Abstract
Basement membranes are a dense, sheet-like form of extracellular matrix (ECM) that underlie epithelia and endothelia, and surround muscle, fat and Schwann cells. Basement membranes separate tissues and protect them from mechanical stress. Although traditionally thought of as a static support structure, a growing body of evidence suggests that dynamic basement membrane deposition and modification instructs coordinated cellular behaviors and acts mechanically to sculpt tissues. In this Commentary, we highlight recent studies that support the idea that far from being a passive matrix, basement membranes play formative roles in shaping tissues.
Introduction
The basement membrane is an ancient and highly conserved form of extracellular matrix (ECM) that arose at the dawn of multicellularity. The evolution of this cell-associated matrix was likely a crucial innovation required for the emergence of metazoans (Hynes, 2012; Ozbek et al., 2010). Most basement membranes are composed of a core set of proteins, including laminin, type IV collagen, the glycoprotein nidogen and the heparan sulfate proteoglycan perlecan (Yurchenco, 2011). Basement membrane assembly is initiated when the laminin heterotrimer binds to cell surfaces through sulfated glycolipids and matrix receptors, such as integrin and α-dystroglycan (Fässler and Meyer, 1995; Henry and Campbell, 1998; McKee et al., 2007; Stephens et al., 1995; Yurchenco and Wadsworth, 2004). At a high local concentration, laminin self assembles into a polymeric network (Li et al., 2003). This lattice serves as a scaffold for further elaboration of the basement membrane, including the addition of a polymeric network of type IV collagen molecules (Pöschl et al., 2004). Type IV collagen has the unique ability to form intermolecular covalent bonds, which is thought to endow the basement membrane with its ability to withstand mechanical stress (Khoshnoodi et al., 2008; Vanacore et al., 2009). Consistent with this notion, loss of the enzyme peroxidasin, which localizes to basement membranes and catalyzes a conserved intermolecular sulfilimine bond between type IV collagen molecules, reduces tissue integrity in Drosophila, Caenorhabditis elegans and zebrafish (Bhave et al., 2012; Fidler et al., 2014; Gotenstein et al., 2010). The biochemical interactions linking the initial laminin meshwork to the cross-linked collagen lattice are unclear. Although nidogen has the ability to bind both collagen and laminin, genetic evidence suggests that it is not essential for basement membrane assembly (Bader et al., 2005; Fox et al., 1991; Kang and Kramer, 2000). Like nidogen, the heparan sulfate proteoglycan perlecan binds both the laminin and collagen networks, and thus might redundantly function with nidogen to link these two lattices (Behrens et al., 2012; Costell et al., 1999). Structurally, the overlaid networks of laminin and collagen are thought to be arranged with the long axis of individual molecules in parallel to the cell surface, creating a dense meshwork with a pore size ranging from ∼10–130 nm (Abrams et al., 2003; Abrams et al., 2000; Yurchenco et al., 1992; Yurchenco and Ruben, 1987). Basement membranes vary in composition in a temporal and tissue-specific manner (see Matrixome Project http://www.matrixome.com/bm/Home/home/home.asp and the human protein atlas, http://www.proteinatlas.org) (Hynes and Naba, 2012; Pontén et al., 2011; Uhlen et al., 2010). As proteomic studies of isolated basement membranes have revealed over 200 core matrix and matrix-associated proteins, the composition or structure of basement membranes can likely be modified in many ways to create specialized or context-specific assemblies (Uechi et al., 2014).
Disruptions in genes encoding basement membrane components have long revealed the importance of basement membranes in normal tissue morphogenesis and resistance to mechanical stress (Ekblom, 1989; Hynes and Naba, 2012; Miner et al., 2004; Pöschl et al., 2004; Urbano et al., 2009). However, elucidating specific functions of basement membrane in tissue structure has been experimentally difficult to establish owing to embryonic lethal phenotypes of basement membrane-encoding genes and lack of visual accessibility. Recently, advances in imaging basement membranes in vivo and more sophisticated genetic tools in Drosophila and C. elegans, have begun to reveal how basement membranes dynamically shape organs. Here, we focus on three recent developments in our understanding of how basement membranes actively shape tissue architecture. First, we discuss basement membrane deposition as a mechanism for instructing polarity during epithelial tube formation. Second, we examine how basement membranes, and in particular type IV collagen, dynamically constricts tissues and shapes tissue structure. Finally, we highlight recent work showing how basement membranes are remodeled through a newly identified adhesion system that links developing tissues. These studies suggest that far from being a static support structure, the basement membrane is, in fact, an active regulator of tissue shape.
Basement membrane deposition instructs cell polarization and tissue shape
One emerging role for basement membranes in regulating tissue shape is through directing alterations in cellular behavior. For example, local deposition of the matrix protein fibronectin plays an instructive role during cleft formation in branching morphogenesis (Daley and Yamada, 2013; Onodera et al., 2010; Sakai et al., 2003). Fibronectin induces the expression of the transcription factor Snail2 and suppresses E-cadherin, leading to cell separation at the cleft (Onodera et al., 2010). Another example of basement membranes altering cell behavior is the role of laminin deposition in coordinating epithelial cell polarity.
Coordinated cell polarity is crucial in forming and shaping tissues (Bryant and Mostov, 2008; Niessen et al., 2012). When embedded in a laminin-rich matrix, a single Madin–Darby canine kidney (MDCK) cell gives rise to a cyst-like monolayer of epithelial cells surrounding a central lumen (McAteer et al., 1988). This process requires coordinated polarity, with all cells establishing an apical luminal membrane domain, separate from the basolateral domain. Prior to establishing apical-basal polarity, MDCK cysts secrete laminin through a Rac1-dependent mechanism (Fig. 1A; O'Brien et al., 2001). Secreted laminin accumulates on the basal side of the cysts, and blocking laminin secretion inhibits the ability of cysts to properly orient polarity (O'Brien et al., 2001). This polarity defect can be rescued by providing the cells with high levels of extracellular laminin (O'Brien et al., 2001). Supporting these findings, histological sectioning has revealed that function-blocking antibodies specific for laminin disrupt the development of polarized kidney tubules in kidney organ cultures and that blocking laminin polymerization in lung organotypic cultures inhibits the formation of polarized epithelial cysts. (Ekblom, 1989; Klein et al., 1988; Schuger et al., 1998). Furthermore, work on embryoid bodies derived from mouse stem cells that lack the laminin γ1 subunit suggests that, in the absence of laminin, the epiblast (an epidermal layer that gives rise to the future embryo) is unable to form a polarized columnar epithelium (Murray and Edgar, 2000). Taken together, these studies suggest that deposition of laminin is required for the coordinated polarity of developing epithelial tissues.
Fig. 1.
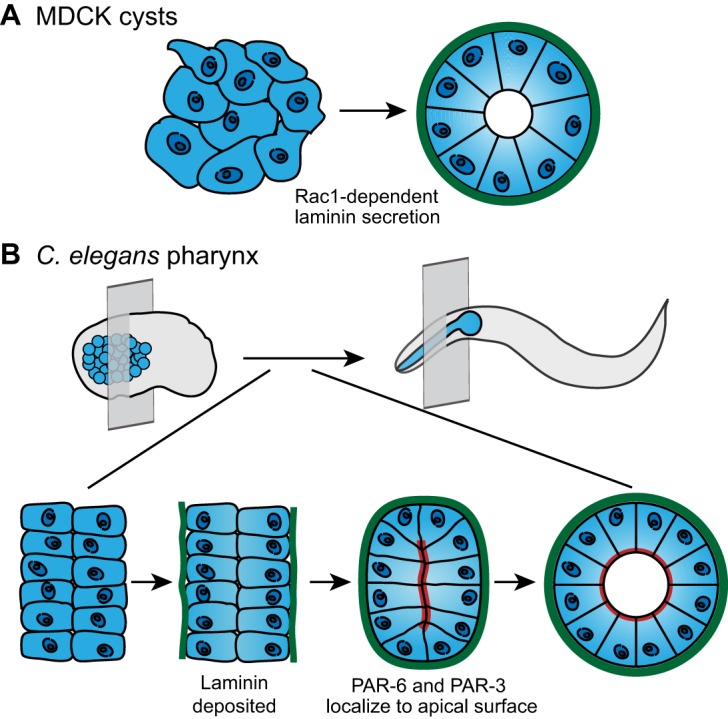
Basement membrane deposition instructs cell polarization and tissue shape. (A) MDCK cells (left) secrete laminin (green) in a Rac1-dependent manner. Laminin accumulates on the basal surface of the cells and directs coordinated apical-basal polarization, which results in formation of a cyst with an apical lumen (right). (B) In the C. elegans embryo (top left), the pharyngeal precursors (blue) organize into two plates of cells (bottom left; perspective indicated by transverse plane in top left). Laminin (green) accumulates on the basal surface of the pharyngeal precursors (middle left). Laminin assembly on the basal cell surface precedes the apical localization of polarity proteins PAR-6 and PAR-3 (red; middle right), as well as apical lumen formation (bottom right). Lumen formation is necessary to shape a functional pharynx in the larva and adult C. elegans (top right). Data are from O'Brien et al. and Rasmussen et al. (O'Brien et al., 2001; Rasmussen et al., 2012) and have been adapted with permission.
The role of laminin in polarizing newly formed epithelial tissues in vivo, however, has remained challenging to experimentally examine because its loss leads to widespread pleiotropic effects in embryos. For example, genetically eliminating any subunit of the most abundant laminin heterotrimer during mouse embryogenesis (α1β1γ1) results in endoderm differentiation defects, widespread apoptosis and embryonic lethality (Alpy et al., 2005; Miner et al., 2004; Smyth et al., 1999). In addition, mice lacking the α5 laminin subunit do not survive until birth, and have prenatal defects in digit separation, glomerulogenesis and neural tube closure (Kikkawa et al., 2003; Miner et al., 1998; Nguyen et al., 2002). Notably, in C. elegans partial loss-of-function alleles for the epi-1 laminin α chain result in disrupted polarity in the muscles, pharynx and epithelia, and laminin is required to establish polarity in the Drosophila endoderm (Huang et al., 2003; Urbano et al., 2009). Because laminin regulates diverse processes, however, it has been difficult to determine whether the absence of polarity is a direct consequence of eliminating laminin in these cases or indirectly caused by a lack of tissue organization due to defects in cell–matrix adhesion, cell proliferation or mechanical stability.
To elucidate the mechanism for coordinating tissue polarity in vivo, a recent study examined the role of laminin in generating the tubular epithelial C. elegans pharynx (Rasmussen et al., 2012). Initially, the mesenchymal pharyngeal precursor cells arrange into two opposing sheets (Fig. 1B). Live imaging of pharyngeal development revealed that prior to polarization, laminin begins to accumulate at the basal surface of pharyngeal precursor cells. The localization of laminin at the basal surface is required for the polarity proteins PAR-3 and PAR-6 to enrich at the opposing apical surface, which occurs ∼15 minutes after the appearance of laminin. In the absence of laminin, PAR-3 and PAR-6 localize randomly, accumulating at the apical surface of the pharynx in some animals and the basal surface in others. Importantly, these polarity cues are specific to laminin. Other basement membrane components, such as type IV collagen and perlecan, are not localized to the pharynx until an hour after laminin, when PAR-6 and PAR-3 are already polarized. Furthermore, genetic reduction or loss of collagen or perlecan does not alter pharyngeal polarity (Graham et al., 1997; Mullen et al., 1999; Rasmussen et al., 2012). Laminin-binding integrins likely recognize laminin as a polarity cue. In MDCK cysts, laminin-binding β1 integrin plays a role in orienting apical-basal polarity in response to ECM cues (Bryant et al., 2014). Consistent with a similar mechanism in the pharynx, PAT-3, the sole C. elegans β-integrin, is localized to the basal surface of the pharyngeal epithelial cells (Baum and Garriga, 1997). Taken together, these data suggest a conserved role for laminin deposition in shaping tissues through instructing coordinated epithelial polarization during development.
Collagen within basement membranes constricts and contours tissues
In addition to coordinating cell behavior, basement membranes also directly regulate tissue shape through patterned physical constrictions. One of the most dramatic examples occurs during tissue elongation in the Drosophila egg chamber (Haigo and Bilder, 2011). The Drosophila egg chamber is composed of two cell types – the germ cells and a surrounding somatic follicular epithelium. The egg chamber undergoes a 2.5-fold increase in aspect ratio and changes from a sphere to an ellipsoid shape along the anterior–posterior axis of the egg during a 20-hour period of oogenesis (Haigo and Bilder, 2011). Strikingly, during the time of elongation, the Drosophila egg chamber rotates within the surrounding basement membrane, which remains static. As the egg chamber rotates, the follicular epithelium deposits strips of matrix containing type IV collagen perpendicular to the axis of elongation (Fig. 2A; Haigo and Bilder, 2011). The oriented fibers act as a molecular ‘corset’ for the growing egg, restricting growth along the dorsal–ventral axis and allowing expansion along the anterior–posterior axis. Accordingly, genetically eliminating type IV collagen expression in the follicular epithelium, by treatment with collagenase, or preventing follicle rotation by clonally eliminating myosin expression, all abrogate egg chamber elongation (Haigo and Bilder, 2011). Taken together, these data show that deposition of oriented collagen fibers can drive tissue elongation by constricting the circumference of a developing tissue, forcing elongation along the perpendicular axis.
Fig. 2.
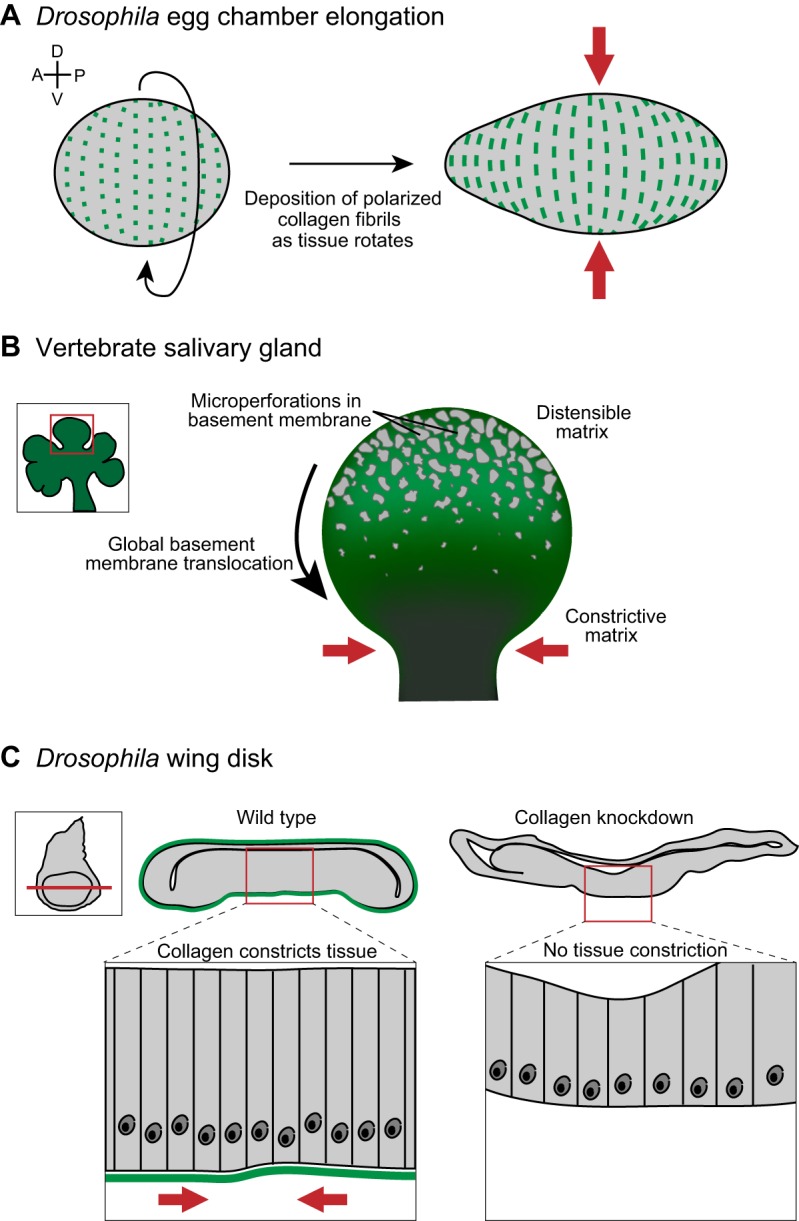
Collagen within basement membranes constricts and contours tissues. (A) Prior to tissue elongation, the Drosophila egg chamber initiates global tissue revolutions, directing the deposition of oriented collagen fibrils (green) as it rotates (left). The collagen fibrils constrict egg chamber growth in the dorsal-ventral (D-V) tissue axis (red arrows), forcing elongation along the anterior-posterior (A-P) axis (right). (B) Inset shows a developing salivary gland, with a single bud (red box in inset) highlighted on the right. Imaging of basement membrane (green) dynamics during salivary gland branching morphogenesis revealed a perforated, distensible matrix at the bud tip, which facilitates tissue growth. During bud tip growth, the basement membrane translocates from the bud tip to the stalk, where basement membrane movement slows and collagen accumulates. This results in a constrictive basement membrane corset around the stalk (red arrows), which stabilizes and likely restricts growth at the stalk. (C) A tissue-specific RNAi strategy revealed that collagen is required to shape the imaginal wing disc in Drosophila (inset). Cross sections (position indicated by red line in inset) through a wild-type (left) and collagen-knockdown (right) wing disc show that the wild-type wing disc is more compact than the collagen-knockdown wing disc. A magnified view (below) illustrates the highly ordered columnar epithelium in wild type compared to a flattened epithelium following collagen knockdown. Data are from Haigo and Bilder, 2011, Harunaga et al. and Pastor-Pareja and Xu (Haigo and Bilder, 2011; Harunaga et al., 2014; Pastor-Pareja and Xu, 2011) and have been adapted with permission.
Tissue rotation accompanied by patterned basement membrane deposition might not be an isolated mechanism for shaping tissues. Mammary acini growing in 3D culture also rotate while assembling basement membrane (Tanner et al., 2012; Wang et al., 2013). In addition, a corset of ECM might also be used to facilitate the anisotropic cell growth required for hypocotyl elongation in Arabidopsis (Paredez et al., 2006). In this case, instead of a rotating epithelia depositing matrix, the cells remain static while cellulose synthase rotates around the cell cortex in a microtubule-dependent manner. The rotating cellulose synthase deposits cellulose fibrils perpendicular to the axis of elongation. This constrains cell expansion, forcing hypocotyl elongation (Paredez et al., 2006).
A collagen corset has also been hypothesized to regulate directional outgrowth and tissue shape in the vertebrate salivary gland (Harunaga et al., 2014). Immunostaining for type IV collagen, laminin and perlecan showed that the basement membrane at the tips of rapidly growing salivary gland end buds contains hundreds of microperforations (Fig. 2B). Time-lapse analysis of cultured salivary glands revealed that cells at the bud tip extend myosin-dependent blebs through the basement membrane, dynamically distending the matrix by enlarging existing microperforations and likely creating additional holes (Harunaga et al., 2014). Photobleaching of landmarks within fluorescently labeled basement membrane in living salivary gland explants revealed that the basement membrane translocates away from the tip towards the duct, where it accumulates in dense aggregates with significantly fewer and smaller perforations. This combination of cell blebbing and movement of the entire basement membrane results in a perforated and highly distensible matrix at the bud tip and in the accumulation of a collagen-rich basement membrane corset around the duct. Reducing the number of microperforations by broadly inhibiting protease activity resulted in less branching, suggesting that the basement membrane dynamics at the bud tip promote branch outgrowth (Harunaga et al., 2014). Microperforation in the basement membrane also occurs at the bud tip of developing lung and kidney branches, suggesting that a similar mechanism regulates tissue architecture in these organs (Harunaga et al., 2014). Further supporting this idea, static localization of matrix components during mammary gland branching has revealed that the mammary duct, but not the bud tip, is surrounded by a thickened layer of collagen which might stabilize the duct and constrain expansion (Hinck and Silberstein, 2005; Silberstein and Daniel, 1982). The observations in the Drosophila egg chamber and the mammalian salivary gland support the idea that selectively modifying the basement membrane to create regions with high or low distensibility can shape organs by constricting and directing tissue growth.
In addition to proteolysis and deposition of oriented collagen, altering the composition of the basement membrane can modify its pliability and regulate tissue shape. A recent study in Drosophila elegantly illustrates how collagen and perlecan might act in opposition to alter the amount of constrictive force imposed by the basement membrane on tissues (Pastor-Pareja and Xu, 2011). Using a temporal and tissue-specific RNA interference (RNAi) approach, these authors selectively inhibited post-embryonic collagen expression. In animals in which post-embryonic collagen levels were reduced, several organs adopted highly aberrant shapes: the imaginal discs, usually consisting of highly ordered columnar epithelia, were expanded laterally or flattened (Fig. 2C); the ventral nerve cord was highly elongated, suggesting a failure to condense; and ducts within the salivary gland were dilated and contained an expanded lumen (Pastor-Pareja and Xu, 2011). These phenotypes further suggest that type IV collagen within basement membrane provides a constricting force on tissues. Notably, the basement membrane component perlecan was found to play an opposing role to collagen in regulating tissue constriction. Overexpressing perlecan resulted in phenotypes that are reminiscent of collagen knockdown, with flattened imaginal discs and an uncondensed ventral nerve cord. Furthermore, reducing the levels of perlecan caused a compaction of the imaginal disc and ventral nerve cord (Pastor-Pareja and Xu, 2011). Importantly, perlecan does not affect collagen incorporation or levels in basement membrane, suggesting it directly counteracts the constricting properties of collagen (Pastor-Pareja and Xu, 2011). Taken together, these data suggest that the addition of collagen into basement membranes shapes organs by providing mechanical constriction while perlecan opposes this tightening.
Collagen maintenance in basement membranes also appears to be regulated and important in retaining tissue architecture. Recent work in C. elegans has shown that the conserved matrix component fibulin is required to preserve collagen in both the gonadal and intestinal tissues – loss of fibulin does not affect collagen function during embryogenesis or early larval development, but does result in the loss of collagen during the end of larval development (Kubota et al., 2012). This loss of collagen is thought to underlie the shortened and widened gonad seen in fibulin mutants, as this phenotype can be recapitulated by shifting a temperature-sensitive collagen mutant to the restrictive temperature following embryogenesis (Kawano et al., 2009; Kubota et al., 2012). Taken together, these data indicate that there are numerous mechanisms that have evolved to regulate collagen incorporation, function and maintenance in basement membranes. These diverse modes of regulation likely reflect the key importance of collagen in shaping and maintaining tissue structure.
Basement membrane linkages connect tissues
Basement membranes encapsulate tissues and are usually bordered by a loose fibrillar interstitial matrix that provides tissues with further mechanical support (Halfter et al., 2013). In some situations, the basement membranes of adjacent tissues contact one another. In these cases, neighboring basement membranes are often remodeled to form stable connections between basement membranes. Alternatively, contact between two basement membranes might precede their removal and direct cell–cell interaction.
Illustrating the importance of basement membrane linkage, basement membrane connections occur at the interface of the vasculature and neighboring tissues in many vital organs (Fig. 3). For example, during glomerular development in the kidney, the epithelial podocyte and endothelial vasculature each synthesize and assemble basement membrane on their extracellular surface (Abrahamson et al., 2009). As development progresses, the two basement membranes first meet as a bi-layered basement membrane, and subsequently fuse into a single basement membrane sheet (Fig. 3A; Abrahamson, 1985). The resulting glomerular basement membrane is shared by both cell types, and defects in the composition and structure of this basement membrane are associated with nephropathies such as Alport Syndrome, where kidney filtration is severely impaired (Hudson et al., 2003). A similar process occurs during alveolar development in the lung, where the basement membrane of the developing vasculature fuses with the basement membrane that is synthesized by the alveolus (Fig. 3B; Vaccaro and Brody, 1981). This creates a tight connection between the vasculature and the alveolus, which is thought to be important for efficient gas exchange. Apparent fusion between basement membranes is also observed in the central nervous system at the blood–brain barrier, where the basement membrane of the endothelial cells merges with the parenchymal basement membrane deposited by the surrounding astrocytes. The fused basement membrane is important for the structure and proper functioning of the blood–brain barrier (Obermeier et al., 2013).
Fig. 3.
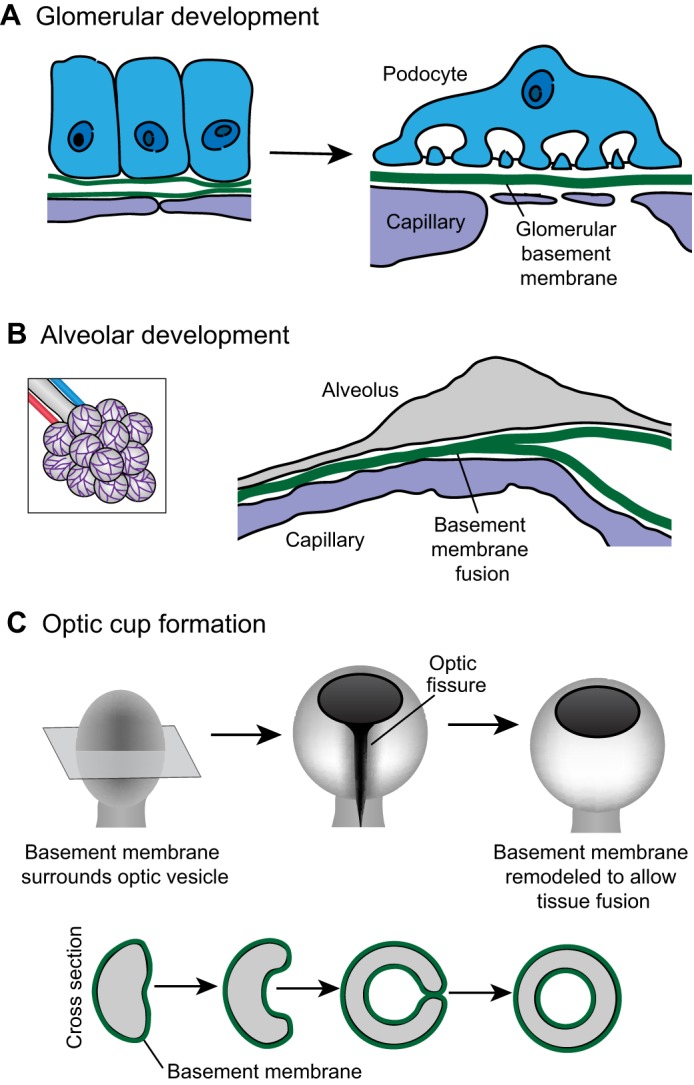
Basement-membrane–basement-membrane interactions. (A) As the kidney develops, the epithelial podocyte (blue) and the vascular endothelium (purple) each synthesize basement membrane (left). These individual basement membranes fuse to form the glomerular basement membrane (right). (B) During development of the alveolus (left; a magnified view is shown on the right), the capillary (purple) basement membrane and the alveolar (gray) basement membrane fuse to form a single sheet of basement membrane (C) The optic cup initiates as an epithelial bud called the optic vesicle (left). Shown in cross section (bottom), the center of the optic vesicle invaginates and the sides of the optic vesicle grow circumferentially until they meet to close the optic fissure and form a continuous circular cup (right). When the basement membrane-encased sides of the optic cup meet (middle right), the basement membrane is remodeled to allow direct cell–cell contract (right). Data are from Vaccaro and Brody (©1981 Vaccaro and Brody. Journal of Cell Biology. 91:427–437. doi: 10.1083/jcb.91.2.427) and St John and Abrahamson (St John and Abrahamson, 2001) and have been adapted with permission.
In addition to fusing into a single sheet of matrix, juxtaposed basement membranes can also be remodeled to allow tissue fusion through direct cell–cell contact. For example, during optic cup formation in vertebrates, the optic bud is initially surrounded by basement membrane. The epithelia of the optic bud flattens and curves such that the two sides of the optic bud meet and fuse to form the optic cup (Fig. 3C; Lamb et al., 2007). Prior to tissue fusion, the basement membrane covering each side of the optic bud is removed, facilitating the formation of a continuous epithelium (Tsuji et al., 2012). Basement membrane contact and removal also occurs during Drosophila imaginal disc eversion (Pastor-Pareja et al., 2004). Imaginal discs are sacs of epithelial cells initially located within the larval body that are then everted and fuse with the larval epidermis to form the adult exoskeleton. Prior to eversion, the basement membrane of the wing disc (the peripodial and stalk cells or PS cells) contacts the larval epidermal basement membrane. Following contact, both basement membranes are removed and the PS cells invade the larval epidermis, fusing the imaginal disc epithelium with the larval epithelium (Pastor-Pareja et al., 2004). Taken together, these studies indicate that interactions between juxtaposed basement membranes occur during the formation of many different tissues and organ systems.
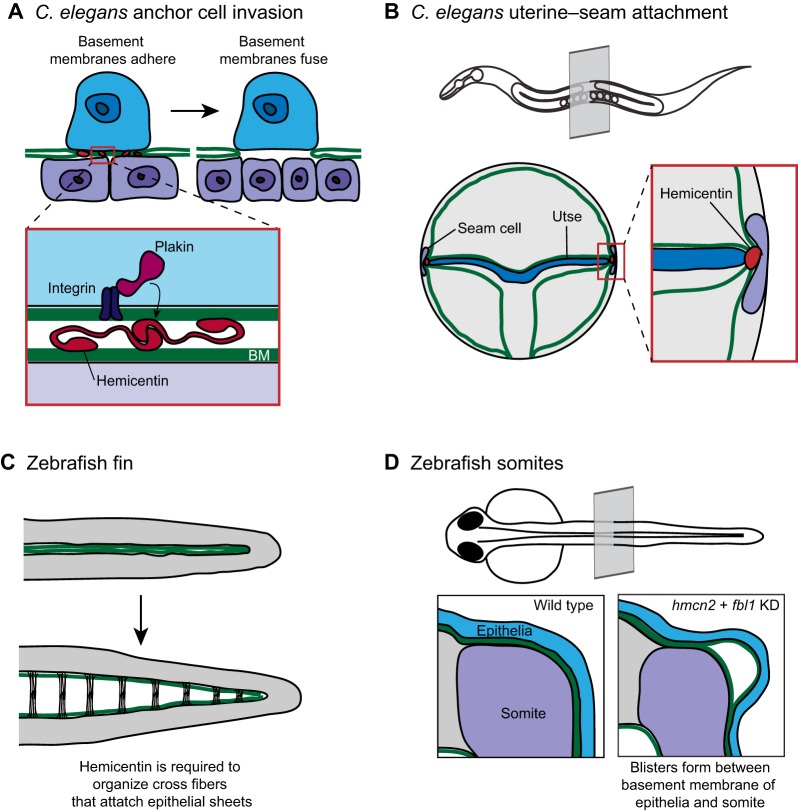
Studies on uterine–vulval attachment in C. elegans have begun to reveal mechanisms that regulate temporal and tissue-specific interactions between basement membranes. Prior to attachment, the uterine and vulval tissues are separated by their respective basement membranes. Similar to Drosophila disc eversion and vertebrate eye formation, the juxtaposed basement membranes that separate the uterine and vulval tissues are removed to facilitate direct tissue fusion. This removal is initiated by a single invasive cell in the uterine tissue, the anchor cell, which breaches the basement membranes (Fig. 4A; Hagedorn et al., 2014; Hagedorn et al., 2013; Ihara et al., 2011; Matus et al., 2014; Sherwood et al., 2005; Sherwood and Sternberg, 2003). Live-cell imaging and electron microscopy of the juxtaposed basement membranes showed a precise attachment between the basement membranes immediately preceding anchor cell invasion. This adhesion is required for rapid and coordinated basement membrane removal (Morrissey et al., 2014). Basement membrane attachment is mediated by the conserved extracellular matrix component hemicentin, which is secreted by the anchor cell prior to invasion (Morrissey et al., 2014; Sherwood et al., 2005; Vogel and Hedgecock, 2001). Hemicentin assembles into punctate aggregates that are associated with the basement membranes under the anchor cell. These hemicentin aggregates might form the physical link between the uterine and vulval basement membranes, as hemicentin has a number of potential selfassociation domains, including a long stretch of tandem immunoglobulin repeats, three EGF-like domains, and a fibulin-like C-terminus. Furthermore, hemicentin contains a von Willebrand A (VWA) domain that might bind to collagen (Fig. 4A; Dong et al., 2006). In addition to hemicentin, VAB-10A, a vertebrate plakin ortholog (Bosher et al., 2003), functions within the anchor cell and is required to connect the uterine and vulval basement membranes. Plakins are cytolinker proteins that connect cytoskeletal elements to one another and to adhesion complexes (Jefferson et al., 2004). The C. elegans integrin heterodimer INA-1/PAT-3, which is most similar to vertebrate laminin-binding integrins (Baum and Garriga, 1997), is also required for basement membrane-basement membrane adhesion. Both VAB-10A and INA-1/PAT-3 localize to the basal cell membrane of the anchor cell, which contacts the basement membrane. Because integrin binds directly to plakin in vertebrates, INA-1/PAT-3 (integrin) might provide the link between VAB-10A and hemicentin (Fig. 4A; Gregory and Brown, 1998; Rezniczek et al., 1998; Seifert et al., 1992). Together, hemicentin, plakin and integrin, form a cell-specific temporally and spatially localized basement-membrane-to-basement-membrane linkage system, which we term a B-LINK (for ‘Basement membrane LINKage’).
Fig. 4.
Hemicentin regulates basement membrane-basement membrane adhesion. (A) The C. elegans uterine anchor cell (blue) breaches the juxtaposed gonadal and ventral basement membranes (green) and contacts the underlying vulval epithelium (purple). Just prior to basement membrane breach (left), the individual basement membranes are connected by a B-LINK adhesion structure with its molecular regulators shown in the inset. Extracellular hemicentin (red) localizes between the basement membranes. Intracellular plakin (magenta) and integrin (dark purple) are required to form a functional B-LINK and might stabilize the adhesion by connecting the B-LINK to the anchor cell cytoskeleton. (B) The hemicentin, plakin and integrin-dependent B-LINK is also required to attach the C. elegans seam cell (purple) to the uterine utse cell (blue). A transverse plane through an adult worm (top) indicates the location of the uterine–seam attachment. (C) The zebrafish fin originates as two abutting sheets of epithelia (top). During fin development, these sheets separate but remain attached through extracellular cross-fibers (bottom). Hemicentin is required to organize the cross fibers that attach the epithelial sheets. (D) A cross section through a zebrafish larva shows the juxtaposed basement membranes surrounding the somite (purple) and the epithelia (blue). Following knockdown of the hemicentin ortholog hmcn2 and the fibulin family member fbl1, blisters develop between the somite basement membrane and the epithelial basement membrane (right). Data from Morrissey et al. and Feitosa et al. (Morrissey et al., 2014; Feitosa et al., 2012) and have been adapted with permission.
Although the B-LINK functions transiently to connect the uterine and vulval basement membranes during anchor cell invasion, it also mediates a long-term tissue connection in C. elegans. A stable attachment involving the B-LINK components hemicentin, plakin and integrin links the C. elegans uterine tissue to the hypodermal seam cell in the adult (Fig. 4B; Morrissey et al., 2014; Vogel and Hedgecock, 2001). Disrupting any of the three B-LINK components results in uterine vulval prolapse, where the uterus detaches from the body cavity and spills out of the worm during egg laying (Morrissey et al., 2014). Thus, B-LINKs are used transiently prior to basement membrane removal and during longer, more stable, basement membrane linkages. These data suggest that the B-LINK system might be used in other cellular contexts to connect neighboring basement membranes.
Supporting the idea that the B-LINK adhesion system might function broadly, the two zebrafish hemicentin orthologs, hmcn1 and hmcn2, appear to mediate basement-membrane–basement-membrane adhesion in the developing fin fold and trunk (Fig. 4C; Carney et al., 2010; Feitosa et al., 2012). Mutations in hmcn1 result in a loss of attachment between the epidermal sheets of the fin fold (Carney et al., 2010). At a later time, hmcn2 in combination with the fibulin fbln1 organize electron-dense cross fibers that might represent a more elaborate or altered B-LINK (Fig. 4C; Feitosa et al., 2012). The hnmc2 gene also regulates a basement membrane connection in the zebrafish trunk. Morpholino-mediated reduction in hmnc2 in combination with reduction of fbln1 results in blistering between the juxtaposed epidermal and somite basement membranes (Fig. 4D; Feitosa et al., 2012). Furthermore, the Drosophila plakin ortholog short stop regulates tissue-tissue adhesion at the muscle–tendon junction, but it is unclear whether this is directly related to the role of plakin in linking basement membranes (Gregory and Brown, 1998; Röper et al., 2002). Human genetic studies have also linked mutations in hemicentin to macular degeneration (Schultz et al., 2003; Thompson et al., 2007). Macular degeneration is often caused by defects in Bruch's membrane, a structure that originates as two juxtaposed basement membranes (Booij et al., 2010), suggesting that a transient or stable B-LINK might be involved in organizing the complex basement membrane architecture in the eye. Taken together, these studies provide evidence that an adhesion complex linking basement membranes might be a common and conserved strategy to regulate diverse tissue structures and morphogenetic processes.
It is likely that many more B-LINK components remain to be discovered. Well-studied adhesion systems, such as focal adhesions, are associated with numerous proteins (Kuo et al., 2011). As Hmcn1 was identified as a regulator of Fraser syndrome, a disease that is attributed in part to transient basement membrane disruptions and blistering below the basement membrane of the epidermis (Short et al., 2007; Smyth and Scambler, 2005), other genes associated with Fraser syndrome might also encode B-LINK components. In addition, further studies in C. elegans using forward genetic analysis will likely reveal additional B-LINK proteins.
Conclusions and future directions
Far from just being a static support matrix, recent in vivo studies have revealed that basement membranes actively shape tissue morphology. Changes to basement membrane deposition, structure and composition are thus essential components of the cellular toolkit used to sculpt diverse tissue architecture. In particular, type IV collagen deposition and modification play a pivotal role in shaping tissue structure. Thus, further studies on how collagen is added, maintained and removed from basement membranes will be especially important. As previous studies have shown that collagen and perlecan act in opposition to regulate the mechanical properties of the basement membrane, determining what other molecular signals or basement membrane components regulate basement membrane pliability will be of interest. Furthermore, recent studies on isolated native basement membranes from adult human tissues have indicated that basement membranes have a distinct sidedness, with the epithelial side of basement membranes having different physical properties and epitope distribution from the stromal side (Halfter et al., 2013). The unique characteristics on each side of the basement membrane appear to be functionally relevant, as epithelial cells adhere only to the epithelial side of the basement membrane and not to the stromal side (Halfter et al., 2013). These studies suggest that there is an intriguing, unexamined complexity that might organize tissue architecture (Halfter and Yip, 2014). Given the essential role of basement membranes in tissue form and function, basement membranes might also play a role in maintaining tissue structure as an organism ages. Indeed, proteins such as fibulin might play an ongoing role in shaping and maintaining tissues (Kubota et al., 2012). In addition, basement membranes show dramatic changes in structure with age (Candiello et al., 2010; Danysh et al., 2008; Khalil-Manesh and Price, 1985; Taylor and Price, 1982). It will be interesting to determine whether age-dependent alterations to basement membranes underlie any changes the structure and function of aged tissues. Overall, the studies highlighted in this Commentary demonstrate that basement membranes are dynamic structures that are modified to affect cell and tissue level function. Furthermore, this work makes clear that the roles and regulation of this fascinating ancient matrix are just beginning to be understood.
Acknowledgements
We thank L. Kelley, M. Clay, D. Keeley and A. Schindler for helpful comments.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by a National Science Foundation Graduate Research Fellowship to M.A.M.; The Pew Scholars Program in the Biomedical Sciences; and the National Institutes of Health [grant numbers GM079320, GM100083 to D.R.S.]. Deposited in PMC for release after 12 months.
References
- Abrahamson DR. Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J. Cell Biol. 1985;100:1988–2000. doi: 10.1083/jcb.100.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St John PL. Cellular origins of type IV collagen networks in developing glomeruli. J. Am. Soc. Nephrol. 2009;20:1471–1479. doi: 10.1681/ASN.2008101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams GA, Schaus SS, Goodman SL, Nealey PF, Murphy CJ. Nanoscale topography of the corneal epithelial basement membrane and Descemet's membrane of the human. Cornea. 2000;19:57–64. doi: 10.1097/00003226--200001000--00012. [DOI] [PubMed] [Google Scholar]
- Abrams GA, Murphy CJ, Wang ZY, Nealey PF, Bjorling DE. Ultrastructural basement membrane topography of the bladder epithelium. Urol. Res. 2003;31:341–346. doi: 10.1007/s00240--003--0347--9. [DOI] [PubMed] [Google Scholar]
- Alpy F, Jivkov I, Sorokin L, Klein A, Arnold C, Huss Y, Kedinger M, Simon-Assmann P, Lefebvre O. Generation of a conditionally null allele of the laminin alpha1 gene. Genesis. 2005;43:59–70. doi: 10.1002/gene.20154. [DOI] [PubMed] [Google Scholar]
- Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, Mokkapati S, Murshed M, Nischt R. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol. Cell. Biol. 2005;25:6846–6856. doi: 10.1128/MCB.25.15.6846--6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum PD, Garriga G. Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron. 1997;19:51–62. doi: 10.1016/S0896--6273(00)80347--5. [DOI] [PubMed] [Google Scholar]
- Behrens DT, Villone D, Koch M, Brunner G, Sorokin L, Robenek H, Bruckner-Tuderman L, Bruckner P, Hansen U. The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J. Biol. Chem. 2012;287:18700–18709. doi: 10.1074/jbc.M111.336073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Cummings CF, Vanacore RM, Kumagai-Cresse C, Ero-Tolliver IA, Rafi M, Kang JS, Pedchenko V, Fessler LI, Fessler JH. et al. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat. Chem. Biol. 2012;8:784–790. doi: 10.1038/nchembio.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij JC, Baas DC, Beisekeeva J, Gorgels TG, Bergen AA. The dynamic nature of Bruch's membrane. Prog. Retin. Eye Res. 2010;29:1–18. doi: 10.1016/j.preteyeres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Bosher JM, Hahn BS, Legouis R, Sookhareea S, Weimer RM, Gansmuller A, Chisholm AD, Rose AM, Bessereau JL, Labouesse M. The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces. J. Cell Biol. 2003;161:757–768. doi: 10.1083/jcb.200302151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Roignot J, Datta A, Overeem AW, Kim M, Yu W, Peng X, Eastburn DJ, Ewald AJ, Werb Z. et al. A molecular switch for the orientation of epithelial cell polarization. Dev. Cell. 2014;31:171–187. doi: 10.1016/j.devcel.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiello J, Cole GJ, Halfter W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010;29:402–410. doi: 10.1016/j.matbio.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Carney TJ, Feitosa NM, Sonntag C, Slanchev K, Kluger J, Kiyozumi D, Gebauer JM, Coffin Talbot J, Kimmel CB, Sekiguchi K. et al. Genetic analysis of fin development in zebrafish identifies furin and hemicentin1 as potential novel fraser syndrome disease genes. PLoS Genet. 2010;6:e1000907. doi: 10.1371/journal.pgen.1000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fässler R. Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Yamada KM. ECM-modulated cellular dynamics as a driving force for tissue morphogenesis. Curr. Opin. Genet. Dev. 2013;23:408–414. doi: 10.1016/j.gde.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysh BP, Czymmek KJ, Olurin PT, Sivak JG, Duncan MK. Contributions of mouse genetic background and age on anterior lens capsule thickness. Anat. Rec. (Hoboken) 2008;291:1619–1627. doi: 10.1002/ar.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Muriel JM, Ramirez S, Hutter H, Hedgecock EM, Breydo L, Baskakov IV, Vogel BE. Hemicentin assembly in the extracellular matrix is mediated by distinct structural modules. J. Biol. Chem. 2006;281:23606–23610. doi: 10.1074/jbc.M513589200. [DOI] [PubMed] [Google Scholar]
- Ekblom P. Developmentally regulated conversion of mesenchyme to epithelium. FASEB J. 1989;3:2141–2150. doi: 10.1096/fasebj.3.10.2666230. [DOI] [PubMed] [Google Scholar]
- Fässler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Feitosa NM, Zhang J, Carney TJ, Metzger M, Korzh V, Bloch W, Hammerschmidt M. Hemicentin 2 and Fibulin 1 are required for epidermal-dermal junction formation and fin mesenchymal cell migration during zebrafish development. Dev. Biol. 2012;369:235–248. doi: 10.1016/j.ydbio.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler AL, Vanacore RM, Chetyrkin SV, Pedchenko VK, Bhave G, Yin VP, Stothers CL, Rose KL, McDonald WH, Clark TA. et al. Aspirnauts. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc. Natl. Acad. Sci. USA. 2014;111:331–336. doi: 10.1073/pnas.1318499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J. et al. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991;10:3137–3146. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotenstein JR, Swale RE, Fukuda T, Wu Z, Giurumescu CA, Goncharov A, Jin Y, Chisholm AD. The C. elegans peroxidasin PXN-2 is essential for embryonic morphogenesis and inhibits adult axon regeneration. Development. 2010;137:3603–3613. doi: 10.1242/dev.049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham PL, Johnson JJ, Wang S, Sibley MH, Gupta MC, Kramer JM. Type IV collagen is detectable in most, but not all, basement membranes of Caenorhabditis elegans and assembles on tissues that do not express it. J. Cell Biol. 1997;137:1171–1183. doi: 10.1083/jcb.137.5.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SL, Brown NH. kakapo, a gene required for adhesion between and within cell layers in Drosophila, encodes a large cytoskeletal linker protein related to plectin and dystrophin. J. Cell Biol. 1998;143:1271–1282. doi: 10.1083/jcb.143.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn EJ, Ziel JW, Morrissey MA, Linden LM, Wang Z, Chi Q, Johnson SA, Sherwood DR. The netrin receptor DCC focuses invadopodia-driven basement membrane transmigration in vivo. J. Cell Biol. 2013;201:903–913. doi: 10.1083/jcb.201301091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn EJ, Kelley LC, Naegeli KM, Wang Z, Chi Q, Sherwood DR. ADF/cofilin promotes invadopodial membrane recycling during cell invasion in vivo. J. Cell Biol. 2014;204:1209–1218. doi: 10.1083/jcb.201312098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–1074. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Yip J. An organizing function of basement membranes in the developing nervous system. Mech. Dev. 2014;133:1–10. doi: 10.1016/j.mod.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Halfter W, Monnier C, Müller D, Oertle P, Uechi G, Balasubramani M, Safi F, Lim R, Loparic M, Henrich PB. The bi-functional organization of human basement membranes. PLoS ONE. 2013;8:e67660. doi: 10.1371/journal.pone.0067660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harunaga JS, Doyle AD, Yamada KM. Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Dev. Biol. 2014;394:197–205. doi: 10.1016/j.ydbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MD, Campbell KP. A role for dystroglycan in basement membrane assembly. Cell. 1998;95:859–870. doi: 10.1016/S0092--8674(00)81708--0. [DOI] [PubMed] [Google Scholar]
- Hinck L, Silberstein GB. Key stages in mammary gland development: the mammary end bud as a motile organ. Breast Cancer Res. 2005;7:245–251. doi: 10.1186/bcr1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Hall DH, Hedgecock EM, Kao G, Karantza V, Vogel BE, Hutter H, Chisholm AD, Yurchenco PD, Wadsworth WG. Laminin alpha subunits and their role in C. elegans development. Development. 2003;130:3343–3358. doi: 10.1242/dev.00481. [DOI] [PubMed] [Google Scholar]
- Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N. Engl. J. Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The evolution of metazoan extracellular matrix. J. Cell Biol. 2012;196:671–679. doi: 10.1083/jcb.201109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Naba A. Overview of the matrisome – an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara S, Hagedorn EJ, Morrissey MA, Chi Q, Motegi F, Kramer JM, Sherwood DR. Basement membrane sliding and targeted adhesion remodels tissue boundaries during uterine-vulval attachment in Caenorhabditis elegans. Nat. Cell Biol. 2011;13:641–651. doi: 10.1038/ncb2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson JJ, Leung CL, Liem RK. Plakins: goliaths that link cell junctions and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2004;5:542–553. doi: 10.1038/nrm1425. [DOI] [PubMed] [Google Scholar]
- Kang SH, Kramer JM. Nidogen is nonessential and not required for normal type IV collagen localization in Caenorhabditis elegans. Mol. Biol. Cell. 2000;11:3911–3923. doi: 10.1091/mbc.11.11.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Zheng H, Merz DC, Kohara Y, Tamai KK, Nishiwaki K, Culotti JG. C. elegans mig-6 encodes papilin isoforms that affect distinct aspects of DTC migration, and interacts genetically with mig-17 and collagen IV. Development. 2009;136:1433–1442. doi: 10.1242/dev.028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil-Manesh F, Price RG. Age-related changes in rat glomerular basement membrane components solubilised with pepsin. Ren. Physiol. 1985;8:120–128. doi: 10.1159/000173043. [DOI] [PubMed] [Google Scholar]
- Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc. Res. Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y, Virtanen I, Miner JH. Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J. Cell Biol. 2003;161:187–196. doi: 10.1083/jcb.200211121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G, Langegger M, Timpl R, Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–341. doi: 10.1016/0092--8674(88)90056--6. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Nagata K, Sugimoto A, Nishiwaki K. Tissue architecture in the Caenorhabditis elegans gonad depends on interactions among fibulin-1, type IV collagen and the ADAMTS extracellular protease. Genetics. 2012;190:1379–1388. doi: 10.1534/genetics.111.133173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 2011;13:383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Collin SP, Pugh EN., Jr Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat. Rev. Neurosci. 2007;8:960–976. doi: 10.1038/nrn2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Edgar D, Fässler R, Wadsworth W, Yurchenco PD. The role of laminin in embryonic cell polarization and tissue organization. Dev. Cell. 2003;4:613–624. doi: 10.1016/S1534--5807(03)00128--X. [DOI] [PubMed] [Google Scholar]
- Matus DQ, Chang E, Makohon-Moore SC, Hagedorn MA, Chi Q, Sherwood DR. Cell division and targeted cell cycle arrest opens and stabilizes basement membrane gaps. Nat. Commun. 2014;5:4184. doi: 10.1038/ncomms5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAteer JA, Dougherty GS, Gardner KD, Jr, Evan AP. Polarized epithelial cysts in vitro: a review of cell and explant culture systems that exhibit epithelial cyst formation. Scanning Microsc. 1988;2:1739–1763. [PubMed] [Google Scholar]
- McKee KK, Harrison D, Capizzi S, Yurchenco PD. Role of laminin terminal globular domains in basement membrane assembly. J. Biol. Chem. 2007;282:21437–21447. doi: 10.1074/jbc.M702963200. [DOI] [PubMed] [Google Scholar]
- Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J. Cell Biol. 1998;143:1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- Morrissey MA, Keeley DP, Hagedorn EJ, McClatchey ST, Chi Q, Hall DH, Sherwood DR. B-LINK: a hemicentin, plakin, and integrin-dependent adhesion system that links tissues by connecting adjacent basement membranes. Dev. Cell. 2014;31:319–331. doi: 10.1016/j.devcel.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen GP, Rogalski TM, Bush JA, Gorji PR, Moerman DG. Complex patterns of alternative splicing mediate the spatial and temporal distribution of perlecan/UNC-52 in Caenorhabditis elegans. Mol. Biol. Cell. 1999;10:3205–3221. doi: 10.1091/mbc.10.10.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P, Edgar D. Regulation of programmed cell death by basement membranes in embryonic development. J. Cell Biol. 2000;150:1215–1221. doi: 10.1083/jcb.150.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NM, Miner JH, Pierce RA, Senior RM. Laminin alpha 5 is required for lobar septation and visceral pleural basement membrane formation in the developing mouse lung. Dev. Biol. 2002;246:231–244. doi: 10.1006/dbio.2002.0658. [DOI] [PubMed] [Google Scholar]
- Niessen MT, Iden S, Niessen CM. The in vivo function of mammalian cell and tissue polarity regulators – how to shape and maintain the epidermal barrier. J. Cell Sci. 2012;125:3501–3510. doi: 10.1242/jcs.092890. [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901--831. [DOI] [PubMed] [Google Scholar]
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera T, Sakai T, Hsu JC, Matsumoto K, Chiorini JA, Yamada KM. Btbd7 regulates epithelial cell dynamics and branching morphogenesis. Science. 2010;329:562–565. doi: 10.1126/science.1191880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbek S, Balasubramanian PG, Chiquet-Ehrismann R, Tucker RP, Adams JC. The evolution of extracellular matrix. Mol. Biol. Cell. 2010;21:4300–4305. doi: 10.1091/mbc.E10--03--0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez AR, Somerville CR, Ehrhardt DW. Visualization of cellulose synthase demonstrates functional association with microtubules. Science. 2006;312:1491–1495. doi: 10.1126/science.1126551. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Xu T. Shaping cells and organs in Drosophila by opposing roles of fat body-secreted Collagen IV and perlecan. Dev. Cell. 2011;21:245–256. doi: 10.1016/j.devcel.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Grawe F, Martín-Blanco E, García-Bellido A. Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev. Cell. 2004;7:387–399. doi: 10.1016/j.devcel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Pontén F, Schwenk JM, Asplund A, Edqvist PH. The Human Protein Atlas as a proteomic resource for biomarker discovery. J. Intern. Med. 2011;270:428–446. doi: 10.1111/j.1365--2796.2011.02427.x. [DOI] [PubMed] [Google Scholar]
- Pöschl E, Schlötzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- Rasmussen JP, Reddy SS, Priess JR. Laminin is required to orient epithelial polarity in the C. elegans pharynx. Development. 2012;139:2050–2060. doi: 10.1242/dev.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezniczek GA, de Pereda JM, Reipert S, Wiche G. Linking integrin alpha6beta4-based cell adhesion to the intermediate filament cytoskeleton: direct interaction between the beta4 subunit and plectin at multiple molecular sites. J. Cell Biol. 1998;141:209–225. doi: 10.1083/jcb.141.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röper K, Gregory SL, Brown NH. The ‘spectraplakins’: cytoskeletal giants with characteristics of both spectrin and plakin families. J. Cell Sci. 2002;115:4215–4225. doi: 10.1242/jcs.00157. [DOI] [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- Schuger L, Yurchenco P, Relan NK, Yang Y. Laminin fragment E4 inhibition studies: basement membrane assembly and embryonic lung epithelial cell polarization requires laminin polymerization. Int. J. Dev. Biol. 1998;42:217–220. [PubMed] [Google Scholar]
- Schultz DW, Klein ML, Humpert AJ, Luzier CW, Persun V, Schain M, Mahan A, Runckel C, Cassera M, Vittal V. et al. Analysis of the ARMD1 locus: evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum. Mol. Genet. 2003;12:3315–3323. doi: 10.1093/hmg/ddg348. [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Lawson D, Wiche G. Immunolocalization of the intermediate filament-associated protein plectin at focal contacts and actin stress fibers. Eur. J. Cell Biol. 1992;59:138–147. [PubMed] [Google Scholar]
- Sherwood DR, Sternberg PW. Anchor cell invasion into the vulval epithelium in C. elegans. Dev. Cell. 2003;5:21–31. doi: 10.1016/S1534--5807(03)00168--0. [DOI] [PubMed] [Google Scholar]
- Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005;121:951–962. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Short K, Wiradjaja F, Smyth I. Let's stick together: the role of the Fras1 and Frem proteins in epidermal adhesion. IUBMB Life. 2007;59:427–435. doi: 10.1080/15216540701510581. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev. Biol. 1982;90:215–222. doi: 10.1016/0012--1606(82)90228--7. [DOI] [PubMed] [Google Scholar]
- Smyth I, Scambler P. The genetics of Fraser syndrome and the blebs mouse mutants. Hum. Mol. Genet. 2005;14 Spec. No 2:R269–R274. doi: 10.1093/hmg/ddi262. [DOI] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J. Cell Biol. 1999;144:151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001;60:1037–1046. doi: 10.1046/j.1523--1755.2001.0600031037.x. [DOI] [PubMed] [Google Scholar]
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Tanner K, Mori H, Mroue R, Bruni-Cardoso A, Bissell MJ. Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc. Natl. Acad. Sci. USA. 2012;109:1973–1978. doi: 10.1073/pnas.1119578109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SA, Price RG. Age-related changes in rat glomerular basement membrane. Int. J. Biochem. 1982;14:201–206. doi: 10.1016/0020--711X(82)90139--2. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Klein BE, Klein R, Xu Z, Capriotti J, Joshi T, Leontiev D, Lee KE, Elston RC, Iyengar SK. Complement factor H and hemicentin-1 in age-related macular degeneration and renal phenotypes. Hum. Mol. Genet. 2007;16:2135–2148. doi: 10.1093/hmg/ddm164. [DOI] [PubMed] [Google Scholar]
- Tsuji N, Kita K, Ozaki K, Narama I, Matsuura T. Organogenesis of mild ocular coloboma in FLS mice: failure of basement membrane disintegration at optic fissure margins. Exp. Eye Res. 2012;94:174–178. doi: 10.1016/j.exer.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Uechi G, Sun Z, Schreiber EM, Halfter W, Balasubramani M. Proteomic view of basement membranes from human retinal blood vessels, inner limiting membranes, and lens capsules. J. Proteome Res. 2014 doi: 10.1021/pr5002065. [DOI] [PubMed] [Google Scholar]
- Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S. et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210--1248. [DOI] [PubMed] [Google Scholar]
- Urbano JM, Torgler CN, Molnar C, Tepass U, López-Varea A, Brown NH, de Celis JF, Martín-Bermudo MD. Drosophila laminins act as key regulators of basement membrane assembly and morphogenesis. Development. 2009;136:4165–4176. doi: 10.1242/dev.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro CA, Brody JS. Structural features of alveolar wall basement membrane in the adult rat lung. J. Cell Biol. 1981;91:427–437. doi: 10.1083/jcb.91.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacore R, Ham AJ, Voehler M, Sanders CR, Conrads TP, Veenstra TD, Sharpless KB, Dawson PE, Hudson BG. A sulfilimine bond identified in collagen IV. Science. 2009;325:1230–1234. doi: 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel BE, Hedgecock EM. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development. 2001;128:883–894. doi: 10.1242/dev.128.6.883. [DOI] [PubMed] [Google Scholar]
- Wang H, Lacoche S, Huang L, Xue B, Muthuswamy SK. Rotational motion during three-dimensional morphogenesis of mammary epithelial acini relates to laminin matrix assembly. Proc. Natl. Acad. Sci. USA. 2013;110:163–168. doi: 10.1073/pnas.1201141110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011;3:a004911. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Ruben GC. Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. J. Cell Biol. 1987;105:2559–2568. doi: 10.1083/jcb.105.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Wadsworth WG. Assembly and tissue functions of early embryonic laminins and netrins. Curr. Opin. Cell Biol. 2004;16:572–579. doi: 10.1016/j.ceb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Cheng YS, Colognato H. Laminin forms an independent network in basement membranes. J. Cell Biol. 1992;117:1119–1133. doi: 10.1083/jcb.117.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]