Abstract
Genetic variants in apolipoprotein L1 (APOL1) confer risk for kidney disease. We sought to better define the phenotype of APOL1-associated nephropathy. The FSGS Clinical Trial involved 138 children and young adults who were randomized to cyclosporin or mycophenolate mofetil plus pulse oral dexamethasone with a primary outcome of proteinuria remission. DNA was available from 94 subjects who were genotyped for APOL1 renal risk variants, with two risk alleles comprising the risk genotype. Two APOL1 risk alleles were present in 27 subjects, of whom four subjects did not self-identify as African American, and 23 of 32 (72%) self-identified African Americans. Individuals with the APOL1 risk genotype tended to present at an older age and had significantly lower baseline eGFR, more segmental glomerulosclerosis and total glomerulosclerosis, and more tubular atrophy/interstitial fibrosis. There were differences in renal histology, particularly more collapsing variants in those with the risk genotype (P=0.02), although this association was confounded by age. APOL1 risk genotype did not affect response to either treatment regimen. Individuals with the risk genotype were more likely to progress to ESRD (P<0.01). In conclusion, APOL1 risk genotypes are common in African-American subjects with primary FSGS and may also be present in individuals who do not self-identify as African American. APOL1 risk status is associated with lower kidney function, more glomerulosclerosis and interstitial fibrosis, and greater propensity to progress to ESRD. The APOL1 risk genotype did not influence proteinuria responses to cyclosporin or mycophenolate mofetil/dexamethasone.
Keywords: FSGS, cyclosporin, genetic renal disease
FSGS is the most common pattern of glomerular injury observed in glucocorticoid-resistant nephrotic syndrome, and it commonly progresses to ESRD.1 Many subjects with primary FSGS do not respond to first-line therapy with glucocorticoids,2,3 and the optimal second-line therapy has been a matter of some debate. To address this issue, the National Institutes of Health–sponsored FSGS Clinical Trial (FSGS-CT) randomized 138 subjects with steroid-resistant FSGS to treatment with cyclosporin versus mycophenolate combined with pulse oral dexamethasone, and all subjects received low-dose oral prednisone.4
Genetic variants in apolipoprotein L1 (APOL1), encoding APOL1, have been shown to be strongly associated with glomerular disease, including FSGS, HIV-associated collapsing glomerulopathy, and hypertension-attributed CKD and end stage kidney disease.5–7 We wished to learn more about the phenotype of APOL1-associated nephropathy, particularly whether subjects with two APOL1 risk alleles manifest particular histologic variants and whether they tend to be responsive or resistant to cyclosporin or mycophenolate mofetil, and we investigated these issues in the context of the FSGS-CT.
Results
As shown in Table 1, the APOL1 risk genotype (the presence of two risk alleles, defined as G1/G1 homozygotes, G2/G2 homozygotes, and G1/G2 compound heterozygotes) was present in 72% of self-identified African-American patients, which is the same frequency previously observed for sporadic FSGS.6 Surprisingly, 6% (four of 62) of individuals who identified themselves as non–African American had two APOL1 risk alleles; these included two of 42 European American non-Hispanics and two of 17 European American Hispanics. Among self-identified Hispanic individuals, APOL1 risk status was present among those who reported African ancestry and those who did not report African ancestry. There were three subjects who self-identified as having Asian, Native American, and other ancestry, none of whom carried APOL1 risk alleles. These results suggest that, among Americans, self-identified race or ethnicity is not a reliable criterion to exclude the possibility that individuals carry APOL1 risk alleles.
Table 1.
Racial and ethnic background and APOL1 risk allele status of the study population
| Racial and Ethnic Background | Zero or One APOL1 Risk Alleles | Two APOL1 Risk Alleles | Subgroup Percentage with Two APOL1 Risk Alleles |
|---|---|---|---|
| African American, non-Hispanic | 8 | 21 | 72 |
| African American, Hispanic | 1 | 2 | 67 |
| European American, non-Hispanic | 40 | 2 | 7 |
| European American, Hispanic | 15 | 2 | 12 |
| Amerindian | 1 | 0 | 0 |
| Asia | 1 | 0 | 0 |
| Other | 1 | 0 | 0 |
| Total | 67 | 27 |
The distributions of APOL1 risk alleles are shown by groups defined by self-identified race and ethnicity. DNA was available from 94 subjects.
Summaries of other demographic, clinical, and histologic data are presented in Table 2 (considering all subjects) and Table 3 (limited to those self-identified as African American). Several observations can be made about findings that reached statistical significance in at least one of these two approaches. FSGS onset occurred at an older age among those with two APOL1 risk alleles when all subjects were considered; among these individuals, the youngest individual was 2 years old, and the others were 9–37 years old, which resembles the peak onset age brackets of 15–39 years for APOL1-associated FSGS as previously reported.6 This observation must be tempered by the fact that the data are right-censored, because the FSGS-CT excluded subjects >40 years of age. Baseline eGFR was significantly lower, and the number of glomeruli showing segmental glomerulosclerosis, global glomerulosclerosis, and total glomerulosclerosis (segmental plus global glomerulosclerosis) and the extent of tubular atrophy and fibrosis were all greater among those with the APOL1 risk genotype, which is consistent with the faster progression rate that has been observed in these individuals.6 Most APOL1-associated FSGS was present in subjects entering the study after age 12 years, and this makes age a confounding variable for the interpretation of some results (Supplemental Table 1). Subjects >12 years old tended to have a lower baseline eGFR and more collapsing glomerulopathy, segmental glomerulosclerosis, total glomerulosclerosis, and interstitial fibrosis, and APOL1 2 risk allele status was associated with the first four of these variables.
Table 2.
Demographic, clinical, and histologic variables by APOL1 risk status (all subjects)
| Variable | Zero or One APOL1 Risk Alleles | Two APOL1 Risk Alleles | P Value |
|---|---|---|---|
| Number of subjects | 67 | 27 | |
| Onset age (yr) | 13 (8, 23) | 17 (13, 27) | 0.03 |
| Enrollment age (yr) | 14 (10, 28) | 17 (13, 27) | 0.07 |
| Baseline eGFR (ml/min per 1.73 m2) | 130 (83, 195) | 94 (68, 109) | 0.003 |
| Baseline serum albumin (g/dl) | 3.1 (2.3, 3.7) | 3.0 (2.1, 3.8) | 0.84 |
| Baseline urine protein-to-creatinine ratio | 3.6 (2.2, 7.1) | 4.7 (2.8, 10.2) | 0.28 |
| Baseline serum suPAR (ng/ml) | 4297 (3402, 5405) | 4020 (3104, 4510) | 0.18 |
| Change in suPAR (ng/ml) | −27 (−1062, +764) | +103 (−532, +1541) | 0.33 |
| FSGS histologic variant | 0.02 | ||
| Collapsing | 3 | 8 | |
| Tip | 10 | 2 | |
| Cellular | 2 | 0 | |
| Perihilar | 7 | 2 | |
| Not otherwise specified | 45 | 15 | |
| Segmental glomerulosclerosis (%) | 20 (9, 31) | 25 (19, 34) | 0.02 |
| Global glomerulosclerosis (%) | 0 (0, 12) | 7 (0, 17) | 0.43 |
| Total glomerulosclerosis (%) | 25 (12, 50) | 44 (24, 62) | 0.02 |
| Tubular atrophy/fibrosis (%) | 10 (5, 25) | 20 (5, 60) | <0.01 |
| Arteriosclerosis (score) | 0 (0, 1) | 0 (0, 1.2) | 0.13 |
| CR (remissions coded as one or two) | 11 | 1 | 0.17 |
| CR or PR sustained at week 52 (remission coded as one, two, or three) | 29 | 7 | 0.16 |
| Reached end stage kidney disease during the follow-up period | 8 | 10 | <0.01 |
Data are presented as medians (25th, 75th percentiles). Note that the total glomerulosclerosis is not the sum of segmental glomerulosclerosis plus global glomerulosclerosis, because the values are medians.
Table 3.
Demographic, clinical, and histologic variables by APOL1 risk status (self-identified African Americans only)
| Variable | Zero or One APOL1 Risk Alleles | Two APOL1 Risk Alleles | P Value |
|---|---|---|---|
| Number of subjects | 9 | 23 | |
| Onset age (yr) | 10 (4.5, 30) | 17 (13, 27) | 0.10 |
| Enrollment age (yr) | 10 (5, 30) | 18 (13, 29) | 0.12 |
| Baseline eGFR (ml/min per 1.73 m2) | 130 (63, 178) | 94 (79, 109) | 0.34 |
| Baseline serum albumin (g/dl) | 2.6 (1.6, 3.2) | 3.3 (2.1, 3.8) | 0.16 |
| Baseline urine protein-to-creatinine ratio | 1.6 (0.7, 2.4) | 1.5 (1.0, 2.3) | 0.87 |
| Baseline serum suPAR | 4474 (3180, 5559) | 3864 (2826, 4472) | 0.25 |
| Change in suPAR (ng/ml) | +580 (+82, +1353) | +60 (−510, +1659) | 0.49 |
| FSGS histologic variant | 0.23 | ||
| Collapsing | 0 | 6 | |
| Tip | 1 | 1 | |
| Cellular | 0 | 0 | |
| Perihilar | 0 | 2 | |
| Not otherwise specified | 8 | 14 | |
| Segmental glomerulosclerosis (%) | 20 (9, 29) | 25 (16, 44) | 0.12 |
| Global glomerulosclerosis (%) | 0 (0, 0) | 7.4 (0. 17) | 0.03 |
| Total glomerulosclerosis (%) | 20 (9, 35) | 36 (20, 62) | <0.05 |
| Tubular atrophy/fibrosis | 10 (2.5, 30) | 20 (6, 70) | 0.31 |
| Arteriosclerosis (score) | 0 (0, 1) | 0 (0, 1) | 0.56 |
| CR (remissions coded as one or two) | 0 | 1 | >0.99 |
| CR or PR sustained at week 52 (remission coded as one, two, or three) | 2 | 5 | >0.99 |
| Reached end stage kidney disease during the follow-up period | 0 | 8 | 0.07 |
Data are presented as medians (25th, 75th percentiles).
With regard to glomerular histology (FSGS variant), there were differences when the data from all subjects were analyzed, driven particularly by an excess of collapsing variant and fewer tip lesion cases among subjects with two APOL1 risk alleles. There was no similar trend when self-identified African Americans were analyzed, possibly because of reduced statistical power. There were no differences between genotype groups with respect to mean levels of soluble urokinase-type plasminogen activator receptor (suPAR), which were elevated in both genotype groups, consistent with a proposed role for suPAR in the pathogenesis of primary and recurrent FSGS after kidney transplantation.8
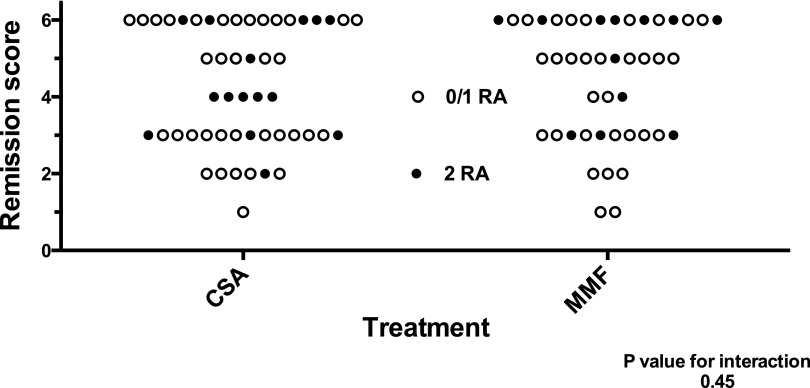
Importantly, there were no differences in complete remission (CR) rate or CR plus partial remission (PR) rate between the APOL1 risk and nonrisk genotype groups, although the numbers are too small to draw firm conclusions. Furthermore, ANOVA analyses looking for an interaction between treatment (cyclosporin versus mycophenolate mofetil) and APOL1 risk genotype in the outcome, defined as remission score, yielded a nonsignificant P value (0.45). This suggests that the APOL1 risk genotype status did not affect an individual’s propensity to respond to these remittive agents (Figure 1). Note that this curve is likely not an entirely accurate reflection of the typical FSGS course, because individuals who progressed to low eGFR early and rapidly would not have been eligible to participate in the FSGS-CT.
Figure 1.
Interaction between APOL1 genotype and treatment response. Randomized treatment with cyclosporin (CSA) or mycophenolate mofetil combined with oral pulse dexamethasone (MMF) was associated with remissions scored as one to six. Each of 94 (total) subjects is depicted as a circle, with open circles denoting those with zero or one APOL1 risk alleles (RAs) and closed circles denoting those with two APOL1 RAs. Analysis for interaction between treatment and genotype using two-way ANOVA yielded a nonsignificant P value (P=0.45), suggesting that the presence or absence of the APOL1 risk genotype was not associated with differential responses to these medications.
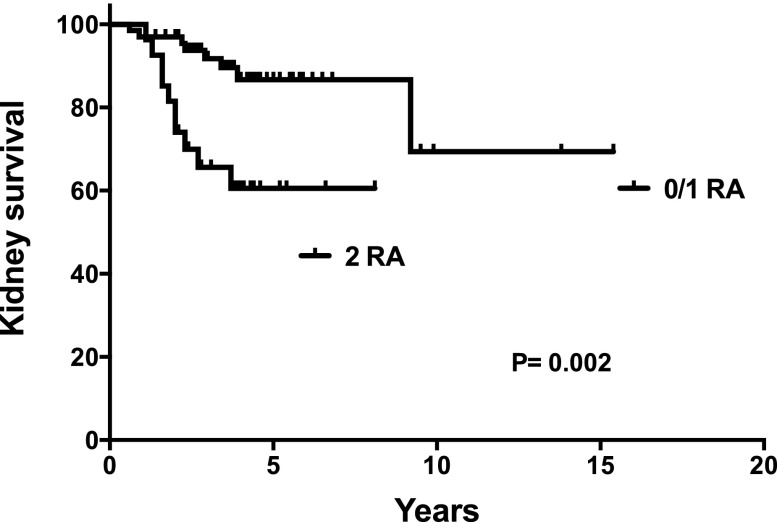
In a follow-up analysis to week 78 of the trial, renal survival assessed from the onset of FSGS was significantly shorter among subjects with two APOL1 risk alleles (Figure 2). We conducted multivariable analysis excluding variables associated with APOL1 risk genotype, including baseline eGFR and tubular atrophy/interstitial fibrosis, to avoid multiple colinearity. Previous work in this cohort has suggested that the outcome of end stage kidney disease/death was associated with lower baseline eGFR, severe nephrotic syndrome, public insurance, and more tubular atrophy/interstitial fibrosis (S. Hogan et al., unpublished data). The multivariable model included premature birth, birth weight, body mass index (scored as underweight/healthy weight, overweight, obese, or severely obese to account for different thresholds in children and adults), insurance (scored as none, public, or private), baseline proteinuria, and APOL1 risk status. In this model, baseline proteinuria (P=0.001) and APOL1 risk status (P=0.02) were the only variables significantly associated with renal survival.
Figure 2.
Renal survival analysis. Renal survival, assessed from FSGS onset to dialysis or kidney transplant and censored for death with kidney function, was significantly shorter for individuals with two APOL1 risk alleles (RAs) compared with others. Analysis includes all 94 subjects.
Discussion
APOL1 kidney risk variants are strongly associated with kidney disease among African Americans,5 and the association is strongest for HIV-associated nephropathy (odds ratio, 29; 95% confidence interval, 13 to 68) and FSGS (odds ratio, 17; 95% confidence interval, 11 to 26).6 Previous studies have established that APOL1-associated FSGS is characterized by a tendency to present between ages 15–39 years old and rapid progression to end stage kidney disease but similar response to glucocorticoids compared with those lacking two APOL1 risk alleles.6 With regard to response to therapy, the only other published data suggest that APOL1-associated hypertension-attributed kidney disease is refractory to therapy with angiotensin-converting enzyme inhibitors.9
This study offers several new insights into the nature of APOL1-associated FSGS. First (most important from a clinical standpoint), although the numbers of subjects are small, the response to cyclosporin and mycophenolate mofetil/dexamethasone was similar in those with and without two APOL1 risk alleles, suggesting that both approaches could be considered when these individuals have not responded to glucocorticoids or for individuals for whom glucocorticoids are contraindicated. Second, the finding that APOL1 risk alleles can be present in subjects who do not self-identify as having African ancestry suggests that, when a clinical role for APOL1 genetic testing has been shown, testing may be indicated in individuals who present with characteristic renal syndromes, regardless of the ancestry that they may report. Third, this is the first systematic analysis of renal histology in APOL1-associated FSGS. The finding of more glomerulosclerosis, tubular atrophy, and interstitial fibrosis is consistent with the tendency toward rapid functional loss that is a reflection of these structural alterations. The distribution of FSGS variants differed between APOL1 risk groups and was driven largely by more collapsing variants in the APOL1 risk group. The propensity of individuals with APOL1 risk to collapsing glomerulopathy has been reported before in the setting of HIV-associated nephropathy10 and lupus nephritis.11 Fourth, there was no association between APOL1 risk status and suPAR levels. It is possible that cytokines, such as suPAR, may interact with APOL1 variants; studies to address this possibility will require analysis of patients with FSGS and appropriate healthy volunteers. Fifth, as previously shown, APOL1-associated FSGS has a propensity to progress rapidly to end stage kidney disease, suggesting that early treatment should be aggressive if the clinical setting warrants.
This study has several limitations; 42 of 138 subjects in the FSGS-CT, representing 30% of all subjects, did not provide DNA for analysis, reducing statistical power. We did not characterize ancestry-informative markers, and therefore, we did not determine the fraction of African ancestry in each subject, although we do not believe that this information would change the analyses or interpretation, because the prevalence of two APOL1 risk alleles in this study was nearly identical to that previously reported.6 Both therapeutic arms used glucocorticoids, and therefore, the results are not strictly applicable to responses that might be seen with cyclosporin or mycophenolate mofetil used as monotherapy for steroid-resistant FSGS.
In summary, APOL1-associated FSGS may occur in individuals with various self-reported ancestries and is associated with more severe kidney fibrosis at diagnosis and more rapid progression to end stage kidney disease, but it has equal propensity to respond to glucocorticoids as well as cyclosporin and mycophenolate as used in this study. The strongest argument for APOL1 genetic testing in clinical practice might be that individuals carrying two risk alleles should be treated aggressively with remittive agents from the initial diagnosis of FSGS. At present, however, there are no data that would indicate that aggressive treatment with available agents retards progressive kidney function loss in these high-risk individuals.
Concise Methods
Subjects and Treatment
The FSGS-CT enrolled children and adults with primary steroid-resistant, biopsy-proven FSGS who were between the ages of 2 and 40 years, had an eGFR>40 ml/min per 1.73 m2, and had a urine protein-to-creatinine ratio>1 g/g. Exclusion criteria included secondary FSGS, diabetes mellitus, and chronic infection (including chronic viral infection) as well as the other criteria as previously described.4 Race and ethnicity (Hispanic ancestry) were determined by self-report of subjects or report by parents; genetic studies for ancestry informative markers were not carried out. Of these subjects, 138 individuals were randomized to receive either cyclosporin or mycophenolate mofetil combined with pulse oral dexamethasone; both groups received low-dose oral prednisone and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. CR was defined as a urine protein-to-creatinine ratio <0.2 g/g, and PR was defined as a 50% reduction, reaching a value of 0.2 to <2.0 g/g. The FSGS-CT defined remission on a six-point ordinal scale, on which one denoted CR during the first 24 weeks that was sustained to week 52, two denoted CR during weeks 27–48, three denoted a PR by week 26 that was sustained to week 78, four and five denoted a transient PR, and six denoted failure to obtain a PR. For the purposes of this study, a CR is equivalent to a score of one or two, and a PR is equivalent to a score of three. Of these subjects, 94 individuals provided DNA for genetic studies.
Clinical and Laboratory Testing
Serum and urine chemistry measurements were made during the treatment period and every 26 weeks after week 78 using standard methods that were performed in a central laboratory (Spectra East Core Laboratory). eGFR was calculated according to the Schwartz equation for subjects <18 years of age and the Cockroft–Gault equation adjusted for body surface area for subjects ≥18 years of age.
Genetic Testing
APOL1 genotyping for 94 subjects from whom DNA was available was performed using a Taqman assay and allele-specific primers6; this approach shows 100% concordance with Sanger sequencing (C.A. Winkler, unpublished data). Exon sequencing of 86 subjects with DNA available as of 2010 included the following genes: NPHS2 sequencing, which identified two subjects with heterozygous mutations (one subject each had R138Q and L139R) and one subject with a heterozygous A242V variant (sequencing carried out independently by L.M.G.-W. and F.H.)12; PLCE1 sequencing, which identified eight novel heterozygous codon variants, of which four variants were missense mutations13; WT1 exons 8 and 9, which identified one Frasier syndrome variant and one novel potential mutation; and INF2 sequencing, with no mutations identified. In summary, Mendelian genetic variants accounted for one and possibly, two patients with FSGS, both with WT1 variants.
Pathology
Kidney biopsy tissue was available for all subjects and reviewed by a central pathology committee, which confirmed the diagnosis of FSGS, made an FSGS histologic variant diagnosis according to the Columbia Classification,14 and carried out quantitative analysis of histologic variables as recently described.15
suPAR
Serum suPAR levels were measured in the FSGS-CT as previously described and reported.16
Statistical Analyses
Data for subjects with zero or one APOL1 risk alleles versus those with two risk alleles are summarized as medians (interquartile ranges). Differences between groups were compared by Mann–Whitney U tests for nonparametric data using two-tailed tests. Analysis of histology (FSGS variants) was by chi-squared testing, whereas analysis of progression to end stage kidney disease was by Fisher exact testing. Cox survival analysis was performed using the Gehan–Breslow–Wilcoxon test and Prism software (GraphPad Software, La Jolla, CA). A P value <0.05 was considered to be significant.
Human Subjects Research Issues
The FSGS-CT protocol was approved by the institutional review boards at the participating institutions, and all subjects gave informed assent or consent. Genetic analysis for APOL1 kidney risk variants was approved by the National Institute of Diabetes and Digestive and Kidney Diseases Institutional Review Board.
Disclosures
None.
Supplementary Material
Acknowledgments
We acknowledge the excellent technical assistance of Elizabeth Binns-Roemer.
The FSGS Clinical Trial was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH), and this work was supported by the NIDDK and National Cancer Institute (NCI) Intramural Research Programs. This project has been funded in whole or part with federal funds from NCI, NIH Contract HHSN26120080001E. This research was supported in part by the Intramural Research Program of the NIH, NCI, Center for Cancer Research.
These data were presented in abstract form at the Annual Meeting of the American Society of Nephrology in San Diego, CA on October 30–November 4, 2012.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, and mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Apolipoprotein L1-Associated Nephropathy and the Future of Renal Diagnostics,” on pages 1232–1235.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013111242/-/DCSupplemental.
References
- 1.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Korbet SM: Treatment of primary FSGS in adults. J Am Soc Nephrol 23: 1769–1776, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Joy MS, Gipson DS, Powell L, MacHardy J, Jennette JC, Vento S, Pan C, Savin V, Eddy A, Fogo AB, Kopp JB, Cattran D, Trachtman H: Phase 1 trial of adalimumab in focal segmental glomerulosclerosis (FSGS): II. Report of the FONT (Novel Therapies for Resistant FSGS) study group. Am J Kidney Dis 55: 50–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gipson DS, Trachtman H, Kaskel FJ, Greene TH, Radeva MK, Gassman JJ, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Hogan SL, Middleton JP, Vehaskari VM, Flynn PA, Powell LM, Vento SM, McMahan JL, Siegel N, D’Agati VD, Friedman AL: Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int 80: 868–878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB, SK Investigators : Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atta MG, Estrella MM, Kuperman M, Foy MC, Fine DM, Racusen LC, Lucas GM, Nelson GW, Warner AC, Winkler CA, Kopp JB: HIV-associated nephropathy patients with and without apolipoprotein L1 gene variants have similar clinical and pathological characteristics. Kidney Int 82: 338–343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larsen CP, Beggs ML, Saeed M, Walker PD: Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 24: 722–725, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li RZS, Guay-Woodford LM: Comprehensive NPHS2 mutational analysis in FSGS Clinical Trial patients and population controls. Presented at the American Society of Nephrology Annual Meeting, Philadelphia, PA, November 4–9, 2008 [Google Scholar]
- 13.Saiswat P, Chernin G, Vega-Warner V, Ovunc B, Saskia H, Hidebrandt H, FSGS-CTSteeringCommittee: PLCE1 mutation analysis in 86 patients of the NIH FSGS Clinical Trial (FSGS-CT). Presented at the American Society of Nephrology Annual Meeting, San Diego, CA, October 27–November 1, 2009 [Google Scholar]
- 14.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 15.D’Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA, Cohen AH, Gipson DS, Gassman JJ, Radeva MK, Moxey-Mims MM, Friedman AL, Kaskel FJ, Trachtman H, Alpers CE, Fogo AB, Greene TH, Nast CC: Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol 8: 399–406, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei C, Trachtman H, Li J, Dong C, Friedman AL, Gassman JJ, McMahan JL, Radeva M, Heil KM, Trautmann A, Anarat A, Emre S, Ghiggeri GM, Ozaltin F, Haffner D, Gipson DS, Kaskel F, Fischer DC, Schaefer F, Reiser J, PodoNet and FSGS CT Study Consortia : Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol 23: 2051–2059, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.