Abstract
A circular covalently closed duplex deoxyribonucleic acid plasmid carrying genes for resistance to kanamycin/neomycin has been identified in Staphylococcus aureus E419. The plasmid has a molecular size of 9.2 × 106 daltons and can be transduced into or can transform competent susceptible strains of S. aureus to kanamycin/neomycin resistance.
Full text
PDF

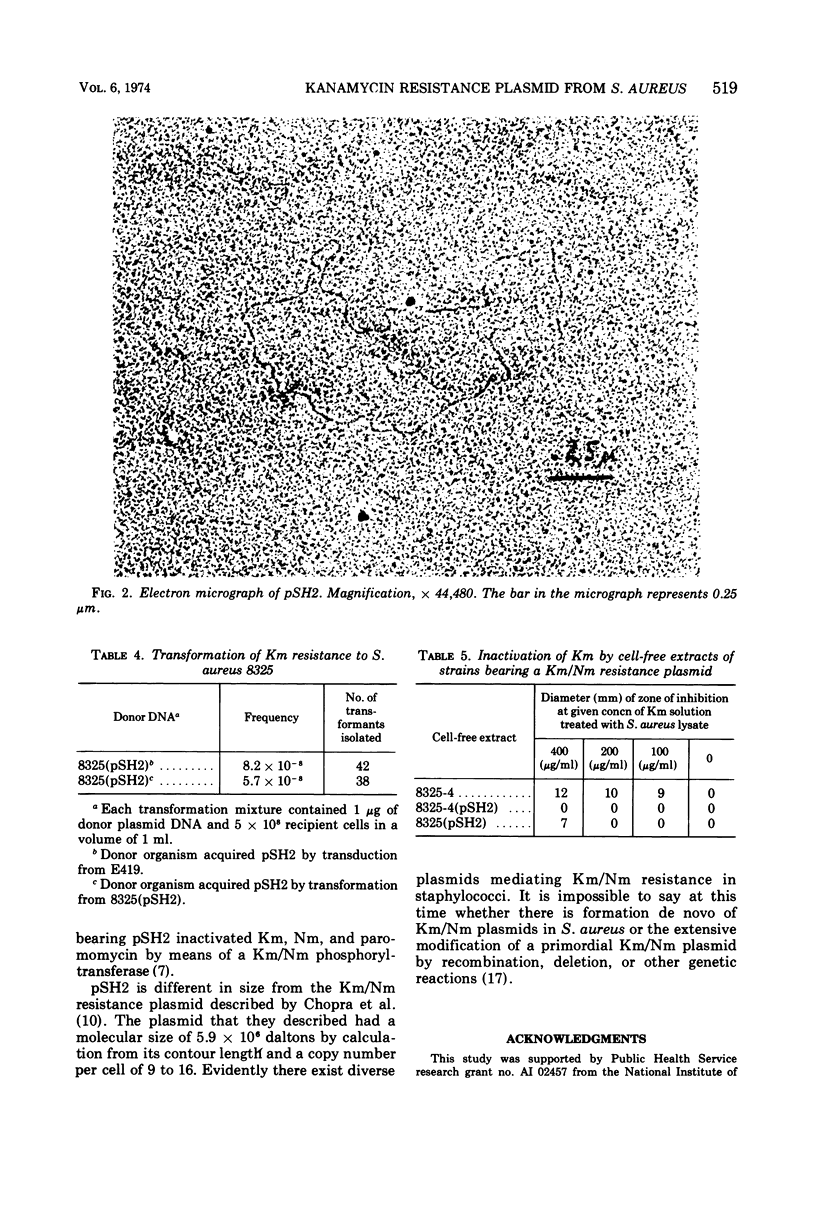

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Annear D. I., Grubb W. B. Unstable resistance to kanamycin, lincomycin and penicillin in a methicillin resistant culture of Staphylococcus aureus. Pathology. 1972 Oct;4(4):247–252. doi: 10.3109/00313027209068949. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- CHABBERT Y. A., BAUDENS J. G., GERBAUD G. R. VARIATIONS SOUS L'INFLUENCE DE L'ACRIFLAVINE ET TRANSDUCTION DE LA R'ESISTANCE A LA KANAMYCINE ET AU CHLORAMPH'ENICOL CHEZ LES STAPHYLOCOQUES. Ann Inst Pasteur (Paris) 1964 Nov;107:678–690. [PubMed] [Google Scholar]
- Chopra I., Bennett P. M., Lacey R. W. A variety of Staphylococcal plasmids present as multiple copies. J Gen Microbiol. 1973 Dec;79(2):343–345. doi: 10.1099/00221287-79-2-343. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Transduction of Methicillin Resistance in Staphylococcus aureus Dependent on an Unusual Specificity of the Recipient Strain. J Bacteriol. 1970 Dec;104(3):1158–1167. doi: 10.1128/jb.104.3.1158-1167.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi O., Miyamoto M., Tanaka N., Umezawa H. Inactivation and phosphorylation of kanamycin by drug-resistant Staphylococcus aureus. Appl Microbiol. 1968 Sep;16(9):1282–1284. doi: 10.1128/am.16.9.1282-1284.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W. Genetic basis, epidemiology, and future significance of antibiotic resistance in Staphylococcus aureus: a review. J Clin Pathol. 1973 Dec;26(12):899–913. doi: 10.1136/jcp.26.12.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W. High-frequency transfer of neomycin resistance between naturally occurring strains of Staphylococcus aureus. J Med Microbiol. 1971 Feb;4(1):73–84. doi: 10.1099/00222615-4-1-73. [DOI] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Lindberg M., Novick R. P. Plasmid-specific transformation in Staphylococcus aureus. J Bacteriol. 1973 Jul;115(1):139–145. doi: 10.1128/jb.115.1.139-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Peyru G., Wexler L. F., Novick R. P. Naturally occurring penicillinase plasmids in Staphylococcus aureus. J Bacteriol. 1969 Apr;98(1):215–221. doi: 10.1128/jb.98.1.215-221.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Absence of circular plasmid deoxyribonucleic acid attributable to a genetic determinant for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):771–777. doi: 10.1128/jb.116.2.771-777.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J. Physical and topological properties of circular DNA. J Gen Physiol. 1966 Jul;49(6):103–125. doi: 10.1085/jgp.49.6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]




