Abstract
New carbocyclic nucleoside analogs with five-membered heterocyclic nucleobases were synthesized and evaluated as potential anti-HIV and anti-HCV agents. Among the synthesized carbocyclic nucleoside analogs, the pyrazole amide 15f exhibited modest selective anti-HIV-1 activity (EC50 = 24 µM).
Keywords: carbocyclic ribonucleoside, antiviral, five-membered nucleobases
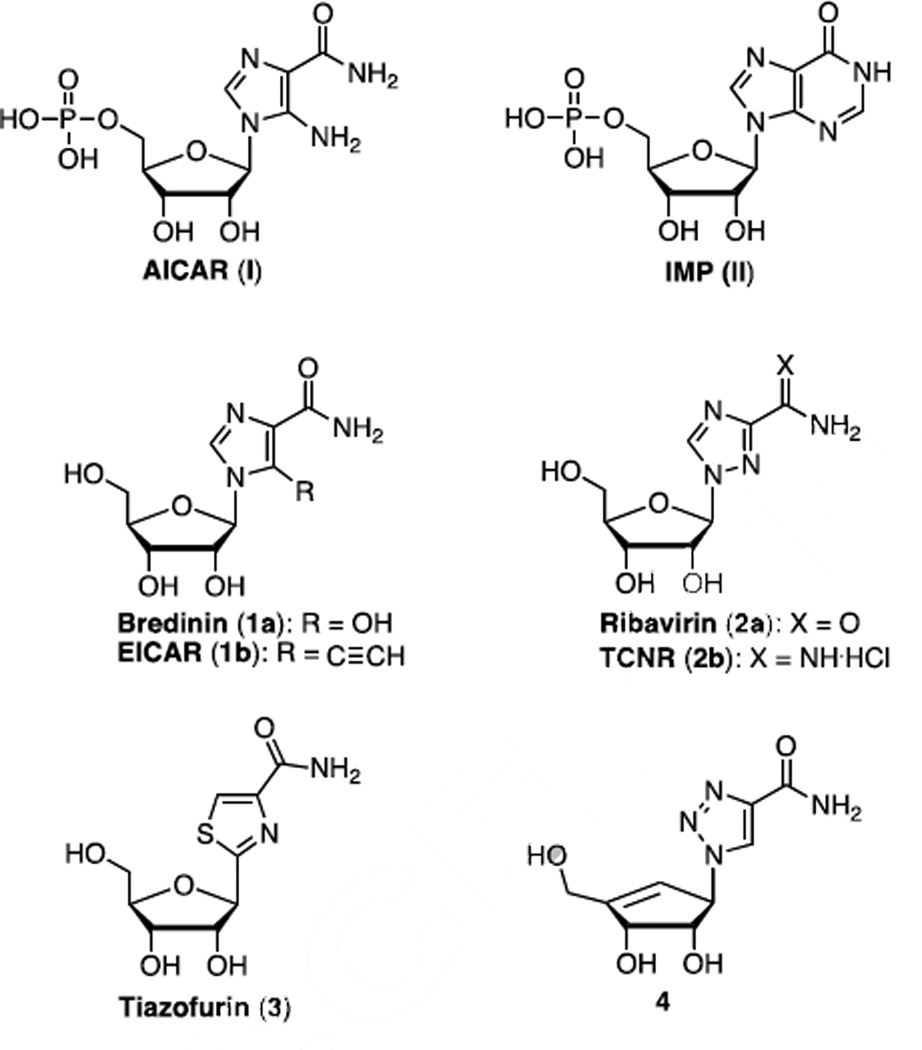
Studies of nucleoside analogs with unmodified naturally occurring nucleo-purine and - pyrimidine bases have been more successful in the development of biologically interesting nucleoside analogs.1 However, a few nucleosides with five-membered heterocyclic nucleobases, such as the 5-amino-1-β-d-ribofuranosylimidazole-4-carboxamide (aminoimidazole carboxamide ribonucleotide, AICAR, I) or the inosine 5’-monophosphate (IMP, II), display useful biological activity (Figure 1). Both AICAR and IMP are important intermediates in the de novo generation of adenine and guanine nucleotides.2 AICAR is well known as an adenosine 5’-monophosphate (AMP)-dependent protein kinase (AMPK) activator.3 It can activate AMPK in times of reduced energy availability (high cellular AMP:ATP ratios) and serves to inhibit anabolic processes.4,5 In addition, AICAR may play a role in tumor suppression6 and induction of cellular apoptosis.3,7
Figure 1.
Structures of AICAR, IMP and related biologically active five-membered heterocyclic nucleobase nucleoside analogs.
Additionally, AICAR is converted to IMP in two steps by AICAR transformylase and IMP cyclohydrolase in purine nucleotide biosynthesis.8 IMP is the precursor for both adenosine and guanosine nucleotides. Inosine monophosphate dehydrogenase (IMPDH)9 catalyzes the oxidation of IMP to xanthosine 5’-monophosphate (XMP) in guanine nucleotide biosynthesis, which is subsequently converted to guanosine monophosphate (GMP) by GMP synthetase.10 Recently, IMPDH has received a great deal of attention as a promising target for the development of anticancer, antiviral, immunosuppressive, and antimicrobial chemotherapy.9
Among the previously reported nucleoside or nucleotide analogs with five-membered heterocyclic nucleobases as activators of AMPK or inhibitors of IMPDH, Bredinin, 1a11, first isolated from the mold Eupenicillium brefeldianum, is an imidazole containing nucleoside analog IMPDH inhibitor that is used clinically as an immunosuppressive agent (Figure 1). EICAR (5-ethyleneimidazole-4-carboxamide, 1b)12 is a 5-alkyne substituted imidazole derivative that shows potent antitumor and antiviral activity. Ribavirin, 2a13, is the most clinically important five-membered heterocyclic nucleoside analog. It possesses a 1,2,4-triazole nucleobase on a ribo-sugar and exhibits broad-spectrum antiviral activity (including HIV, hepatitis (A, B and C) virus, influenza, respiratory syncytial virus (RSV), measles and mumps). Taribavirin (viramidine, 1-β-d-ribofuranosyl-1H-1,2,4-triazole-3-carboxamidine, TCNR, 2b)14 is a prodrug of ribavirin with improved liver-targeting pharmacokinetics. It has a shorter half-life, but displays significant antiviral activity against HSV, rhino and parainfluenza viruses. Tiazofurin (1,2-(β-d-ribofuranosyl) thiazole-4-carboxamide, 3, a synthetic C-nucleoside derivative, displayed potent anticancer activity.15 An early discovery compound, 1,2,3-triazole carbocyclic nucleoside 416, exhibited antiviral activity versus vaccinia virus. With these five-membered heterocyclic nucleobase containing nucleoside analogs as a backdrop, we designed novel carbocyclic nucleoside analogs as potential antiviral agents.
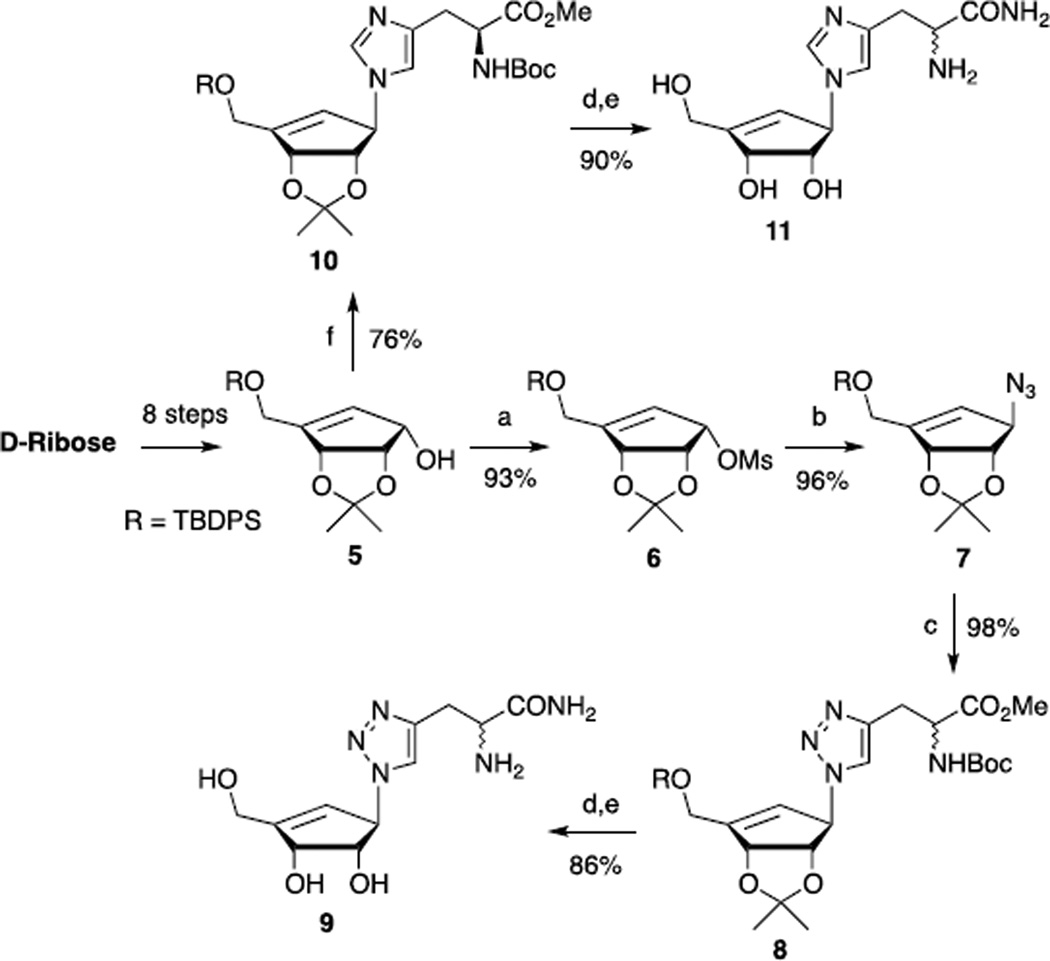
Recently, the azide/alkyne cycloaddition reaction17 has become one of the most synthetically useful tools in the formation of the 1,2,3-triazole heterocyclic ring system. A variety of reaction conditions for this particular click reaction18 have been reported by multiple groups.19 For our work, we envisioned the use of the known cyclopentenol 516 to prepare the cyclopentenyl azide 7 for subsequent synthesis of carbocyclic analogs containing five-membered heterocyclic bases (Scheme 1).
Scheme 1.
Reagents and reaction conditions: a) see reference 16 for synthesis of 5, MsCl, Et3N, CH2Cl2, 0 °C, 3h; b) NaN3, DMF, 70 °C, 8h; c) d,l-methyl 2-(t-butoxycarbonylamino)pent-4-ynoate, CuI, Et3N, THF, rt, 12h; d) NH3/MeOH, rt, 12h; e) HCl/MeOH, rt, 6h; f) N-Boc-L-histidine methyl ester, DIAD, PPh3, THF, rt, 12h.
The hydroxyl group of compound 5 was reacted with methanesulfonyl chloride (MsCl) to provide the sulfonyl analog 6, followed by treatment of sodium azide to give its corresponding azide derivative 7 in 89 % yield (2 steps).
The 1,3-dipolar cycloaddition reaction of compound 7 with D,L-methyl 2-(tert-butoxycarbonylamino) pent-4-ynoate in the presence of CuI and triethylamine gave carbocyclic 1,2,3-triazole derivative 8 in 98% yield.20 The triazole ester 8 was transformed to its corresponding amide with saturated methanolic ammonia, followed by removal of the tert-butyldiphenylsilyl (TBDPS), isopropylidene, and tert-butoxycarbonyl (Boc) deprotection groups by methanolic hydrogen chloride to afford the carbocyclic 1,2,3-triazole- propanamide analog 9 in 86% yield over 2 steps (Scheme 1).
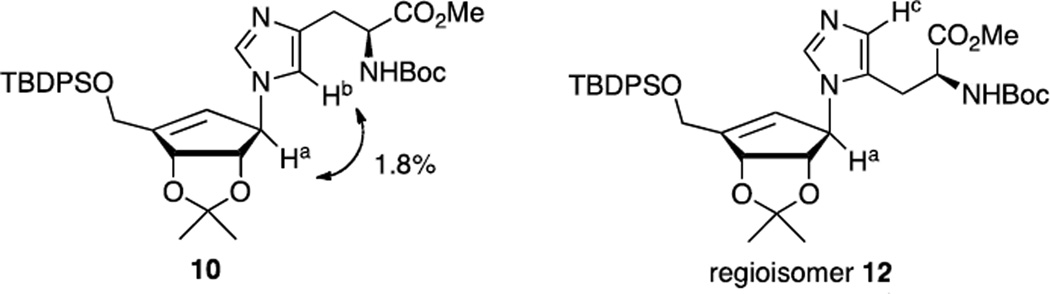
The Mitsunobu reaction21 of compound 5 with NBoc-L-histamine methyl ester provided carbocyclic imidazole analog 10 in 76% yield, which was subsequently treated with methanolic ammonia then methanolic hydrogen chloride to gave the carbocyclic imidazole propanamide 11 in 90% yield as a D/L-mixture at the histidine chiral center. The regioselectivity of 10 was determined on the basis of Nuclear Overhauser Effect (1D-NOE). The 1D-NOE showed a significant interaction between Ha and Hb that is only possible with 10 (π-isomer), as the Ha and Hc NOE with τ-isomer 12 would not be observed (Figure 2).
Figure 2.
1D-NOE spectra of compound 10 and its isomer 12.
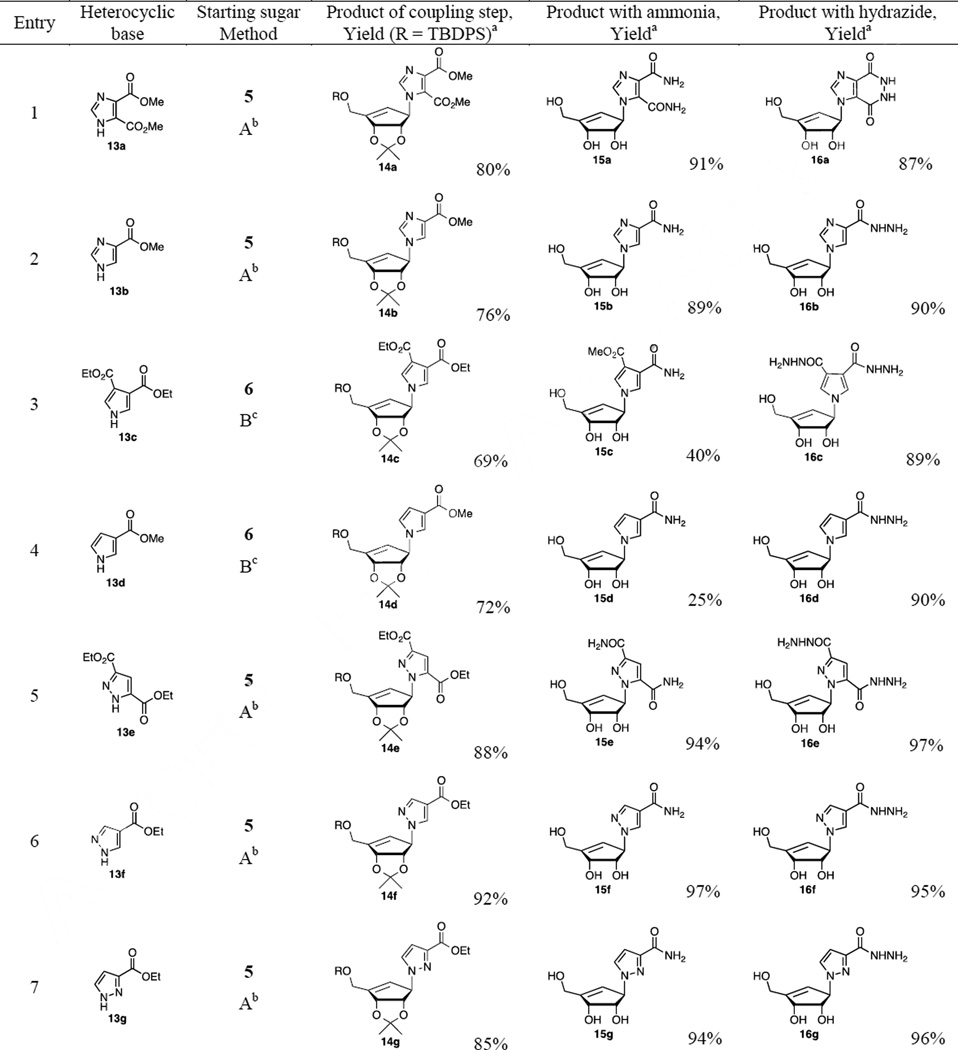
Furthermore, the chiral cyclopentenyl alcohol 5 and sulfonyl derivative 6 were utilized for the synthesis of additional new five-membered heterocyclic nucleoside analogs. The displacement reaction of 5 (or 6) with commercially available five-membered heterocyclic analogs such as pyrroles, imidazoles, and pyrazoles esters with either a Mitsunobu reaction (method A for 5) or nucleophilic conditions of sodium hydride in dimethylformamide (method B for 6) afforded their corresponding derivatives 14a–h (Table 1). The reaction of 5 separately with 4,5-dimethylester imidazole carboxylate 13a, 4-methylester imidazole carboxylate 13b, 3,5-diethylester pyrazole carboxylate 13e, 4-ethylester pyrazole carboxylate 13f, ethyl 3-pyrazolecarboxylate 13g and ethyl 3-(trifluoromethyl)-1H-pyrazole-4-carboxylate 13h in the presence of triphenylphosphine and diisopropyl azodicarboxylate in tetrahydrofuran provided their corresponding methyl ester imidazole nucleoside 14a (80%), 14b (76%) and ethyl ester pyrazole nucleosides 14e (88%), 14f (92%), 14g (85%) and 14h (62%), respectively. Unexpectedly, the Mitsunobu reactions of 5 with ethyl ester pyrrole carboxylates (13c and 13d) afforded only a trace amount of their corresponding pyrrole nucleoside derivatives 14c and 14d. Therefore, compound 6 was employed as an alternative approach for the efficient synthesis of pyrrole nucleoside derivatives. The displacement reactions of 6 with 3,5-diethyl ester pyrrole carboxylate 13c and 5-methyl ester pyrrole carboxylate 13d were performed under method B conditions to obtain the pyrrole nucleoside derivative 14c (69%) and 14d (72%), respectively (entry 3 and 4). The imidazole esters 14a–b and pyrazole esters 14e–h were transformed to their corresponding amides with saturated methanolic ammonia, followed by deprotection of TBDPS and isopropylidene groups by using methanolic hydrogen chloride solution to afford the desired compound 15a–b and 15e–h22 in good yields. However, subsequent removal of TBDPS and isopropylidene groups of 14c–d with methanolic hydrogen chloride, and treatment of deprotected esters with methanolic ammonia solution gave amide 15c in 40% yield and amide 15d in 25% yield (2 steps) as the reaction of the pyrrole esters (14c–d) with the methanolic ammonia provided the amides in low yield. Additionally, the ester intermediates 14a–h were converted to their corresponding cyclic (pyridazine) or acyclic (hydraizde) derivatives 16a–h. The treatment of 14a–h with hydrazine in methanol, followed by deprotection of TBDPS and isopropylidene with methanolic hydrogen chloride to provide pyridazine analog 16a (87%), as well as hydrazide derivatives (16b–h)23 in high yields as summarized in Table 1. The newly synthesized carbocyclic five-membered heterocyclic nucleosides (9, 11, 15a–h, and 16ah) were evaluated for their antiviral activity versus both HIV-1LAI and HCV (genotype 1b). For HCV activity, compounds were evaluated for their ability to inhibit HCV RNA replication using an Huh7 cell based subgenomic replicon assay.24 Cytotoxicity in Huh7 cells was determined simultaneously with anti-HCV activity by extraction and amplification of both HCV RNA and Huh7 ribosomal RNA (rRNA). In addition, all compounds were evaluated against HIV-1 in human peripheral blood mononuclear (PBM) cells and cytotoxicity was determined in PBM, CEM and Vero cells.25
Table 1.
Results of coupling reactions and synthesis of carbocyclic nucleoside analogs
 |
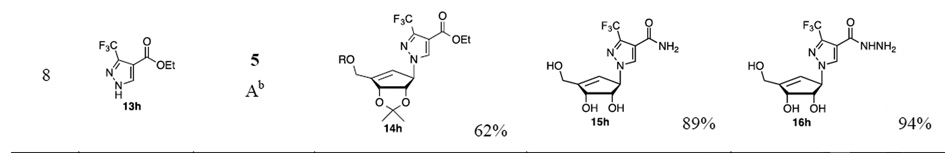 |
The yield was obtained after silica gel column chromatography.
Mitsunobu reaction was used (method A).
NaH/DMF was used (method B)
Unfortunately, most of the nucleoside analogs did not show antiviral activity versus HIV-1 or HCV. Only the pyrazole amide 15f showed mild antiviral activity versus HIV-1 (median effective concentration or EC50 = 24 µM). None of these nucleoside analogs displayed cytotoxicity in PBM, CEM or Vero cells up to 100 µM.
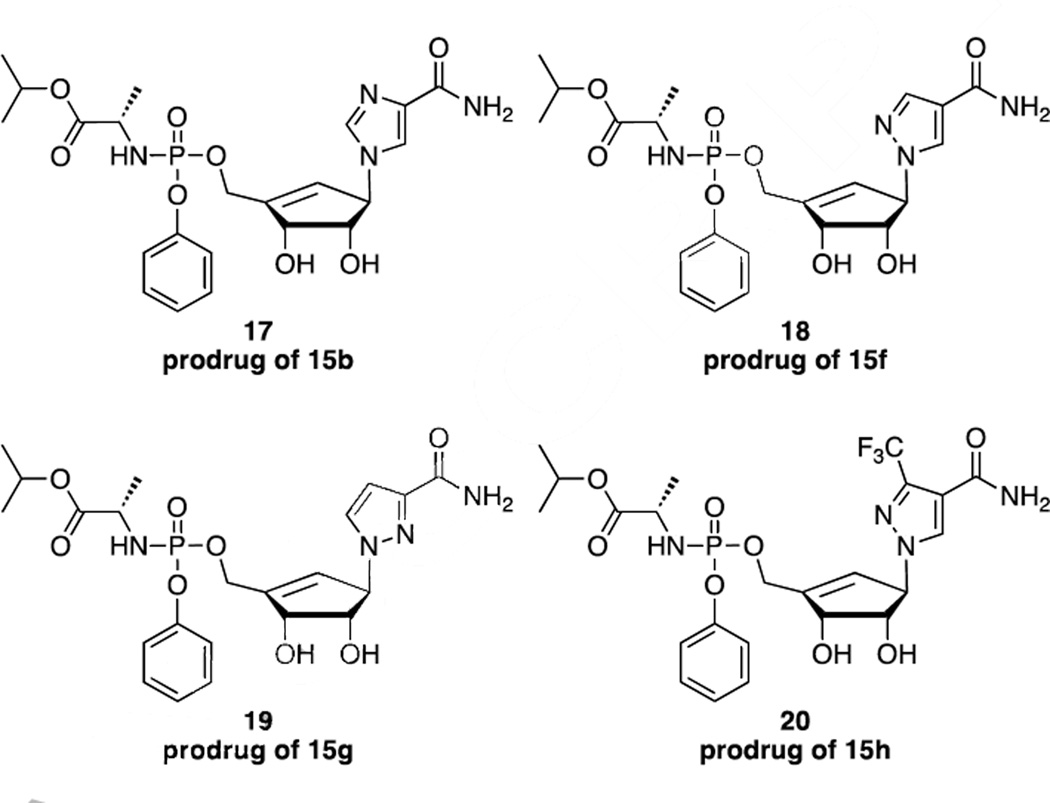
Isopropyl alanine phenoxy phosphoramidate prodrugs were prepared for nucleoside analogs 15b, 15f, 15g and 15h and evaluated for antiviral activity and cytotoxicity to determine if the phosphorylation of these nucleoside analogs was the limiting factor for their antiviral activity (Figure 3).
Figure 3.
Phosphoramidate prodrugs of nucleoside analogs 15b, 15f, 15g and 15h.
These monophosphate prodrugs did not improve the antiviral activity versus HIV-1 or HCV, but also did not increase cytotoxicity versus PBM, CEM or Vero cells. Further studies of potential antiviral activity against other RNA viruses is progressing and will be reported in due course.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grant 5P30-AI-50409 (CFAR), and by the Department of Veterans Affairs. Dr. Schinazi is the founder and a major shareholder of RFS Pharma, LLC. Emory received no funding from RFS Pharma, LLC to perform this work and vice versa. We thank Mervi Detorio, Catherine M. Montero and Tamara R. McBrayer for excellent technical assistance and biological testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data
Supplementary data (details experimental procedure and characterization data of selected compounds and copies of1H NMR and13C NMR spectra are given in supplementary material) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tetlet….
References and notes
- 1.Simons C. Nucleoside Mimetics: Their Chemistry and Biological properties. Cardiff University:UK: Gordon and Breach Science Publishers; 2000. [Google Scholar]
- 2.(a) Shive W, Ackermann WW, Gordon M, Getzendaner ME, Eakin RE. J. Am. Chem. Soc. 1947;69:725–726. doi: 10.1021/ja01195a521. [DOI] [PubMed] [Google Scholar]; (b) Flaks JG, Erwin MJ, Buchanan JM. J. Biol. Chem. 1957;288:201–213. [PubMed] [Google Scholar]; (c) Lowy BA, Cook JL, London IM. J. Biol. Chem. 1961;236:1442–1445. [PubMed] [Google Scholar]
- 3.(a) Hardi DG, Mackintosh RW. BioEssays. 1992;14:699–704. doi: 10.1002/bies.950141011. [DOI] [PubMed] [Google Scholar]; (b) Kemp BE, Mitchelhill DS, Michell BJ, Chen Z-P, Witters LA. Trends. Biochem. Sci. 1999;24:22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]; (c) Caring D. Trends. Biochem. Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]; (d) Motoshima H, Goldstein BJ, Igata M, Araki E. J. Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. FEBS Lett. 1994;353:33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]; (b) Fryer SA, Parbu-patel A, Carling D. J. Biol. Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 5.(a) Merrill GF, Kurth EJ, Hardie DG, Winder WW. Am. J. Physiol. 1997:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]; (b) Hong SP, Leiper FC, Woods A, Carlson M. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8839–8834. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hardi DG, Scott JW, Pan DA, Hudson ER. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Connors KE, Yang D-Q. Mol. Cell. Biochem. 2007;306:239–245. doi: 10.1007/s11010-007-9575-6. [DOI] [PubMed] [Google Scholar]
- 7. Rattan R, Giri S, Singh AK, Singh I. J. Biol. Chem. 2005;280:39582–39593. doi: 10.1074/jbc.M507443200. and references therein.
- 8.Ni L, Guan K, Zalkin H, Dixon JE. Gene. 1991;106:197–205. doi: 10.1016/0378-1119(91)90199-l. [DOI] [PubMed] [Google Scholar]
- 9.(a) Pankiewicz KW, Patterson SE, Black PL, Jayaram HN, Risal D, Golgstein BM, Stuyver LJ, Schinzai RF. Curr. Med. Chem. 2004;11:887–900. doi: 10.2174/0929867043455648. [DOI] [PubMed] [Google Scholar]; (b) Nair V, Shu Q. Med. Res. Rev. 2008;28:219–232. doi: 10.1002/med.20104. [DOI] [PubMed] [Google Scholar]; (c) Hedstrom L. Chem. Rev. 2009;109:2903–2928. doi: 10.1021/cr900021w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Jackson RC, Weber G, Morris HP. Nature. 1975;256:331–333. doi: 10.1038/256331a0. [DOI] [PubMed] [Google Scholar]; (b) Nair V, Shu Q. Antiviral Chem. Chemother. 2007;18:245–258. doi: 10.1177/095632020701800501. [DOI] [PubMed] [Google Scholar]
- 11.(a) Inou T, Kusaba R, Takahashi I, Sugimoto H, Kuzuhara K, Yamada Y, Yamauchi J, Otsubo O. Transplantation Proc. 1981;13:135–138. [PubMed] [Google Scholar]; (b) Mizuno K, Tsujino M, Takada M, Hayashi M, Atsmi K, Asano K, Matsuda T. J. Antibiot. 1974;27:775–782. doi: 10.7164/antibiotics.27.775. [DOI] [PubMed] [Google Scholar]
- 12.(a) Minakawa N, Takeda T, Sasaki T, Matsuda A, Ueda T. J. Med. Chem. 1991;34:778–786. doi: 10.1021/jm00106a045. [DOI] [PubMed] [Google Scholar]; (b) Balzarini J, Lee C-K, Herdewijn P, De Clercq E. J. Biol. Chem. 1991;266:21509–21514. [PubMed] [Google Scholar]
- 13.(a) Sidwell RW, Huffman JH, Khare GP, Allen LB, Witkowski JT, Robins RK. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]; (b) Witkowski JT, Robins RK, Sidwell RW, Simon LN. J. Med. Chem. 1972;15:1150–1154. doi: 10.1021/jm00281a014. [DOI] [PubMed] [Google Scholar]
- 14.(a) Willis RC, Robins RK, Seegmiller JE. Mol. Pharmacol. 1980;18:287–297. [PubMed] [Google Scholar]; (b) Witkowski JT, Robins RK, Sidwell RW, Simon LN. J. Med. Chem. 1973;16:935–937. doi: 10.1021/jm00266a014. [DOI] [PubMed] [Google Scholar]
- 15.(a) Fuertes M, Garcia Lopez T, Garcia Monoz G, Stud M. J. Org. Chem. 1976;41:4074. [Google Scholar]; (b) Ramasamy KS, Lau JYN. Nucleosides Nucleotides Nucleic Acids. 2001;20:1329–1331. doi: 10.1081/NCN-100002548. [DOI] [PubMed] [Google Scholar]
- 16.(a) Cho JH, Amblard F, Coats SJ, Schinazi RF. J. Org. Chem. 2013;78:723–727. doi: 10.1021/jo302038d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cho JH, Bernard DL, Sidwell RW, Kern ER, Chu CK. J. Med. Chem. 2006;49:1140–1148. doi: 10.1021/jm0509750. [DOI] [PubMed] [Google Scholar]
- 17.(a) Meldal M, Tornϕe CW. Chem. Rev. 2008;108:2952. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]; (b) Amblard F, Cho JH, Schinazi RF. Chem. Rev. 2009;109:4207–4220. doi: 10.1021/cr9001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J. Am, Chem. Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. and references therein.
- 19.(a) Angell YL, Burgess K. Chem. Soc. Rev. 2007;36:1674. doi: 10.1039/b701444a. [DOI] [PubMed] [Google Scholar]; (b) Jewett JC, Bertozzi CR. Chem. Soc. Rev. 2009;38:3449–3462. [Google Scholar]; (c) Iha RK, Wooley KL, Nyström AM, Burke DJ, Kade MJ, Hawker CJ. Chem. Rev. 2009;109:5620–5658. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mamidyala SK, Finn MG. Chem. Soc. Rev. 2010;39:1252–1261. doi: 10.1039/b901969n. [DOI] [PubMed] [Google Scholar]; (e) Kappe CO, Van der Eycken EV. Chem. Soc. Rev. 2010;39:1280–1290. doi: 10.1039/b901973c. [DOI] [PubMed] [Google Scholar]; (f) Hein JE, Fokin VV. Chem. Soc. Rev. 2010;39:1302–1315. doi: 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Appukkuttan P, Mehta VP, Van der Eycken EV. Chem. Soc. Rev. 2010;39:1467–1477. doi: 10.1039/b815717k. [DOI] [PubMed] [Google Scholar]; (h) Thirumurugan P, Matosiuk D, Jozwiak K. Chem. Rev. 2013;113:4905–1479. doi: 10.1021/cr200409f. [DOI] [PubMed] [Google Scholar]
- 20.Compound 8: 1H NMR (400 MHz, CDCl3) δ 7.68 (m, 4H), 7.43 (m, 6H), 7.23 (d, J = 3.6 Hz, 1H), 5.09 (s, 1H), 5.57 (s, 1H), 5.54 (d, J = 8.8 Hz, 1H), 5.17 (t, J = 5.6 Hz, 1H), 4.68 (d, J = 4.8 Hz, 1H), 4.63 (m, 1H), 4.44 (m, 2H), 3.74 (s, 3H), 3.24 (d, J = 4.8 Hz, 2H), 1.44 (s, 9H), 1.39 (s, 1H), 1.30 (s, 1H), 1.08 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.9, 151.8, 135.5, 133.1, 129.9, 127.8, 121.6, 120.8, 112.6, 84.8, 83.4, 69.9, 61.2, 52.9, 52.4, 28.3, 27.3, 26.8, 25.9, 19.2; MS-ESI+ m/z 677 (M+H)+; HRMS-ESI+: m/z calcd. for C36H49N4O7Si (M+H)+ 677.3369, found 677.3365.
- 21.(a) Mitsunobu O. Synthesis. 1981:1–28. [Google Scholar]; (b) Swamy KCK, Kumar NNB, Balaraman E, Kumar KVPP. Chem. Rev. 2009;109:2551–2651. doi: 10.1021/cr800278z. [DOI] [PubMed] [Google Scholar]
- 22.Compound 15f: 1H NMR (400 MHz, CD3OD) δ 8.17 (s, 1H), 7.91 (s, 1H), 5.69 (q, J = 2.0 Hz, 1H), 5.14 (d, J = 2.0 Hz, 1H), 4.41 (d, J = 5.2 Hz, 1H), 4.14 (q, J = 1.6 Hz, 2H), 4.08 (t, J = 5.2 Hz, 1H); 13C NMR (100 MHz, CD3OD) δ 151.5, 141.3, 133.6, 125.4, 125.1, 116.8, 79.1, 74.3, 73.6, 60.4; MS-ESI+ m/z 240 (M+H)+; HRMS-ESI+: m/z calcd. for C10H14N3O4 (M+H)+ 240.0980, found 240.0979.
- 23.Compound 16f: 1H NMR (400 MHz, CD3OD) δ 8.09 (s, 1H), 7.79 (s, 1H), 5.62 (q, J = 1.6 Hz, 1H), 5.08 (d, J = 2.4 Hz, 1H), 4.34 (d, J = 5.2 Hz, 1H), 4.06 (q, J = 2.0, 2H), 4.00 (t, J = 5.2 Hz, 1H); 13C NMR (100 MHz, CD3OD) δ 163.7, 151.3, 140.9, 133.1, 125.5, 115.4, 79.0, 74.2, 73.6, 60.4; MS-ESI+ m/z 255 (M+H)+; HRMS-ESI+: m/z calcd. for C10H15N4O4 (M+H)+ 255.1089, found 255.1088.
- 24.Stuyver LJ, Whitaker T, McBrayer TR, Hernandez-Santiago BI, Lostia S, Tharnish PM, Ramesh M, Chu CK, Jordan R, Shi J, Rachakonda S, Watanabe KA, Otto MJ, Schinazi RF. Antimicrob. Agents Chemother. 2003;47:244–254. doi: 10.1128/AAC.47.1.244-254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(a) Schinazi RF, Sommadossi JP, Saalmann V, Cannon DL, Xie M-W, Hart GC, Smith GA, Hahn EF. Antimicrob. Agents Chemother. 1990;34:1061–1067. doi: 10.1128/aac.34.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Stuyver LJ, Lostia S, Adams M, Mathew JS, Pai BS, Grier S, Tharnish PM, Choi Y, Chong Y, Choo H, Chu CK, Otto MJ, Schinazi RF. Antimicrob. Agents Chemother. 2002;46:3854–3860. doi: 10.1128/AAC.46.12.3854-3860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.