test). (C) TGF-β signaling activity as assessed by Smad2 and Smad3 phosphorylation under basal conditions or in response to TGF-β1 or BMP7 ligand treatment in shSC and shBmal1 cells. (D) BMP signaling as assessed by Smad1/5 and p38 phosphorylation under basal conditions or in response to TGF-β1 or BMP7 ligand treatment in shSC and shBmal1 cells. (E) BMP signaling as assessed by a BMP-responsive luciferase reporter, BRE2-Luc, under basal or BMP4-stimulated conditions (n = 4). RLU, relative luciferase units; Ctrl, control. ##P<0.01 under basal conditions; **P<0.01 under BMP4-treated conditions (Student's t-test). All quantitative data show the mean±s.e.m.
The finding of attenuated TGF-β pathway activity together with augmented BMP signaling in Bmal1-deficient cells suggests that these mechanisms might contribute to the enhanced differentiation observed in these cells. Therefore, we tested whether activation of TGF-β or blockade of BMP signaling can rescue (i.e. suppress) the enhanced brown adipogenic phenotype of Bmal1-deficient cells. As shown in Fig. 7A, TGF-β1 treatment of normal brown preadipocytes effectively inhibited their adipogenic differentiation as indicated by weaker lipid (BODIPY) and mitochondrial (MitoTracker) staining relative to vehicle-treated controls, as expected. Notably, TGF-β1 also blocked the differentiation of Bmal1-deficient cells as efficiently as for the controls. Consistent with these findings on morphological differentiation, TGF-β1 robustly suppressed the induction of brown-adipocyte-specific marker genes, including Ucp-1, Dio2 and Prdm16, in shBmal1 cells to the same degree as seen in the shSC cells (Fig. 7B), suggesting that activation of the TGF-β1 pathway can rescue the effect of Bmal1 depletion on brown adipogenesis. We found that TGF-β signaling activities of Bmal1-deficient cells were severely impaired as compared to shSC-treated controls, although TGF-β1 can still induce weak activation of 3×TP-Luc (twofold), SBE4-Luc (sixfold) and Smad3 phosphorylation compared to the non-stimulated condition (Fig. 6A–C). So it is possible that TGF-β1-induced signaling in Bmal1-knockdown cells, although significantly diminished, is sufficient to suppress adipogenic differentiation. Furthermore, blockade of the BMP signaling cascade by noggin, a specific BMP inhibitor, inhibits the induction of brown adipocyte markers in Bmal1-deficient cells similarly to the TGF-β1 treatment, suggesting that inhibiting BMP pathway activation is also sufficient to abrogate enhanced differentiation induced by Bmal1 depletion. Interestingly, the combined effects of TGF-β1 and noggin on suppressing the induction of brown adipocyte marker genes are similar to those of TGF-β1 or noggin alone, suggesting either a saturated response to TGF-β1 or noggin alone in these cells, or that these pathways are interdependent through potential crosstalk. The regulation by TGF-β1 and noggin of adipogenic genes in Bmal1-deficient cells is distinct from that of the brown-adipocyte-specific genes, as demonstrated in Fig. 7C. The downregulation of Cebpa in response to these agents occurs to the same extent as that seen for brown-adipocyte-specific markers. In contrast, Pparg is suppressed by TGF-β1 but not noggin in Bmal1-deficient cells, whereas Cebpb displays increased expression in the presence of these ligands, possibly reflecting differential ligand sensitivities of these genes in brown adipocytes. Taken together, these results indicate that Bmal1-mediated regulation of TGF-β as well as BMP pathways contributes to its function in modulating brown adipogenesis.
Fig. 7.
TGF pathway activation or BMP pathway blockade suppresses enhanced adipogenesis of Bmal1-deficient brown preadipocytes. (A) Representative images of lipid (BODIPY) and mitochondrial (Mitotracker) staining of day-3-differentiated shSC- and shBmal1-expressing HIB-1B cells in the absence or presence of TGF-β1 treatment. Ctr, control. Scale bars: 50 µm. (B,C) RT-qPCR analysis of TGF-β1, noggin or TGF-β1 plus Noggin (T+N) treatment on brown-adipocyte-specific gene expression (B) or adipogenic gene expression (C) of day-3-differentiated shSC and shBmal1 cells. Cells were treated with TGF-β1 (2 ng/ml) or noggin (100 ng/ml) for 8 hours prior to the induction of differentiation and treatment was maintained throughout differentiation (n = 3). Data show the mean±s.e.m. #P<0.05; ##P<0.01 (treatment versus non-treated control); *P<0.01; **P<0.05 (shBmal1 versus shSC).
Bmal1 exerts direct transcriptional control of TGF-β pathway genes and confers circadian regulation
Bmal1 is an essential transcriptional activator of the core clock regulatory loop, and has been implicated in transcriptional control of developmental signaling pathways including the TGF-β or BMP cascades (Janich et al., 2011). Therefore, prompted by findings from the microarray study, we examined whether components of the TGF-β or BMP pathways are direct transcriptional targets of Bmal1. Through computational screening of putative Bmal1-binding sites, the canonical E-box elements (5′-CACGTG-3′) (Rey et al., 2011), in the proximal promoter regions (−2 kb+first intron) of key genes in these pathways, we identified a number of TGF-β pathway genes harboring the E-boxes, including the ligands Tgfb1 and Tgfb2, the receptor Tgfbr2 and the signal transducer Smad3 (supplementary material Table S2). However, our computational analysis failed to identify E-box elements among the genes of the BMP pathway examined, including BMPs, Smad1 and Smad5.
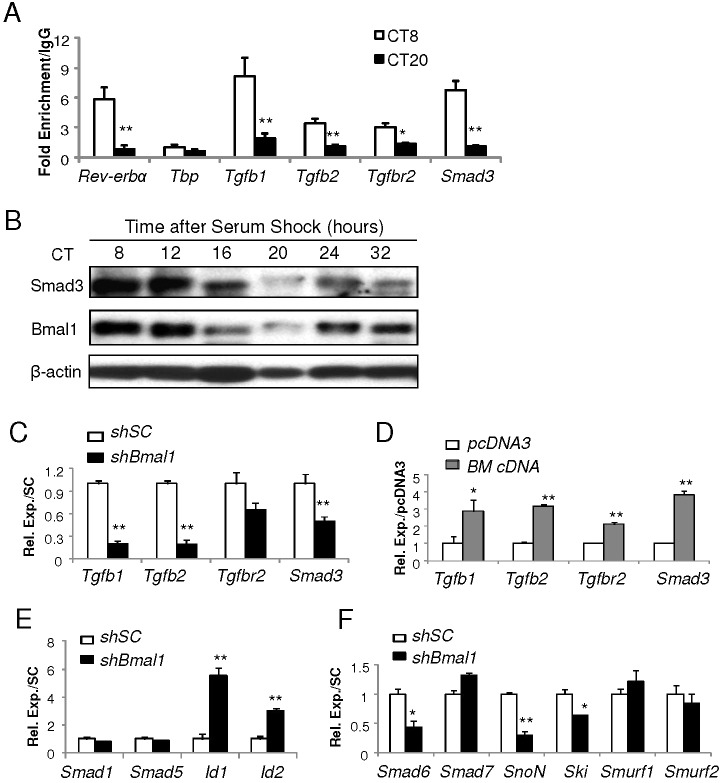
To determine Bmal1 occupancy of these potential binding sites and its potential circadian time-dependent association with target promoters, we performed chromatin immunoprecipitation (ChIP) analysis in HIB1B cells under serum shock conditions (Balsalobre et al., 1998), an established method to elicit cell-intrinsic clock oscillation and synchronize circadian gene rhythmicity (Guo et al., 2012; Janich et al., 2011). As shown in Fig. 8A, robust recruitment of Bmal1 to a known target promoter, Rev-erbα, is detected at circadian time (CT)8 upon serum shock, whereas this activity is nearly absent at CT20. This rhythmic promoter occupancy by Bmal1 coincides with its protein peak observed at CT8 and trough at CT20 (Fig. 8B). Bmal1 enrichment on identified sites of Tgfb1 and Smad3 promoters occurred in a circadian-dependent manner similar to that occurring at Rev-erbα, with stronger chromatin association occurring at CT8 (Fig. 8A). In contrast, other candidate target promoters, Tgfb2 and Tgfbr2, exhibited only modestly enriched Bmal1 association. Notably, serum shock in HIB1B cells elicits oscillation of Smad3 protein with a rhythmic profile similar to that of Bmal1 (Fig. 8B), suggesting an intrinsic temporal regulation of the key signaling transducer of the TGF-β cascade. Likely due to direct Bmal1-mediated regulation, silencing of Bmal1 blunted (Fig. 8C), whereas its forced expression augmented the expression of the identified target genes in the TGF-β pathway – Tgfb1, Tgfb2, Tgfbr2 and Smad3 (Fig. 8D). In line with the results from our initial screening indicating the absence of potential Bmal1-response elements on BMP signaling genes, mRNA expression of Smad1 and Smad5 was not affected by Bmal1 knockdown (Fig. 8E). However, the canonical BMP signaling targets Id1 and Id2 were markedly upregulated in Bmal1-deficient cells, additional evidence for augmented BMP activity in these cells as revealed by the findings of Smad5 phosphorylation and BMP reporter activity discussed above. Given that intracellular BMP signal transduction is subjected to negative-feedback regulation (Kavsak et al., 2000; Kawamura et al., 2012), we further analyzed whether loss of Bmal1 could affect negative regulatory mechanisms involved in the BMP signaling pathway to affect its activity. Interestingly, as demonstrated by expression analysis of BMP pathway inhibitory molecules (Fig. 8F), the levels of Smad6, SnoN (also known as Skil) and Ski transcripts are significantly reduced in Bmal1-deficient cells. This finding of inhibition of the expression of BMP negative regulators with loss of Bmal1 suggests that a relief of inhibition of the BMP pathway might account for the enhanced BMP activity in these cells.
Fig. 8.
Bmal1 exerts direct transcriptional control on genes of the TGF-β pathway. (A) Chromatin immunoprecipitation qPCR (ChIP-qPCR) analysis of Bmal1 occupancy on identified TGF-β pathway gene promoters at 8 and 20 hours after serum shock in HIB-1B cells. Bmal1 occupancy of the Rev-erbα promoter E-box (a known target) is included as a positive control and that of Tbp as a negative control. Values are presented as the fold enrichment of the percentage of total input over IgG control (n = 4). CT, circadian time, with time immediately after serum shock taken as CT0. *P<0.05; **P<0.01 (CT8 versus CT20). (B) Immunoblot analysis of Bmal1 and Smad3 protein oscillation induced by serum shock from CT8 to 32. (C–F) RT-qPCR gene expression analysis of components of TGF-β and BMP pathways in Bmal1-knockdown (C,E,F), or Bmal1-overexpressing HIB-1B cells (D). n = 3. All quantitative data show the mean±s.e.m. *P<0.05; **P<0.01 (shBmal1 versus shSC, or BM cDNA versus pcDNA3).
DISCUSSION
The unique energy-dissipating function of brown adipose tissue and its presence in humans holds promise as a potential therapeutic target against obesity (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009). Accumulating evidence suggests that disruption of the clock mechanism leads to various metabolic abnormalities, particularly obesity and insulin resistance (Pan et al., 2011; Roenneberg et al., 2012; Sahar and Sassone-Corsi, 2012). Although diverse aspects of metabolism are subjected to circadian regulation (Bass and Takahashi, 2010; Green et al., 2008), whether the adipocyte-intrinsic timekeeping mechanism participates in brown fat cell development is not known. Our study demonstrates that the essential transcription activator of the molecular clock, Bmal1, exerts direct temporal control of the TGF-β pathway, a key inhibitory signal of brown fat formation (Fournier et al., 2012; Koncarevic et al., 2012; Yadav et al., 2011). Together with reciprocally altered BMP activity, these developmental signaling mechanisms driven by Bmal1 modulate brown adipogenesis and consequently affect adaptive thermogenesis in vivo. As crucial negative and positive signals involved in brown adipogenesis, respectively (Fournier et al., 2012; Koncarevic et al., 2012; Kuo et al., 2014; Qian et al., 2013; Tseng et al., 2008; Yadav et al., 2011), our finding of circadian regulation of TGF-β and BMP activities implicates a temporal regulatory control of adipocyte lineage commitment and differentiation.
The TGF-β and BMP pathways are known to suppress (Fournier et al., 2012; Koncarevic et al., 2012; Yadav et al., 2011) and drive (Kuo et al., 2014; Qian et al., 2013; Tseng et al., 2008) brown adipocyte differentiation, respectively. BMP7 promotes both early commitment of mesenchymal precursors to the brown adipocyte lineage (Tseng et al., 2008) and subsequent differentiation (Cao et al., 2004; Sellayah et al., 2011). In contrast, TGF-β signaling exerts inhibitory effects on BAT development, with ablation of Smad3, the ultimate signal transducer of the TGF-β pathway, resulting in selective induction of brown-adipocyte-specific marker genes and enhanced mitochondrial function (Yadav et al., 2011). In addition, inhibition of activin receptor IIB and myostatin, both signaling through Smad3 (Fournier et al., 2012; Kim et al., 2012; Koncarevic et al., 2012; Zhang et al., 2012), were recently reported to promote brown adipogenesis. We have previously shown that Bmal1 suppresses white adipocyte differentiation (Guo et al., 2012). The current finding of a Bmal1 inhibitory effect on brown adipogenesis is in parallel with its role in white adipocyte formation, although the molecular pathways mediating these effects are distinct. We found that Bmal1 modulates Wnt signaling in white adipose tissue (Guo et al., 2012), whereas in brown fat, this core clock regulator exerts transcriptional control on the TGF-β pathway. Interestingly, recent studies indicate that the circadian clock can act on various signaling cascades, including the TGF-β and BMP pathways, to modulate epidermal stem cell activation in accordance with environmental stimulatory cues (Janich et al., 2011; Karpowicz et al., 2013). These findings, in aggregate, likely reflect the tissue specificity and the diverse array of developmental signals involved in transmitting a circadian timing cue to fine-tune tissue homeostasis.
Findings of the direct association of Bmal1 with the promoters of key components of the TGF-β pathway, such as Tgfbr2 and Smad3, indicate that the TGF-β signaling cascade could be under a coordinated circadian control in brown preadipocytes. Likely owing to this circadian element present in the Smad3 promoter, a robust rhythmic oscillation of Smad3 protein in phase with Bmal1 is elicited upon serum shock. In addition, we found that Bmal1 deficiency leads to significantly attenuated Smad3 activation and reporter activity, indicating that TGF-β signaling transduction could be subjected to temporal regulation as well. Indeed, circadian expression of Smad3 was detected in human gingival fibroblasts, mesenchymal stem cells and mouse liver (Sato et al., 2012). Furthermore, in epidermal stem cells (Janich et al., 2011; Karpowicz et al., 2013) and the central clock (the suprachiasmatic nuclei; Beynon et al., 2009), a circadian pattern of TGF-β signaling activity as indicated by Smad3 phosphorylation has been reported. Given the importance of TGF-β signaling in stem cell proliferation and differentiation (Gaarenstroom and Hill, 2014), circadian modulation of this pathway might confer appropriate responses to temporal cues involved in controlling stem cell behavior and tissue homeostasis. Although our current study defines a specific role of Bmal1 in the brown fat differentiation program, its broader impact on various important TGF-β-regulated biological processes remains to be elucidated.
An interesting observation from our study is the reciprocally altered BMP signaling with altered TGF-β pathway activity in Bmal1 loss- or gain-of-function studies. Based on several lines of evidence, changes in BMP activity might have occurred secondary to the direct regulatory effect of Bmal1 on TGF-β pathway. First, we failed to identify canonical E-box elements in genes directly involved in BMP signal transduction through extensive promoter screening, although it is possible that there could be non-canonical elements or additional genes not screened. Secondly, searches in available Bmal1 ChIP-Seq datasets (Cho et al., 2012; Koike et al., 2012; Rey et al., 2011) for binding peaks among BMP pathway genes also yield negative findings. Most importantly, a mutually antagonistic relationship between TGF-β and BMP signal transduction has been well-characterized (Shi and Massagué, 2003), and loss of endogenous TGF-β signaling can augment BMP activity. Ski (Ehnert et al., 2012; Wang et al., 2000) and SnoN (Kawamura et al., 2012) are known BMP inhibitory molecules that can be induced by TGF-β to mediate the antagonism between these signaling events, whereas Smad6 is a ubiquitin ligase specific for BMP-activated Smad1/5/8 degradation. In Bmal1-deficient cells, as the attenuated TGF-β activity is accompanied by downregulation of SnoN, Ski and Smad6, a relief of these inhibitory mechanisms might have contributed to enhanced BMP signaling transduction. Moreover, we observed that TGF-β alone, or BMP blockade, is sufficient to suppress adipogenic induction in these cells (Fig. 7), but these effects are not additive, further suggesting potential interdependence of the two pathways. These observations support a model that direct transcriptional control of the TGF-β pathway by Bmal1 might consequently alter BMP signaling, and together they exert concerted actions to drive brown adipogenesis.
Despite the established link between circadian disruption and metabolic abnormalities such as obesity and insulin resistance (Roenneberg et al., 2012; Sahar and Sassone-Corsi, 2012), the precise molecular mechanisms responsible for these effects are yet to be defined. As our study demonstrates, circadian-controlled brown fat thermogenic capacity might contribute to this phenomenon. Two recent reports indicate that circadian regulators Rev-erbα (Gerhart-Hines et al., 2013) and Per2 (Chappuis et al., 2013) both contribute to the regulation of brown adipose tissue function. In particular, our findings are in agreement with the study by Chappuis et al., as mice lacking Per2, a repressor of Bmal1, display sensitivity to cold, whereas Bmal1 ablation leads to resistance to cold. Based on the opposing regulation of Per2 and Bmal1 in the core clock circuit, our current study, together with these previous reports, suggests the participation of a coordinated temporal control mechanism in modulating thermogenic capacity. Whereas previous studies mainly focus on clock genes in BAT function, our study addresses an additional layer of temporal control in the brown fat concerning brown adipocyte differentiation. Nonetheless, specific strategies, such as circadian shift regimens, will be needed in the future to assess the direct impact of altered clock regulation on BAT function and its contribution to metabolic diseases. Given that certain clock regulators, such as Rev-erbα, are amenable to pharmacological manipulation by synthetic ligands (Solt et al., 2012), the clock circuit represents a potential target for therapeutic interventions.
Compared to our extensive knowledge of white adipocyte development, the current understanding of regulatory mechanisms governing BAT formation is only emerging. Our study elucidates a previously unappreciated temporal regulatory mechanism involved in brown adipocyte development that ultimately contributes to fine-tuning of adaptive thermogenesis. Future efforts to determine how this mechanism affects systemic metabolic homeostasis might lead to the discovery of new therapies against widespread circadian-disruption-induced metabolic disorders.
MATERIALS AND METHODS
Animals
Animals were maintained in the Methodist Hospital Research Institute mice facility under a constant 12∶12 light-dark cycle, with light on at 7:00 (ZT0). Room temperature was controlled at 22°C. All experimental protocols were approved by the IACUC animal care research committee of the Houston Methodist Research Institute, and carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. Bmal1−/−, Bmal1fl/fl and ap2-Cre transgenic mice were obtained from the Jackson Laboratory (Storch et al., 2007). Mice were fed ad lib on standard chow diet (AIN-76A, Research Diets) with free access to water. Tissue samples were obtained at ambient temperature, unless indicated otherwise, and at the same time of the day to ensure reproducible circadian gene expression.
Cold-tolerance test
12-week-old mice were placed in individual cages with free access to water without food or sedation. Rectal temperature was measured with a probe connected to a high-precision thermometer. Body temperature was measured twice at 09.00 before the mice were subjected to 4°C for 24 hours, with temperature monitored at the indicated times.
Cell culture
C3H10T1/2 and HIB1B cell lines were obtained from ATCC and maintained in 10% FBS DMEM. Stable cell lines were constructed and selected as described previously (Guo et al., 2012) using shRNA constructs from Open Biosystems. C3H10T1/2 differentiation to brown adipocytes was conducted as described previously (Tseng et al., 2008) but without BMP7 pretreatment. Briefly, cells at confluency were cultured in brown induction medium containing insulin (20 nM), T3 (1 nM), isobutylmethylxanthine (0.5 mM), dexamethasone (5 mM) and rosiglitazone (1 mg/ml) for 3 days. Cells were then switched to maintenance medium supplemented with insulin and T3 only for 9 days. The fully differentiated brown adipocyte phenotype with significant lipid accumulation and Ucp-1 expression occurs at 9 days (D9) after induction. Differentiation of HIB-1B cells and primary brown adipocytes was induced using the same induction medium for 2 days and maintenance medium for 2–4 days.
Primary brown adipocyte and preadipocyte isolation and immortalization
Primary brown adipocytes and preadipocytes were isolated from the interscapular brown adipose tissue pad of 6-week-old mice as described previously (Timmons et al., 2007). Briefly, tissues were digested with type I collagenase in the presence of 1% BSA at 37°C for 30 minutes. The suspension was filtered and centrifuged, and the top fat layer was collected as adipocytes and the pellet containing the stromal vascular fraction was resuspended and plated. Preadipocytes were passaged once prior to adipogenic differentiation. Immortalization of isolated primary preadipocytes was performed using retroviral SV-40 Large T antigen transformation and puromycin selection as described previously (Tseng et al., 2008).
Serum shock synchronization of the cellular clock
Serum shock to synchronize cells in culture was performed as described previously (Guo et al., 2012). Briefly, confluent cultures were incubated in serum-free medium overnight and subjected to 20% FBS serum shock for 1 hour. The medium was then removed and replaced with 10% serum normal culture medium. This was considered circadian time (CT) 0, and samples were collected at the indicated times after serum shock.
RNA extraction and quantitative reverse-transcriptase PCR analysis
RNeasy miniprep kits (Qiagen) were used to isolate total RNA from snap-frozen tissues or cells. Tissue samples were collected at the times indicated, and cell samples were obtained under non-synchronized normal culture conditions. cDNA was generated using q-Script cDNA Supermix kit (Quanta Biosciences), and quantitative PCR was performed using a Roche 480 Light Cycler with Perfecta SYBR Green Supermix (Quanta Biosciences) as described previously (Guo et al., 2012). Relative expression levels were determined using the comparative Ct method to normalize target genes to the 36B4 (also known as Rplp0) internal control, and compared to experimental controls as indicated.
Immunoblot analysis
Total protein (40–50 µg) was used for the analysis as described previously (Chatterjee et al., 2013). Smad2, Smad3 or Smad1/5 and p38 phosphorylation were assessed at 1 hour after the indicated ligand treatment (TGF-β1, 10 ng/ml; BMP7 100 ng/ml). The primary antibodies that were used are listed in supplementary material Table S4.
ChIP-qPCR analysis
Immunoprecipitation was performed using Bmal1 (AB93806) or control rabbit IgG plus Protein A/G beads as described previously (Chatterjee et al., 2013). Briefly, cells were fixed with formaldehyde, lysed and sonicated to shear the chromatin. The immunoprecipitated chromatin fragments were eluted, treated with proteinase K and purified using the Qiaquick PCR purification kit (Qiagen). Real-time PCR using Perfecta SYBR Green Supermix (Quanta Biosciences) was carried out with an equal volume (4 µl) of each reaction with specific primers. Negative control primers for TBP were also included. The primer sequences used are listed in supplementary material Table S3. Values are expressed as the fold enrichment of the percentage of input normalized to IgG control.
Oil Red O, BODIPY and Mitotracker staining
Oil Red O staining was carried out in fixed cells using 0.5% Oil Red O for 1 hour as described previously (Guo et al., 2012). BODIPY staining (Molecular Probes, Carlsbad, CA) was carried out at a concentration of 1 mg/ml for 30 minutes. Mitotracker (100 nM, Molecular Probes) staining was performed according to the manufacturer's protocol and applied to cells in culture at 37°C for 30 minutes before fixation. Microscopy images were captured on a Nikon 80i microscope with a color camera and processed using Nikon NIS Elements acqusition software. BODIPY and Mitotracker images were captured using excitation/emission wavelength at 590-650/700 and 465-495/535 nm, respectively using appropriate exposure times.
Luciferase reporter assays
Cells were grown to 80% confluency and transiently transfected using FuGENE 6 (Roche) in four replicates as described previously (Beynon et al., 2009). TGF-β1 (2 ng/ml) or BMP4 (100 ng/ml) were added 16 hours after transfection and luciferase activity was measured using the Dual-Glo luciferase assay system (Promega) 24 hours following ligand treatment. Reporter luciferase values were normalized to Renilla readings and expressed as the fold induction over controls. TGF-β luciferase reporters, 3×TP-Luc (Wrana et al., 1992) and SBE4-Luc (Zhou et al., 1998) were obtained from Addgene, and the BRE2-Luc reporter (Korchynskyi and ten Dijke, 2002) was a kind gift from Dr Peter Ten Dijke (Leiden University Medical Center, The Netherlands).
Statistical analysis
Data are expressed as the mean±s.e.m. Differences between groups in the cold-tolerance tests were analyzed by one-way analysis of variance (ANOVA). Statistical differences of other experiments were assessed by using Student's t-test.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
K.M. and D.N. designed and performed the experiments, analyzed the data and wrote the manuscript. B.G., S.C., R.L. and H.Y. performed experiments on adipocytes, M.C. performed experiments on brown fat development, Z.Z. and D.N. carried out circadian actogram analysis.
Funding
We thank The Houston Methodist Research Institute (HMRI) for start-up support and the Center for Diabetes Research for technical assistance. This project is supported by the American Heart Association [grant number 12SDG12080076]; and the American Diabetes Association [grant numbers 1-13-BS-118 to K.M. and 7-12-BS-210]; and the National Institutes of Health [grant number DK097160-01] to V.Y. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.167643/-/DC1
Reference
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/S0092--8674(00)81199--X. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon AL, Thome J, Coogan AN. Age and time of day influences on the expression of transforming growth factor-beta and phosphorylated SMAD3 in the mouse suprachiasmatic and paraventricular nuclei. Neuroimmunomodulation. 2009;16:392–399. doi: 10.1159/000228914. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/S0092--8674(00)00205--1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 2004;24:3057–3067. doi: 10.1128/MCB.24.7.3057--3067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappuis S, Ripperger JA, Schnell A, Rando G, Jud C, Wahli W, Albrecht U. Role of the circadian clock gene Per2 in adaptation to cold temperature. Mol. Metab. 2013;2:184–193. doi: 10.1016/j.molmet.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Nam D, Guo B, Kim JM, Winnier GE, Lee J, Berdeaux R, Yechoor VK, Ma K. Brain and muscle Arnt-like 1 is a key regulator of myogenesis. J. Cell Sci. 2013;126:2213–2224. doi: 10.1242/jcs.120519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK. et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010;72:517–549. doi: 10.1146/annurev--physiol--021909--135821. [DOI] [PubMed] [Google Scholar]
- Ehnert S, Zhao J, Pscherer S, Freude T, Dooley S, Kolk A, Stöckle U, Nussler AK, Hube R. Transforming growth factor β1 inhibits bone morphogenic protein (BMP)-2 and BMP-7 signaling via upregulation of Ski-related novel protein N (SnoN): possible mechanism for the failure of BMP therapy? BMC Med. 2012;10:101. doi: 10.1186/1741--7015--10--101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine C, Dubois G, Duguay Y, Helledie T, Vu-Dac N, Gervois P, Soncin F, Mandrup S, Fruchart JC, Fruchart-Najib J. et al. The orphan nuclear receptor Rev-Erbalpha is a peroxisome proliferator-activated receptor (PPAR) gamma target gene and promotes PPARgamma-induced adipocyte differentiation. J. Biol. Chem. 2003;278:37672–37680. doi: 10.1074/jbc.M304664200. [DOI] [PubMed] [Google Scholar]
- Fournier B, Murray B, Gutzwiller S, Marcaletti S, Marcellin D, Bergling S, Brachat S, Persohn E, Pierrel E, Bombard F. et al. Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol. Cell. Biol. 2012;32:2871–2879. doi: 10.1128/MCB.06575--11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaarenstroom T, Hill CS. TGF-β signaling to chromatin: how Smads regulate transcription during self-renewal and differentiation. Semin. Cell Dev. Biol. 2014;32:107–118. doi: 10.1016/j.semcdb.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Feng D, Emmett MJ, Everett LJ, Loro E, Briggs ER, Bugge A, Hou C, Ferrara C, Seale P. et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature. 2013;503:410–413. doi: 10.1038/nature12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Chatterjee S, Li L, Kim JM, Lee J, Yechoor VK, Minze LJ, Hsueh W, Ma K. The clock gene, brain and muscle Arnt-like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J. 2012;26:3453–3463. doi: 10.1096/fj.12-205781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. USA. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janich P, Pascual G, Merlos-Suárez A, Batlle E, Ripperger J, Albrecht U, Cheng HY, Obrietan K, Di Croce L, Benitah SA. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209–214. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- Karpowicz P, Zhang Y, Hogenesch JB, Emery P, Perrimon N. The circadian clock gates the intestinal stem cell regenerative state. Cell Reports. 2013;3:996–1004. doi: 10.1016/j.celrep.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol. Cell. 2000;6:1365–1375. doi: 10.1016/S1097--2765(00)00134--9. [DOI] [PubMed] [Google Scholar]
- Kawamura I, Maeda S, Imamura K, Setoguchi T, Yokouchi M, Ishidou Y, Komiya S. SnoN suppresses maturation of chondrocytes by mediating signal cross-talk between transforming growth factor-β and bone morphogenetic protein pathways. J. Biol. Chem. 2012;287:29101–29113. doi: 10.1074/jbc.M112.349415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WK, Choi HR, Park SG, Ko Y, Bae KH, Lee SC. Myostatin inhibits brown adipocyte differentiation via regulation of Smad3-mediated β-catenin stabilization. Int. J. Biochem. Cell Biol. 2012;44:327–334. doi: 10.1016/j.biocel.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncarevic A, Kajimura S, Cornwall-Brady M, Andreucci A, Pullen A, Sako D, Kumar R, Grinberg AV, Liharska K, Ucran JA. et al. A novel therapeutic approach to treating obesity through modulation of TGFβ signaling. Endocrinology. 2012;153:3133–3146. doi: 10.1210/en.2012--1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- Kuo MM, Kim S, Tseng CY, Jeon YH, Choe S, Lee DK. BMP-9 as a potent brown adipogenic inducer with anti-obesity capacity. Biomaterials. 2014;35:3172–3179. doi: 10.1016/j.biomaterials.2013.12.063. [DOI] [PubMed] [Google Scholar]
- Massagué J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11:268–272. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/S0092--8674(02)00722--5. [DOI] [PubMed] [Google Scholar]
- Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature. 2014;510:76–83. doi: 10.1038/nature13477. [DOI] [PubMed] [Google Scholar]
- Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, Xue RD, Yu HY, Guo L, Gao HD. et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl. Acad. Sci. USA. 2013;110:E798–E807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 2011;9:e1000595. doi: 10.1371/journal.pbio.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr. Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar S, Sassone-Corsi P. Regulation of metabolism: the circadian clock dictates the time. Trends Endocrinol. Metab. 2012;23:1–8. doi: 10.1016/j.tem.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09--0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Sato H, Jin D, Bhawal UK, Wu Y, Noshiro M, Kawamoto T, Fujimoto K, Seino H, Morohashi S. et al. Smad3 and Snail show circadian expression in human gingival fibroblasts, human mesenchymal stem cell, and in mouse liver. Biochem. Biophys. Res. Commun. 2012;419:441–446. doi: 10.1016/j.bbrc.2012.02.076. [DOI] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14:478–490. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/S0092--8674(03)00432--X. [DOI] [PubMed] [Google Scholar]
- Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R. et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, Raviola E, Pawlyk B, Li T, Weitz CJ. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K. et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl. Acad. Sci. USA. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y. et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Wang J, Lazar MA. Bifunctional role of Rev-erbalpha in adipocyte differentiation. Mol. Cell. Biol. 2008;28:2213–2220. doi: 10.1128/MCB.01608--07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Mariani FV, Harland RM, Luo K. Ski represses bone morphogenic protein signaling in Xenopus and mammalian cells. Proc. Natl. Acad. Sci. USA. 2000;97:14394–14399. doi: 10.1073/pnas.97.26.14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Cárcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092--8674(92)90395--S. [DOI] [PubMed] [Google Scholar]
- Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C. et al. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, McFarlane C, Lokireddy S, Masuda S, Ge X, Gluckman PD, Sharma M, Kambadur R. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia. 2012;55:183–193. doi: 10.1007/s00125--011--2304--4. [DOI] [PubMed] [Google Scholar]
- Zhou S, Zawel L, Lengauer C, Kinzler KW, Vogelstein B. Characterization of human FAST-1, a TGF beta and activin signal transducer. Mol. Cell. 1998;2:121–127. doi: 10.1016/S1097--2765(00)80120--3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.