Abstract
The epithelial-to-mesenchymal transition (EMT) is important for the formation of migratory neural crest cells during development and is co-opted in human diseases such as cancer metastasis. Chick premigratory cranial neural crest cells lose intercellular contacts, mediated in part by Cadherin-6B (Cad6B), migrate extensively, and later form a variety of adult derivatives. Importantly, modulation of Cad6B is crucial for proper neural crest cell EMT. Although Cad6B possesses a long half-life, it is rapidly lost from premigratory neural crest cell membranes, suggesting the existence of post-translational mechanisms during EMT. We have identified a motif in the Cad6B cytoplasmic tail that enhances Cad6B internalization and reduces the stability of Cad6B upon its mutation. Furthermore, we demonstrate for the first time that Cad6B is removed from premigratory neural crest cells through cell surface internalization events that include clathrin-mediated endocytosis and macropinocytosis. Both of these processes are dependent upon the function of dynamin, and inhibition of Cad6B internalization abrogates neural crest cell EMT and migration. Collectively, our findings reveal the significance of post-translational events in controlling cadherins during neural crest cell EMT and migration.
KEY WORDS: Cadherin, EMT, Neural crest, Macropinocytosis, Endocytosis
INTRODUCTION
Neural crest cells are a multipotent cell population arising at the border of the neural and non-neural ectoderm. Initially immotile in the dorsal region of the chick neural tube and termed premigratory neural crest cells, these cells undergo an epithelial-to-mesenchymal transition (EMT) to emerge from the neural tube. Migratory neural crest cells later differentiate into a variety of specialized adult derivatives such as the neurons and glia of peripheral nervous system, craniofacial tissues, portions of the heart and melanocytes (Hall, 2009; Dupin and Le Douarin, 2014). EMT plays a central role not only during normal embryonic development and adult homeostasis, but also in pathological conditions such as cancer metastasis and fibrosis (Thiery et al., 2009; Nieto, 2011). EMT requires a decrease in cell–cell adhesion, loss of apicobasal polarity, upregulation of mesenchymal markers and, finally, initiation of migration (Hay, 1995; Nieto, 2011). As such, neural crest cells are a popular model to study EMT because they provide a relevant, in vivo system in which to examine molecular mechanisms underlying EMT and migration that are directly translatable to aberrant EMTs occurring during human disease (Hay, 1995; Theveneau and Mayor, 2012; Kulesa et al., 2013).
Chick premigratory cranial neural crest cells express several cell adhesion molecules, including those of adherens and tight junctions (Nakagawa and Takeichi, 1995; Coles et al., 2007; Wu et al., 2011; Dady et al., 2012; Fishwick et al., 2012). Many of these proteins are undetectable upon initiation of EMT and early migration, suggesting that their downregulation is important (Nakagawa and Takeichi, 1995; Coles et al., 2007; Wu et al., 2011; Dady et al., 2012; Fishwick et al., 2012). Cadherins are central components of adherens junctions, and, along with nectin and afadins, form the ‘adhesion belt’ through interactions with circumferential F-actin, linking cells into a continuous sheet and separating the apical and basolateral membranes (Farquhar and Palade, 1963; Takai et al., 2008; Meng and Takeichi, 2009). Chick premigratory cranial neural crest cells express at least three cadherins: Cadherin-6B (Cad6B), N-cadherin and E-cadherin (Hatta and Takeichi, 1986; Duband et al., 1988; Nakagawa and Takeichi, 1995; Nakagawa and Takeichi, 1998; Dady et al., 2012). Expression of E-cadherin is high in prospective neural crest cells prior to neurulation, but as neurulation progresses, E-cadherin is gradually reduced and only retained until early stages of neural crest cell delamination. N-cadherin protein, however, is expressed during neurulation but is lost before EMT in premigratory cranial neural crest cells (Dady et al., 2012; Rogers et al., 2013). In contrast to E-cadherin, Cad6B is uniquely restricted to the premigratory cranial neural crest cell population. Cad6B protein is observed in the neural folds, gradually increases as premigratory neural crest cells prepare for EMT, and is completely downregulated as neural crest cells undergo EMT and migrate (Nakagawa and Takeichi, 1995; Nakagawa and Takeichi, 1998; Taneyhill, 2008). A reduction in Cad6B is crucial for the emergence of cranial neural crest cells from the neural tube, as Cad6B overexpression or knockdown inhibits or enhances this process, respectively (Coles et al., 2007).
Cadherins are removed from cellular plasma membranes during EMT through multiple post-translational mechanisms, including proteolytic processing and endocytosis (McCusker and Alfandari, 2009; Ulrich and Heisenberg, 2009; Kowalczyk and Nanes, 2012). Upon endocytosis, cadherins are either recycled back to the plasma membrane (Le et al., 1999; Classen et al., 2005; Desclozeaux et al., 2008) or degraded in lysosomes (Xiao et al., 2003b; Palacios et al., 2005). Cadherins can be internalized through clathrin-dependent and -independent endocytosis (Le et al., 1999; Akhtar and Hotchin, 2001; Paterson et al., 2003; Bryant et al., 2005; Palacios et al., 2005; Xiao et al., 2005; Bryant et al., 2007; Toyoshima et al., 2007). Indeed, the cytoplasmic domain of several cadherins harbors motifs that have been demonstrated to regulate clathrin-mediated endocytosis (Miyashita and Ozawa, 2007b; Chiasson et al., 2009; Ishiyama et al., 2010; Nanes et al., 2012). In addition to endocytosis, macropinocytosis, in which whole adherens junctions are internalized, also regulates cell surface cadherin levels (Paterson et al., 2003; Bryant et al., 2007; Sharma and Henderson, 2007; Solis et al., 2012). Furthermore, both clathrin-mediated endocytosis and macropinocytosis can rely upon dynamin for vesicle scission from the plasma membrane (Jarrett et al., 2002; Orth et al., 2002; Palacios et al., 2002; Cao et al., 2007).
We recently showed that ADAM-mediated proteolysis of Cad6B is crucial to remove Cad6B protein from the plasma membrane of premigratory cranial neural crest cells and facilitate EMT (Schiffmacher et al., 2014). In this study, we explored whether endocytosis plays an additional role during cranial neural crest cell EMT. We now show for the first time that premigratory cranial neural crest cells internalize Cad6B during EMT through clathrin-mediated endocytosis and macropinocytosis, the latter of which likely involves removal of whole adherens junctions from the plasma membrane. Furthermore, both of these processes depend upon the function of dynamin, and loss of Cad6B internalization prevents neural crest cell EMT and migration. Taken together, our results highlight a crucial role for cadherin internalization during an in vivo EMT in cranial neural crest cells.
RESULTS
Cad6B undergoes internalization and localizes to the cytoplasm in vitro, in vivo and ex vivo
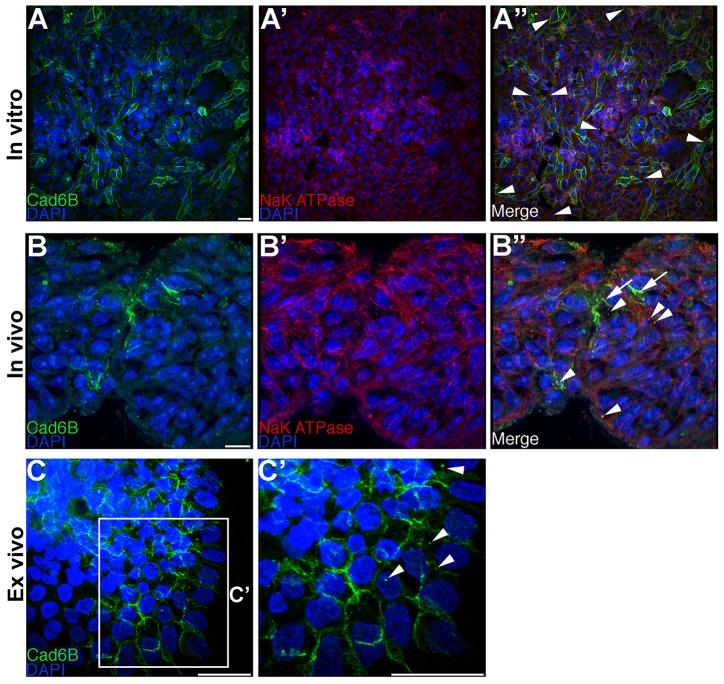
To explore the possibility that Cad6B might undergo internalization by endocytosis, FlpInCHO-Cad6B cell lines were generated that stably express wild-type Cad6B [tagged with a hemagglutinin (HA) epitope at the C-terminus] from a single genomic locus (FlpIn-wtC6B) (Schiffmacher et al., 2014). This approach was taken to minimize any deleterious effects that might occur owing to Cad6B overexpression (Levenberg et al., 1999; Bryant et al., 2005), and CHO cells were chosen because they do not express any cadherins (Hong et al., 2010). We first determined whether Cad6B undergoes basal levels of internalization in these cells by performing indirect immunofluorescence using antibodies that recognize the N-terminal extracellular domain of Cad6B (NT-6B) and Na+K+ ATPase, a marker of the plasma membrane (Van Dyke, 2004) (other antibodies raised to proteins marking the endocytic or lysosomal compartments did not work on chick tissue, data not shown). Because Na+K+ ATPase plays an important role in the acidification of endosomes (Cain et al., 1989; Fuchs et al., 1989; Feldmann et al., 2007) and localizes to Rab5-positive endosomes (Feldmann et al., 2007), we reasoned that cytoplasm-localized Na+K+ ATPase could serve as a marker for early endosomes. Through confocal microscopy analysis of Z-stack images, we observed Cad6B on the plasma membrane and in the cytoplasm in apparent puncta, where it colocalized with Na+K+ ATPase (Fig. 1A–A″, arrowheads). These results suggest that Cad6B undergoes internalization and localizes to the cytoplasm in this cell line. To ascertain whether internalization plays a role in the post-translational downregulation of Cad6B in vivo, we performed immunohistochemistry for Cad6B and Na+K+ ATPase on 6-somite-stage (ss) and 7-ss embryos, when premigratory cranial neural crest cells begin to reduce Cad6B levels during EMT (Taneyhill et al., 2007; Schiffmacher et al., 2014). In addition to Cad6B marking cell membranes (Fig. 1B–B″, arrows), we observed Cad6B in cytoplasmic puncta colocalizing with Na+K+ATPase in premigratory cranial neural crest cells (Fig. 1B″, arrowheads), pointing to the possibility that Cad6B is internalized in these precursors prior to and/or during EMT. To address this further, we conducted cranial neural crest cell explant assays (Taneyhill et al., 2007) and examined Cad6B in neural crest cells undergoing EMT ex vivo in culture. Immunostaining for Cad6B revealed several puncta in the cytosol of emerging neural crest cells, corroborating our in vivo and in vitro observations (Fig. 1C,C′, arrowheads).
Fig. 1.
Cad6B localizes to the cytoplasm. (A–A″) FlpIn-wtCad6B cells were fixed and immunostained for Cad6B (green) and Na+K+ ATPase (red). Panels represent the 3D composite of several Z-stack images acquired with the confocal. Cad6B colocalizes with several cytoplasmic puncta containing Na+K+ ATPase (A″, arrowheads). (B–B″) Embryos possessing neural crest cells initiating EMT were fixed and immunostained for Cad6B (green) and Na+K+ ATPase (red). Cad6B colocalizes with Na+K+ ATPase-positive puncta (B″, arrowheads), with membrane Cad6B denoted by arrows. (C,C′) Dorsal neural folds possessing premigratory neural crest cells were explanted, allowed to undergo EMT, fixed and immunostained for Cad6B. C′ is a higher magnification view of the boxed region in C. Cad6B localizes to the cytoplasm (C′, arrowheads). Scale bars: 10 µm.
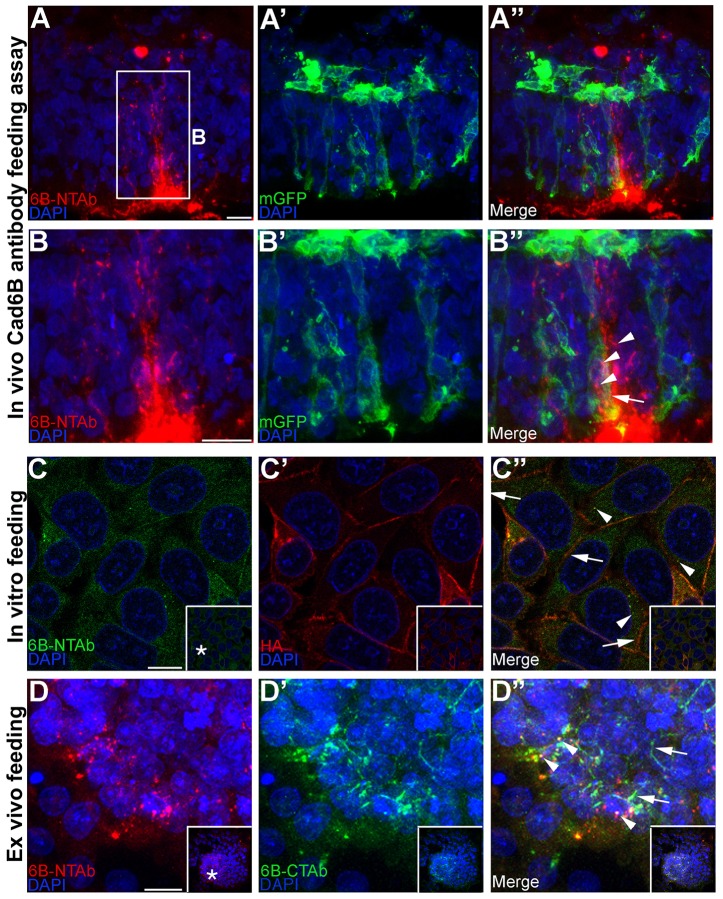
To determine whether the cytoplasmic puncta observed in vivo were of endocytic or exocytic nature, we performed Cad6B antibody feeding assays (Arancibia-Carcamo, 2006), in which we introduced the NT-6B antibody into the lumen of embryos electroporated with a construct encoding membrane-associated GFP (to label premigratory neural crest cell plasma membranes). Because the NT-6B antibody recognizes the lumen-exposed extracellular domain of Cad6B, the Cad6B antibody–antigen complex should be processed in the same way as endogenous Cad6B protein when premigratory cranial neural crest cells undergo EMT. Through confocal imaging, we noted Cad6B in several cytosolic puncta (Fig. 2B″, arrowheads) within the confines of a membrane-GFP-expressing delaminating neural crest cell (Fig. 2B″, arrow), implying that the puncta are likely to be of endocytic rather than exocytic nature. To eliminate the possibility that the binding of the NT-6B antibody was stimulating endocytosis, we performed the same antibody assay in vitro (Fig. 2C–C″) and ex vivo (Fig. 2D–D″). The plasma-membrane-bound Cad6B antibody–antigen complex (non-internalized fraction) was distinguished from the internalized fraction by washing the cells with a low pH buffer, which strips off any plasma-membrane-bound antibodies but does not disturb the internalized pool (Arancibia-Carcamo, 2006). Plasma-membrane-bound Cad6B was distinguished from the internalized Cad6B antibody–antigen complex by performing immunofluorescence for the HA tag at the C-terminal end of Cad6B. When the assay was performed at 4°C, most of the Cad6B antibody was stripped off, indicating the continued presence of Cad6B on the membrane (supplementary material Fig. S1A), without affecting membrane distribution of Cad6B, as shown by the HA immunoreactivity (supplementary material Fig. S1A″, arrows). Performing the assay at 37°C resulted in the presence of several internalized NT-6B–Cad6B cytoplasmic puncta (Fig. 2C″, arrowheads), but did not stimulate global endocytosis of Cad6B protein at 37°C, as indicated by the presence of retained HA (Cad6B) immunoreactivity on the plasma membrane (Fig. 2C″, arrows). The same assay was then performed with explants (supplementary material Fig. S1B–B″; Fig. 2D–D″). Here, the plasma-membrane-bound fraction of Cad6B was differentiated from the endocytosed Cad6B antibody–antigen complex with an antibody that recognizes the C-terminal domain of endogenous Cad6B (CT-6B). Explants remaining at 4°C that were subjected to the low pH wash had virtually all Cad6B antibody bound to plasma-membrane-bound Cad6B removed without affecting general Cad6B membrane distribution (supplementary material Fig. S1B′,B″, arrows). Incubating the explants at 37°C, however, led to the presence of several NT-6B–Cad6B cytosolic puncta (Fig. 2D″, arrowheads) and the persistence of some membrane-bound Cad6B (Fig. 2D″, arrows). These data suggest that addition of the NT-6B antibody does not stimulate significant internalization of Cad6B, and they corroborate our earlier observation that the puncta are likely endocytic rather than exocytic in nature. Taken together, these observations reveal that Cad6B undergoes internalization in cultured cells and in premigratory cranial neural crest cells in vivo and ex vivo.
Fig. 2.
Cad6B puncta are endocytic rather than exocytic in nature. (A–A″) Cad6B antibody recognizing the Cad6B extracellular domain (NT-6B) was introduced into the lumen of embryos electroporated with membrane-associated GFP, incubated to allow for EMT, fixed and immunostained with the NT-6B antibody (red) and GFP (green). (B–B″) High magnification image of the boxed region in A. Internalized NT-6B antibody–Cad6B complexes are seen in the cytoplasm (B″, arrowheads) within the confines of GFP-expressing plasma membrane (B″, arrow) in a delaminating neural crest cell. The NT-6B antibody was added to medium of FlpIn-wtCad6B cells (C–C″) and dorsal neural fold explants (D–D″), followed by incubation to allow for EMT, a low pH buffer wash, fixation and immunostaining for NT-6B (C,D) and HA (C′) or CT-6B (D′). Internalized NT-6B antibody–Cad6B complexes localize to the cytoplasm (C″,D″, arrowheads), and Cad6B is still observed on the plasma membrane (C″,D″, arrows). All images except C–C″ represent the 3D composite of several Z-stack images acquired with the confocal. Inset boxes in C–D″ show the original image, with the asterisks in C and D indicating the location of the higher magnification field in the main panels. Scale bars: 10 µm.
Cad6B possesses a functional endocytic motif in its cytoplasmic domain
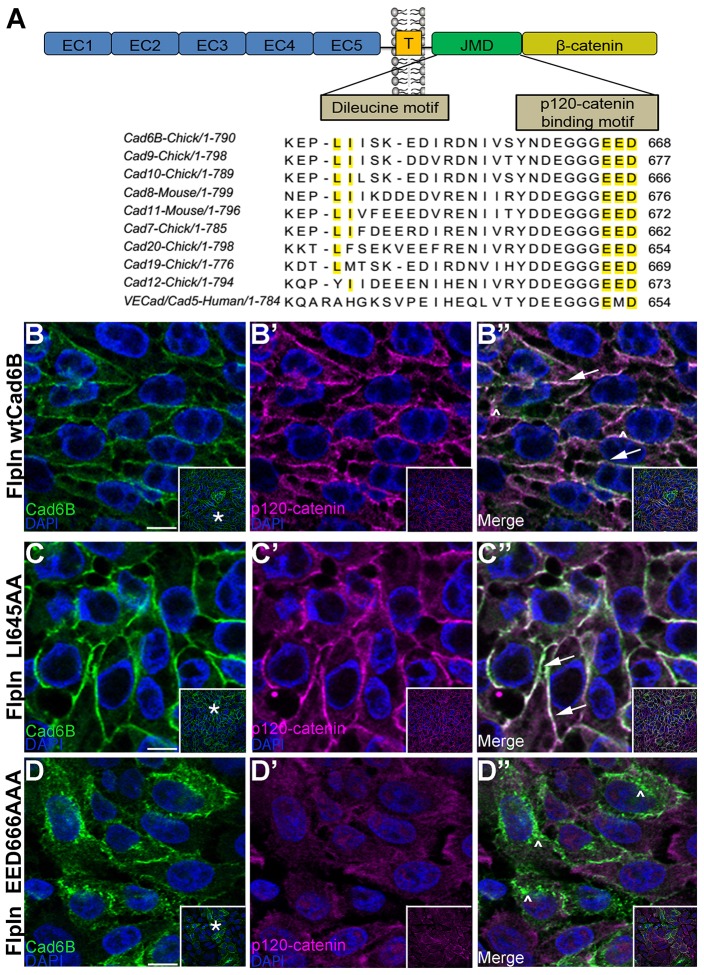
Several cadherins possess motifs within their C-terminal intracellular domains that regulate their internalization and degradation (Xiao et al., 2005; Miyashita and Ozawa, 2007a; Miyashita and Ozawa, 2007b; Tai et al., 2007; Hong et al., 2010; Kowalczyk and Nanes, 2012; Nanes et al., 2012). We compared the amino acid sequence of the cytoplasmic domain of Cad6B to that of several type II cadherins through the ClustalW algorithm (http://www.ebi.ac.uk/Tools/msa/clustalw2/), and in doing so identified two motifs that could potentially regulate Cad6B internalization (Fig. 3A, yellow highlight). Mutation of the dileucine motif (LI) has been shown to negatively influence endocytosis (Miyashita and Ozawa, 2007b; Hong et al., 2010), whereas changes in the p120-catenin binding motif (EED) can promote endocytosis owing to the resulting inability of p120-catenin to bind and prevent endocytosis (Ireton et al., 2002; Xiao et al., 2003a; Xiao et al., 2005; Miyashita and Ozawa, 2007b; Ishiyama et al., 2010; Nanes et al., 2012). To investigate the function of these two motifs, we performed site-directed mutagenesis to create two Cad6B mutant constructs, LI645AA and EED666AAA, and expressed each independently from a single genomic locus in the FlpIn-CHO cell lines as described above. We hypothesized that mutating the LI or EED residues would decrease or augment endocytosis, respectively. Immunofluorescence analysis with the NT-6B antibody qualitatively revealed that, compared to wild-type Cad6B (Fig. 3B–B″, arrows) and the LI645AA mutant (Fig. 3C–C″, arrows), the EED666AAA mutant localized predominantly to the cytoplasm, indicating that these residues might modulate Cad6B distribution (Fig. 3D–D″, carets). Furthermore, wild-type Cad6B and the LI mutant colocalize with p120-catenin at the cell membrane (Fig. 3B″,C″, arrows), whereas p120-catenin is observed diffusely throughout the cytoplasm in the EED mutant. Finally, we note intracellular puncta that are Cad6B-positive but p120-catenin-negative (Fig. 3B″,D″, carets).
Fig. 3.
Cad6B possesses putative endocytic motifs in its cytoplasmic domain. (A) A portion of the alignment of the Cad6B juxtamembrane domain with several type II cadherins. The dileucine and putative p120-catenin binding motifs are highlighted (yellow). EC, Extracellular domain; T, transmembrane domain; JMD, juxtamembrane domain. FlpIn cells expressing wtCad6B (B–B″), LI645AA (C–C″) and EED666AAA (D–D″) were fixed and immunostained for Cad6B (green) and p120-catenin (purple). Panels represent single confocal plane images. Arrows point to membrane-bound Cad6B and p120-catenin, and carets indicate Cad6B-positive, p120-catenin-negative cytoplasmic puncta. Inset boxes in B–D″ show the original image, with the asterisks in B,C,D indicating the location of the higher magnification field in the main panels. Scale bars: 10 µm.
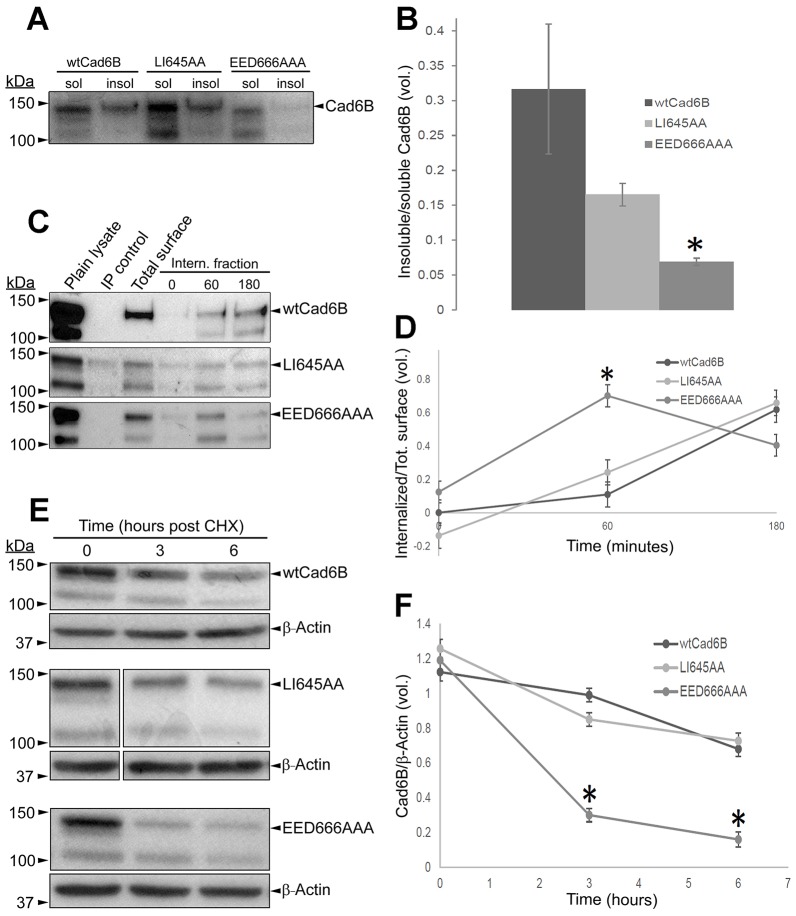
To evaluate the ability of these cells to form stable cytoskeletal-associated cell–cell junctions, we investigated the proportion of Triton-X-100-soluble and -insoluble Cad6B (Fig. 4A,B). We observed a statistically significant reduction in the proportion of actin-anchored Cad6B in the EED666AA mutant (P<0.05), suggesting that these cells are deficient in forming stable cell–cell adhesions. We then quantified the steady-state level of internalization of wild-type Cad6B and of the mutants through biotinylation assays. Although wild-type Cad6B and LI645AA did not show significant differences in endocytosis, the EED666AAA mutant underwent rapid endocytosis by 60 minutes post-biotinylation (Fig. 4C,D, P<0.05). Surprisingly, the levels of endocytosed Cad6B in the EED666AAA mutant decreased at 180 minutes post-biotinylation. We reasoned that the absence of these residues might negatively affect Cad6B stability, making it more susceptible to degradation and resulting in decreased levels of endocytosed protein at 180 minutes. To investigate this possibility, we inhibited de novo protein synthesis through cycloheximide treatment of cells expressing wild-type or mutant Cad6B and analyzed levels of Cad6B. Compared to wild-type Cad6B and LI645AA, the EED666AAA mutant underwent rapid reduction in the levels of Cad6B, substantiating our hypothesis (Fig. 4E,F, P<0.001). Collectively, these observations reveal that Cad6B undergoes endocytosis and possesses a functional motif that negatively regulates its endocytosis in vitro.
Fig. 4.
Cad6B possess a functional endocytic motif. (A) Soluble (sol) and insoluble (insol) fractions from FlpIn-wtCad6B, -LI645AA and -EED666AAA cells were subjected to SDS-PAGE. (B) Densitometric ratios of insoluble:soluble Cad6B from triplicate blots. (C) FlpIn cells expressing Cad6B constructs were surface-biotinylated and incubated at 37°C, followed by biotin immunoprecipitation (IP) with Streptavidin–agarose and SDS-PAGE. (D) Quantification of the internalized biotinylated fraction (internalized biotinylated protein/total surface biotinylated protein). (E) FlpIn cells expressing Cad6B constructs were treated with cycloheximide (CHX), lysed and subjected to SDS-PAGE. Irrelevant lanes between time 0 and the other two time points were cropped out of the LI645AA immunoblot. (F) Quantification of the densitometric ratios (Cad6B:actin) from E. Data show the mean±s.e.m.; *P<0.05 (B,D), *P<0.001 (F).
In vitro and in vivo internalization of Cad6B is mediated, in part, through clathrin-dependent endocytosis
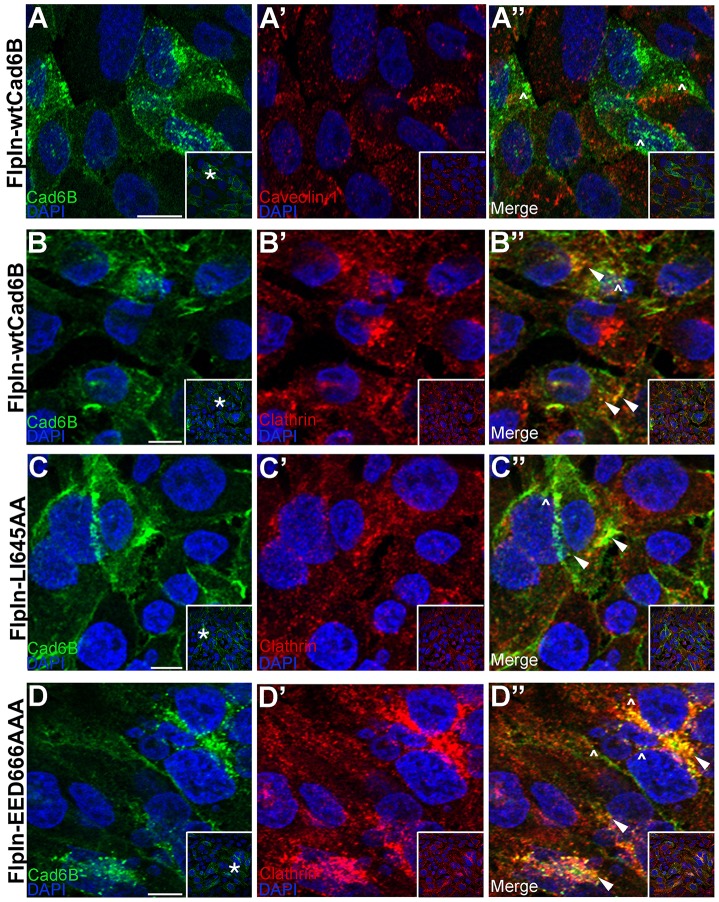
Our biochemical and immunostaining data suggest that Cad6B is internalized through endocytosis. Cadherins can be internalized through both clathrin-dependent and -independent endocytic pathways (Le et al., 1999; Akhtar and Hotchin, 2001; Paterson et al., 2003; Bryant et al., 2005; Palacios et al., 2005; Xiao et al., 2005; Bryant et al., 2007; Toyoshima et al., 2007). To determine through which pathway Cad6B is endocytosed, we performed immunohistochemistry to document the distribution of Cad6B with respect to caveolin-1 and clathrin. We noted that intracellular Cad6B did not colocalize with caveolin-1 (Fig. 5A–A″, carets) but partially colocalized with clathrin in vitro (Fig. 5B–B″, arrowheads). This colocalization was corroborated by examining Cad6B and clathrin subcellular distribution in the LI645AA (Fig. 5C–C″, arrowheads) and EED666AAA (Fig. 5D–D″, arrowheads) mutant cell lines. Intriguingly, though, all cell lines showed some intracellular puncta that were Cad6B-positive but devoid of clathrin immunoreactivity (Fig. 5B″,C″,D″, carets).
Fig. 5.
Cad6B does not colocalize with caveolin-1 but partially colocalizes with clathrin in vitro. (A–A″) FlpInwtCad6B cells were fixed and immunostained for Cad6B (green) and caveolin-1 (red). Carets show absence of Cad6B and caveolin-1 colocalization. FlpIn cells expressing wtCad6B (B–B″), LI645AA (C–C″) and EED666AAA (D–D″) were fixed, and immunostaining was performed for Cad6B (green) and clathrin (red). Panels represent single confocal plane images. Arrowheads in B″,C″,D″ show Cad6B and clathrin colocalization, and carets point to Cad6B puncta not colocalizing with clathrin. Inset boxes show the original image, with the asterisks indicating the location of the higher magnification field in the main panels. Scale bars: 10 µm.
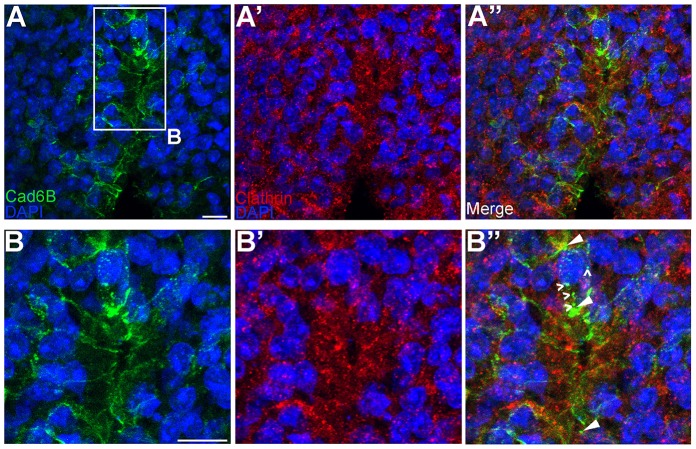
To confirm these findings in vivo, embryos at stages where premigratory cranial neural crest cells are undergoing EMT were immunostained for Cad6B and clathrin (Fig. 6). As observed in vitro, Cad6B colocalized with clathrin in vivo (Fig. 6B–B″, arrowheads). Once again, though, we noted some Cad6B-positive puncta that lacked clathrin (Fig. 6B″, carets). Taken together, these data indicate that Cad6B undergoes clathrin-mediated endocytosis in vitro and in vivo during EMT. The presence of clathrin-negative, Cad6B-positive puncta, however, is suggestive of additional mechanism(s) by which Cad6B could be internalized.
Fig. 6.
Cad6B partially colocalizes with clathrin in vivo. (A–A″) Embryos in which neural crest cells are actively undergoing EMT were fixed and immunostained for Cad6B (green) and clathrin (red). (B–B″) A higher magnification view of the boxed region in A. Panels represent the 3D composite of several Z-stack images acquired with the confocal. Arrowheads in B″ indicate Cad6B and clathrin colocalization, and carets show Cad6B-positive, clathrin-negative puncta. Scale bars: 10 µm.
Cad6B undergoes dynamin-dependent, clathrin-mediated endocytosis and macropinocytosis in neural crest cells undergoing EMT
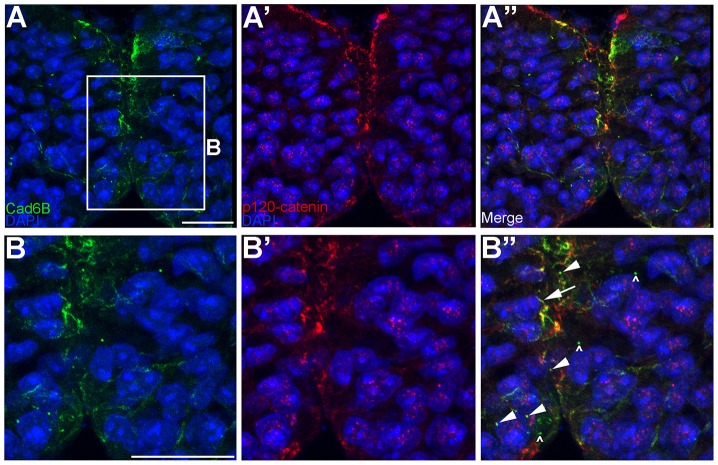
Our cell line data revealed that Cad6B colocalizes with p120-catenin in some intracellular puncta (Fig. 3B″,C″,D″, arrowheads). To corroborate this in vivo, we performed immunohistochemistry for Cad6B and p120-catenin on chick cranial transverse sections. Our results showed that Cad6B and p120-catenin colocalized to cytoplasmic puncta or vesicles and at the plasma membrane (Fig. 7, arrowheads, arrow, respectively; p120-catenin-negative, Cad6B-positive puncta are indicated by carets). Endocytosis of cadherins, however, relies upon the removal of the p120-catenin protein (Davis et al., 2003; Xiao et al., 2003a; Hoshino et al., 2005). These colocalization data suggest that, in some instances, whole complexes containing Cad6B and catenins, rather than individual Cad6B molecules, could be internalized through a process such as macropinocytosis. Collectively, these results indicate the existence of at least two mechanisms (clathrin-mediated endocytosis and macropinocytosis) by which premigratory cranial neural crest cells reduce levels of surface Cad6B.
Fig. 7.
Cad6B partially colocalizes with p120-catenin in cytosolic puncta in vivo. (A–A″) Embryos in which neural crest cells are actively undergoing EMT were fixed and immunostained for Cad6B (green) and p120-catenin (red). (B–B″) A higher magnification view of the boxed region in A. Panels represent the 3D composite of several Z-stack images acquired with the confocal. Arrowheads in B″ point to colocalized Cad6B and p120-catenin in the cytosol, the arrow shows colocalized Cad6B and p120-catenin at the membrane and carets show Cad6B-positive, p120-catenin-negative puncta. Scale bars: 10 µm.
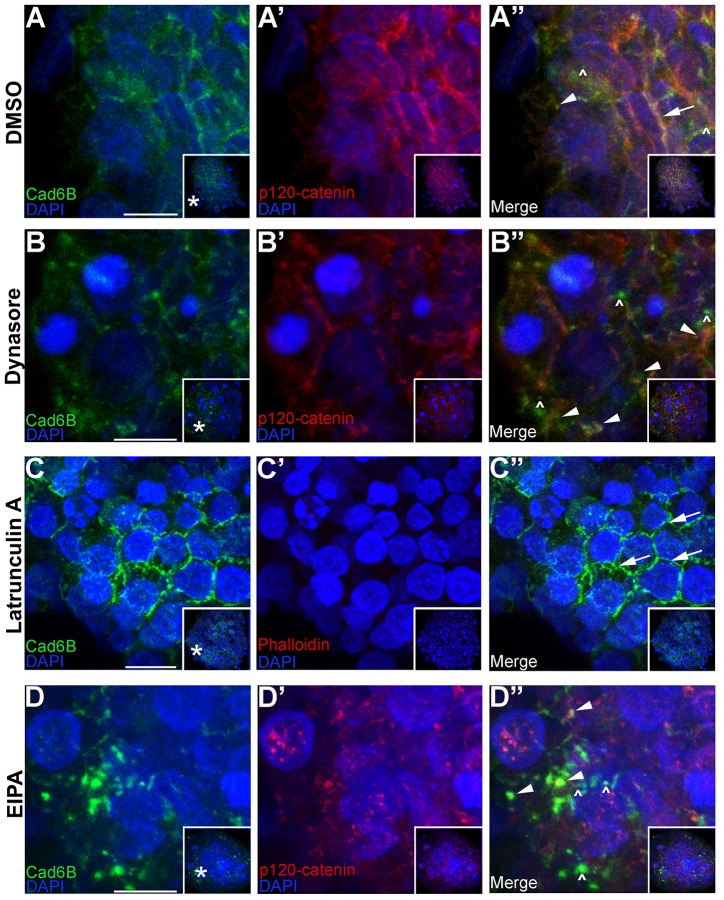
To test these hypothesized mechanisms, we pharmacologically inhibited endocytosis and macropinocytosis in neural crest cell explants. We first assessed a role for dynamin by treating explants with dynasore, a dynamin inhibitor (Macia et al., 2006). Our results show that, compared to control explants (Fig. 8A–A″, arrowhead, arrow indicates Cad6B membrane staining), dynamin inhibition blocked neural crest cell EMT and migration (in 23/24 explants), as evidenced by brightfield images and phalloidin staining (arrows in supplementary material Fig. S2, see supplementary material Fig. S2B,B′; Coles et al., 2007; Taneyhill et al., 2007; Jhingory et al., 2010). Control explants underwent EMT and migrated normally (supplementary material Fig. S2A,A′, 18/21 explants examined). Moreover, dynasore treatment led to a statistically significant 2.2-fold increase in the accumulation of multiple, large intracellular and membrane-bound Cad6B puncta of ≥2 µm compared to control explants (Fig. 8B–B″, arrowheads; supplementary material Fig. S2G). To determine a function for macropinocytosis in internalizing Cad6B during neural crest cell EMT, we prevented macropinosome formation by blocking actin polymerization (Mercer and Helenius, 2009) using latrunculin A (Spector et al., 1983; Mercer and Helenius, 2009). Latrunculin A treatment of explants eliminated actin polymerization as evidenced by the absence of phalloidin staining (Fig. 8C′) compared to control explants (supplementary material Fig. S2A′). Importantly, addition of latrunculin A severely decreased the presence or number of Cad6B puncta (Fig. 8C–C″), with the majority of Cad6B still localized to the plasma membrane (Fig. 8C″, arrows), in contrast to Cad6B distribution observed in control explants (Fig. 8A–A″, arrows, arrowheads). Furthermore, latrunculin A treatment inhibited EMT (supplementary material Fig. S2C,C′, 12/12 explants examined) compared to normal EMT and migration in control explants. We next tested the sensitivity of Cad6B internalization to the amiloride EIPA, which is a macropinocytosis inhibitor (Mercer and Helenius, 2008; Koivusalo et al., 2010). EIPA treatment resulted in the appearance of multiple, large Cad6B cytoplasmic puncta (2.5-fold increase in the number of large Cad6B puncta; supplementary material Fig. S2G) colocalizing with p120-catenin (Fig. 8D–D″, arrowheads) and abolished EMT (supplementary material Fig. S2D,D′; 18/23 explants showed an absence of EMT and migration compared to normal EMT and migration in 18/21 control explants). Finally, the size of the accumulated puncta (1.5–3 µm, ∼700 puncta measured) is also indicative of macropinocytosis (Swanson, 1989; Hewlett et al., 1994; Schnatwinkel et al., 2004).
Fig. 8.
Cad6B undergoes dynamin-dependent macropinocytosis. Vehicle (DMSO, A–A″), dynasore (B–B″), latrunculin A (C–C″) or EIPA (D–D″) was added to the explant medium. Explants were incubated to allow for EMT, fixed and immunostained for Cad6B (green) and p120-catenin (red; A′,A″,B′,B″,D′,D″) or stained with phalloidin (red; C′,C″). The arrow and arrowhead in A″ represent Cad6B and p120-catenin co-staining at the membrane and cytoplasm, respectively. Arrowheads in B″,D″ show large Cad6B and p120-catenin double-positive cytoplasmic puncta. Arrows in C″ indicate membrane-associated Cad6B. Carets in A″,B″,D″ point to Cad6B-positive, p120-catenin-negative puncta. Panels represent the 3D composite of several Z-stack images acquired with the confocal. Insets show the original image, with the asterisks in A–D indicating the location of the higher magnification field in the main panels. Scale bars: 10 µm.
To rule out the possibility of non-specific effects of dynasore and EIPA on Cad6B and EMT, we used two additional chemicals that block targets of dynasore and EIPA – dansylcadaverine, a clathrin-specific inhibitor (for dynasore) (Davies et al., 1980; Bradley et al., 1993; Wang and Liu, 2003), and NSC23766, a Rac1-specific inhibitor (for EIPA) (Gao et al., 2004). Indeed, addition of these inhibitors partially recapitulated the phenotype observed upon treatment of explants with either EIPA or dynasore, including a reduction in EMT in the case of NSC23766 (14/14 explants; compare supplementary material Fig. S2D,D′ and F,F′), whereas the majority of explants (12/15) underwent normal EMT in the presence of dansylcadaverine (compare supplementary material Fig. S2B,B′ and E,E′). In addition, treatment with dansylcadaverine or NSC23766 caused accumulation of Cad6B puncta (supplementary material Fig. S3A–B′, arrows), although this was not statistically significant (supplementary material Fig. S2G). These data strongly suggest that Cad6B undergoes macropinocytosis and clathrin-dependent endocytosis in premigratory neural crest cells.
We then examined the distribution of p120-catenin with respect to Cad6B upon inhibitor treatment. Surprisingly, many of the Cad6B cytoplasmic puncta observed upon dynasore (Fig. 8B″, carets) or EIPA (Fig. 8D″, carets) treatment were p120-catenin-negative, in contrast to our expectation that these would possess both Cad6B and p120-catenin. To investigate whether these observations were due to extraneous inhibitor effects, we examined Cad6B and p120-catenin colocalization in untreated cells, explants and embryos. We noticed in several instances that Cad6B did not colocalize with p120-catenin in puncta in untreated cells (Fig. 3B″,D″, carets), explants (Fig. 8A″, carets) and embryos (Fig. 7B″, carets), implying that these are either endocytic vesicles or that p120-catenin dissociates later from the puncta post-macropinocytosis. Taken together, our results reveal that Cad6B is internalized during cranial neural crest cell EMT through dynamin-dependent, clathrin-mediated endocytosis and macropinocytosis, with the absence of internalization severely impacting the ability of cranial neural crest cells to undergo EMT and migrate.
DISCUSSION
Regulation of cadherin proteins is crucial during development and disease (Lim and Thiery, 2012; Gheldof, 2013). In this study, we provide the first evidence for dynamin-dependent cadherin endocytosis and macropinocytosis during an in vivo EMT. By following the processing of endogenous Cad6B protein using an antibody, we demonstrate that Cad6B undergoes internalization in premigratory and early migratory neural crest cells. Interestingly, we note no differences in the degree of endocytosis during specific stages of EMT, and this internalization process might be conserved, as we observe internalized Cad6B in both chick hindbrain and trunk premigratory neural crest cells (supplementary material Fig. S4). We further show that Cad6B possesses a functional motif in its cytoplasmic domain that negatively regulates Cad6B endocytosis, likely due to the presence of bound p120-catenin. Partial colocalization of internalized Cad6B with clathrin suggests that Cad6B undergoes endocytosis through a clathrin-mediated pathway, and retention of Cad6B on the plasma membrane in large puncta or vesicles upon addition of a dynamin inhibitor further substantiates this observation. Interestingly, many intracellular puncta are, in fact, devoid of clathrin but instead are double-positive for Cad6B and p120-catenin, suggesting that whole adherens junctions are internalized through an additional mechanism such as macropinocytosis. A reduction in Cad6B puncta in neural crest cells treated with latrunculin A confirms this hypothesis. In addition, results from EIPA treatment lend further credence to a role for macropinocytosis in Cad6B internalization, a process that also depends upon dynamin. Importantly, treatment with dynasore, latrunculin A or EIPA blocked neural crest cell EMT and migration, suggesting that downregulation of Cad6B through endocytosis and macropinocytosis is crucial for EMT. Nevertheless, the use of such chemical inhibitors could have broad-spectrum effects on cell physiology and function (reviewed in Ivanov, 2008) and could affect Cad6B distribution and/or EMT. For example, EIPA can alter morphology and the intracellular distribution of early and late endosomes in HeLa cells (Fretz et al., 2006). Dynasore, which affects clathrin-mediated endocytosis, can also have minor effects on caveolar endocytic pathways (Macia et al., 2006). To rule out any non-specific effects with regards to these compounds, Cad6B localization was investigated in the presence of more specific inhibitors (dansylcadaverine for clathrin, NSC23766 for Rac1). The use of these inhibitors partially recapitulated the Cad6B accumulation phenotype noted in the presence of dynasore and EIPA, although the effects on EMT varied. Thus, the accumulation of large Cad6B puncta, even under these more specific inhibitor conditions, validates the phenotypes observed after EIPA and dynasore treatment. Lastly, these internalization processes appear to be specific to Cad6B, as no effects on N-cadherin were observed, owing to the absence of N-cadherin in cranial neural crest cells during EMT (Dady et al., 2012; Rogers et al., 2013). Therefore, a combination of endocytosis and macropinocytosis, together with proteolysis (Schiffmacher et al., 2014), might be required to fully remove Cad6B from the plasma membrane of premigratory cranial neural crest cells undergoing EMT.
The mechanism(s) by which Cad6B is removed from premigratory neural crest cell membranes has broad implications for cell biology, tissue homeostasis and pathogenic conditions resulting from deregulated cadherins (Gheldof, 2013). We have previously shown that one mechanism by which Cad6B is lost from premigratory cranial neural crest cells is proteolytic cleavage (Schiffmacher et al., 2014). Nevertheless, we still observed Cad6B localized to cytoplasmic puncta in premigratory and early migratory cranial neural crest cells, suggestive of a potential role for Cad6B internalization as neural crest cells undergo EMT. The possibility that internalization of Cad6B plays an important role during EMT is not without precedence, as cadherins undergo endocytosis during a variety of other cell biological processes (Cavey and Lecuit, 2009; Kowalczyk and Nanes, 2012; Collinet and Lecuit, 2013), but cadherin internalization during EMT has not been reported until now.
Cadherins possess endocytosis motifs in their cytoplasmic domain that regulate their plasma-membrane-bound state (Xiao et al., 2005; Miyashita and Ozawa, 2007a; Miyashita and Ozawa, 2007b; Tai et al., 2007; Hong et al., 2010; Ishiyama et al., 2010; Kowalczyk and Nanes, 2012; Nanes et al., 2012). Our experiments reveal that Cad6B possesses a putative p120-catenin binding motif in its cytoplasmic domain that functions to negatively regulate Cad6B endocytosis. p120-catenin modulates cadherin endocytosis by ‘masking’ the dileucine motif (Miyashita and Ozawa, 2007b; Ishiyama et al., 2010; Nanes et al., 2012). Dissociation of p120-catenin from the cadherin exposes the dileucine motif, which in turn is recognized by cytoplasmic adaptor proteins of the clathrin-mediated endocytic pathway and leads to cadherin endocytosis (Miyashita and Ozawa, 2007b; Ishiyama et al., 2010; Nanes et al., 2012). Mutating this putative p120-catenin binding motif to alanine enhanced endocytosis of Cad6B, in agreement with results for E-cadherin (Xiao et al., 2003a; Miyashita and Ozawa, 2007b). Furthermore, this mutant was deficient in forming stable cell–cell adhesions and possessed reduced overall stability. Enhanced endocytosis could translate into fewer opportunities for this mutant Cad6B to form stable interactions with the cytoskeleton, further augmenting its endocytosis and decreasing its stability, as observed for other cadherins in vitro (Collinet and Lecuit, 2013). Alternatively, association with p120-catenin could be required for cadherins to interact with the cytoskeleton (Hoshino et al., 2005), which might lead to similar effects upon the absence of p120-catenin binding.
The dileucine motif in Cad6B, which is actually an LI motif and corresponds to the consensus (DE)XXXL(LI) or the DXXLL, is also crucial for endocytosis (Bonifacino and Traub, 2003). This motif (normally masked by p120-catenin) binds several proteins required for clathrin-mediated endocytosis (Ishiyama et al., 2010), and thus the absence of this motif was hypothesized to negatively affect Cad6B endocytosis. Mutating the LI residues in Cad6B, however, did not significantly affect Cad6B internalization and its association with the cytoskeleton. This is in contrast to what has been observed with E-cadherin (Miyashita and Ozawa, 2007a) and N-cadherin (Tai et al., 2007), and could be due to the lack of acidic residues that usually precede this dileucine motif in these other cadherins (Bonifacino and Traub, 2003). Furthermore, the lysine at position 748 in E-cadherin controls E-cadherin endocytosis in vitro, and complete loss of endocytosis was only observed when both the dileucine motif and lysine residue were mutated (Hong et al., 2010). Because Cad6B has a conserved lysine in a similar position, mutating it along with the LI motif might be necessary to block Cad6B endocytosis, and thus colocalization with clathrin should serve as indirect evidence for the role of the LI motif and lysine during Cad6B endocytosis. Nonetheless, many intracellular puncta observed in vivo and in vitro were not double-positive for clathrin and Cad6B, implying additional mechanisms of Cad6B internalization.
Our results now reveal that, besides clathrin-mediated endocytosis, Cad6B undergoes internalization as part of whole adherens junctions through macropinocytosis. The use of macropinocytosis to remove cadherins from membranes has been shown previously in vitro for E-cadherin molecules not actively engaged in cell–cell adhesion (Bryant et al., 2007) and for N-cadherin at the leading edge of migratory cells (Sharma and Henderson, 2007), but to our knowledge has never been documented during an in vivo EMT. As such, our work is the first to report the significance of cadherin internalization during neural crest cell EMT. Importantly, this study raises additional questions regarding the fate of internalized cadherins and catenins during neural crest cell EMT. For example, the absence of p120-catenin in some intracellular puncta might be indicative of the need to release p120-catenin so that it can perform other signaling roles that impinge upon neural crest cell EMT (Bellovin et al., 2005; Yanagisawa and Anastasiadis, 2006; Cheung et al., 2010; Johnson et al., 2010). Furthermore, it is intriguing that cranial neural crest cells employ multiple mechanisms (internalization and proteolysis) to reduce surface Cad6B levels during EMT. One possibility is that these mechanisms are used in different subpopulations of cranial neural crest cells (Lee et al., 2013; Ridenour et al., 2014). Alternatively, this rapid reduction in Cad6B protein might be necessary to release catenins so they can signal (i.e. transcription) and/or form complexes with other mesenchymal cadherins to mediate migration. Finally, en masse removal of Cad6B from premigratory cranial neural crest cells could be occurring simply to eliminate physical barriers (e.g. adherens junctions) that initially hold premigratory neural crest cells together prior to their delamination and EMT.
In summary, our results provide crucial insight into additional molecular mechanisms underlying the post-translational downregulation of cadherins in premigratory cranial neural crest cells. Our studies are the first to delineate the importance of Cad6B internalization during an in vivo EMT in the neural crest. Notably, this internalization of Cad6B occurs through at least two mechanisms, clathrin-mediated endocytosis and macropinocytosis, both of which are dynamin-dependent processes. Furthermore, Cad6B internalization is crucial for cranial neural crest cells to undergo EMT and properly migrate. Collectively, our work highlights how cranial neural crest cells employ multiple means to effectively clear this important cadherin from their plasma membranes and initiate EMT in the developing vertebrate embryo.
MATERIALS AND METHODS
Chick embryos
Fertilized chicken eggs were obtained from B & E Farms (York, PA) and incubated at 38°C in humidified incubators (EggCartons.com, Manchaug, MA). Embryos were staged by the number of pairs of somites (somite stage, ss) according to Hamburger–Hamilton (Hamburger and Hamilton, 1992). All animal experiments were performed according to approved guidelines.
Neural crest cell explant assays
Neural crest cell explants were prepared as described previously (Coles et al., 2007; Taneyhill et al., 2007; Jhingory et al., 2010). Briefly, dorsal neural folds (containing premigratory neural crest cells) from chick embryo midbrains were dissected out into PB-1 standard medium and placed into chamber slides coated with a 1∶100 dilution of poly-L-lysine (P5899, Sigma, St Louis, MO) and fibronectin (356008, Corning, NY). Cultures were incubated in serum-free Dulbecco's Modified Eagle's Medium (DMEM, 10-013-CV, CellGro, Manassas, VA) supplemented with a 1∶100 dilution of N-2 (17502-048, Life Technologies, Carlsbad, CA) at 37°C for varying times. For inhibitor assays, tissue was directly explanted into chamber slides containing 0.5% (v/v) DMSO, dynasore (100 µM; 304448-55-3, Adipogen, San Diego, CA), latrunculin A (300 nM; L5163, Sigma-Aldrich, St Louis, MO), EIPA (50 µM; 3378, Tocris Bioscience, Sunnyvale, CA), dansylcadaverine (100 µM; sc-214851, Santa Cruz Biotechnology, Santa Cruz, CA) and NSC23766 (150 µM; sc-204823, Santa Cruz Biotechnology, Santa Cruz, CA). Explants were incubated for 3.5 hours at 37°C to allow for neural crest cells to undergo EMT, followed by fixation and immunostaining (described below). Putative macropinocytic vesicles in explants were visualized in three dimensions using the Zen software (Zeiss), with vesicle length measured at its greatest extent using the software Measurement tool.
Cloning of Cad6B mutants
Full-length Cad6B cloned in pCIG (Coles et al., 2007) was subcloned into pCI-H2B-RFP (a gift from Dr. Marianne Bronner; California Institute of Technology, Pasadena, CA) along with a hemagglutinin (HA) epitope tag at its C-terminus through standard cloning procedures. To create endocytic mutants, Cad6B in pCI-H2B-RFP was mutagenized using the QuikChange II XL Site-Directed Mutagenesis Kit (200521, Agilent Technologies, Santa Clara, CA). All constructs were sequenced to ensure sequence accuracy.
FlpIn cell culture and reagents
CHO cells stably expressing a single integrated copy of wild-type and various endocytic mutants of Cad6B were created as described previously using the FlpIn system (K6010-02, R75807, Life Technologies, Carlsbad, CA; Schiffmacher et al., 2014). Briefly, HA-tagged wild-type or mutant Cad6B was directionally subcloned from pCI-H2B-RFP (above) into the pcDNA5/FRT vector and co-transfected with pOG44 into FlpIn-CHO cells. After transfection, cells were trypsinized and plated at 20–25% confluency in F12 medium supplemented with 1 mM L-glutamine and 600 µg/ml hygromycin for selection of positive transfectants (cells were subsequently grown in this medium). Individual colonies were transferred to 96-well plates and sequentially passaged into 48-, 24- and 12-well plates. Colonies were screened for Cad6B expression by immunohistochemistry, and those with maximum transfection efficiency were eventually passaged to 10-cm plates and expression was verified by immunoblotting. Cells were grown at 37°C in Ham's F12 growth medium supplemented with 10% fetal bovine serum (S11150, Atlanta Biologicals, Flowery Branch, GA), 600 µg/ml hygromycin (30-240-R, CellGro, Manassas, VA), 1 mM L-glutamine (25-005, CellGro, Manassas, VA) and a 1∶100 dilution of 1∶1 penicillin-streptomycin (30-002-CI, CellGro, Manassas, VA).
Immunohistochemistry
Embryos at 6 and 7 ss were collected and fixed in 4% paraformaldehyde (PFA) for 30 minutes at room temperature. Embryos were permeabilized in fresh Tris-buffered saline (TBS) containing 0.2% Triton X-100 (TBST) and blocked in this plus 5% fetal bovine serum (FBS) for 1 hour at room temperature. Immunostaining was performed overnight at 4°C for Cad6B (Jhingory et al., 2010; Schiffmacher et al., 2014). Explants were fixed by serial dilution of the medium with 4% PFA for 20 minutes at room temperature, permeabilized with fresh TBST, blocked with 5% FBS in TBST, and incubated with the Cad6B primary antibody overnight at 4°C and secondary antibody for 3 hours at room temperature. Cells were fixed in either 4% PFA for 20 minutes at room temperature or in cold 5% ethanol/95% acetic acid for 20 minutes at −20°C. For cells fixed in 4% PFA, permeabilization was carried out with TBST and immunostaining was performed as for explants. Immunostaining on cells fixed in ethanol/acetic acid was performed similarly as for explants but in TBS buffer without detergent.
The following primary antibodies and concentrations were used in experiments: Cad6B (1∶100 in whole-mount and 1∶250 on sections and cells; CCD6B-1, Developmental Studies Hybridoma Bank, OH; referred to as NT-6B in the text), caveolin-1 (1∶500; ab2910, Abcam, Cambridge, UK), HA (1∶750; 3F10, Roche, Basel, Switzerland), clathrin (1∶750; ab21679, Abcam, Cambridge, UK), Na+K+ ATPase (1∶750; ab76020, Abcam, Cambridge, UK), p120-catenin (1∶500; sc-373751, Santa Cruz Biotechnology, Santa Cruz, CA), β-catenin (1∶500; AHO0462, Life Technologies, Carlsbad, CA), GFP (1∶1000; R10367, Life Technologies, Carlsbad, CA), and K-cad (1∶200; ab64917, Abcam, Cambridge, UK; referred to as CT-6B in the text). Alexa-Fluor-488-conjugated phalloidin (Life Technologies, Carlsbad, CA) was used at 1∶50. Appropriate fluorescently conjugated secondary antibodies (Alexa Fluor 488, 594, 647; Life Technologies, Carlsbad, CA) were used at the following concentrations for each primary antibody: Cad6B (1∶250 in whole-mount and 1∶500 on cells), HA (1∶1000), clathrin (1∶1000), Na+K+ ATPase (1∶1000), p120 (1∶1000), β-catenin (1∶1000), GFP (1∶1500) and K-cad (1∶750).
Cad6B antibody feeding assays
The feeding assay was performed in vivo as described previously (Arancibia-Carcamo, 2006) with the following modifications. The neural tube lumen of 5-ss embryos was filled with CCD6B-1 antibody after electroporation of a membrane-GFP construct to label plasma membranes (a kind gift from Dr Paul Kulesa, Stowers Institute of Medical Research, Kansas City, MO). After incubation to allow for EMT, the embryos were fixed and immunostaining was performed for Cad6B and GFP as described above.
For the in vitro feeding assay, FlpIn-wtCad6B cells were washed three times with ice-cold fresh PBS2+ (PBS supplemented with 1.5 mM MgCl2 and 0.2 mM CaCl2) and incubated with a 1∶50 dilution of NT-6B antibody in medium at 4°C for 1 hour. The cells were washed three times with ice-cold PBS2+, replaced with warm medium and incubated at 37°C for 1 hour. Following this, cells were brought back to 4°C and subjected to three acid washes (0.1 M glycine, pH 2.0) for 5 minutes at 4°C. The cells were washed twice with PBS and fixed, and immunostaining was performed as described above.
To perform the feeding assay ex vivo, cranial dorsal neural folds from 5-ss embryos were explanted as described above and incubated at 37°C for 90 minutes to allow tissue to attach to the chamber slide. The medium was serially diluted with ice-cold fresh PBS2+, and explants were incubated at 4°C for 5 minutes. PBS2+ was then serially diluted with ice-cold explant medium containing a 1∶50 dilution of CCD6B-1 antibody and incubated at 4°C for 1 hour. Following serial dilution with ice-cold PBS2+, fresh warm explant medium was added to the explants, which were then incubated at 37°C for 3 hours. The explants were brought back to 4°C and, following serial dilution of the explant medium with ice-cold PBS2+, non-internalized antibody (bound to the plasma membrane) was removed by treatment with the low pH buffer described above for 15 minutes at 4°C temperature. The explants were fixed and permeabilized, and immunostaining was performed as described above.
Protein extraction and immunoblotting
Protein extraction and immunoblotting was performed as described previously (Schiffmacher et al., 2014). Briefly, cells were scraped in ice-cold PBS2+ and pelleted with low-speed centrifugation. Cell pellets were lysed in twice their volume of lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% IGEPAL CA-630) supplemented with protease inhibitors for 30 minutes at 4°C with periodic mixing. Following separation of supernatant from insoluble material through high-speed centrifugation, supernatant protein concentration was quantified by using a Bradford assay. Equivalent amounts of protein per sample were boiled at 95°C for 5 minutes in 4× reducing Laemmli sample buffer, processed by SDS-PAGE and then transferred to PVDF membrane. Membranes were blocked in 5% non-fat milk in PBS supplemented with 0.1% Tween and incubated overnight at 4°C with the following primary antibodies diluted in blocking solution: Cad6B (1∶80, CCD6B-1), β-actin (1∶1000; sc-47778, Santa Cruz Biotechnology, Santa Cruz, CA) and HA (1∶1000; 3F10, Roche, Basel, Switzerland). Membranes were washed and incubated with species- and isotype-specific horseradish-peroxidase-conjugated secondary antibodies (40 ng/ml; Jackson ImmunoResearch, West Grove, PA) in blocking solution for 1 hour at room temperature. Antibody detection was performed using Supersignal West Pico or Femto chemiluminescent substrate (Thermo Pierce Scientific, Waltham, MA) following washes and visualized using a ChemiDoc XRS system (Bio-Rad, Hercules, CA). Band volumes (intensities) were calculated from unmodified immunoblot images using Image Lab software (Bio-Rad, Hercules, CA) and analyzed by repeated measures analysis of variance assuming a compound symmetric covariance matrix for time and an unstructured covariance matrix for band intensity based on AIC values within the PROC MIXED procedure (SAS statistical software, SAS Institute, Cary, NC). Levels were deemed significantly different when P<0.05 based on Fisher's LSD multiple mean comparison test. Confidence intervals were calculated for the cytoskeleton (CSK) extraction assays and were deemed statistically significant if they did not overlap.
Cell surface biotinylation
Confluent layers of cells were brought to 4°C and washed twice with ice-cold PBS2+. After removal of PBS2+, EZ-Link Sulfo-NHS-Biotin (21326; Thermo Scientific, Waltham, MA) dissolved in ice-cold PBS2+ was added to cells at 1 mg/ml, and cells were incubated on a rocking platform for 30 minutes at 4°C. Excess biotin was quenched by washing three times for 5 minutes each, with ice-cold PBS2+ supplemented with 100 mM glycine and 0.5% BSA. After rinsing off residual quenching buffer, cells were scraped off the dish in ice-cold PBS2+, pelleted at 900 g for 5 minutes at 4°C, and set aside as the positive control (‘Total Surface’ fraction). Biotin was then stripped off of cells from another plate by washing cells three times for 10 minutes each at 4°C using a stripping buffer [75 mM NaCl, 75 mM NaOH, 50 mM L-glutathione (0399, Amresco, Solon, OH) supplemented with 1% BSA], followed by cell scraping in ice-cold PBS2+, and the cell pellets were set aside (negative control). Warm growth medium (see above) was added to cells in the remaining two plates, and cells were incubated at 37°C for 60 and 180 minutes. At the indicated time points, cells were brought back to 4°C, and any remaining biotin from cell-surface-bound, non-endocytosed proteins was removed with the biotin stripping conditions described above. Cells were lysed and subjected to a Bradford protein analysis as described previously. Streptavidin–agarose resin (20347, Thermo Scientific, Waltham, MA) pre-blocked with 4% BSA was incubated with 175–200 µg of total protein lysate overnight at 4°C to immunoprecipitate biotin-labeled proteins. After washing three times with 1× lysis buffer, 4× Laemmli sample buffer was added to the resin, and samples were boiled at 95°C for 5 minutes and loaded onto a 7.5% PAGE gel. Electrophoresis, immunoblotting and band intensity quantification for Cad6B was performed as described above.
Triton X-100 extraction of soluble and insoluble proteins
Ice-cold cytoskeleton extraction buffer (CSK) (50 mM NaCl, 10 mM PIPES pH 6.8, 3 mM MgCl2, 0.5% Triton X-100, 300 mM sucrose), supplemented with 1.2 mM PMSF (36978; Thermo Pierce, Waltham, MA) and 1× dilution of cOmplete Protease Inhibitor Cocktail (04693124001; Roche, Basel, Switzerland), was added to equivalent numbers of cells and incubated on a rocking platform for 30 minutes at 4°C. The extraction buffer was collected and centrifuged at maximum speed for 5 minutes at 4°C, and the supernatant was designated the Triton X-100 soluble fraction. The cells remaining on the plate were scraped and pelleted in ice-cold PBS2+, and 2× Laemmli sample buffer was directly added to the cell pellet. Following brief pulses of sonication to shear genomic DNA, the lysate was centrifuged at maximum speed for 5 minutes at 4°C, and the supernatant was designated the Triton X-100 insoluble fraction. Both fractions were boiled at 95°C for 5 minutes and subjected to electrophoresis, immunoblotting and band intensity quantification as described above.
Confocal microscopy
All images were acquired with the LSM Zeiss 710 microscope (Carl Zeiss Microscopy, Thornwood, NY) at the University of Maryland Imaging Core Facility. Where possible, the laser power, gain and offset were kept consistent for different channels, and the pinhole was always set to one airy unit. The Z-section optical images were acquired between 0.25 and 0.4 µm per optical section and reconstituted in three-dimensional (3D) composites using the Zen software (Zeiss).
Supplementary Material
Acknowledgements
We thank Dr Paul Kulesa (Stowers Institute of Medical Research) for the membrane GFP construct; Dr Marianne Bronner (California Institute of Technology) for the pCI-H2B-RFP construct; Dr Ashley Franklin (Point Defiance Zoo and Aquarium) for assistance with statistics; Dr Shruthi Rangarajan for help with figures; and Ms Lizbeth Hu for technical assistance.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
All authors contributed to the design of the experiments. R.P. and L.A.T. wrote the manuscript. R.P. performed all of the experiments in the manuscript, with L.A.T. also carrying out some of the inhibitor (or control)-treated explant assays. All authors read and approved the final manuscript.
Funding
This work was supported by the National Institutes of Health [grant number R00HD055034 to L.A.T.]; and a Sigma Xi Grants-In-Aid of Research and the University of Maryland Ann G. Wylie Dissertation Fellowship to R. P. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.164426/-/DC1
References
- Akhtar N, Hotchin NA. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol. Biol. Cell. 2001;12:847–862. doi: 10.1091/mbc.12.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia-Carcamo IL, Fairfax BP, Moss SJ, Kittler JT. Studying the localization, surface stability and endocytosis of neurotransmitter receptors by antibody labeling and biotinylation approaches. In: Kittler J A, Moss S J, editors. The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. Boca RatonFL: Taylor & Francis Group, LLC; 2006. p. 91. [PubMed] [Google Scholar]
- Bellovin DI, Bates RC, Muzikansky A, Rimm DL, Mercurio AM. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res. 2005;65:10938–10945. doi: 10.1158/0008--5472.CAN--05--1947. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Bradley JR, Johnson DR, Pober JS. Four different classes of inhibitors of receptor-mediated endocytosis decrease tumor necrosis factor-induced gene expression in human endothelial cells. J. Immunol. 1993;150:5544–5555. [PubMed] [Google Scholar]
- Bryant DM, Wylie FG, Stow JL. Regulation of endocytosis, nuclear translocation, and signaling of fibroblast growth factor receptor 1 by E-cadherin. Mol. Biol. Cell. 2005;16:14–23. doi: 10.1091/mbc.E04--09--0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Kerr MC, Hammond LA, Joseph SR, Mostov KE, Teasdale RD, Stow JL. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J. Cell Sci. 2007;120:1818–1828. doi: 10.1242/jcs.000653. [DOI] [PubMed] [Google Scholar]
- Cain CC, Sipe DM, Murphy RF. Regulation of endocytic pH by the Na+,K+-ATPase in living cells. Proc. Natl. Acad. Sci. USA. 1989;86:544–548. doi: 10.1073/pnas.86.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Chen J, Awoniyi M, Henley JR, McNiven MA. Dynamin 2 mediates fluid-phase micropinocytosis in epithelial cells. J. Cell Sci. 2007;120:4167–4177. doi: 10.1242/jcs.010686. [DOI] [PubMed] [Google Scholar]
- Cavey M, Lecuit T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb. Perspect. Biol. 2009;1:a002998. doi: 10.1101/cshperspect.a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung LW, Leung PC, Wong AS. Cadherin switching and activation of p120 catenin signaling are mediators of gonadotropin-releasing hormone to promote tumor cell migration and invasion in ovarian cancer. Oncogene. 2010;29:2427–2440. doi: 10.1038/onc.2009.523. [DOI] [PubMed] [Google Scholar]
- Chiasson CM, Wittich KB, Vincent PA, Faundez V, Kowalczyk AP. p120-catenin inhibits VE-cadherin internalization through a Rho-independent mechanism. Mol. Biol. Cell. 2009;20:1970–1980. doi: 10.1091/mbc.E08--07--0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev. Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Coles EG, Taneyhill LA, Bronner-Fraser M. A critical role for Cadherin6B in regulating avian neural crest emigration. Dev. Biol. 2007;312:533–544. doi: 10.1016/j.ydbio.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinet C, Lecuit T. Stability and dynamics of cell-cell junctions. Prog. Mol. Biol. Transl. Sci. 2013;116:25–47. doi: 10.1016/B978--0--12--394311--8.00002--9. [DOI] [PubMed] [Google Scholar]
- Dady A, Blavet C, Duband JL. Timing and kinetics of E- to N-cadherin switch during neurulation in the avian embryo. Dev. Dyn. 2012;241:1333–1349. doi: 10.1002/dvdy.23813. [DOI] [PubMed] [Google Scholar]
- Davies PJ, Davies DR, Levitzki A, Maxfield FR, Milhaud P, Willingham MC, Pastan IH. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature. 1980;283:162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclozeaux M, Venturato J, Wylie FG, Kay JG, Joseph SR, Le HT, Stow JL. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am. J. Physiol. 2008;295:C545–C556. doi: 10.1152/ajpcell.00097.2008. [DOI] [PubMed] [Google Scholar]
- Duband JL, Volberg T, Sabanay I, Thiery JP, Geiger B. Spatial and temporal distribution of the adherens-junction-associated adhesion molecule A-CAM during avian embryogenesis. Development. 1988;103:325–344. doi: 10.1242/dev.103.2.325. [DOI] [PubMed] [Google Scholar]
- Dupin E, Le Douarin NM. The neural crest, a fourth germ layer of the vertebrate embryo: significance in chordate evolution. In: Trainor P, editor. Neural Crest Cells: Evolution, Development and Disease. Amsterdam: Elsevier; 2014. pp. 4–22. [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J. Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann T, Glukmann V, Medvenev E, Shpolansky U, Galili D, Lichtstein D, Rosen H. Role of endosomal Na+-K+-ATPase and cardiac steroids in the regulation of endocytosis. Am. J. Physiol. 2007;293:C885–C896. doi: 10.1152/ajpcell.00602.2006. [DOI] [PubMed] [Google Scholar]
- Fishwick KJ, Neiderer TE, Jhingory S, Bronner ME, Taneyhill LA. The tight junction protein claudin-1 influences cranial neural crest cell emigration. Mech. Dev. 2012;129:275–283. doi: 10.1016/j.mod.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretz M, Jin J, Conibere R, Penning NA, Al-Taei S, Storm G, Futaki S, Takeuchi T, Nakase I, Jones AT. Effects of Na+/H+ exchanger inhibitors on subcellular localisation of endocytic organelles and intracellular dynamics of protein transduction domains HIV-TAT peptide and octaarginine. J. Control. Release. 2006;116:247–254. doi: 10.1016/j.jconrel.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Fuchs R, Schmid S, Mellman I. A possible role for Na+,K+-ATPase in regulating ATP-dependent endosome acidification. Proc. Natl. Acad. Sci. USA. 1989;86:539–543. doi: 10.1073/pnas.86.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheldof A, Berx G. Cadherins and epithelial-to-mesenchymal transition. Prog. Mol. Biol. Transl. Sci. 2013;116:317–336. doi: 10.1016/B978-0-12-394311-8.00014-5. [DOI] [PubMed] [Google Scholar]
- Hall BK. The Neural Crest and Neural Crest Cells in Vertebrate Development and Evolution. New YorkNY: Springer; 2009. [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat. (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J. Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Troyanovsky RB, Troyanovsky SM. Spontaneous assembly and active disassembly balance adherens junction homeostasis. Proc. Natl. Acad. Sci. USA. 2010;107:3528–3533. doi: 10.1073/pnas.0911027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J. Biol. Chem. 2005;280:24095–24103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L. et al. A novel role for p120 catenin in E-cadherin function. J. Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, Ikura M. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell. 2010;141:117–128. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Ivanov IA. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? In: Ivanov I A, editor. Exocytosis and Endocytosis. TotowaNJ: Humana Press; 2008. pp. 15–33. [DOI] [PubMed] [Google Scholar]
- Jarrett O, Stow JL, Yap AS, Key B. Dynamin-dependent endocytosis is necessary for convergent-extension movements in Xenopus animal cap explants. Int. J. Dev. Biol. 2002;46:467–473. [PubMed] [Google Scholar]
- Jhingory S, Wu CY, Taneyhill LA. Novel insight into the function and regulation of alphaN-catenin by Snail2 during chick neural crest cell migration. Dev. Biol. 2010;344:896–910. doi: 10.1016/j.ydbio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E, Seachrist DD, DeLeon-Rodriguez CM, Lozada KL, Miedler J, Abdul-Karim FW, Keri RA. HER2/ErbB2-induced breast cancer cell migration and invasion require p120 catenin activation of Rac1 and Cdc42. J. Biol. Chem. 2010;285:29491–29501. doi: 10.1074/jbc.M110.136770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, Touret N, Hahn KM, Grinstein S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010;188:547–563. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk AP, Nanes BA. Adherens junction turnover: regulating adhesion through cadherin endocytosis, degradation, and recycling. Subcell. Biochem. 2012;60:197–222. doi: 10.1007/978--94--007--4186--7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesa PM, Morrison JA, Bailey CM. The neural crest and cancer: a developmental spin on melanoma. Cells Tissues Organs. 2013;198:12–21. doi: 10.1159/000348418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 1999;146:219–232. doi: 10.1083/jcb.146.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RT, Nagai H, Nakaya Y, Sheng G, Trainor PA, Weston JA, Thiery JP. Cell delamination in the mesencephalic neural fold and its implication for the origin of ectomesenchyme. Development. 2013;140:4890–4902. doi: 10.1242/dev.094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenberg S, Yarden A, Kam Z, Geiger B. p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene. 1999;18:869–876. doi: 10.1038/sj.onc.1202396. [DOI] [PubMed] [Google Scholar]
- Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139:3471–3486. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- McCusker CD, Alfandari D. Life after proteolysis: Exploring the signaling capabilities of classical cadherin cleavage fragments. Commun. Integr. Biol. 2009;2:155–157. doi: 10.4161/cib.7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb. Perspect. Biol. 2009;1:a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509--510. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Ozawa M. A dileucine motif in its cytoplasmic domain directs beta-catenin-uncoupled E-cadherin to the lysosome. J. Cell Sci. 2007a;120:4395–4406. doi: 10.1242/jcs.03489. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Ozawa M. Increased internalization of p120-uncoupled E-cadherin and a requirement for a dileucine motif in the cytoplasmic domain for endocytosis of the protein. J. Biol. Chem. 2007b;282:11540–11548. doi: 10.1074/jbc.M608351200. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development. 1995;121:1321–1332. doi: 10.1242/dev.121.5.1321. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, Kowalczyk AP. p120-catenin binding masks an endocytic signal conserved in classical cadherins. J. Cell Biol. 2012;199:365–380. doi: 10.1083/jcb.201205029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu. Rev. Cell Dev. Biol. 2011;27:347–376. doi: 10.1146/annurev--cellbio--092910--154036. [DOI] [PubMed] [Google Scholar]
- Orth JD, Krueger EW, Cao H, McNiven MA. The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc. Natl. Acad. Sci. USA. 2002;99:167–172. doi: 10.1073/pnas.012607899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios F, Schweitzer JK, Boshans RL, D'Souza-Schorey C. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat. Cell Biol. 2002;4:929–936. doi: 10.1038/ncb881. [DOI] [PubMed] [Google Scholar]
- Palacios F, Tushir JS, Fujita Y, D'Souza-Schorey C. Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol. Cell. Biol. 2005;25:389–402. doi: 10.1128/MCB.25.1.389--402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS. Characterization of E-cadherin endocytosis in isolated MCF-7 and chinese hamster ovary cells: the initial fate of unbound E-cadherin. J. Biol. Chem. 2003;278:21050–21057. doi: 10.1074/jbc.M300082200. [DOI] [PubMed] [Google Scholar]
- Ridenour DA, McLennan R, Teddy JM, Semerad CL, Haug JS, Kulesa PM. The neural crest cell cycle is related to phases of migration in the head. Development. 2014;141:1095–1103. doi: 10.1242/dev.098855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CD, Saxena A, Bronner ME. Sip1 mediates an E-cadherin-to-N-cadherin switch during cranial neural crest EMT. J. Cell Biol. 2013;203:835–847. doi: 10.1083/jcb.201305050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmacher AT, Padmanabhan R, Jhingory S, Taneyhill LA. Cadherin-6B is proteolytically processed during epithelial-to-mesenchymal transitions of the cranial neural crest. Mol. Biol. Cell. 2014;25:41–54. doi: 10.1091/mbc.E13--08--0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnatwinkel C, Christoforidis S, Lindsay MR, Uttenweiler-Joseph S, Wilm M, Parton RG, Zerial M. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol. 2004;2:e261. doi: 10.1371/journal.pbio.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Henderson BR. IQ-domain GTPase-activating protein 1 regulates beta-catenin at membrane ruffles and its role in macropinocytosis of N-cadherin and adenomatous polyposis coli. J. Biol. Chem. 2007;282:8545–8556. doi: 10.1074/jbc.M610272200. [DOI] [PubMed] [Google Scholar]
- Solis GP, Schrock Y, Hülsbusch N, Wiechers M, Plattner H, Stuermer CA. Reggies/flotillins regulate E-cadherin-mediated cell contact formation by affecting EGFR trafficking. Mol. Biol. Cell. 2012;23:1812–1825. doi: 10.1091/mbc.E11--12--1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- Swanson JA. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J. Cell Sci. 1989;94:135–142. doi: 10.1242/jcs.94.1.135. [DOI] [PubMed] [Google Scholar]
- Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54:771–785. doi: 10.1016/j.neuron.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Takai Y, Ikeda W, Ogita H, Rikitake Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu. Rev. Cell Dev. Biol. 2008;24:309–342. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- Taneyhill LA. To adhere or not to adhere: the role of Cadherins in neural crest development. Cell Adh. Migr. 2008;2:223–230. doi: 10.4161/cam.2.4.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneyhill LA, Coles EG, Bronner-Fraser M. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development. 2007;134:1481–1490. doi: 10.1242/dev.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E, Mayor R. Neural crest migration: interplay between chemorepellents, chemoattractants, contact inhibition, epithelial-mesenchymal transition, and collective cell migration. Wiley Interdiscip. Rev. Dev. Biol. 2012;1:435–445. doi: 10.1002/wdev.28. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Toyoshima M, Tanaka N, Aoki J, Tanaka Y, Murata K, Kyuuma M, Kobayashi H, Ishii N, Yaegashi N, Sugamura K. Inhibition of tumor growth and metastasis by depletion of vesicular sorting protein Hrs: its regulatory role on E-cadherin and beta-catenin. Cancer Res. 2007;67:5162–5171. doi: 10.1158/0008-5472.CAN-06-2756. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Heisenberg CP. Trafficking and cell migration. Traffic. 2009;10:811–818. doi: 10.1111/j.1600--0854.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Van Dyke RW. Heterotrimeric G protein subunits are located on rat liver endosomes. BMC Physiol. 2004;4:1. doi: 10.1186/1472--6793--4--1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu XJ. A G protein-coupled receptor kinase induces Xenopus oocyte maturation. J. Biol. Chem. 2003;278:15809–15814. doi: 10.1074/jbc.M300320200. [DOI] [PubMed] [Google Scholar]
- Wu CY, Jhingory S, Taneyhill LA. The tight junction scaffolding protein cingulin regulates neural crest cell migration. Dev. Dyn. 2011;240:2309–2323. doi: 10.1002/dvdy.22735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 2003a;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Allison DF, Kottke MD, Summers S, Sorescu GP, Faundez V, Kowalczyk AP. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J. Biol. Chem. 2003b;278:19199–19208. doi: 10.1074/jbc.M211746200. [DOI] [PubMed] [Google Scholar]
- Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol. Biol. Cell. 2005;16:5141–5151. doi: 10.1091/mbc.E05--05--0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Anastasiadis PZ. p120 catenin is essential for mesenchymal cadherin-mediated regulation of cell motility and invasiveness. J. Cell Biol. 2006;174:1087–1096. doi: 10.1083/jcb.200605022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.