Abstract
There has been a long-known association between high dietary sodium intake and hypertension, as well as the increased risk of cardiovascular disease. Reduction of sodium intake is a major challenge for public health. Recently, there have been several controversial large population-based studies regarding the current recommendation for dietary sodium intake. Although these studies were performed in a large population, they aroused controversies because they had a flaw in the study design and methods. In addition, knowledge of the advantages and disadvantages of the methods is essential in order to obtain an accurate estimation of sodium intake. I have reviewed the current literatures on the association between sodium intake and cardiovascular events, as well as the methods for the estimation of sodium intake.
Keywords: Sodium, dietary; Hypertension; Cardiovascular diseases; Urine specimen collection
Introduction
High sodium intake is a huge problem throughout the world. High sodium intake is a significant contributing factor that causes blood pressure (BP) elevation, along with increased risk of cardiovascular disease.1),2),3) World Health Organization recommends an overall reduction in sodium intake to less than 2000 mg/day of sodium.4) However, there have recently been some controversies about whether sodium intake reduction to the recommended level would be detrimental rather than helpful.5),6),7) Studies reporting that low sodium intake is associated with high cardiovascular morbidity and mortality have limitations. The current article will review and discuss two recent issues: 1) sodium intake and cardiovascular disease and 2) measurement methods used in the research on sodium intake.
Sodium Intake and Cardiovascular Events
High sodium intake and hypertension
The association between high sodium intake and hypertension has been well documented. The international co-operative study on the relation of BP to electrolyte excretion in population (INTERSALT) study evaluated this association, and it was the first large, multinational analysis of sodium intake, BP, and other comorbidities. The INTERSALT study estimated the average daily sodium intake by measuring the 24-hour urinary sodium excretion along with BP of participants from 52 communities.3) Through this collaborative effort, the researchers found a significant association between urinary sodium excretion and systolic BP in individual participants as well as in the pooled analysis. Furthermore, the association between the median 24-hour urinary sodium excretion and the adjusted slope of systolic BP with age in 52 centers was linear and significant. The linear association was persistent after the exclusion of 4 isolated populations who had extremely low urinary sodium excretion.8) Supporting the INTERSALT study, the European Prospective Investigation into Cancer in Norfolk study also found an association of urinary sodium excretion with BP difference.9) Along with these large-scale epidemiologic studies, many intervention trials have shown that BP is indeed lowered by decreasing the sodium intake.
In a randomized, double-blind, placebo-controlled trial, MacGregor et al.10) demonstrated that a reduction in dietary sodium intake from 162±9 mmol/day to 86±9 mmol/day can lower systolic BP by approximately 7.1 mm Hg. In another controlled trial, the Dietary Approaches to Stop Hypertension (DASH)-Sodium trial, the authors compared three levels of sodium intake; 50, 100, and 150 mmol/day for 30 days.11) The DASH diet with 50 mmol/day sodium level lowered the mean systolic BP by 7.1 mm Hg than the control diet with 150 mmol/day sodium level in participants without hypertension, and by 11.5 mm Hg in participants with hypertension. Population-based intervention of sodium intake reduction performed in Finland reduced the incidence of stroke by 75% and coronary artery disease mortality by 80%.12)
Similarly, numerous other meta-analyses have shown a positive effect of sodium intake reduction on lowering of BP.13),14) One meta-analysis published in the BMJ by He et al.,14) demonstrated that a mean decrease in urinary sodium excretion by 75 mmol/day was accompanied by a mean decrease in systolic BP by 4.18 mm Hg and a mean decrease in diastolic BP by 2.06 mm Hg.14) In the trials of hypertension prevention phase I (TOHP I) and phase II (TOHP II), the participants were randomly assigned to reduce sodium intake for 18 months (TOHP I) or 36-48 months (TOHP II).15),16) Compared to the control group, the intervention group reduced their sodium intake by 44 mmol/day (TOHP I) and 33 mmol/day (TOHP II), respectively. Although the decrease in BP was small (1.7/0.9 mm Hg in TOHP I and 1.2/0.7 mm Hg in TOHP II), after 10 to 15 years of completion of the original trial, the participants in the intervention group had an approximately 25% lower incidence of cardiovascular events after adjustment for confounders (relative risk: 0.75, 95% confidence interval: 0.57-0.99, p=0.04).17)
High sodium intake and cardiovascular disease (Table 1)
Table 1. Reviewed studies on the association of high sodium intake with cardiovascular disease and the measurement methods.
| Studies | Limitations |
|---|---|
| Association between sodium intake and cardiovascular events | |
| Stolarz-Skrzypek et al.5) | Subject selection bias (combination of different cohorts) |
| Lack of consideration of changes in sodium intake | |
| Lack of consideration of baseline nutritional status | |
| O'Donnell et al.6) | Estimation of sodium intake from spot urine |
| Lack of consideration of changes in sodium intake | |
| Subject selection bias (high risk subjects) | |
| O'Donnell et al.7) | Estimation of sodium intake from spot urine |
| Lack of consideration of changes in sodium intake | |
| Subject selection bias | |
| Meta-analysis | |
| Graudal et al.13) | Lack of consideration of long-term sodium intake reduction |
| Taylor et al.18) | Subject selection bias (inclusion of patients with heart failure and exclusion of the TONE study) |
| Spot urine collection method | |
| Kawasaki et al.70) | Large limits of agreement (approximately 100 mmol)85) |
| Tendency for under- or over-estimation of the 24-hour urinary sodium excretion75),85) | |
| Significant bias in the measured and estimated 24-hour urinary sodium excretion in the PURE study population75) | |
| Dependency on the time of spot urine collection | |
| Tanaka et al.71) | Large limits of agreement (approximately 100 mmol)85) |
| Tendency for under- or over-estimation of the 24-hour urinary sodium excretion84),85) | |
| Significant bias in the measured and estimated 24-hour urinary sodium excretion in the external validation71),85) |
TONE: trial of nonpharmacologic interventions in the elderly, PURE: prospective urban rural epidemiology
On the contrary, a meta-analysis (that was withdrawn) had reported that salt intake reduction has no clear benefit in reducing the mortality or cardiovascular morbidity.18),19) In addition, an analysis of cohort data showed that the highest cardiovascular mortality was found in subjects who had the lowest 24-hour urinary sodium excretion at baseline.5) In this study, the authors found a paradoxical correlation such that people with the highest sodium intake had the lowest cardiovascular mortality despite the positive association between high BP and increase in sodium intake. The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) study as well as the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) and the Prospective Urban Rural Epidemiology (PURE) study showed a J-shaped association between estimated urinary sodium excretion and cardiovascular events.6),7) According to the results of these studies, the recommendation for sodium intake of less than 2000 mg/day may be harmful because it has the possibility of increasing cardiovascular morbidity and mortality.
This result and the controversy surrounding it has created a strong demand for additional, large, prospective, long-term, randomized controlled trials in order to demonstrate the beneficial effect of sodium intake reduction and to further elucidate the counterintuitive finding that reducing sodium intake is harmful.20) However, such studies are difficult to perform due to many factors such as difficulty in determining the complexity of food and the exact proportion of sodium actually consumed by an individual on a daily basis. Furthermore, it is very difficult to accurately and consistently have the study participants restrict food intake according to a specific regimen for many years' duration. Furthermore, the analysis of the ONTARGET and TRANSCEND trial data,6) along with data from the PURE study7) indicated some flaws that were in common with other similar studies. Moreover, these studies had methodological flaws that will be discussed further in the next section.
In another meta-analysis by He et al.21) the authors obtained a different result compared to that obtained by Taylor and colleagues.18) Taylor and colleagues had excluded the Trial of Nonpharmacologic Interventions in the Elderly (TONE)22) and had included the study by Paterna et al.23) He and MacGregor21) had included the TONE study and had excluded the study by Paterna et al.23) since the study by Paterna et al.23) specifically evaluated the effect of sodium intake reduction in patients with heart failure. Different methods for performing the meta-analyses yielded completely different results among these studies. The meta-analysis by He et al.21) had decreased bias in the analysis because the participants of the heart failure trial were in a state of severe sodium and water depletion due to aggressive diuretic therapy and fluid restriction. The flaw in the study by Paterna et al.23) was that they did not adjust the dose of diuretics along with the implementation of a more aggressive sodium restriction, which resulted in overtreatment with worse outcomes in individuals in the sodium reduction group.
In the combined cohort analysis by Stolarz-Skrzypek et al.5) there is a lack of discussion or insight into potential confounders that may have led to the finding that higher sodium intake levels lead to a lower cardiovascular mortality. First, the time of recruitment of each cohort is different which may confound the data. In the Flemish Study on Environment, Genes, and Health Outcomes (FLEMENGHO), the participants were recruited from 1985 to 1990, and from June of 1996 to January of 2004, additional families were recruited. The recruitment of participants in the European Project on Genes in Hypertension (EPOGH) started in 1999 and ended in 2001. One thought is that since the recruitment was performed at such varied time periods, the advances in medicine and treatment that could have caused Stolarz and colleagues5) to observe a false decrease in morbidity and mortality in the high sodium intake group. The changes in treatment include the introduction of statins and antiplatelet agents to the therapeutic regimen in patients with known cardiovascular or coronary disease. The fact that the authors did not report the follow-up duration for each cohort in the outcome analysis, the longer follow-up duration of FLEMENGHO may be reflected in the higher rate of cardiovascular events compared to that in EPOGH (7.89% vs. 2.38%, repectively). Secondly, the outcome analysis was solely based on the baseline level of 24-hour sodium excretion, and not on the change in the 24-hour sodium excretion throughout the study period. Furthermore, they did not provide any information about the intervention and how the participants decreased their sodium intake, which was included in the analysis or discussion. They did not discuss the methods used to control the sodium intake whether or not the participants inadvertently increased their sodium intake during the follow-up period although they were supposed to be on a low sodium diet at baseline. They could not exclude the possibility that some of the cardiovascular events were related to an increase in sodium intake during the study period. Third, they did not evaluate the reason why participants had a low sodium intake at baseline and they failed to mention the nutritional status since poor nutritional balance often has a detrimental effect on health. The recommended sodium intake is calculated on the basis of adequate daily intake of nutrients in a balanced state. Thus, low sodium intake during a poor nutritional balance state (i.e., starvation) is quite a different matter. In such a person, it is easy to expect a poor cardiovascular outcome.
The analysis of the ONTARGET and TRANSCEND trial data,6) along with that of the data from the PURE study7) also showed some of the same flaws as in the study by Stolarz-Skrzypek et al.5) In addition, their study had methodological flaws that will be discussed in the following section.
Lastly, the BP response observed in relation to dietary sodium intake is not homogeneous within the population, and it has been shown that sodium-sensitive individuals demonstrate a greater change in BP with alteration in their sodium intake. Sodium sensitivity has been found to be an independent predictor of cardiovascular events.24),25) Subjects with hypertension and sodium sensitivity had the lowest cumulative survival rate. The association between sodium sensitivity and mortality was independent of elevated BP.25) Although the exact prevalence of sodium sensitivity is unknown, it is expected that more than 50% of hypertensive subjects have sodium sensitivity.26),27) Different BP responses to the same amount of sodium intake may be an explanation for the inconsistent result regarding the association between high sodium intake and cardiovascular events. However, it does not always mean that individuals with sodium resistance are free from complications associated with high sodium intake. It has been shown that morning BP surge is an independent risk factor for cardiovascular events.28),29) Individuals with sodium resistance showed an elevation of morning BP after high sodium intake.30)
Low sodium intake and high renin level
High renin activity is associated with high cardiovascular morbidity and mortality.31),32),33) Accordingly, it has been known that a reduction in sodium intake can elevate plasma renin activity.34) In a meta-analysis by Graudal et al.13) restriction of sodium intake was found to be associated with activation of the renin-angiotensin-aldosterone system. Thus, it has been postulated that activation of the renin-angiotensin-aldosterone system due to reduction of sodium intake may be the reason for the higher incidence of morbidity and mortality.35),36),37) However, the elevation of plasma renin activity by reducing sodium intake is only apparent in short-term interventional studies which are mostly less than 3 months.38) There is currently no long-term study of more than 3 months showing persistent elevation of plasma renin activity after reduction in sodium intake. Additionally, we found no association between sodium intake and plasma renin activity within the general population in a cross-sectional study.39) There is a possibility that plasma renin activity might return to normal when sodium intake is restricted over a longer period of time; however, there is still no study that confirms whether or not there is such an effect.
Measurement Methods of Sodium Intake
Accurate estimation of an individual's or a population's average sodium intake is essential to study the association between sodium intake and health and to establish a proper sodium intake reduction policy. Dietary surveys and urine collection methods are commonly used for the estimation of sodium intake. Prior to any study that use the assessment of sodium intake, the researchers should know the advantages and disadvantages of each method in detail (Table 2).
Table 2. Advantages and disadvantages of sodium intake measurement methods.
| Advantages | Disadvantages | |
|---|---|---|
| Dietary survey methods | Low to moderate burden | Difficult to accurately assess the amount of sodium added or lost during cooking and eating |
| Simultaneous measurement of other nutrients and energy | Inaccurate or incomplete food consumption tables and use of different tables between countries | |
| Available in a large population survey | Reporting and coding errors | |
| Variable content in manufactured or processed foods | ||
| High intra-observer and inter-observer variability | ||
| Variable food culture | ||
| 24-hour urine collection method | Enables comparison with other countries | High burden |
| Independent of cooking and eating habits, and food culture | Dependency on the season and weather | |
| Low intra-observer and inter-observer variability | Incomplete collection | |
| Low inter-survey variability | Dependency on the physical condition or renal function | |
| Spot urine method | Easy | Dependency on the time of collection |
| Convenient | Inaccuracy of the calculation formula | |
| Can be used easily in daily clinical practice and mass survey |
Dietary survey methods
Dietary survey methods are widely used because of the convenience and easy applicability to the population. The method requires the use of questionnaires or interviews to obtain specific information on food intake, which can then be converted into nutrient intake by using the food composition table. These surveys include dietary record, 24-hour dietary recall, food frequency, brief dietary assessment instruments, and diet history which can be used alone, in combination, or with modifications that are tailored for specific studies. The disadvantages of dietary survey methods are inaccurate estimation of dietary intake because of the inherent limitations such as reporting errors, inaccurate or incomplete food composition tables, missing data, and coding errors.40),41),42)
The actual amount of sodium intake can be affected by the variable proportion of sodium added during cooking or eating and the variable content of sodium in manufactured or processed foods.43),44) In addition, sodium loss during cooking (due to washing) or eating (sodium in the soup left behind in the dishes or bowel) should be considered, but it is extremely difficult to incorporate this sodium loss into any type of survey method.
There is also often a discrepancy between the sodium intake measured by dietary survey methods and 24-hour urinary collection method, and in one study, patients showed a tendency to underestimate their sodium intake by 30 to 50%.45) The analysis of data from the TONE study also revealed a larger under-estimation of sodium intake by 24-hour dietary recall than by 24-hour urine collection.40) Furthermore, the correlation between the dietary survey method and the 24-hour urine collection method in the estimation of sodium intake was not good (r=0.3) although it was statistically significant.40),46)
Another disadvantage of the dietary survey method is that a direct comparison of sodium intake between countries is difficult because they use different food composition tables. Analysis of data from the international collaborative study of macronutrients, micronutrients and BP (INTERMAP) study clarified the difference between sodium intake measured by a 24-hour dietary recall and that measured by two 24-hour urine collections.47) The differences between countries were not consistent and ranged from 0.3% to 30%, indicating a significant estimation error by the dietary recall method. The validity of food composition tables is inconsistent and also considered to be an important source of errors.42),48) There are no validation tests or standards for food composition tables, and due to the cultural and linguistic differences it would be nearly impossible to create uniform food composition tables throughout the world. The most recent Korean National Health and Nutrition Examination Survey has modified the food composition table by implementing recalculation of sodium intake, which differs from that in previous surveys. Use of the modified food composition table will yield a different result of sodium intake from that in a previous survey.
The 24-hour urine collection method
Approximately 85 to 90% of ingested sodium is excreted in urine,49) while the rest is lost either in feces or sweat due to exercise and high-temperature climates.50) Unlike the dietary survey method, the urine collection method is not influenced by interviewers, different cooking methods, eating habits, and diverse survey methods. Furthermore, it enables the comparison of data between countries, or between old and new data. Thus, the urine collection method is more reasonable and likely to be more accurate in comparing sodium intake between populations and studies, allowing for a greater ability to design collaborative multi-national studies or large-scale meta-analyses.
However, the major disadvantage of the 24-hour urine collection method is that it requires a high level of participant compliance as well as education on the complete collection method. Thus, performing measurements in more than 1000 people in a limited time period may be exceedingly difficult. Since there is a large day-to-day variation in sodium intake, a single collection of 24-hour urine may not be enough for estimation of individual sodium intake. At least nine to fourteen 24-hour urine collection specimens have been recommended in order to obtain the highest yield and the most accurate results.51),52) However, when performing an epidemiologic study in a large population, obtaining multiple collections of 24-hour urine is difficult and frequently impractical. On the other hand, a single 24-hour urine collection from a large number of people may improve the accuracy of estimation of sodium intake in the population.53)
Another disadvantage is that urine collection should be performed in a manner that will prevent loss of urine, for example forgetting to void in the sample container. The INTERSALT study was entirely dependent on the participant's report on the determination of complete urine collection, in which the participant may forget to report loss of urine.3),54) Due to this limitation, biochemical methods such as urine creatinine and para-aminobenzoic acid (PABA) excretion have been used to determine complete urine collection. In a study by Bingham and Cummings55) the mean recovery of PABA over 24-hours in urine was 93±4% in 33 free living volunteers when it was given at a dose of 240 mg a day. The range in individual recovery from maximum to minimum was 15% of the mean. In other sodium intake surveys in the UK, PABA was used to determine complete collection of 24-hour urine, which will contain 85-110% of the PABA marker in order to be considered complete.56),57) However, PABA is unavailable in some countries and it elevates the cost of the survey. Another drawback of using PABA is that PABA has also been known to cause a hypersensitivity reaction in susceptible individuals, although the incidence is very low.58)
Instead of PABA, urinary creatinine excretion has been used as a marker for the determination of complete urine collection since it is easy to use and is not very expensive. However, urinary creatinine excretion is highly dependent on the amount of ingested protein59) and the individual's lean body mass.60) Various formulae for creatinine excretion have been used to check for adequate collection of 24-hour urine.61),62),63),64),65),66) Among them, the formula suggested by Knuimann et al.61) has the highest sensitivity and specificity.67) In our survey in 2011, we used urinary creatinine excretion in addition to the participant's report for the loss of urine.66) The percentage of valid urine collection based on our criteria was 74.2%, which seemed to be lower when compared to that in other studies. The percentage of valid urine collection depends on the criteria for the creatinine-based determination method used. When we applied the method used in a Finnish study,62) the percentage of valid urine collection in our study was 96.6%. When the Portuguese adult population study criteria was used, the percentage of valid urine collection was 93.5%.63) Irrespective of the creatinine-based methods used, there was no difference in the estimated sodium intake in the study population.66)
Regardless of the aforementioned limitations, the 24-hour urine collection method is becoming the "gold standard" method in the estimation of sodium intake.53) In our survey in 2011, among the 21 countries surveyed, 9 countries used the 24-hour urine collection method, 3 countries used a combination of the dietary survey and 24-hour urine collection method, 3 countries used the spot urine collection method, and 6 countries used only the dietary survey method.68) In another survey, less than half of the 30 surveyed countries used the 24-hour urine collection method.69)
Spot urine collection method (Table 1)
Because of the inconvenience and high participant burden of the 24-hour urine collection, a less demanding method has been sought after. As a potential solution, estimation of the 24-hour urinary sodium excretion by using the calculation from a randomly or timely collected spot urine sample has been suggested.70),71),72),73) These equations showed a fairly high correlation between the calculated 24-hour urinary sodium excretion and the measured 24-hour urinary sodium excretion.
Several studies have used the calculated 24-hour urinary sodium excretion from spot urine for evaluation of the relationship between sodium intake and cardiovascular disease or central aortic BP.6),74) Two recent studies evaluated the association of urinary sodium and potassium excretion with cardiovascular events and overall mortality.6),7) They calculated the 24-hour urinary sodium and potassium excretion from a morning fasting urine sample using the Kawasaki formula,70) and reported an interesting J-shaped association between the estimated urinary sodium excretion from the single morning fasting urine sample and cardiovascular events. They also reported the result of the validation analysis of the Kawasaki equation which they used for an accurate calculation.75) Although they suggested that the Kawasaki equation is the most valid and least biased method, the serious drawback of the spot urine method for estimation of the 24-hour urinary sodium excretion should be considered.
One potential drawback of the spot urine collection method is that its accuracy is dependent on the time of urine collection, since there is a large variation in urinary sodium excretion throughout the day. This large variation exists not only between individuals, but it is also observed to fluctuate throughout the day in a single person.76),77),78) In addition, this variation is also dependent on the amount of sodium ingested.79) Due to this variation, the time of spot urine collection may significantly influence the accuracy of the estimating equation and result in skewed or incorrect data. Although the Kawasaki equation was developed for the second morning urine,70) O'Donnell et al.6),7) used the first morning or fasting morning urine in the Kawasaki equation instead of the second morning urine. The Tanaka equation was developed for the randomly collected urine.71) Kawamura et al.80) suggested that second morning urine is better than randomly collected urine when using the Kawasaki equation. However, Mann and Gerber73) recommended collection of spot urine samples at the midpoint of 24-hour urine collection (for example in the late afternoon) for the highest correlation between the measured and estimated 24-hour urinary sodium excretion.
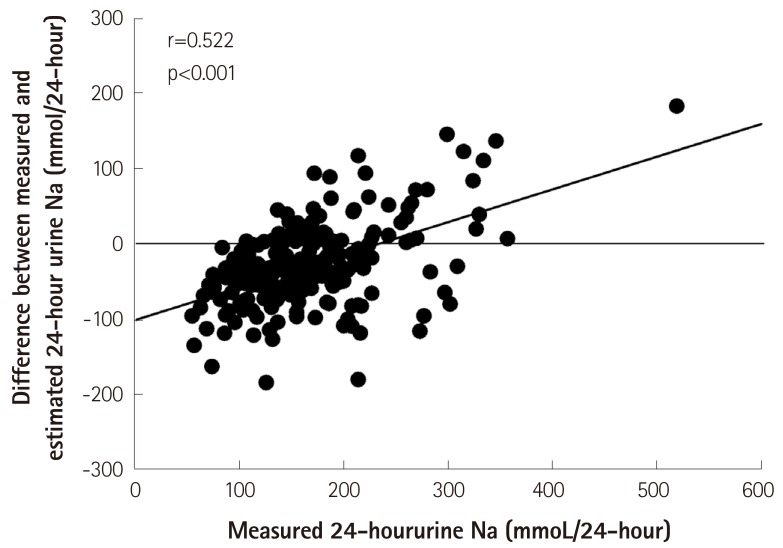
For validating any particular method, the correlation analysis is not enough for assessing the agreement between methods. A significant correlation between the measured and estimated 24-hour urinary sodium excretion does not always mean that both methods can be used as substitutes for each other. Similar to our study, the validation test of the Tanaka equation in an external population showed a significant difference between the estimated and measured 24-hour urinary sodium excretion (p<0.001).71) There was also a significant difference between the measured and estimated 24-hour urinary sodium excretion in the validation test among the PURE study population.75) Furthermore, when using the Bland-Altman method for comparison of the two methods, the limits of agreement and percentage errors should be considered. 81),82) Ji et al. as well as ourselves used the Bland-Altman method to determine the agreement between the spot urine collection method and the 24-hour urine collection method for the estimation of sodium intake.83),84),85) All equations showed large limits of agreement approximately more than 100 mmol.72),75),83),85) Furthermore, the equations used for calculation of the 24-hour urinary sodium excretion from spot urine have a tendency for under- or over-estimation of the 24-hour urinary sodium excretion according to the level of an individual's sodium intake (Fig. 1).85)
Fig. 1. Under- or over-estimation of the 24-hour urinary sodium excretion by the Kawasaki equation, depending on the level of measured 24-hour urinary sodium excretion.85) Adopted from Rhee MY, Kim JH, Shin SJ, et al. Nutrients 2014;6:2360-75.
Despite the tendency of these calculations for under- or over-estimation of the 24-hour sodium excretion and their large limits of agreement, Mente et al.75) concluded that estimation of the 24-hour urinary sodium excretion from a single morning fasting urine specimen by using the Kawasaki equation is also suitable for large population studies, and they used the spot urine method in their study.7)
Due to the various drawbacks of the spot urine collection method and the equations used to estimate the 24-hour urinary sodium excretion, there is still no single calculation or method that is able to accurately estimate the 24-hour urinary sodium excretion among the individuals in a study.
Conclusion
Although the recent studies argued that the current recommendation for dietary sodium intake should be changed, the association between low sodium intake and high risk of cardiovascular events is not evident because of the various drawbacks of the methods. Until now, there is no doubt that high sodium intake elevates BP and it may eventually lead to high risk of cardiovascular events. Furthermore, using the accurate method and knowing its limitations in the measurement of sodium intake is an essential step towards proper understanding of hazardous effects of high sodium intake, which will promote sodium intake reduction in humans.
Acknowledgments
This research was supported by grants (13162MFDS106) from the Korea Ministry of Food and Drug Safety in 2013.
Footnotes
The author has no financial conflicts of interest.
References
- 1.Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He FJ, MacGregor GA. Salt, blood pressure and cardiovascular disease. Curr Opin Cardiol. 2007;22:298–305. doi: 10.1097/HCO.0b013e32814f1d8c. [DOI] [PubMed] [Google Scholar]
- 3.Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Guideline: Sodium intake for adults and children. Geneva, Switzerland: World Health Organization; 2012. [PubMed] [Google Scholar]
- 5.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–1785. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 6.O'Donnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–2238. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 7.O'Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 8.Elliott P, Stamler J, Nichols R, et al. Intersalt Cooperative Research Group. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. BMJ. 1996;312:1249–1253. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khaw KT, Bingham S, Welch A, et al. Blood pressure and urinary sodium in men and women: the Norfolk Cohort of the European Prospective Investigation into Cancer (EPIC-Norfolk) Am J Clin Nutr. 2004;80:1397–1403. doi: 10.1093/ajcn/80.5.1397. [DOI] [PubMed] [Google Scholar]
- 10.MacGregor GA, Markandu ND, Best FE, et al. Double-blind randomised crossover trial of moderate sodium restriction in essential hypertension. Lancet. 1982;1:351–355. doi: 10.1016/s0140-6736(82)91389-7. [DOI] [PubMed] [Google Scholar]
- 11.Sacks FM, Svetkey LP, Vollmer WM, et al. DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 12.Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis. 2006;49:59–75. doi: 10.1016/j.pcad.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Graudal NA, Hubeck-Graudal T, Jürgens G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review) Am J Hypertens. 2012;25:1–15. doi: 10.1038/ajh.2011.210. [DOI] [PubMed] [Google Scholar]
- 14.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- 15.The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA. 1992;267:1213–1220. doi: 10.1001/jama.1992.03480090061028. [DOI] [PubMed] [Google Scholar]
- 16.The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. Arch Intern Med. 1997;157:657–667. [PubMed] [Google Scholar]
- 17.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease: a meta-analysis of randomized controlled trials (Cochrane review) Am J Hypertens. 2011;24:843–853. doi: 10.1038/ajh.2011.115. [DOI] [PubMed] [Google Scholar]
- 19.Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S. WITHDRAWN: Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;9:CD009217. doi: 10.1002/14651858.CD009217.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Alderman MH. Reducing dietary sodium: the case for caution. JAMA. 2010;303:448–449. doi: 10.1001/jama.2010.69. [DOI] [PubMed] [Google Scholar]
- 21.He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet. 2011;378:380–382. doi: 10.1016/S0140-6736(11)61174-4. [DOI] [PubMed] [Google Scholar]
- 22.Whelton PK, Appel LJ, Espeland MA, et al. TONE Collaborative Research Group. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE) JAMA. 1998;279:839–846. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 23.Paterna S, Gaspare P, Fasullo S, Sarullo FM, Di Pasquale P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci (Lond) 2008;114:221–230. doi: 10.1042/CS20070193. [DOI] [PubMed] [Google Scholar]
- 24.Morimoto A, Uzu T, Fujii T, et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–1737. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 25.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37(2 Pt 2):429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 26.Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr. 2006;25(3 Suppl):247S–255S. doi: 10.1080/07315724.2006.10719574. [DOI] [PubMed] [Google Scholar]
- 27.Shin SJ, Lim CY, Rhee MY, et al. Characteristics of sodium sensitivity in Korean populations. J Korean Med Sci. 2011;26:1061–1067. doi: 10.3346/jkms.2011.26.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metoki H, Ohkubo T, Kikuya M, et al. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension. 2006;47:149–154. doi: 10.1161/01.HYP.0000198541.12640.0f. [DOI] [PubMed] [Google Scholar]
- 29.Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 30.Rhee MY, Lim CY, Shin SJ, et al. Elevation of morning blood pressure in sodium resistant subjects by high sodium diet. J Korean Med Sci. 2013;28:555–563. doi: 10.3346/jkms.2013.28.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med. 1991;324:1098–1104. doi: 10.1056/NEJM199104183241605. [DOI] [PubMed] [Google Scholar]
- 32.de Boer RA, Schroten NF, Bakker SJ, et al. Plasma renin and outcome in the community: data from PREVEND. Eur Heart J. 2012;33:2351–2359. doi: 10.1093/eurheartj/ehs198. [DOI] [PubMed] [Google Scholar]
- 33.Verma S, Gupta M, Holmes DT, et al. Plasma renin activity predicts cardiovascular mortality in the Heart Outcomes Prevention Evaluation (HOPE) study. Eur Heart J. 2011;32:2135–2142. doi: 10.1093/eurheartj/ehr066. [DOI] [PubMed] [Google Scholar]
- 34.Brunner HR, Laragh JH, Baer L, et al. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med. 1972;286:441–449. doi: 10.1056/NEJM197203022860901. [DOI] [PubMed] [Google Scholar]
- 35.Grassi G, Dell'Oro R, Seravalle G, Foglia G, Trevano FQ, Mancia G. Short- and long-term neuroadrenergic effects of moderate dietary sodium restriction in essential hypertension. Circulation. 2002;106:1957–1961. doi: 10.1161/01.cir.0000033519.45615.c7. [DOI] [PubMed] [Google Scholar]
- 36.Alderman MH, Madhavan S, Cohen H, Sealey JE, Laragh JH. Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension. 1995;25:1144–1152. doi: 10.1161/01.hyp.25.6.1144. [DOI] [PubMed] [Google Scholar]
- 37.Tikellis C, Pickering RJ, Tsorotes D, et al. Activation of the Renin-Angiotensin system mediates the effects of dietary salt intake on atherogenesis in the apolipoprotein E knockout mouse. Hypertension. 2012;60:98–105. doi: 10.1161/HYPERTENSIONAHA.112.191767. [DOI] [PubMed] [Google Scholar]
- 38.Kotchen TA, Cowley AW, Jr, Frohlich ED. Salt in health and disease--a delicate balance. N Engl J Med. 2013;368:1229–1237. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 39.Rhee MY, Kim JH, Shin SJ, Lim CY, Kim SW, Nah DY. Relationship between plasma renin activity and 24-hour urinary sodium excretion: low sodium intake is dangerous by elevation of renin? Eur Heart J. 2013;34:1117. Abstract. [Google Scholar]
- 40.Espeland MA, Kumanyika S, Wilson AC, et al. Statistical issues in analyzing 24-hour dietary recall and 24-hour urine collection data for sodium and potassium intakes. Am J Epidemiol. 2001;153:996–1006. doi: 10.1093/aje/153.10.996. [DOI] [PubMed] [Google Scholar]
- 41.Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38:791–813. doi: 10.1093/ije/dyp139. [DOI] [PubMed] [Google Scholar]
- 42.Kwon YJ, Rhee MY, Kim JY, et al. Differences between analyzed and estimated sodium contents of food composition table or food exchange list. J Korean Soc Food Sci Nutr. 2010;39:535–541. [Google Scholar]
- 43.James WP, Ralph A, Sanchez-Castillo CP. The dominance of salt in manufactured food in the sodium intake of affluent societies. Lancet. 1987;1:426–429. doi: 10.1016/s0140-6736(87)90127-9. [DOI] [PubMed] [Google Scholar]
- 44.Mattes RD, Donnelly D. Relative contributions of dietary sodium sources. J Am Coll Nutr. 1991;10:383–393. doi: 10.1080/07315724.1991.10718167. [DOI] [PubMed] [Google Scholar]
- 45.Leiba A, Vald A, Peleg E, Shamiss A, Grossman E. Does dietary recall adequately assess sodium, potassium, and calcium intake in hypertensive patients? Nutrition. 2005;21:462–466. doi: 10.1016/j.nut.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 46.Reinivuo H, Valsta LM, Laatikainen T, Tuomilehto J, Pietinen P. Sodium in the Finnish diet: II trends in dietary sodium intake and comparison between intake and 24-h excretion of sodium. Eur J Clin Nutr. 2006;60:1160–1167. doi: 10.1038/sj.ejcn.1602431. [DOI] [PubMed] [Google Scholar]
- 47.Stamler J, Elliott P, Chan Q INTERMAP Research Group. INTERMAP appendix table, tables of contents (tables A) J Hum Hypertens. 2003;17:665–758. [Google Scholar]
- 48.Yoshita K, Miura K, Okayama A, et al. A validation study on food composition tables for the international cooperative INTERMAP study in Japan. Environ Health Prev Med. 2005;10:150–156. doi: 10.1007/BF02900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holbrook JT, Patterson KY, Bodner JE, et al. Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr. 1984;40:786–793. doi: 10.1093/ajcn/40.4.786. [DOI] [PubMed] [Google Scholar]
- 50.Kirby CR, Convertino VA. Plasma aldosterone and sweat sodium concentrations after exercise and heat acclimation. J Appl Physiol (1985) 1986;61:967–970. doi: 10.1152/jappl.1986.61.3.967. [DOI] [PubMed] [Google Scholar]
- 51.Luft FC, Fineberg NS, Sloan RS. Estimating dietary sodium intake in individuals receiving a randomly fluctuating intake. Hypertension. 1982;4:805–808. doi: 10.1161/01.hyp.4.6.805. [DOI] [PubMed] [Google Scholar]
- 52.Liu K, Cooper R, McKeever J, et al. Assessment of the association between habitual salt intake and high blood pressure: methodological problems. Am J Epidemiol. 1979;110:219–226. doi: 10.1093/oxfordjournals.aje.a112806. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. Reducing Salt Intake in Populations: Report of a WHO Forum and Technical Meeting. Paris, France: World Health Organization; 2007. [Google Scholar]
- 54.The INTERSALT Co-operative Research Group. INTERSALT Study an international co-operative study on the relation of blood pressure to electrolyte excretion in populations. I. Design and methods. J Hypertens. 1986;4:781–787. doi: 10.1097/00004872-198612000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Bingham S, Cummings JH. The use of 4-aminobenzoic acid as a marker to validate the completeness of 24 h urine collections in man. Clin Sci (Lond) 1983;64:629–635. doi: 10.1042/cs0640629. [DOI] [PubMed] [Google Scholar]
- 56.National Centre for Social Research. An assessment of dietary sodium levels among adults (aged 19-64) in the UK general population in 2008, based on analysis of dietary sodium in 24 hour urine samples. UK, London: Food Standards Agency; 2008. [Google Scholar]
- 57.Scottish Centre for Social Research. A survey of 24-hour urinary sodium excretion in a representative sample of the Scottish population as a measure of salt intake. London, UK: Scottish Centre for Social Research; 2011. [Google Scholar]
- 58.Henderson L, Irving K, Gregory J, et al. Volume 3: Vitamin and mineral intake and urinary analytes. London: TSO; 2003. National Diet and Nutrition Survey: adults aged 19 to 64 years. [Google Scholar]
- 59.Lykken GI, Jacob RA, Munoz JM, Sandstead HH. A mathematical model of creatine metabolism in normal males--comparison between theory and experiment. Am J Clin Nutr. 1980;33:2674–2685. doi: 10.1093/ajcn/33.12.2674. [DOI] [PubMed] [Google Scholar]
- 60.Forbes GB, Bruining GJ. Urinary creatinine excretion and lean body mass. Am J Clin Nutr. 1976;29:1359–1366. doi: 10.1093/ajcn/29.12.1359. [DOI] [PubMed] [Google Scholar]
- 61.Knuiman JT, Hautvast JG, van der Heyden L, et al. A multi-centre study on completeness of urine collection in 11 European centres. I. Some problems with the use of creatinine and 4-aminobenzoic acid as markers of the completeness of collection. Hum Nutr Clin Nutr. 1986;40:229–237. [PubMed] [Google Scholar]
- 62.Laatikainen T, Pietinen P, Valsta L, Sundvall J, Reinivuo H, Tuomilehto J. Sodium in the Finnish diet: 20-year trends in urinary sodium excretion among the adult population. Eur J Clin Nutr. 2006;60:965–970. doi: 10.1038/sj.ejcn.1602406. [DOI] [PubMed] [Google Scholar]
- 63.Polónia J, Maldonado J, Ramos R, et al. Estimation of salt intake by urinary sodium excretion in a Portuguese adult population and its relationship to arterial stiffness. Rev Port Cardiol. 2006;25:801–817. [PubMed] [Google Scholar]
- 64.Ortega RM, López-Sobaler AM, Ballesteros JM, et al. Estimation of salt intake by 24 h urinary sodium excretion in a representative sample of Spanish adults. Br J Nutr. 2011;105:787–794. doi: 10.1017/S000711451000423X. [DOI] [PubMed] [Google Scholar]
- 65.Ribič CH, Zakotnik JM, Vertnik L, Vegnuti M, Cappuccio FP. Salt intake of the Slovene population assessed by 24 h urinary sodium excretion. Public Health Nutr. 2010;13:1803–1809. doi: 10.1017/S136898001000025X. [DOI] [PubMed] [Google Scholar]
- 66.Rhee MY, Shin SJ, Park SH, Kim SW. Sodium intake of a city population in Korea estimated by 24-h urine collection method. Eur J Clin Nutr. 2013;67:875–880. doi: 10.1038/ejcn.2013.87. [DOI] [PubMed] [Google Scholar]
- 67.Murakami K, Sasaki S, Takahashi Y, et al. Sensitivity and specificity of published strategies using urinary creatinine to identify incomplete 24-h urine collection. Nutrition. 2008;24:16–22. doi: 10.1016/j.nut.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Survey for natrium intake, and analysis of the relationship between natrium intake and health by urine collection method. Osong, Korea: Korea Ministry of Food and Drug Safety; 2012. [Google Scholar]
- 69.Hawkes C, Webster J. National approaches to monitoring population salt intake: a trade-off between accuracy and practicality? PLoS One. 2012;7:e46727. doi: 10.1371/journal.pone.0046727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol. 1993;20:7–14. doi: 10.1111/j.1440-1681.1993.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103. doi: 10.1038/sj.jhh.1001307. [DOI] [PubMed] [Google Scholar]
- 72.Brown IJ, Dyer AR, Chan Q, et al. Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: the INTERSALT study. Am J Epidemiol. 2013;177:1180–1192. doi: 10.1093/aje/kwt066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mann SJ, Gerber LM. Estimation of 24-hour sodium excretion from spot urine samples. J Clin Hypertens (Greenwich) 2010;12:174–180. doi: 10.1111/j.1751-7176.2009.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park S, Park JB, Lakatta EG. Association of central hemodynamics with estimated 24-h urinary sodium in patients with hypertension. J Hypertens. 2011;29:1502–1507. doi: 10.1097/HJH.0b013e3283486311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mente A, O'Donnell MJ, Dagenais G, et al. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24-h measures in 11 countries. J Hypertens. 2014;32:1005–1014. doi: 10.1097/HJH.0000000000000122. discussion 1015. [DOI] [PubMed] [Google Scholar]
- 76.Stanbury SW, Thomson AE. Diurnal variation in electrolyte excretion. Clin Sci (Lond) 1951;10:267–293. [PubMed] [Google Scholar]
- 77.Yamori Y, Kihara M, Fujikawa J, et al. Dietary risk factors of stroke and hypertension in Japan -- Part 1: Methodological assessment of urinalysis for dietary salt and protein intakes. Jpn Circ J. 1982;46:933–938. doi: 10.1253/jcj.46.933. [DOI] [PubMed] [Google Scholar]
- 78.Yamori Y, Kihara M, Fujikawa J, et al. Dietary risk factors of stroke and hypertension in Japan -- Part 2: Validity of urinalysis for dietary salt and protein intakes under a field condition. Jpn Circ J. 1982;46:939–943. doi: 10.1253/jcj.46.939. [DOI] [PubMed] [Google Scholar]
- 79.Wesson LG., Jr Electrolyte excretion in relation to diurnal cycles of renal function. Medicine (Baltimore) 1964;43:547–592. doi: 10.1097/00005792-196409000-00002. [DOI] [PubMed] [Google Scholar]
- 80.Kawamura M, Kawasaki T. Clinical application of the second morning urine method for estimating salt intake in patients with hypertension. Clin Exp Hypertens. 2014 doi: 10.3109/10641963.2014.913601. [Epub ahead of print]. http://dx.doi:10.3109/10641963.2014.913601. [DOI] [PubMed] [Google Scholar]
- 81.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–1087. doi: 10.1016/s0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- 82.Hanneman SK. Design, analysis, and interpretation of method-comparison studies. AACN Adv Crit Care. 2008;19:223–234. doi: 10.1097/01.AACN.0000318125.41512.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 84.Ji C, Miller MA, Venezia A, Strazzullo P, Cappuccio FP. Comparisons of spot vs 24-h urine samples for estimating population salt intake: validation study in two independent samples of adults in Britain and Italy. Nutr Metab Cardiovasc Dis. 2014;24:140–147. doi: 10.1016/j.numecd.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 85.Rhee MY, Kim JH, Shin SJ, et al. Estimation of 24-hour urinary sodium excretion using spot urine samples. Nutrients. 2014;6:2360–2375. doi: 10.3390/nu6062360. [DOI] [PMC free article] [PubMed] [Google Scholar]