Abstract
In total, 1 in 1000 individuals carries a germline mutation in the PKD1 or PKD2 gene, which leads to autosomal dominant polycystic kidney disease (ADPKD). Cysts can form early in life and progressively increase in number and size during adulthood. Extensive research has led to the presumption that somatic inactivation of the remaining allele initiates the formation of cysts, and the progression is further accelerated by renal injury. However, this hypothesis is primarily on the basis of animal studies, in which the gene is inactivated simultaneously in large percentages of kidney cells. To mimic human ADPKD in mice more precisely, we reduced the percentage of Pkd1-deficient kidney cells to 8%. Notably, no pathologic changes occurred for 6 months after Pkd1 deletion, and additional renal injury increased the likelihood of cyst formation but never triggered rapid PKD. In mildly affected mice, cysts were not randomly distributed throughout the kidney but formed in clusters, which could be explained by increased PKD-related signaling in not only cystic epithelial cells but also, healthy-appearing tubules near cysts. In the majority of mice, these changes preceded a rapid and massive onset of severe PKD that was remarkably similar to human ADPKD. Our data suggest that initial cysts are the principal trigger for a snowball effect driving the formation of new cysts, leading to the progression of severe PKD. In addition, this approach is a suitable model for mimicking human ADPKD and can be used for preclinical testing.
Keywords: autosomal dominant polycystic kidney disease, polycystic kidney disease, renal injury, signaling, genetic renal disease
Individuals that carry a heterozygous mutation in polycystic kidney disease 1 (PKD1) or PKD2 develop autosomal dominant polycystic kidney disease (ADPKD). ADPKD is mainly characterized by focal cyst formation in the kidneys, leading to progressive enlargement of the kidneys, fibrosis of the remaining parenchyma, and renal failure at middle age.1 The formation of cysts can already begin in utero, and new cysts can also form during adulthood.2,3 Cysts form in a minority of nephrons, which suggests that a heterozygous mutation in PKD1 or PKD2 is not sufficient to induce cyst formation. Evidence suggests that for cyst formation to occur, it is required that during life the second allele is also lost by for instance, a somatic mutation. Indeed, epithelial cells isolated from individual cysts are clonal in origin and frequently have lost the remaining normal allele by a somatic mutation.4 In mice, although homozygous inactivation of Pkd1 or Pkd2 leads to cyst formation and embryonic lethality, heterozygous knockout (KO) mice have no overt phenotype.5 Moreover, mice carrying a combination of one Pkd2-null allele and one unstable Pkd2-WS25 allele develop renal cysts.6 The window of Pkd1 expression must be regulated rather tightly, because models with reduced (down to <20%) or elevated (by 2- to 15-fold) Pkd1 levels also develop PKD.7–10
To circumvent embryonic lethality, Pkd1 conditional KO mice have been generated in which Pkd1 or Pkd2 is inactivated. These mice develop PKD and revealed that the context of renal tissue has a profound effect on the rate of disease progression. Deletion of Pkd1 before postnatal day 13 (PN13) results in rapid disease onset, whereas deleting Pkd1 in adult mice results in a much slower progression.11,12 Kidneys from these adult mice only exhibit mild pathologic changes, such as tubular dilations during the first 2–3 months after Pkd1 KO, after which the disease progresses to end stage renal failure because of massive enlargement of the kidneys.11 The disease progression accelerates considerably after acute renal injury, which led to the view that renal injury is an important trigger for cyst formation in the adult kidney.13–15 However, this view is primarily on the basis of studies with adult mice, in which Pkd1 is deleted in much larger proportions of cells than in patients with ADPKD. To mimic human ADPKD more precisely, we reduced the number of Pkd1-deficient kidney cells in tamoxifen-inducible kidney-specific Pkd1 deletion (iKsp-Pkd1del) mice down to 8%.11 The data reveal that scattered Pkd1 deletion leads to a long dormant period followed by a relatively rapid onset of severe PKD in the majority of mice. Our data suggest that this sudden shift was primarily caused by the initial cysts that triggered a cystic snowball effect driving the formation of new cysts. In addition, our approach is a suitable model for mimicking human ADPKD and will provide more accurate predictions of clinical outcome.
Results
Scattered Pkd1 Deletion in 8% of Kidney Cells in Mice Does Not Lead to PKD within 6 Months
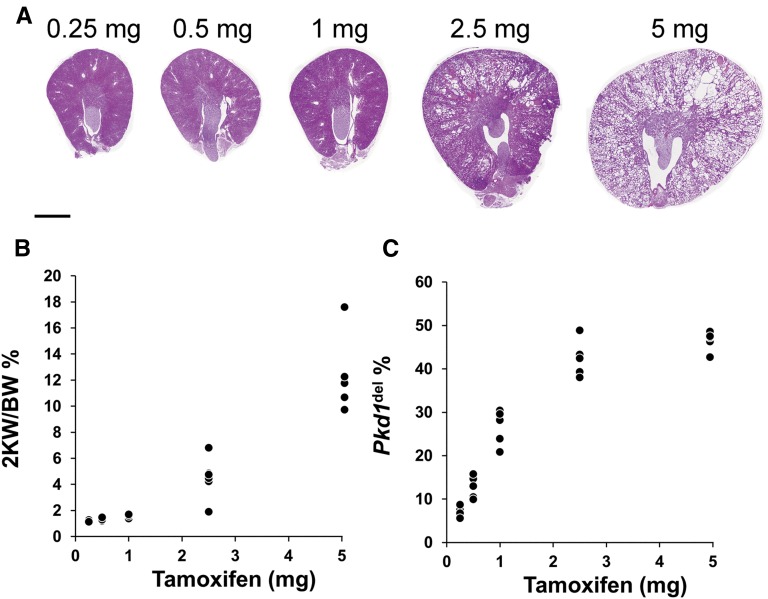
We previously developed a conditional mouse model (iKsp-Pkd1del) in which high-dose (5 mg) tamoxifen administration at PN40 leads to Cre-mediated conversion of Pkd1lox to Pkd1del alleles in >40% of the kidney cells and severe PKD within 4–5 months (Figure 1).11,16–18 To mimic human ADPKD more precisely, we treated iKsp-Pkd1lox,lox mice with a range of tamoxifen doses and quantified the number of Pkd1-deficient cells by using extension multiplex ligation-dependent probe amplification (eMLPA) (Supplemental Figure 1).17 Mice treated with 2.5 mg tamoxifen developed a moderate cystic phenotype accompanied by increased kidney-to-body weight ratios (2KW/BW), and reducing the dose further did not lead to cystogenesis (Figure 1, A and B). However, even at the lowest dose tested (0.25 mg), 5%–10% of the Pkd1lox DNA had been converted to Pkd1del DNA (Figure 1C). Six months after receiving low-dose (i.e., 0.25 mg) tamoxifen treatment, there was no indication of cyst formation in the analyzed sections (Figure 2A). In addition, other than a few clearly dilated tubules (3 of 1311), the tubular diameters were still unaltered (Figure 2B).
Figure 1.
Cre-mediated recombination efficiency and renal pathology at different doses of tamoxifen. (A) Representative images of hematoxylin and eosin-stained kidney sections of iKsp-Pkd1lox,lox mice treated with the indicated tamoxifen doses. All high dose (5 mg)-treated mice entered renal failure between 16 and 20 weeks after the treatment (blood urea concentration>20 mmol/L). All other mice were euthanized 20 weeks after treatment with the indicated tamoxifen dose. Scale bar, 2 mm. (B) The ratio of the kidney weight to body weight expressed as a percentage (2 KW/BW percentage; y axis), plotted against tamoxifen dose (x axis). (C) The Pkd1del DNA percentage (y axis) measured using eMLPA (Supplemental Figure 1) plotted against tamoxifen dose (x axis).17
Figure 2.
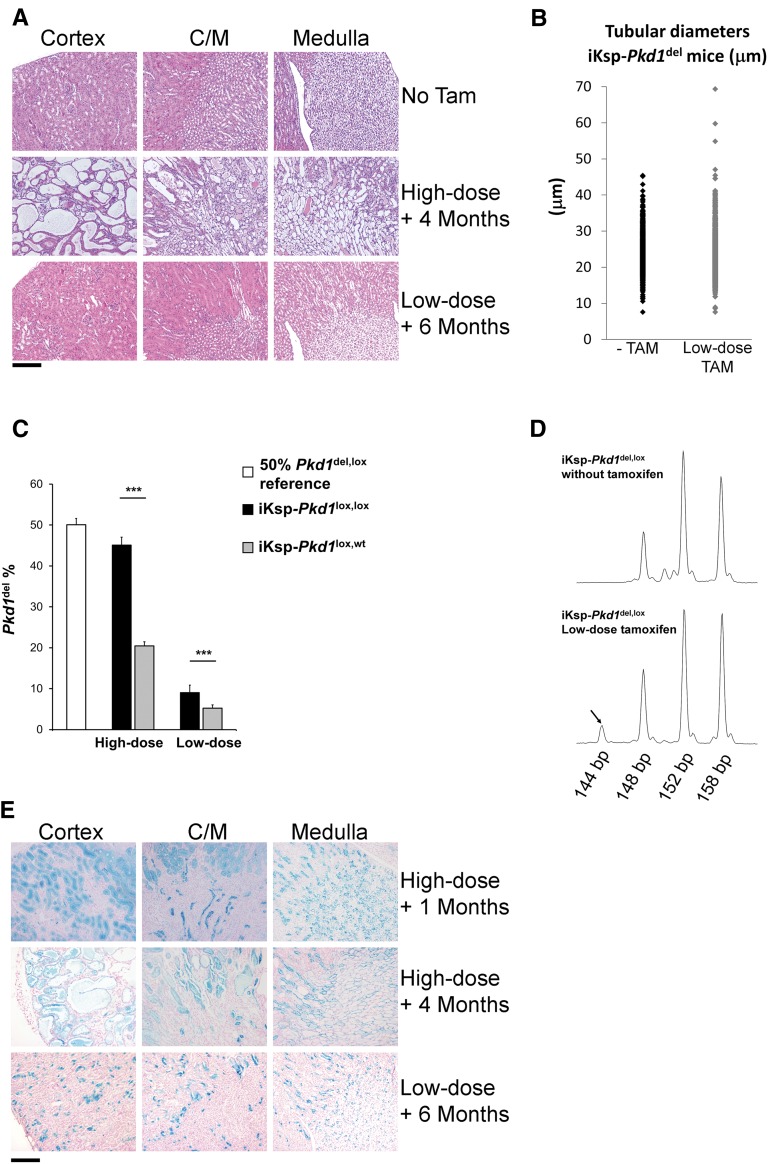
Pkd1 inactivation in a small number of renal epithelial cells does not initiate pathologic changes in the kidney. (A) Examination of the renal cortex, corticomedullary junction (C/M), and medulla from hematoxylin and eosin-stained kidney sections from iKsp-Pkd1lox.lox mice reveals clear cyst formation 4 months after high-dose tamoxifen treatment (5 mg), whereas no cysts are visible 6 months after low-dose tamoxifen treatment (0.25 mg). Tam, tamoxifen. Scale bar, 200 μm (B) Cortical tubule diameters of iKsp-Pkd1del mice 4–6 months after low-dose tamoxifen (low-dose TAM; n=1311 tubules from seven mice) and aged-matched iKsp-Pkd1del mice not treated with tamoxifen (−TAM; n=1087 tubules from five mice). Only 3 of 1311 tubules were clearly dilated in the low-dose TAM group. (C) eMLPA17 (Supplemental Figure 1) on DNA from kidney samples; mice that carry two Pkd1lox alleles (iKsp-Pkd1loxlox; high dose, n=7; low dose, n=12) contain two times the amount of Pkd1del DNA compared with mice that carry a single Pkd1lox allele (iKsp-Pkd1lox,wt; high dose, n=9; low dose, n=6), indicating that, when Cre is activated in cells with two floxed Pkd1 alleles, both alleles will be recombined to create Pkd1del alleles. The peak ratios of kidneys obtained from mice with one germline Pkd1del allele and one Pkd1lox allele (Pkd1del,lox; n=8 kidneys) were used as a 50% reference. ***P<0.001 (t test). (D) Examples of peaks from fluorescent signals obtained from eMLPA traces from iKsp-Pkd1del,lox mice that carried one Pkd1lox and one Pkd1del allele. In mice that did not receive tamoxifen, only the peaks that originate from the Pkd1lox (148 and 152 bp) and the Pkd1del (158 bp) alleles can be observed. Low-dose tamoxifen treatment produced an additional peak (144 bp; arrow) that originated from the deletion circle (Supplemental Figure 1), which confirms that part of the Pkd1lox DNA underwent Cre-mediated recombination, resulting in homozygous Pkd1del,del cells. (E) X-Gal blue staining of kidney sections from iKsp-Pkd1lox,lox mice crossbred with an LacZ reporter mouse to visually determine cells in which Cre had been active. High-dose tamoxifen treatment caused generalized Cre activation, and severe PKD developed 4 months after treatment (top panel, 1 month after high-dose tamoxifen; middle panel, 4 months after high-dose tamoxifen). (Bottom panel) Low-dose tamoxifen did not initiate pathologic changes, despite activating Cre in a substantial number of renal epithelial cells. Scale bar, 200 μm.
We confirmed homozygous inactivation of Pkd1 using eMLPA, because regardless of the tamoxifen dose, iKsp-Pkd1lox,lox mice contain two times as much Pkd1del DNA as iKsp-Pkd1lox,wt mice (Figure 2C). In addition, low-dose tamoxifen treatment of iKsp-Pkd1del,lox mice resulted in recombination of the remaining functional Pkd1lox allele (Figure 2D, Supplemental Figure 1). Six months after low-dose tamoxifen treatment, these iKsp-Pkd1del,lox mice, carrying the homozygous Pkd1 deletion, also lacked a cystic phenotype (Supplemental Figure 2).
To visually determine the location of the Pkd1-deficient cells, we crossbred iKsp-Pkd1lox,lox mice with LacZ reporter (R26R) mice.19 High-dose tamoxifen resulted in widespread Cre activation and the development of severe PKD 4 months after treatment (Figure 2E). In contrast, low dose-treated mice had a mosaic pattern of Pkd1-deficient cells, and this pattern was stable over time; again, these mice displayed no overt pathologic changes, even after 6 months (Figure 2E, Supplemental Figure 3).
Renal Injury after Scattered Pkd1 Deletion Is Insufficient to Induce Rapid PKD
Renal injury has been shown to accelerate PKD progression in several animal models for PKD.13–15,20 Injury-induced tubular epithelial cell proliferation caused by treatment with the nephrotoxic compound 1,2-dichlorovinyl-cysteine (DCVC) accelerates PKD in high-dose tamoxifen-treated iKsp-Pkd1del,lox mice.14 In addition, hypertrophic responses to reduced renal mass after unilateral nephrectomy can have similar effects in ift88 mice.20 Here, we found that unilateral nephrectomy also accelerated PKD in high-dose tamoxifen-treated iKsp-Pkd1lox,lox mice (Supplemental Figure 4).
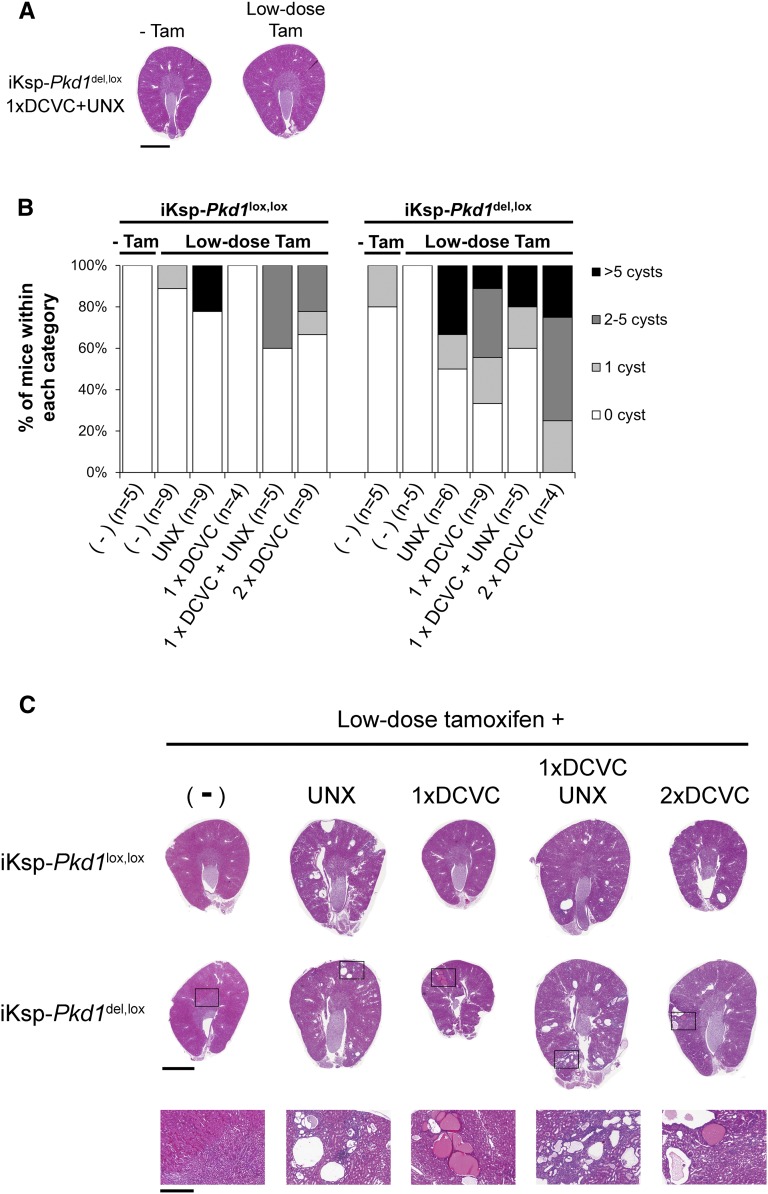
On the basis of these findings, we asked whether renal injury is required to initiate cyst formation in low dose-treated mice. Surprisingly, although the prevalence of cyst formation was higher in mice that underwent nephrectomy and/or DCVC injections, the analyzed renal sections of 57% of a total of 51 low dose-treated mice with renal injury had no cysts 4–6 months after the procedure (Figure 3, A and B). Even the most severely affected mice only developed a few clusters of cysts, whereas most of the renal parenchyma appeared normal, indicating that the vast majority of Pkd1-deficient cells had not been triggered to form cysts under these conditions (Figure 3C).
Figure 3.
Renal injury does not initiate rapid PKD in low-dose tamoxifen-treated mice. (A) Kidneys from two different 7-month-old iKsp-Pkd1del,lox mice that underwent 1× DCVC injection and unilateral nephrectomy (UNX) at 7–9 weeks of age. (Left panel) Without tamoxifen (−Tam). (Right panel) Low-dose tamoxifen at PN40 before renal injury (low-dose Tam). No cysts could be observed in these kidney sections. Scale bar, 2 mm. (B) Each hematoxylin and eosin-stained section was examined for the presence of cysts (defined as larger than three glomeruli) by an investigator who was blinded to the treatment. Mice were then subdivided in groups (0, 1, 2–5 cysts, or >5 cysts). Mice that received no tamoxifen treatment (−Tam) are also plotted. The prevalence of cyst formation increased in mice that were subjected to additional renal injury. Low-dose tamoxifen-treated mice with renal injury (all treatments) versus low-dose tamoxifen-treated mice without renal injury (Mann–Whitney U test; P<0.05). More iKsp-Pkd1del,lox mice had cysts than iKsp-Pkd1lox,lox mice (Mann–Whitney U test; P<0.05). (C) Hematoxylin and eosin-stained kidney sections of the most severely affected (on the basis of the number of cysts counted in each section) low-dose tamoxifen-treated iKsp-Pkd1lox,lox or iKsp-Pkd1del,lox mice that underwent no additional procedure (−), unilateral nephrectomy (UNX), one DCVC injection (1× DCVC), one DCVC injection and unilateral nephrectomy (1× DCVC UNX), or two DCVC injections (2× DCVC); the mice were euthanized 4–6 months after Pkd1 deletion. None of the mice developed severe PKD. Enlargements of the indicated areas are shown in bottom panels. Note that cysts generally form in clusters. Scale bar, 2 mm in top and middle panels; 0.5 mm in bottom panel.
The prevalence of cyst formation was higher—albeit still relatively rare—on a Pkd1del,lox background, supporting the idea that haploinsufficiency of surrounding cells may enhance cyst formation and progression (Figure 3B).11,21 Thus, within a period of 6 months after scattered Pkd1 deletion, the adult kidney remains largely resistant to cyst formation, even after renal injury.
A Long Dormant Period Is Followed by a Phenotypic Switch toward a Rapid Onset of Severe PKD
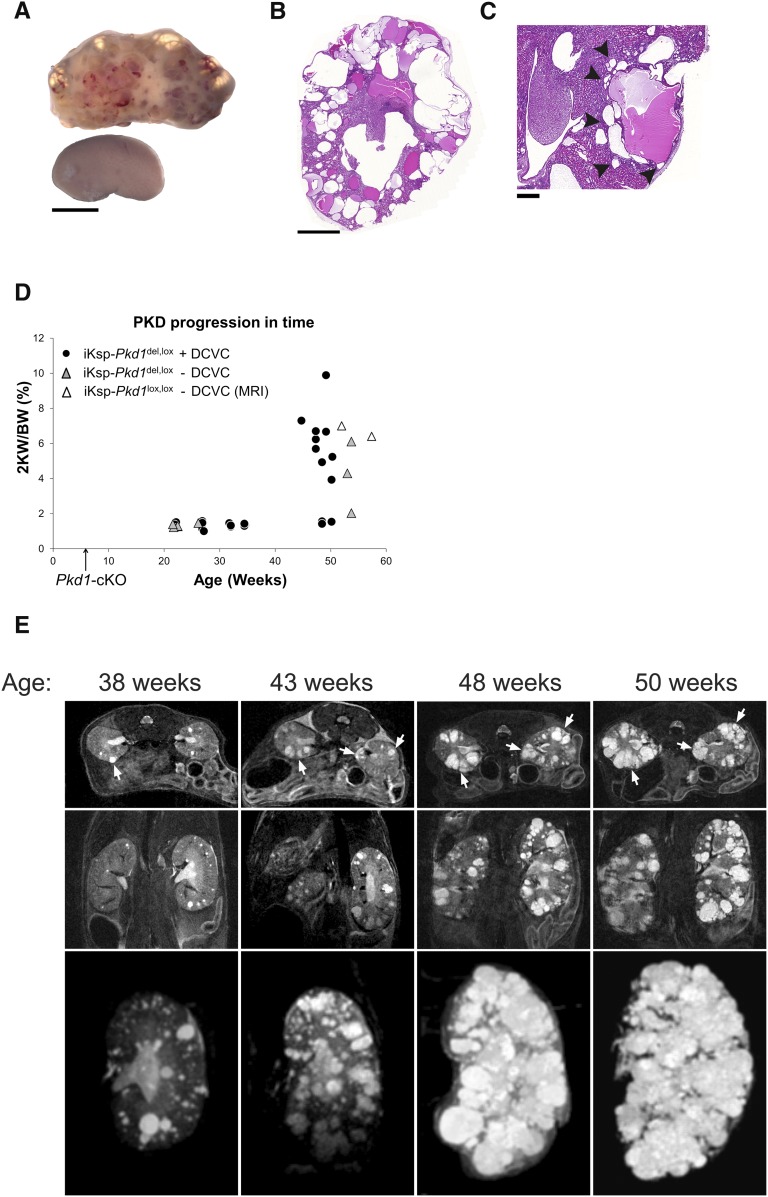
Because scattered Pkd1 deletion with or without renal injury did not lead to severe PKD within the first 6 months, we followed a number of these mice further in time. Strikingly, the 2 kidney weight/body weight percent more than doubled (2- to 6-fold) in 13 of 17 mice euthanized at age 47–57 weeks and correlated with increased cystic index and a decline in renal function (Figure 4, A–D, Supplemental Figure 5). Segment-specific marker staining revealed cysts derived from the proximal and distal region of the nephron and—to a lesser extent—the collecting duct (Supplemental Figure 6). Compared with the mice without DCVC treatment, mice with DCVC treatment tended to develop PKD slightly sooner (Figure 4D). Although this difference was not statistically significant, the trend is consistent with the increased prevalence of cysts caused by renal injury in the mice that had been euthanized 4–6 months after scattered Pkd1 deletion (Figure 3B).
Figure 4.
Within 6–11 months after Pkd1 deletion, mice develop a phenotype that closely mimics human ADPKD. (A) Images of a typical polycystic kidney 10 months after Pkd1 deletion (by low-dose tamoxifen treatment) and an age-matched wild-type control kidney. Scale bar, 5 mm. (B) Hematoxylin and eosin-stained kidney section from a severely affected mouse (blood urea=51.2 mmol/L) 10 months after low-dose tamoxifen treatment. Scale bar, 2 mm. (C) Hematoxylin and eosin-stained kidney section from a moderate to severely affected mouse (blood urea=22 mmol/L) with focal clustered cyst formation. Note the many small cysts around the larger cysts (examples indicated by arrowheads). Scale bar, 500 μm. (D) The ratio of the combined kidney weight to the total body weight was calculated as a percentage (2 KW/BW) and plotted against age for iKsp-Pkd1del,lox mice that were treated with low-dose tamoxifen with or without DCVC treatment. Two iKsp-Pkd1lox,lox mice that were followed by MRI analysis (Figure 4E, Supplemental Figure 8A) are also included in the graph. The time of the low-dose tamoxifen treatment is indicated by the arrow (Pkd1-cKO). (E) MRI analysis of one iKsp-Pkd1lox,lox mouse treated with low-dose tamoxifen at 6 weeks of age (PN40–PN42). MRI analysis was performed at 38, 43, 48, and 50 weeks of age. (Top panel) Transverse T2-weighted scans. (Middle panel) Coronal T2-weighted scan. (Bottom panel) Maximum projection of all cysts in the left kidney. Some cysts developed within 32 weeks after low-dose tamoxifen treatment (age=38 weeks); the phenotype progressed to severe PKD within 12 weeks thereafter (age=50 weeks). The arrows point at clusters of cysts that are growing in both size and number of visible cysts. The mouse was euthanized at an age of 52 weeks, with a 2 KW/BW percentage of 7.0 (also indicated in D) and blood urea of 23.4 mmol/L.
To further confirm the delayed onset of severe PKD, we performed magnetic resonance imaging (MRI) analysis on two low-dose tamoxifen-treated iKsp-Pkd1lox,lox mice (also shown in Figure 4D). Again, the vast majority of cysts only appeared in the later stages of PKD progression, whereas in the first 32 weeks after scattered Pkd1 deletion, only some cysts were formed (Figure 4E, Supplemental Figure 8A).
Collectively, these data suggest that, after a long dormant period, additional factors can trigger a sudden shift from low- to high-frequency cyst formation.
The Switch toward a Rapid Onset of Severe PKD Is Not Caused by Aging or Increased Proliferation
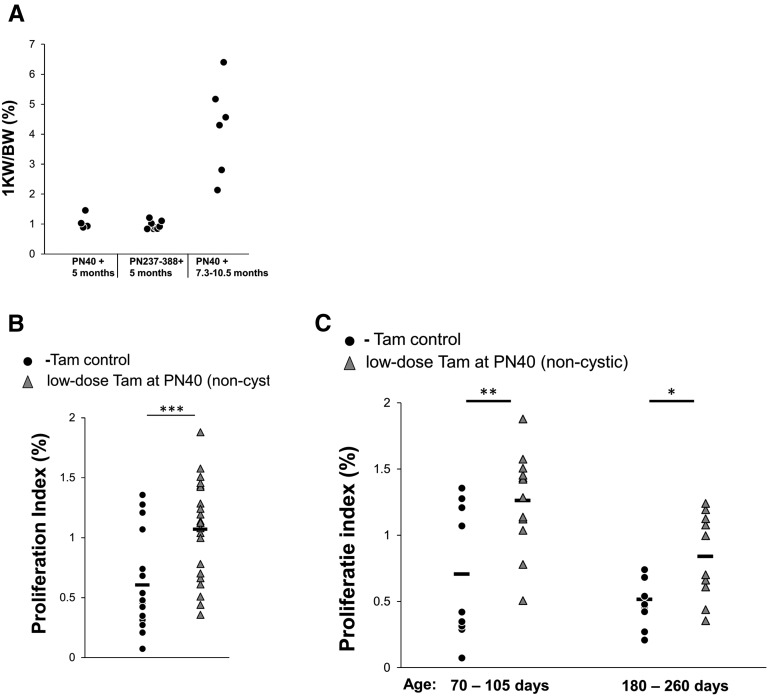
We investigated whether the sudden shift from low- to high-frequency cyst formation was triggered by possible altered kidney homeostasis caused by general aging. However, aged mice (up to an age of 388 days [PN388]) that received low-dose tamoxifen and nephrectomy did not develop severe PKD within 5 months (Figure 5A), excluding the possibility that the severe onset of PKD was caused by age-related events. In addition, we confirmed once more that these mice developed severe PKD after a long dormant period (Figure 5A, Supplemental Figure 7).
Figure 5.
The rapid onset of PKD is not caused by aging or increased proliferation because of Pkd1 deletion. (A) PN40 and aged iKsp-Pkd1lox,lox mice (PN237–PN388) were treated with low-dose tamoxifen and unilateral nephrectomy; after 5 months, the ratio of the remaining kidney weight to total body weight (1 KW/BW) was similar between the two groups. However, by 7.3–10.5 months after Pkd1 deletion and unilateral nephrectomy, all of the PN40 mice developed renal failure (defined as a blood urea level>20 mmol/L) with increased 1 KW/BW ratio caused by severe PKD. (B) Proliferation index from renal sections without cysts from PN40 iKsp-Pkd1lox,lox mice that were treated with low-dose tamoxifen (Tam; n=22 mice) or left untreated (n=19 mice; −Tam control). Each data point represents the proliferation index of one renal section from one mouse. ***P<0.001 (t test). (C) The same data from B subdivided in different age groups (age=70–105 and 180–260 days). For both age groups, proliferation was higher in low dose-treated noncystic mice compared with untreated mice. **P<0.01; *P<0.05 (t test).
A plethora of studies have described complex networks of signaling pathways that—without Polycystin-1—lead to increased cell proliferation, which may be one of the factors that drives cyst formation.22 We tested whether proliferation was increased before the onset of severe PKD by analyzing the occurrence of cells that were positive for Ki-67, a marker of proliferation. However, already 1–2 months after scattered Pkd1 deletion, the mice had a modest increase in proliferation, and this increase was stable over time, indicating that the change in proliferation was not restricted to the period just before the onset of severe PKD (Figure 5, B and C). In addition, we analyzed apoptosis by Caspase-3 staining but found only a few apoptotic cells per renal section, which was too low to be correlated with the proliferation index (not shown). However, the pattern of Pkd1-deficient cells remained stable over time (Supplemental Figure 3). These data suggest that scattered Pkd1 deletion rapidly triggers a modest increase in proliferation, which does not immediately affect the tubular architecture.
Evidence for a Cystic Snowball Effect
After scattered Pkd1 deletion, the cysts tended to grow in clusters rather than being equidistantly scattered (Figures 3C and 4, C and E, Supplemental Figure 8, A and B). These clusters only appeared in the last 10–12 weeks of the disease, long after the tamoxifen treatment (Figure 4E, Supplemental Figure 8A). Although we cannot exclude that cysts from some clusters originated simultaneously from regions with more closely packed Pkd1-deficient cells, the location of small cysts and dilated tubules near larger cysts (Figures 3C and 4C, Supplemental Figures 6 and 8, A and B) suggests that the initiation of the cysts within clusters occurred at different moments.
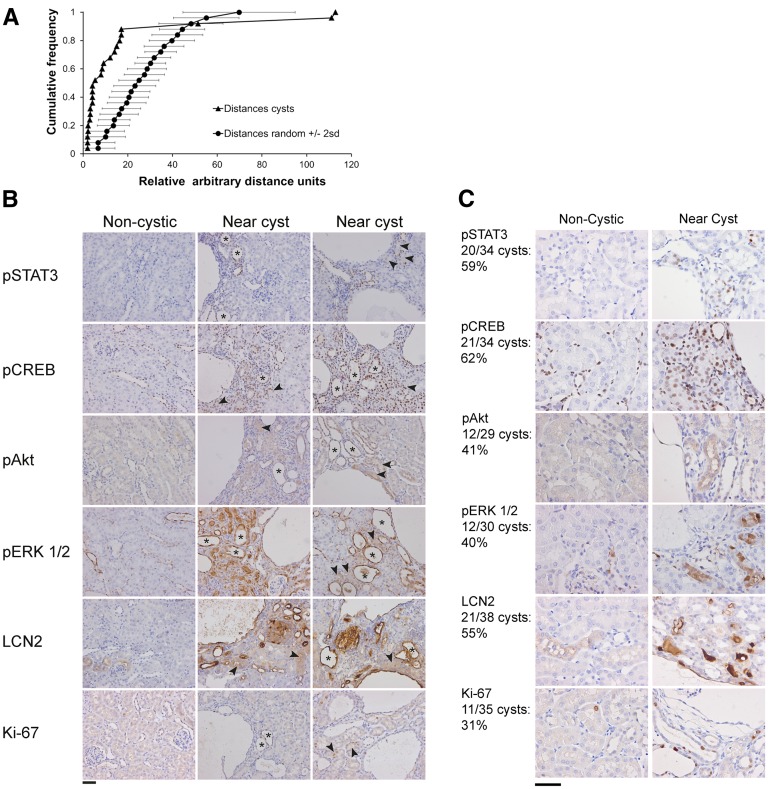
To determine if the distribution of cysts, indeed, followed a clustered pattern rather than a random pattern, we applied the nearest neighbor analysis on seven mildly affected mice that were euthanized 4–6 months after scattered Pkd1 deletion and renal injury (from the analysis in Figure 3B). The distances of each cyst with its nearest neighboring cyst were plotted in a cumulative frequency plot and compared with a similar type of cumulative frequency plot that was generated from 20 simulations of an equal number of randomly distributed points within an equally sized area. One mouse did not show a statistically significant clustering, which is likely explained by the low number of cysts in that mouse (Supplemental Figure 8C). The other six mice displayed a distribution of cysts typical for a clustered pattern (Figure 6A, Supplemental Figure 8C).
Figure 6.
Clustered cyst formation and altered signaling near cysts. (A) Nearest neighbor analysis of a kidney section from a low-dose tamoxifen-treated iKsp-Pkd1del,lox mouse with unilateral nephrectomy and DCVC treatments euthanized 6 months after tamoxifen treatment (hematoxylin and eosin image is shown in Figure 3C). The graph depicts two plots. One plot is a cumulative plot generated from measurements of distances between nearest neighboring cysts (distances cysts), and one plot is an average of 20 simulations of similar types of cumulative plots generated from nearest distances of an equal number of points that were randomly distributed over an equally sized area (distances random). Horizontal error bars indicate 2 SDs of the random simulations. Note that the plot of the measured distances between nearest neighboring cysts is located at the left of the random simulations, indicating a relatively high number of short distances typical for a clustered distribution. (B) Images of renal sections from this mouse were immunostained using antibodies specific for pSTAT3, pCREB, pAKT, pERK1/2, LCN2, and Ki-67. Regions without cysts have low expression of these proteins and show normal renal histology (left panel, noncystic). Regions with cysts have increased expression of these proteins (center and right panels, near cyst). Note that many tubules near cysts are dilated and have high expression of these proteins (examples indicated by asterisks). Also, many normal-appearing tubules near cysts have increased expression (examples indicated by arrowheads). Scale bar, 50 μm. (C) Renal sections at different locations of the kidneys from seven other mildly affected mice (i.e., containing only one or a few cysts per renal section) showed a similar staining pattern in and around 31%–62% of the cysts/clusters. The numbers are indicated at the left of C. Scale bar, 50 μm.
We hypothesized that preferential cyst formation near existing cysts is driven by PKD-related signaling pathways that are locally and persistently activated in surrounding tissue near cysts. Therefore, we performed immunohistochemistry to analyze phospho-STAT3 (pSTAT3), phospho-CREB, phospho-AKT, phospho-ERK1/2, and LCN2 expressions, which have been shown to be involved in PKD.18,23–33 The clustered distribution of cysts in an iKsp-Pkd1del,lox mouse that was treated with low-dose tamoxifen, unilateral nephrectomy, and DCVC and euthanized 6 months after tamoxifen treatment (clustering is shown in Figure 6A), indeed, co-occurred with increased expression of these proteins specifically near cysts or clusters of cysts (Figure 6B). We quantified the number of cysts/clusters that showed this effect in renal sections at two different locations in the kidneys of seven other mildly affected mice. Although regions around smaller and isolated cysts often did not show increased expression of these proteins, in total, the expression around 31%–62% of cysts was clearly elevated (Figure 6C).
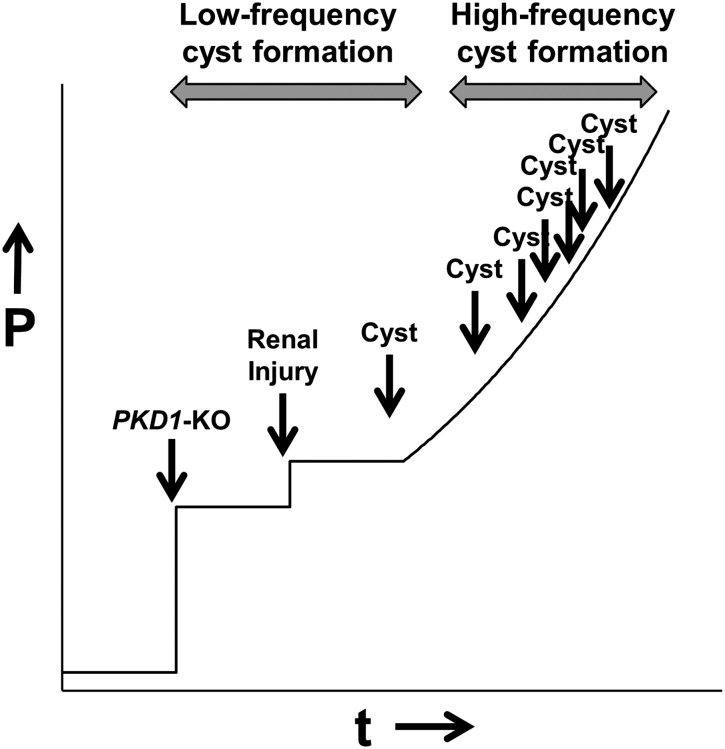
Collectively, our data suggest the following model. The likelihood of cyst formation after PKD1-KO is low but sufficient to induce cyst formation at low frequency. The frequency of cyst formation is increased by acute renal injury but remains low. However, the persistent stress imposed by initial cysts on surrounding tissue triggers a snowball effect, in which local aberrant PKD-related signaling increases the likelihood of new cyst formation, which ultimately leads to accelerated progression to end stage PKD (Figure 7).
Figure 7.
Model of cyst formation in the adult kidney. Although PKD1-KO at a certain time point (t; x-axis) increases the probability (P; y axis) of cystogenesis, cystogenesis is rare. Even when the adult kidney is challenged by renal injury, which further increases the probability, cyst formation occurs only incidentally (low-frequency cyst formation). However, the initial cysts impose continuous stress onto surrounding tissue, which locally increases the probability and frequency of new cyst formation, leading to a snowball effect (high-frequency cyst formation). As time proceeds, larger regions of the kidney become increasingly susceptible to cyst formation, which ultimately leads to severe PKD.
Discussion
In animal models of PKD and patients with ADPKD, the rate of cyst growth is much faster during development than during adulthood.2,11,12 However, given the many microscopic cysts that have been detected in kidneys of adult patients with ADPKD, it is likely that new cysts also form during adulthood.3 In patients, the time interval between the moment that a cell loses functional Polycystin signaling and the moment at which this cell starts to form a cyst is not known. In this study, we reduced the percentage of Pkd1-deficient cells in adult mouse kidneys to 8%, which surprisingly did not lead to a PKD phenotype within 6 months, suggesting that this time interval can be much longer than is expected from previous data.11
We and others have found that the relatively slow progression of cyst growth in adult animal models is accelerated by additional renal injury, which suggests that renal injury is an important trigger for cyst formation in the adult kidney.13–15,20 It is likely that a combination of several processes (i.e., cell death, proliferation, epithelial dedifferentiation, altered planar cell polarity, and inflammation) underlie the injury-induced acceleration of cystogenesis.14,34 Although several types of renal injuries in mice with scattered Pkd1 deletion led to increased likelihood of cyst formation (Figure 3B), these injuries never triggered a rapid disease onset, and most of the renal parenchyma remained normal. The lack of a rapid disease onset after renal injury in our scattered Pkd1 deletion model, therefore, differs from previous data. Two explanations might underlie this difference. (1) Because the number of Pkd1-deficient cells is lower than in other studies, it is less likely that cells are subjected to both loss of Pkd1 and injury. (2) Despite a low number of renal epithelial cells without functional Pkd1, the majority of cells with functional Pkd1 is able to maintain tubular architecture, even during short pulses of tubular injury. Either way, scattered Pkd1 deletion led to a low frequency of cyst formation, which was slightly increased by renal injury.
After this dormant period with low-frequency cyst formation, the majority of mice rapidly developed severe PKD. We excluded the possibility that this shift toward more susceptibility for cyst formation is triggered by advanced age or progressive changes in proliferation rates over time (Figure 5).
It has been shown before that the presence of cysts affects neighboring noncystic tubules, which then become obstructed and have increased proliferation and apoptosis.35–37 We hypothesized that the initial cysts may cause a snowball effect by triggering resident Pkd1-deficient cells in neighboring tubules to form new cysts. If our presumption is true, this would implicate that (1) the continuous stress by cysts on their surroundings leads to altered signaling in tubules near cysts, (2) cysts generally form in clusters, and (3) the progression is faster at an advanced stage. Our data show that, although the mosaic pattern of Cre activation occurred everywhere throughout the kidney, which was shown by crossbreeding experiments with LacZ reporter mice, some areas were clearly cystic, whereas other regions were virtually unaffected. We cannot exclude that some clusters of cysts originated from regions with more closely concentrated Pkd1-deficient cells, possibly because of uneven distribution of tamoxifen. However, from our histologic data, it seems unlikely that small dilated tubules just over 50 μm were formed simultaneously with cysts having diameters of >1000 μm. Longitudinal MRI analysis also showed that the number of cysts particularly increased in the last 10–12 weeks of disease progression and that these cysts were formed near existing isolated cysts that were visible at 32 weeks after scattered Pkd1 deletion (Figure 4E, Supplemental Figure 8A). Collectively, these data suggest that existing cysts locally confer susceptibility for new cyst formation in their vicinity.
If true, it is expected that molecular changes underlie the local increased susceptibility near existing cysts. A number of mildly affected mice with scattered Pkd1 deletion, which had few cysts and were euthanized before the onset of severe PKD, allowed us to test this hypothesis. We compared the expression of a number of PKD-related proteins between cystic and noncystic regions. pSTAT3 has been shown to be elevated in PKD, and its inhibition alleviated cyst formation in mice.18,23,24 LCN2, a marker for renal injury that is highly expressed in cystic kidneys, is likely also involved in cystic growth, because crossbreeding of juvenile cystic kidney (jck) mice with Lcn2 KO mice reduced cystic growth.25–27 Also, activation of AKT and ERK1/2 has been associated with cystic disease, and analysis of phosphorylated CREB has been used before as a readout for cAMP, an important second messenger involved in PKD.28–33 In addition, Ki-67 expression was analyzed as a marker for proliferation. Indeed, the expression of these proteins was frequently elevated in tissue near cysts compared with tissue more distant to cysts. Also, dilated tubules and small cysts that preferentially located near larger cysts had increased PKD-related signaling (Figure 6). Smaller isolated cysts generally did not show altered signaling in their vicinity, but we cannot rule out that, as small cysts increase in size over time, similar effects will take place.
Collectively, scattered Pkd1 deletion leads to a phenotype that differs considerably from previously reported mouse models with high percentages of Pkd1-deficient cells, in which all renal tubules slowly but progressively dilate after Pkd1 inactivation, leading to massively enlarged kidneys. The model presented here provides evidence for the existence of a cystic snowball effect, in which the first cysts pose continuous stress onto surrounding tissue, resulting in aberrant PKD-related signaling. Over time, this will dramatically increase the likelihood of new cyst formation, leading to accelerated cystogenesis and severe PKD (Figure 7). The stochastic nature of this process is inherently associated with large variability. Although this may complicate preclinical testing, the phenotype in these mice is remarkably similar to the phenotype in patients with ADPKD.
Data from the Consortium of Radiologic Imaging Studies showed that, although cysts may form at different ages and grow at different rates, total kidney volume follows an exponential growth curve.38,39 The number of cysts and the growth rate can vary between patients, giving rise to large but predictable interpatient variability. The variation in the mice shown in Figure 4D might reflect a similar pattern. The cystic snowball effect implies that the number of initial cysts is important, because these cysts will ultimately trigger the formation of many more cysts, giving rise to multiple clusters of growing cysts. Therefore, variation in the initial number of cysts might result in different total kidney volume growth rates, giving rise to the pattern of data points in Figure 4D. In addition, mice that also had been treated with DCVC tended to have increased kidney weights earlier than mice without additional DCVC treatment, which might be a reflection of the higher prevalence of initial cysts that were observed in injured mice that were euthanized 4–6 months after scattered Pkd1 deletion (Figure 3B).
To date, preclinical models have been invaluable in identifying many important signaling cascades that were then targeted in a number of clinical trials.40–43 However, to predict the outcome of a therapeutic approach with higher precision, the model must mimic human ADPKD as closely as possible. Our study shows that this can be achieved by reducing the number of adult Pkd1-deficient cells in the kidneys, and this strategy can likely be applied to other rodent models that use conditional Pkd1 or Pkd2 inactivation. These models will, therefore, help guide the development and refinement of improved therapeutic strategies and provide more accurate predictions of clinical outcome.
Concise Methods
Mice and Treatments
Inducible kidney-specific Pkd1 deletion mice (iKsp-Pkd1del) were on a Pkd1del,lox, Pkd1lox,lox, or Pkd1lox,wt background.11 The Pkd1lox allele has Lox-P sites flanking exons 2–11, and this region is deleted in the Pkd1del allele. Mice were also crossbred with LacZ reporter (R26R) mice.19 Only male mice were used in all experiments. Oral tamoxifen administration (high dose, 5 mg; low dose, 0.25 mg) was performed as previously described on 3 consecutive days at PN40–PN42, unless otherwise indicated.16 DCVC administration consisted of a single intraperitoneal injection of 15 mg/kg as described previously.14 For unilateral nephrectomy, mice were anesthetized with 4% isoflurane and maintained at 1.5%–2% isoflurane. After a small incision in the right lumbar region, the renal hilum of the right kidney was tied off; then, the kidney was removed, and the wound was closed. A group of mice underwent a sham operation; these mice underwent the same procedure, but kidneys were left undisturbed.
The first renal injury procedure was carried out 6–10 days after the tamoxifen administration. In mice with two renal injuries, the second procedure was always carried out within 5 weeks after tamoxifen administration.
Blood sampling and blood urea measurements were performed using Reflotron technology (Kerkhof Medical Service) as described previously.14 All mice were on a full C57BL/6 genetic background. The local animal experimental committee of the Leiden University Medical Center and the Commission Biotechnology in Animals of the Dutch Ministry of Agriculture approved the experiments performed.
Immunohistochemical Analyses
Renal sections (4 μm) were prepared from paraffin-embedded kidneys that had been fixed in buffered 4% formaldehyde solution and stained with hematoxylin and eosin using standard procedures.
The tubular diameters were measured using the Panoramic Viewer 1.15 software from 3DHistech Ltd. by drawing a line perpendicular to the basal membranes of two opposite nuclei of randomly chosen tubules throughout the cortex.
The cystic index was measured from total scans from hematoxylin and eosin-stained kidney sections as the percentage of cystic area relative to total tissue as determined by Image J software (public domain software; National Institutes of Health). Before the measurements, signal from protein casts within cysts that might influence the measurements was removed using Photoshop software.
The quantification of the number of cysts (defined as larger than three glomeruli) in Figure 3 was done by an investigator who was blinded to the treatment.
For X-gal blue staining, 3-mm sections from frozen kidneys were cut manually with a scalpel and fixed two times for 20 minutes in 0.2% glutaraldehyde, 1.5% formaldehyde, 2 mM MgCl2, 5 mM EGTA, and 100 mM sodium phosphate (pH 7.3). The sections were washed three times in 2 mM MgCl2 and 100 mM sodium phosphate (pH 7.3) and stained O/N (in the dark at room temperature) in X-gal staining solution [5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 1 mg/ml X-gal, 2 mM MgCl2, and 100 mM sodium phosphate, pH 7.3]. After postfixation in buffered 4% formaldehyde solution and paraffin embedding, the thick sections were further cut into 4-μm-thick sections. The first few sections could be used and were counterstained with Nuclear Fast Red solution. For each time point after tamoxifen administration, two to three mice were stained for LacZ activity.
For immunohistochemical analysis, sections were deparaffinized and subjected to heat-mediated antigen retrieval (10 mM/1 mM Tris/EDTA) [pH 9.0] for anti-p (Tyr705) STAT3 [no. 9145; Cell Signaling] and LCN2 [no. AF1857; R&D Systems] and 10 mM citrate buffer [pH 6.0] for rabbit anti–Ki-67 [no. NCL-Ki67p; Bio Connect Services], antiphospho-Akt [Ser473], antiphospho-ERK1/2 [Thr202/204], antiphospho-CREB [Ser133], and anticleaved Caspase-3 from Cell Signaling Technology (nos. 4060, 4370, 9198, and 9661, respectively). Sections were blocked with 0.1% H2O2 for 20 minutes for endogenous peroxidase activity and preincubated for 1 hour with 5% normal goat serum in 1% BSA in PBS. Next, the sections were incubated O/N with anti-pSTAT3 (1:75), anti-LCN2 (1:150), anti–Ki-67 (1:3000), rabbit antimegalin (gp330; 1:500; Pathology LUMC, Leiden, The Netherlands), goat anti-Tamm Horsfall protein (uromodulin, 1:4000; Organon Teknika-Cappel), rabbit antiaquaporin-2 (1:4000; no. 178612; Calbiochem), anti-pAkt (1:50), anti-pERK1/2 (1:400), anti-pCREB (1:800), or anti–Caspase-3 (1:300) in 1% BSA in PBS. After incubation with rabbit Envision horseradish peroxidase (no. K4011; Dako) or rabbit anti-goat horseradish peroxidase (1:100; no. P0449; Dako), immune reactions were revealed using diaminobenzidine as a chromogen, counterstained with hematoxylin, dehydrated, and mounted. To determine PKD-related signaling in and around cysts, two or three sections at different locations within the kidney were used per staining.
Nearest Neighbor Analyses
The nearest neighbor analysis as applied here is a modification of the method described by Clark and Evans.44 Relative distances of each cyst with its nearest neighboring cyst within a kidney section were measured using Photoshop software. For each analysis, 20 random simulations of an equal number of points in an equally sized rectangle (this size is equal to the size of the cortical region of the section in which cysts can be expected) were generated using Excel software, and the nearest distances of each point to its nearest neighboring point were calculated using Excel software. Each graph depicts two cumulative plots: one plot that has been generated from the actual measured distances of the cysts and their nearest neighboring cysts and one plot that has been generated from the averages of the cumulative plots of the randomly simulated nearest distances. The error bars indicate 2 SDs of 20 simulations. Cumulative plots of measured distances that are located at the left of the plot of the random simulations indicate a relatively high number of short distances between the cysts, which is typical for a clustered distribution. When the plot of the measured distances is also located outside of the region within 2 SDs of the random simulations, the distribution in considered to be clustered and not random. Sections with five or more cysts from different regions of the kidneys from seven mildly affected mice (from the analysis of Figure 3B) were used to perform the analysis, and the analysis of one section per animal is shown in Figure 6A and Supplemental Figure 8C.
eMLPA
The position of the probes used for hybridization to DNA isolated from kidneys is outlined in Supplemental Figure 1 and was described previously.17 DNA was isolated using Nucleobond AXG 100 columns according to the manufacturer’s protocol (no. 740545; Macherey-Nagel). After a denaturing step of the DNA (5 minutes at 95°C), hybridization was carried out for 4 hours at 60°C in 5 μl containing MLPA buffer (MRC-Holland), 4 nM each probe, and 250 ng denatured DNA. Extension and ligation reactions were performed simultaneously for 15 minutes at 54°C by adding 40 μl prewarmed solution containing 2 mM dNTPs, 1 unit Stoffel Taq polymerase (Applied Biosystems), 3 μl Buffer A, 3 μl buffer B, and 1 μl Ligase-65 (MRC-Holland). After 5 minutes at 95°C to inactivate the ligase, 5 μl each sample was used in a 25-μl PCR reaction with 31 rounds of amplification. Samples were run on a 3730 DNA analyzer, and relative peak ratios were used to determine Cre-mediated recombination efficiency (Peak Scanner v1.0 software; Applied Biosystems). Each hybridization was carried out at least four times, and for each experiment, eight Pkd1del,lox samples were included, from which the median of the obtained peak ratios served as a 50% Pkd1del DNA reference.
Proliferation Index
Sections were stained with anti–Ki-67 as described above to visualize proliferating cells. Six images (100×) throughout the cortex from one renal section per mouse were taken. Counting was carried out by an investigator who was blinded to the treatment. The total numbers of nuclei were counted using freely available ImageJ software, and Ki-67–positive nuclei were counted manually. The ratio of Ki-67–positive nuclei to the total number of nuclei was calculated as a percentage.
MRI Analyses
MRI measurements were performed with a 7 T Bruker Pharmascan Biospin (Burker, Ettingen, Germany). Mice were anesthetized with 3% isoflurane and maintained at 1.5%–2%. The mice were imaged with a RARE imaging sequence (TR=11.21 milliseconds, effective TE=44.84 milliseconds). A sufficient number of slices was imaged, with a slice thickness of 0.35 mm, a matrix size of 300×300, and a field of view of 3×3 cm. Respiratory triggering was used to minimize motion artifacts caused by breathing. Two low-dose tamoxifen-treated iKsp-Pkd1lox,lox mice were imaged at the indicated time points (Figure 4E, Supplemental Figure 8A).
Statistical Analyses
Differences in Pkd1del DNA content in iKsp-Pkd1lox,lox versus iKsp-Pkd1lox,wt mice were tested using two-tailed t tests. The difference in onset of renal failure between nephrectomized and sham-operated mice that received high-dose (5 mg) tamoxifen was tested with the generalized Wilcoxon test. Comparison of the prevalence of cyst formation between mice that underwent renal injury or not was tested with Mann–Witney U tests. Differences in proliferation indices were tested with two-tailed t tests.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Ernst Suidgeest for valuable help with the magnetic resonance imaging measurements, Michel Mulder and Ilma Rietbroek for help with the unilateral nephrectomy procedure, Bob van de Water for providing the 1,2-dichlorovinyl-cysteine, Ron Wolterbeek and Erik van Zwet for help with the statistical analysis, and the referees for valuable suggestions that helped us improve the manuscript.
This research is supported by Dutch Kidney Foundation Grant JP11.34 and Dutch Technology Foundation Stichting Technische Wetenschappen Project 11823, which is part of The Netherlands Organization for Scientific Research.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013080864/-/DCSupplemental.
References
- 1.Grantham JJ: Pathogenesis of renal cyst expansion: Opportunities for therapy. Am J Kidney Dis 23: 210–218, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Grantham JJ, Cook LT, Wetzel LH, Cadnapaphornchai MA, Bae KT: Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol 5: 889–896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grantham JJ, Mulamalla S, Grantham CJ, Wallace DP, Cook LT, Wetzel LH, Fields TA, Bae KT: Detected renal cysts are tips of the iceberg in adults with ADPKD. Clin J Am Soc Nephrol 7: 1087–1093, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian F, Watnick TJ, Onuchic LF, Germino GG: The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell 87: 979–987, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, Larson C, Brent G, Zhou J: Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet 17: 179–181, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Wu G, D’Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Jr., Kucherlapati R, Edelmann W, Somlo S: Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93: 177–188, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Pritchard L, Sloane-Stanley JA, Sharpe JA, Aspinwall R, Lu W, Buckle V, Strmecki L, Walker D, Ward CJ, Alpers CE, Zhou J, Wood WG, Harris PC: A human PKD1 transgene generates functional polycystin-1 in mice and is associated with a cystic phenotype. Hum Mol Genet 9: 2617–2627, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, Leonhard WN, van de Wal A, Ward CJ, Verbeek S, Deruiter MC, Breuning MH, de Heer E, Peters DJ: Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet 13: 3069–3077, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Jiang ST, Chiou YY, Wang E, Lin HK, Lin YT, Chi YC, Wang CK, Tang MJ, Li H: Defining a link with autosomal-dominant polycystic kidney disease in mice with congenitally low expression of Pkd1. Am J Pathol 168: 205–220, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thivierge C, Kurbegovic A, Couillard M, Guillaume R, Coté O, Trudel M: Overexpression of PKD1 causes polycystic kidney disease. Mol Cell Biol 26: 1538–1548, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lantinga-van Leeuwen IS, Leonhard WN, van der Wal A, Breuning MH, de Heer E, Peters DJ: Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet 16: 3188–3196, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG: A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med 13: 1490–1495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P: Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Happé H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, de Heer E, Peters DJ: Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet 18: 2532–2542, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, Zhou J: Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 18: 2523–2531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lantinga-van Leeuwen IS, Leonhard WN, van de Wal A, Breuning MH, Verbeek S, de Heer E, Peters DJ: Transgenic mice expressing tamoxifen-inducible Cre for somatic gene modification in renal epithelial cells. Genesis 44: 225–232, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Leonhard WN, Roelfsema JH, Lantinga-van Leeuwen IS, Breuning MH, Peters DJ: Quantification of Cre-mediated recombination by a novel strategy reveals a stable extra-chromosomal deletion-circle in mice. BMC Biotechnol 8: 18, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonhard WN, van der Wal A, Novalic Z, Kunnen SJ, Gansevoort RT, Breuning MH, de Heer E, Peters DJM: Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: In vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol 300: F1193–F1202, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Soriano P: Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Bell PD, Fitzgibbon W, Sas K, Stenbit AE, Amria M, Houston A, Reichert R, Gilley S, Siegal GP, Bissler J, Bilgen M, Chou PC, Guay-Woodford L, Yoder B, Haycraft CJ, Siroky B: Loss of primary cilia upregulates renal hypertrophic signaling and promotes cystogenesis. J Am Soc Nephrol 22: 839–848, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastos AP, Piontek K, Silva AM, Martini D, Menezes LF, Fonseca JM, Fonseca II, Germino GG, Onuchic LF: Pkd1 haploinsufficiency increases renal damage and induces microcyst formation following ischemia/reperfusion. J Am Soc Nephrol 20: 2389–2402, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres VE, Harris PC: Autosomal dominant polycystic kidney disease: The last 3 years. Kidney Int 76: 149–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talbot JJ, Shillingford JM, Vasanth S, Doerr N, Mukherjee S, Kinter MT, Watnick T, Weimbs T: Polycystin-1 regulates STAT activity by a dual mechanism. Proc Natl Acad Sci U S A 108: 7985–7990, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takakura A, Nelson EA, Haque N, Humphreys BD, Zandi-Nejad K, Frank DA, Zhou J: Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet 20: 4143–4154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, Buemi M: Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol 27: 373–378, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Meijer E, Boertien WE, Nauta FL, Bakker SJ, van Oeveren W, Rook M, van der Jagt EJ, van Goor H, Peters DJ, Navis G, de Jong PE, Gansevoort RT: Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: A cross-sectional analysis. Am J Kidney Dis 56: 883–895, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F: Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest 120: 4065–4076, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belibi F, Ravichandran K, Zafar I, He Z, Edelstein CL: mTORC1/2 and rapamycin in female Han:SPRD rats with polycystic kidney disease. Am J Physiol Renal Physiol 300: F236–F244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novalic Z, van der Wal AM, Leonhard WN, Koehl G, Breuning MH, Geissler EK, de Heer E, Peters DJ: Dose-dependent effects of sirolimus on mTOR signaling and polycystic kidney disease. J Am Soc Nephrol 23: 842–853, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Omori S, Hida M, Fujita H, Takahashi H, Tanimura S, Kohno M, Awazu M: Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J Am Soc Nephrol 17: 1604–1614, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S: Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17: 1505–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gattone VH, 2nd, Wang X, Harris PC, Torres VE: Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S: Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45: 1004–1012, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma N, Malarkey EB, Berbari NF, O’Connor AK, Vanden Heuvel GB, Mrug M, Yoder BK: Proximal tubule proliferation is insufficient to induce rapid cyst formation after cilia disruption. J Am Soc Nephrol 24: 456–464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grantham JJ, Mulamalla S, Swenson-Fields KI: Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 7: 556–566, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Woo D: Apoptosis and loss of renal tissue in polycystic kidney diseases. N Engl J Med 333: 18–25, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Nadasdy T, Laszik Z, Lajoie G, Blick KE, Wheeler DE, Silva FG: Proliferative activity of cyst epithelium in human renal cystic diseases. J Am Soc Nephrol 5: 1462–1468, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr., Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP, CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Grantham JJ, Cook LT, Torres VE, Bost JE, Chapman AB, Harris PC, Guay-Woodford LM, Bae KT: Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int 73: 108–116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 41.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP: Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hörl WH, Obermüller N, Arns W, Pavenstädt H, Gaedeke J, Büchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU: Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363: 830–840, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Ruggenenti P, Remuzzi A, Ondei P, Fasolini G, Antiga L, Ene-Iordache B, Remuzzi G, Epstein FH: Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int 68: 206–216, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Clark PJ, Evans FC: Distance to nearest neighbor as a measure of spatial relationships in populations. Ecology 35: 445–453, 1954 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.