Abstract
Although renal hyperfiltration (RHF) or an abnormal increase in GFR has been associated with many lifestyles and clinical conditions, including diabetes, its clinical consequence is not clear. RHF is frequently considered to be the result of overestimating true GFR in subjects with muscle wasting. To evaluate the association between RHF and mortality, 43,503 adult Koreans who underwent voluntary health screening at Seoul National University Hospital between March of 1995 and May of 2006 with baseline GFR≥60 ml/min per 1.73 m2 were followed up for mortality until December 31, 2012. GFR was estimated with the Chronic Kidney Disease Epidemiology Collaboration creatinine equation, and RHF was defined as GFR>95th percentile after adjustment for age, sex, muscle mass, and history of diabetes and/or hypertension medication. Muscle mass was measured with bioimpedance analysis at baseline. During the median follow-up of 12.4 years, 1743 deaths occurred. The odds ratio of RHF in participants with the highest quartile of muscle mass was 1.31 (95% confidence interval [95% CI], 1.11 to 1.54) compared with the lowest quartile after adjusting for confounding factors, including body mass index. The hazard ratio of all-cause mortality for RHF was 1.37 (95% CI, 1.11 to 1.70) by Cox proportional hazards model with adjustment for known risk factors, including smoking. These data suggest RHF may be associated with increased all-cause mortality in an apparently healthy population. The possibility of RHF as a novel marker of all-cause mortality should be confirmed.
Keywords: glomerular hyperfiltration, mortality, GFR, CKD, epidemiology and outcomes
Although CKD is a well known risk factor for all-cause or cardiovascular mortality,1 the clinical consequences of an abnormally high GFR or renal hyperfiltration (RHF) have not been adequately evaluated.
On the basis of several cross-sectional studies, RHF is known to be associated with various medical conditions, such as diabetes,2,3 hypertension,4 obesity,5 prehypertension, and prediabetes,6 as well as lifestyle factors, such as smoking,7 lack of physical activity,8 and low aerobic fitness.9 Although these conditions are well known risk factors for early mortality,10 the clinical implications of RHF remain unclear.
Several cohort studies and meta-analyses have reported a J-shaped association between GFR and all-cause and cardiovascular mortality. However, the increased mortality associated with a higher GFR was commonly regarded as an overestimation of GFR because of muscle wasting in a high-risk group.11–17 The disappearance of the J-shaped association between GFR and mortality within a younger age group in the higher GFR range is considered as supporting evidence for the overestimation of the true GFR because of muscle wasting in high-risk elderly people.16 The various definitions of RHF also makes it difficult to compare results among studies. Some studies have defined high GFR as above a certain absolute value without considering age, sex, or muscle mass. Because both muscle mass and GFR decrease as age increases, both age and muscle mass should be considered in the definition of high GFR or RHF.18–20 In addition, cigarette smoking—one of the most important risk factors for mortality—has been associated with RHF.7 The differential effect of smoking on the association between RHF and mortality should be evaluated.17 No study has yet evaluated the long-term clinical consequences of RHF taking age, muscle mass, and smoking status into account.
To elucidate the long-term clinical implications of RHF as a novel predictor of survival, we investigated the association between age-, sex-, muscle mass-, and history of diabetes and/or hypertension medication-adjusted RHF and all-cause mortality within a large adult population. We further examined the role of smoking on the association between RHF and mortality.
Results
Table 1 summarizes the general characteristics of the participants in this study. Baseline eGFR was 77.5±11.0 ml/min per 1.73 m2 in the deceased group and 80.4±11.5 ml/min per 1.73 m2 in the survivor group (P<0.001). RHF was observed in 5.9% of the deceased and 5.0% of survivors (P=0.09). After a total follow-up period of 527,260 person-years (median follow-up duration of 12.4 years), 1743 deaths were observed, including 854 cancer, 330 cardiovascular, 62 infectious, 46 diabetic, and 45 hepatic deaths (Table 2).
Table 1.
General characteristics of participants at the time of the health screening
| Characteristics | Alive (n=41,760) | Deceased (n=1743) | Total (n=43,503) | P Valuea |
|---|---|---|---|---|
| Men, % | 21,086 (50.5) | 1204 (69.1) | 22,290 (51.2) | <0.001 |
| Age at screening (yr) | 48.9±10.3 | 56.9±10.5 | 49.2±10.4 | <0.001 |
| Smoking, % | ||||
| Nonsmoker | 55.0 | 37.9 | 54.3 | <0.001 |
| Former smoker | 18.1 | 22.2 | 18.3 | |
| Current smoker | 26.9 | 40.0 | 27.5 | |
| Regular exercise, % | 35.0 | 30.8 | 34.8 | <0.001 |
| Regular alcohol intake, % | 48.1 | 49.3 | 48.1 | 0.35 |
| History of antihypertensive therapy, % | 8.8 | 14.7 | 9.0 | <0.001 |
| History of diabetes therapy, % | 5.7 | 12.2 | 6.0 | <0.001 |
| Systolic BP (mmHg) | 128.9±20.4 | 135.2±23.7 | 129.2±20.6 | <0.001 |
| Diastolic BP (mmHg) | 79.0±12.5 | 81.4±13.5 | 79.1±12.5 | <0.001 |
| BMI (kg/m2) | 23.9±3.0 | 23.8±3.1 | 23.9±3.0 | 0.04 |
| <18.5, % | 2.9 | 4.5 | 2.9 | <0.001 |
| >25.0, % | 34.3 | 33.0 | 34.3 | |
| Serum creatinineb (mg/dl) | 0.98±0.15 | 1.00±0.14 | 0.98±0.15 | <0.001 |
| FSG (mg/dl) | 98.3±24.3 | 108.0±38.9 | 98.7±25.2 | <0.001 |
| Serum cholesterol (mg/dl) | 200.9±36.6 | 201.4±41.7 | 200.9±36.8 | 0.61 |
| Serum triglyceride (mg/dl) | 135.4±94.8 | 145.9±103.5 | 135.8±95.2 | <0.001 |
| Serum HDL-cholesterol (mg/dl) | 52.8±13.5 | 50.6±13.7 | 52.8±13.5 | <0.001 |
| eGFRc (ml/min per 1.73 m2) | 80.4±11.5 | 77.5±11.0 | 80.3±11.5 | <0.001 |
| RHF,d % | 5.0 | 5.9 | 5.0 | 0.09 |
| Albuminuria,e % | 3.3 | 6.8 | 3.4 | <0.001 |
| LBMf | 47.4±8.8 | 48.3±8.8 | 47.4±8.8 | <0.001 |
| 1st Quartile, % | 24.4 | 36.0 | 24.9 | <0.001 |
| 2nd Quartile, % | 25.0 | 23.8 | 25.0 | <0.001 |
| 3rd Quartile, % | 25.4 | 20.1 | 25.2 | <0.001 |
| 4th Quartile, % | 25.1 | 20.1 | 24.9 | <0.001 |
Chi-squared tests for discrete variables and t tests for continuous variables.
Recalibrated to isotope dilution mass spectrometry (details in Concise Methods).
eGFR using the CKD-EPI equation.
eGFR higher than the age-, sex-, muscle mass-, and history of diabetes and/or hypertension medication-adjusted 95th percentile (details in Concise Methods).
Dipstick albuminuria 1+ or higher.
LBM by bioimpedance analysis.
Table 2.
Association between RHF and all-cause and cause-specific mortality
| Causes of Death | Cases Per Person-Yr | Incidence Densitya | aHR (95% CI)b |
|---|---|---|---|
| All cause | |||
| All participants | 1743/527,260 | 330.6 | 1.37 (1.11 to 1.70) |
| Nonsmokerc | 626/268,406 | 233.2 | 1.58 (1.13 to 2.22) |
| Former smokerc | 366/86,773 | 421.8 | 1.73 (1.10 to 2.73) |
| Current smokerc | 660/138,767 | 475.6 | 1.08 (0.77 to 1.52) |
| Cause of death | |||
| Cancer | 854/527,260 | 162.0 | 1.14 (0.81 to 1.60) |
| Cardiovascular | 330/527,260 | 62.6 | 1.66 (1.04 to 2.66) |
| Infectious | 62/527,260 | 11.8 | 2.71 (1.07 to 6.88) |
| Diabetic | 46/527,260 | 8.7 | 1.17 (0.28 to 4.89) |
| Hepatic | 45/527,260 | 8.5 | 3.08 (1.20 to 7.92) |
| Other | 406/527,260 | 77.0 | 1.48 (0.98 to 2.25) |
eGFR using the CKD-EPI equation above the age-, sex-, muscle mass-, and history of diabetes and/or hypertension medication-adjusted 95th percentile (details in Concise Methods).
Incidence density (per 100,000 person-yr).
aHR and 95% CI of the participants with RHF compared with those without RHF determined by the Cox proportional hazards model adjusted for age, sex, smoking, regular exercise, regular alcohol ingestion, history of diabetes and/or hypertension medication, BMI, systolic BP, FSG, serum triglyceride, serum HDL-cholesterol, and albuminuria. In the stratified analysis for smoking, smoking history was excluded from the adjusting variables.
Missing in 3316 participants. P for interaction=0.25.
The association between lean body mass (LBM) and RHF was analyzed with a logistic regression adjusted for possible confounding variables, such as age, sex, regular exercise, smoking, regular alcohol ingestion, history of diabetes and/or hypertension medication, body mass index (BMI), systolic BP, fasting serum glucose (FSG), serum triglyceride, serum HDL-cholesterol, and albuminuria. Higher LBM was associated with higher odds of RHF (odds ratio [OR], 1.31; 95% confidence interval [95% CI], 1.11 to 1.54 for the fourth quartile compared with the lowest quartile; P for trend<0.001) (Table 3). When sex-specific BMI quartile groups were included in the model, the highest BMI quartile group was also independently associated with higher odds of RHF compared with the lowest quartile (OR, 1.25; 95% CI, 1.05 to 1.49; data not shown) after adjustment for the above confounding variables, including LBM. Higher LBM was associated with higher BMI in both men and women after adjusting for age, smoking, regular exercise, and regular alcohol ingestion (P for trend<0.001 in both men and women) (Table 3).
Table 3.
Association of LBM with BMI and RHF
| LBMa Quartiles | Estimated Mean BMIb (95% CI) | RHF, % | OR for RHFc (95% CI) | |
|---|---|---|---|---|
| Men | Women | |||
| 1st | 21.7 (21.6 to 21.7) | 20.9 (20.8 to 21.0) | 411/9939 (4.1%) | Reference |
| 2nd | 23.5 (23.5 to 23.6) | 22.5 (22.4 to 22.6) | 417/10,059 (4.1%) | 0.91 (0.78 to 1.05) |
| 3rd | 24.8 (24.7 to 24.9) | 23.7 (23.6 to 23.8) | 508/10,108 (5.0%) | 1.07 (0.93 to 1.25) |
| 4th | 26.8 (26.7 to 26.8) | 25.9 (25.8 to 26.0) | 677/10,143 (6.7%) | 1.31 (1.11 to 1.54) |
eGFR using the CKD-EPI equation above the age-, sex-, muscle mass-, and history of diabetes and/or hypertension medication-adjusted 95th percentile (details in Concise Methods).
LBM was measured by bioimpedance analysis and classified into sex-specific quartile groups.
Estimated by the general linear model and adjusted for age, smoking, regular exercise, and regular alcohol ingestion. P for trend<0.001 in both men and women.
OR by multivariate logistic regression analysis adjusted for age, sex, smoking status, regular exercise, regular alcohol intake, history of therapy for diabetes and/or hypertension, BMI, systolic BP, FSG, serum triglyceride, serum HDL-cholesterol, and albuminuria. P for trend<0.001.
RHF was associated with increased all-cause mortality on the basis of the Cox proportional hazards model adjusted for possible confounding variables, such as age, sex, smoking, regular exercise, regular alcohol ingestion, history of diabetes and/or hypertension medication, BMI, systolic BP, FSG, serum triglyceride, serum HDL-cholesterol, and albuminuria (adjusted hazard ratio [aHR], 1.37; 95% CI, 1.11 to 1.70) (Table 2). When BMI quartile groups were included in the model instead of BMI as a continuous variable, the significance of the association did not change (aHR, 1.36; 95% CI, 1.10 to 1.68; data not shown). When RHF was defined using eGFR residuals of multiple linear regressions with BMI instead of LBM, the association between RHF and all-cause mortality was also significant (aHR, 1.46; 95% CI, 1.18 to 1.81; data not shown). Because age-, sex-, BMI-, and history of diabetes and/or hypertension medication-adjusted RHF cannot clearly address the issue of possible overestimation of GFR because of muscle wasting in the association between RHF and mortality, we decided to use age-, sex-, LBM-, and history of diabetes and/or hypertension medication-adjusted RHF for additional analysis.
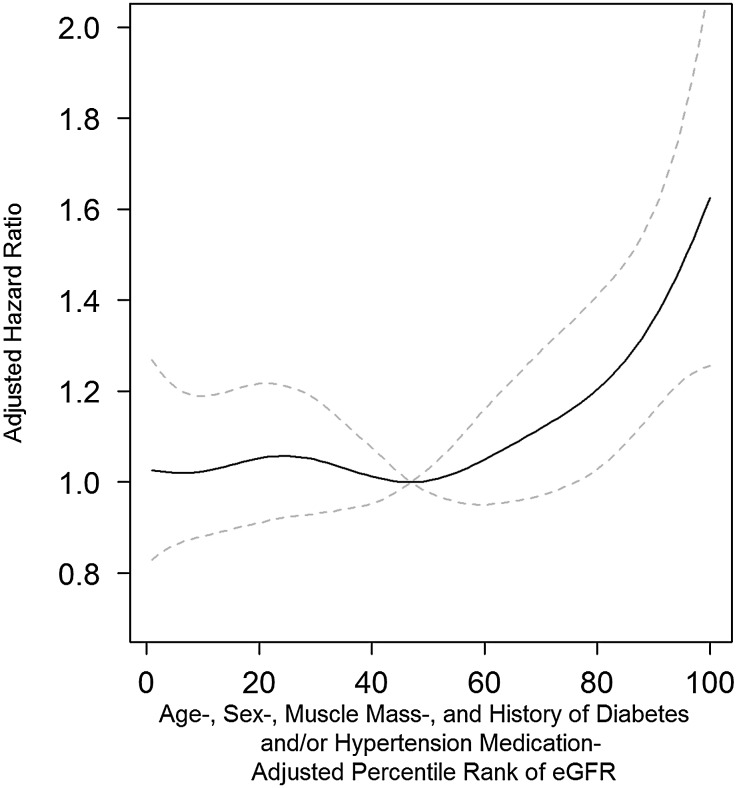
When the relationship between all-cause mortality and the percentile rank of the residuals from the multiple linear regression analysis of eGFR was evaluated with a semiparametric smoothing model with adjustments for age, sex, smoking, regular exercise, regular alcohol ingestion, history of diabetes and/or hypertension medication, BMI, systolic BP, FSG, serum triglyceride, serum HDL-cholesterol, and albuminuria, the aHR was lowest around the 50th percentile and increased thereafter (Figure 1). RHF was associated with higher cardiovascular, infectious, and hepatic mortality (aHR, 1.66; 95% CI, 1.04 to 2.66; aHR, 2.71; 95% CI, 1.07 to 6.88; and aHR, 3.08; 95% CI, 1.20 to 7.92, respectively) (Figure 2, Table 2). Neither the exclusion of participants with a history of diabetes and/or hypertension (all-cause mortality aHR, 1.54; 95% CI, 1.22 to 1.95 after exclusion) nor the exclusion of those with BMI<18.5 kg/m2 (aHR, 1.32; 95% CI, 1.06 to 1.65 after exclusion) changed the level of significance of the association. For the deceased group, the estimated mean age at death of the participants without RHF was 65.4 years (95% CI, 64.5 to 66.3 years) compared with 65.3 years (95% CI, 64.1 to 66.6 years) for those with RHF after adjustment for possible confounding variables at baseline, such as age, sex, smoking, regular exercise, regular alcohol ingestion, history of diabetes and/or hypertension medication, systolic BP, BMI, FSG, serum triglyceride, serum HDL-cholesterol, and albuminuria (P=0.87; data not shown).
Figure 1.
Association of high eGFR with increased all-cause mortality. The age-, sex-, muscle mass-, and history of diabetes and hypertension medication-adjusted percentile rank of eGFR was associated with higher all-cause mortality after adjustment for age, sex, smoking, regular exercise, regular alcohol ingestion, previous history of diabetes and/or hypertension, BMI, systolic BP, FSG, serum triglyceride, serum HDL-cholesterol, and albuminuria. An additive multivariate Cox regression model was used to compute pointwise estimates and CIs of HR curves of the residuals of eGFR. Penalized splines were used as the smoothing technique, and degrees of freedom of splines were selected on the basis of the lowest Akaike Information Criteria. The solid line represents the aHR, and the dashed lines are 95% CIs.
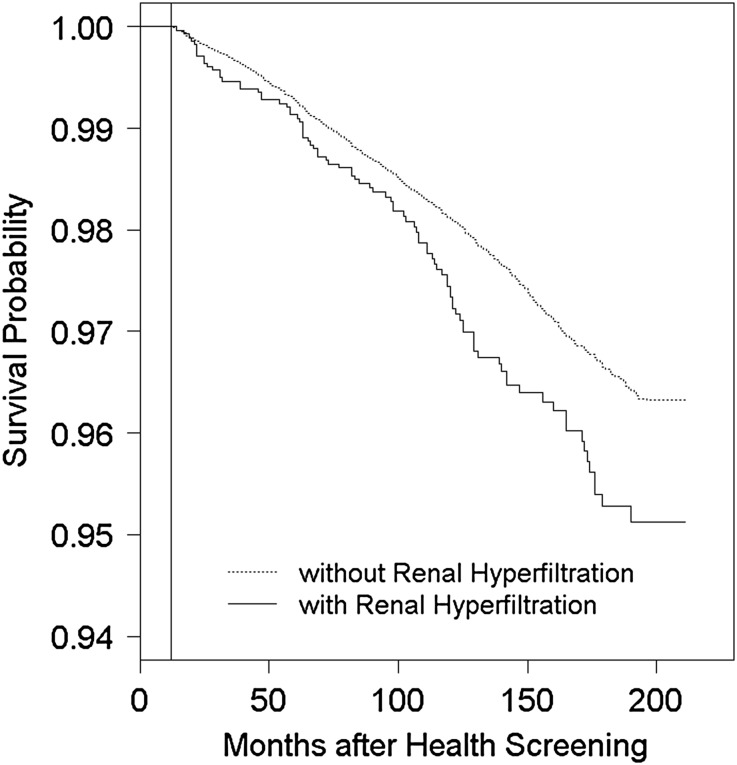
Figure 2.
Association between RHF and increased all-cause mortality. The Cox proportional hazards model was adjusted for age, sex, smoking, regular exercise, regular alcohol ingestion, previous history of diabetes and/or hypertension, BMI, systolic BP, FSG, serum triglyceride, serum HDL-cholesterol, and albuminuria. The vertical line represents 12 months after health screening. The definition of RHF is given in Concise Methods.
To assess the differential effect of smoking status—one of the important risk factors for death—on the association between RHF and mortality, the participants were placed in subgroups according to smoking status. In both nonsmokers and former smokers, RHF was significantly associated with higher all-cause mortality (aHR, 1.58; 95% CI, 1.13 to 2.22 and aHR, 1.73; 95% CI, 1.10 to 2.73, respectively), and this association was not observed in current smokers (aHR, 1.08; 95% CI, 0.77 to 1.52) (Table 2).
To determine a possible link mediating the increased risk of mortality in the RHF group, the risk of developing major illnesses in 23,824 participants who visited the hospital more than one time at least 12 months after the initial health screening was evaluated. The odds of receiving follow-up were not associated with RHF, which was shown by a multivariate logistic regression analysis (OR, 1.07; 95% CI, 0.97 to 1.18; data not shown) after adjustment for possible confounding variables, such as age, sex, smoking status, regular exercise, regular alcohol ingestion, history of diabetes and/or hypertension medication, BMI, FSG, serum triglyceride, serum HDL-cholesterol, and albuminuria. The Cox proportional hazards model adjusted for possible confounding variables found a higher risk of developing hypertension (aHR, 1.30; 95% CI, 1.01 to 1.67) and cardiovascular diseases (aHR, 1.17; 95% CI, 1.01 to 1.35) for subjects with RHF at baseline. The risk of diabetes was not associated with RHF (Table 4). Although a significant association was not observed between RHF and the risk of chronic renal disorders, the yearly rate of decline in eGFR was significantly higher in participants with RHF compared with those without RHF (estimated mean=−2.52; 95% CI, −2.78 to −2.26 with RHF versus estimated mean=0.20; 95% CI, 0.03 to 0.36 without RHF; P<0.001), and a higher likelihood of a rapid decline in eGFR (>5 ml/min per 1.73 m2 per year) was observed for subjects with RHF (OR, 4.49; 95% CI, 3.63 to 5.56) after adjustment for age, sex, smoking, regular exercise, regular alcohol ingestion, history of diabetes and/or hypertension medication, systolic BP, BMI, FSG, serum triglyceride, serum HDL-cholesterol, and albuminuria. After the median follow-up of 8 years, the mean eGFR of participants with RHF was lower than that of those without RHF (−2.4 ml/min per 1.73 m2; 95% CI, −1.1 to −3.6 ml/min per 1.73 m2; P<0.001) after adjustment for the above baseline variables, baseline eGFR, and follow-up duration (data not shown).
Table 4.
Association between RHF at health screening and risk of disease development
| Morbidities | Case Per Person-Yr | Incidence Densitya | aHR (95% CI)b |
|---|---|---|---|
| Hypertensionc | 1091/145,949 | 797.7 | 1.30 (1.01 to 1.67) |
| Diabetesc | 3578/192,796 | 1855.8 | 1.17 (0.91 to 1.51) |
| Cardiovascular diseasesc | 3849/227,784 | 1689.8 | 1.17 (1.01 to 1.35) |
| Chronic renal disordersc,d | 366/216,478 | 169.1 | 1.31 (0.86 to 2.00) |
eGFR using the CKD-EPI equation above the age-, sex-, muscle mass-, and history of diabetes and/or hypertension medication-adjusted 95th percentile (details in Concise Methods).
Incidence density (per 100,000 person-yr).
aHR and 95% CI of the participants with RHF compared with those without RHF determined by the Cox proportional hazards model adjusted for possible confounding variables at baseline, such as age, sex, smoking, regular exercise, regular alcohol ingestion, history of diabetes and/or hypertension medication, BMI, systolic BP, FSG, serum triglyceride, serum HDL-cholesterol, and albuminuria (details in Concise Methods).
Morbidities diagnosed at baseline or within 12 mo after health screening were excluded (details in Concise Methods).
Definition in Concise Methods.
Discussion
In this study, we observed a significant association between age-, sex-, muscle mass-, and history of diabetes and/or hypertension medication-adjusted RHF and higher all-cause and cardiovascular mortality in a large apparently healthy adult population after a median follow-up period of 12.4 years. The confounding effect of muscle wasting could be reasonably excluded, because the association between RHF and mortality was independent of age, sex, and muscle mass. The association was also independent of smoking status.
These observations are consistent with previous studies reporting a J-shaped association between serum creatinine-based eGFR and mortality.11–17 The J-shaped association between eGFR and mortality has been explained by the overestimation of the true GFR in individuals in the high-risk group because of muscle wasting. Previous studies defined high GFR as above a certain absolute value, irrespective of age, sex, and muscle mass, and could not exclude the possibility of the confounding effects of muscle wasting in the high-risk group or the misclassification of normal GFR in young subjects as higher GFR or higher GFR in older subjects as normal GFR. Astor et al.11 reported the disappearance of the J-shaped association between higher serum creatinine-based eGFR and mortality when they estimated GFR using the serum cystatin-based equation, which was thought to be less subject to the influence by muscle mass. In that study, Astor et al.11 defined RHF as an eGFR>120 ml/min per 1.73 m2, irrespective of the age of the participant. The possibility that normal GFR in young subjects could be misclassified as higher GFR and lead to the disappearance of the J-shaped association between higher serum cystatin-based GFR and mortality could not be excluded.18 Hallan et al.16 observed a J-shaped association between higher serum creatinine-based eGFR and mortality in the older (≥55 years old) but not the young group (18–54 years old). Hallan et al.16 compared the eGFR of these groups using the same absolute value without considering the age-dependent decline in GFR.
Therefore, the age factor and muscle mass should be considered when defining higher GFR or RHF. Melsom et al.19 proposed an age- and body size-adjusted definition of RHF on the basis of measured GFR and observed an association between RHF and impaired fasting glucose in a cross-sectional study. However, direct measurement of GFR may be impractical in a clinical or epidemiologic setting. To the best of our knowledge, our study is the first report on the clinical consequences of age-, sex-, muscle mass-, and history of diabetes and/or hypertension medication-adjusted RHF in an apparently healthy population.
Because eGFR was on the basis of serum creatinine in this study, the possibility of overestimating the true GFR in subjects with muscle wasting could not be completely excluded. To define RHF in this study, we used eGFR adjusted for age, sex, muscle mass, and medication for diabetes and/or hypertension. Muscle mass was positively correlated with the prevalence of RHF independent of BMI, which could be explained by the increased GFR in response to the higher metabolic demands resulting from higher muscle mass. BMI was also independently associated with a higher prevalence of RHF. When the participants with BMI<18.5 kg/m2 with a greater possibility of muscle wasting than those with BMI≥18.5 kg/m2 were excluded, this association did not change. We measured LBM by bioimpedance analysis, a standard method for measuring body components, especially in an epidemiologic setting.21,22 Although bioimpedance analysis generally provides fairly accurate estimates of LBM, it tends to underestimate LBM compared with double energy x-ray absorptiometry in obese subjects with BMI>35 kg/m2.23 In this study, only 93 participants (0.2%) had BMI>35 kg/m2, and this underestimation did not seem to influence the results. Peripheral edema or hydration status may also lead to an overestimation of LBM. Because no participants had symptomatic edema at baseline and all participants underwent bioimpedance analysis after an overnight fast lasting at least 12 hours, the possibility of overestimating LBM because of peripheral edema or differences in hydration status seems to be quite low. Furthermore, excluding 16 participants who developed heart failure within 12 months after health screening did not change the significance level of the association between RHF and mortality.
Smoking was suggested to play a role in the association between higher GFR and higher mortality, because habitual smoking could increase both GFR and mortality.17 In our study, the participants were divided according to smoking status, and the association between RHF and higher mortality was significant in both nonsmokers and former smokers. However, this association was no longer significant in current smokers, and the interaction between RHF and smoking status was not significant either. This finding might be explained by the effect of smoking obscuring the modest effect of RHF on mortality. Therefore, the positive relationship between RHF and total death was not affected by smoking status.
The explanation for the association between RHF and increased all-cause and cardiovascular mortality remains unclear. RHF was also associated with an increased risk of developing hypertension or cardiovascular diseases and not associated with an increased risk of developing diabetes. In this study, impaired FSG was independently associated with increased odds of RHF (data not shown). It suggested that RHF might be one of the pathophysiologic mechanisms of diabetic complications and not a cause of diabetes. Although the association between chronic renal disorders and RHF was not significant, the association between RHF and the rapid decline in eGFR suggested a shared pathophysiology between RHF and CKD in the association with cardiovascular disease and mortality. Therefore, as an early stage of CKD, RHF can be associated with premature mortality attributed to diabetic or nondiabetic CKD.24 The activation of many mechanisms, including the renin-angiotensin system, by various conditions, such as diabetes, hypertension, obesity, and other lifestyle factors, may be associated with both RHF and premature mortality.25,26 The association between RHF and increased infectious or hepatic mortality can be explained by either shared underlying risk factors, such as obesity,27,28 or the risk resulting from the progression of RHF to CKD.29,30 Future studies on the underlying mechanisms of the association between RHF and increased all-cause or cause-specific mortality are necessary to find an effective strategy for preventing premature mortality.
This study has several limitations. First, GFR was estimated using the serum creatinine-based Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation rather than directly measured. Although the CKD-EPI equation used in this study is a standard method of estimating GFR, especially in an epidemiologic setting, and performs better than the Modification of Diet in Renal Disease study equation in subjects with normal or above-normal renal function, it does not seem to completely avoid overestimating GFR, especially in subjects with muscle wasting. Therefore, in this study, we defined RHF after adjustment for age, sex, history of diabetes and/or hypertension medication, and muscle mass, which was measured by bioimpedance analysis. Second, the single ethnic origin of this study’s participants may limit the ability to generalize the results. The BMI of the population in this study was consistent with previous studies and lower than that of other ethnic groups. Future studies are necessary to confirm the association between RHF and increased mortality in other ethnic groups with higher BMI.31 Third, the sample size of this study was relatively large but not sufficient for additional analysis of the causes of death and disease risk because of the low mortality and disease incidence in a relatively healthy study population. Fourth, the results of this study are derived from a retrospective study without structured protocols conducted in a single center. Therefore, the generalization of these results, especially for the risk of developing diseases, should be made cautiously.
Despite these limitations, this study has strengths. First, RHF was defined with age-, sex-, and muscle mass-adjusted criteria to minimize the possibility of false-positive classification of RHF in subjects with muscle wasting. Furthermore, to the best of our knowledge, this is the first report suggesting an association between RHF and all-cause and cardiovascular mortality independent of muscle wasting. Second, this study observed that the association between RHF and increased mortality was independent of smoking status, which is a known risk factor for both all-cause mortality and RHF.
In conclusion, age-, sex-, muscle mass-, and history of diabetes and/or hypertension medication-adjusted RHF was found to be associated with all-cause and cardiovascular mortality in an apparently healthy adult population, and this association was independent of smoking status. The possibility of using RHF as a marker for all-cause and cardiovascular mortality must be confirmed by future studies.
Concise Methods
Setting and Participants
Between March of 1995 and May of 2006, 65,096 health screenings were performed at the Health Promotion Center of the Seoul National University Hospital. We excluded 16,990 repeated screenings and 2153 screenings with incomplete data. After additional exclusion of 2450 screenings from participants younger than 20 years of age, with eGFR values<60 ml/min per 1.73 m2, or with survival durations of ≤12 months after the health screening, we analyzed the health records for the first screening visits of 43,503 adults. The Institutional Review Board of the Seoul National University Hospital approved the study protocol.
Information on the participant’s history of smoking, alcohol consumption, exercise, diabetes, hypertension, and medication for diabetes and/or hypertension was obtained using a structured, self-reported questionnaire and validated by trained nurses. Trained physicians interviewed and examined all participants just before the health screening, and no participants had symptomatic peripheral edema. Smoking status was classified into three categories: current smokers, former smokers, and nonsmokers. Participants who smoked at least one cigarette per day at the time of the health screening were classified as current smokers. Participants who reported that they did not smoke at the time of screening but had previously smoked were classified as former smokers. Participants who drank alcoholic beverages at least one time per week were classified as regular drinkers. Regular exercise was defined as exercise>30 minutes at least three times per week.
Height and weight were measured after an overnight fast and after voiding urine. BMI was calculated by dividing weight (in kilograms) by height (in meters) squared. LBM at the baseline was measured with bioimpedance analysis using Inbody 2.0 (Biospace, Seoul, Korea). The body was measured in five segments: right upper and lower extremities, left upper and lower extremities, and trunk. The LBM of the upper extremities, trunk, and lower extremities was estimated by multiplying the values for the water volumes of each compartment by 1.37 under the assumptions that the density of LBM was 1.1 kg/m3 at 37°C and that the water content of LBM was 73%.22
BP was measured using an automated BP measurement device (Jawon, Busan, Korea) after resting for at least 20 minutes in a sitting position. Blood samples were drawn after a 12-hour overnight fast. Serum creatinine was measured using the Jaffe alkaline picrate method with a Hitachi Clinical Analyzer 747 (Hitachi Ltd., Tokyo, Japan) until February of 2000 and a Toshiba 200FR (Toshiba Medical Systems Co., Tokyo, Japan) thereafter. The results from the two analyzers were recalibrated with the experimentally derived conversion equation.9 The serum creatinine measurement was not standardized to isotope dilution mass spectrometry, and we reduced the serum creatinine levels by 5% as previously proposed.15 All tests were conducted in a clinical laboratory of Seoul National University Hospital, which is inspected and surveyed annually by the Korean Association of Quality Assurance for Clinical Laboratories. eGFR was calculated with the CKD-EPI equation on the basis of serum creatinine.32 Albuminuria was determined semiquantitatively with a single spot urine dipstick analysis (YD Diagnostics, Yong-In, Korea) performed on morning urine samples after overnight fasting and reported as negative, trace, 1+, 2+, 3+, or 4+. Albuminuria was defined as 1+ or higher.
RHF was defined as previously suggested with some modifications.19 Briefly, the residuals were calculated from a multiple linear regression analysis, where logarithm-transformed eGFR was a dependent variable and sex, LBM, previous history of medication for diabetes and/or hypertension, and logarithm-transformed age were independent variables. An additive multivariate Cox regression model was used to compute pointwise estimates and CIs of aHR curves of mortality associated with the percentile rank of the residuals of eGFR after controlling for age, sex, smoking status, regular alcohol consumption, regular exercise, previous history of diabetes and/or hypertension medication, BMI, systolic BP, FSG, serum HDL-cholesterol, serum triglyceride, and albuminuria. Penalized splines were used as the smoothing technique, and the degrees of freedom of splines were selected on the basis of the lowest Akaike Information Criteria.33 The age-, sex-, muscle mass-, and history of diabetes and hypertension medication-adjusted residuals of eGFR were divided into semidecile groups (five percentile intervals) according to percentile rank. Then, the association between percentile rank groups and all-cause mortality was analyzed with the Cox proportional hazards model. The aHR for a group of adjusted residuals above the 95th percentile was highest among the semidecile groups compared with a group of adjusted residuals at the 5th percentile or lower (aHR, 1.26; 95% CI, 0.92 to 1.71). Therefore, we defined RHF as eGFR with residuals above the 95th percentile and analyzed the association between RHF and mortality using the Cox proportional hazards model adjusting for confounding variables.
Outcome and Follow-Up
The outcome of interest was all-cause mortality. The participants were followed up for mortality until December 31, 2012. Death was confirmed through record linkage with the death certificate data from the National Statistical Office of Korea using the personal identification number assigned at birth. The cause of death was classified according to the International Classification of Disease, 10th revision (ICD-10). Chronic renal disorders were defined as (1) primary renal disorders, except acute nephritic syndrome, hereditary nephropathy, acute renal failure, nephrolithiasis, and urinary tract infection (N01–N06, N08, N10–N16, N18, N19, and N25–N29 by ICD-10), (2) renal diseases associated with hypertension (I12 and I13 by ICD-10), and (3) renal diseases associated with diabetes (E10.2, E11.2, E12.2, E13.2, and E14.2 by ICD-10).
Among 43,503 participants, the disease codes from the medical records of 23,824 participants who visited the hospital more than one time at least 12 months after the baseline health screening were analyzed, and the risks of developing hypertension, diabetes, cardiovascular diseases, and chronic renal disorders were evaluated with the Cox proportional hazards model after adjusting for possible confounding variables, such as age, sex, smoking, regular exercise, regular alcohol ingestion, previous history of diabetes and/or hypertension, BMI, systolic BP, FSG, serum triglyceride, serum HDL-cholesterol, and albuminuria. Morbidities diagnosed within 12 months after health screening were excluded when analyzing those diseases. Participants with a history of hypertension or baseline BP≥140/90 mmHg were excluded from the analysis of hypertension, and participants with a history of diabetes or baseline FSG≥126 mg/dl were excluded from the analysis of diabetes. Participants with albuminuria at baseline were excluded from the analysis of chronic renal disorders.
Statistical Analyses
Statistical analyses were conducted with SPSS IBM 19 (IBM, Chicago, IL) and R software 3.0.2 (http://www.R-project.org). The distribution of clinical parameters between the deceased and survivor groups was compared with a t test for continuous variables and a chi-squared test for discrete variables. Two-sided P values<0.05 were considered statistically significant. The association between RHF and mortality was analyzed with the Cox proportional hazard model adjusted for possible confounding variables, such as age, sex, smoking, regular exercise, regular alcohol ingestion, previous history of diabetes and/or hypertension, BMI, systolic BP, FSG, serum triglyceride, serum HDL-cholesterol, and albuminuria. The survival curves according to the presence of RHF were modeled with the Cox proportional hazards model using R software 3.0.2.34
Disclosures
None.
Acknowledgments
This work was supported by the National Research Foundation of Korea funded by the Korean Government (Ministry of Education and Science Technology) Grant 2010-0028631 and Seoul National University Hospital Research Fund Grant 04-2013-0540.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR, Chronic Kidney Disease Prognosis Consortium : Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: A meta-analysis. BMJ 346: f324, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mogensen CE, Christensen CK: Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 311: 89–93, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, Hirschman GH, Myers BD, Diabetic Renal Disease Study Group : Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N Engl J Med 335: 1636–1642, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Schmieder RE, Messerli FH, Garavaglia G, Nunez B: Glomerular hyperfiltration indicates early target organ damage in essential hypertension. JAMA 264: 2775–2780, 1990 [PubMed] [Google Scholar]
- 5.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y: The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 14: 1480–1486, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S: Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant 27: 1821–1825, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Maeda I, Hayashi T, Sato KK, Koh H, Harita N, Nakamura Y, Endo G, Kambe H, Fukuda K: Cigarette smoking and the association with glomerular hyperfiltration and proteinuria in healthy middle-aged men. Clin J Am Soc Nephrol 6: 2462–2469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melsom T, Mathisen UD, Eilertsen BA, Ingebretsen OC, Jenssen T, Njølstad I, Solbu MD, Toft I, Eriksen BO: Physical exercise, fasting glucose, and renal hyperfiltration in the general population: The Renal Iohexol Clearance Survey in Tromsø 6 (RENIS-T6). Clin J Am Soc Nephrol 7: 1801–1810, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park M, Ko Y, Song SH, Kim S, Yoon HJ: Association of low aerobic fitness with hyperfiltration and albuminuria in men. Med Sci Sports Exerc 45: 217–223, 2013 [DOI] [PubMed] [Google Scholar]
- 10.WHO : Risk reduction can add 5-10 years to healthy life expectancy. Bull World Health Organ 80: 991, 2002 [PMC free article] [PubMed] [Google Scholar]
- 11.Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J: Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol 20: 2214–2222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J: Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 55: 648–659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmoodi BK, Matsushita K, Woodward M, Blankestijn PJ, Cirillo M, Ohkubo T, Rossing P, Sarnak MJ, Stengel B, Yamagishi K, Yamashita K, Zhang L, Coresh J, de Jong PE, Astor BC, Chronic Kidney Disease Prognosis Consortium : Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: A meta-analysis. Lancet 380: 1649–1661, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG, Chronic Kidney Disease Prognosis Consortium : Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 380: 1662–1673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen CP, Coresh J, Gansevoort RT, Hemmelgarn BR, Levey AS, Chronic Kidney Disease Prognosis Consortium : Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 307: 1941–1951, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, Kleefstra N, Naimark D, Roderick P, Tonelli M, Wetzels JF, Astor BC, Gansevoort RT, Levin A, Wen CP, Coresh J, Chronic Kidney Disease Prognosis Consortium : Age and association of kidney measures with mortality and end-stage renal disease. JAMA 308: 2349–2360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donfrancesco C, Palleschi S, Palmieri L, Rossi B, Lo Noce C, Pannozzo F, Spoto B, Tripepi G, Zoccali C, Giampaoli S: Estimated glomerular filtration rate, all-cause mortality and cardiovascular diseases incidence in a low risk population: The MATISS study. PLoS ONE 8: e78475, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunder-Plassmann G, Hörl WH: A critical appraisal for definition of hyperfiltration. Am J Kidney Dis 43: 396–397, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Melsom T, Mathisen UD, Ingebretsen OC, Jenssen TG, Njølstad I, Solbu MD, Toft I, Eriksen BO: Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes Care 34: 1546–1551, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman MJ, Ghate SR, Mavros P, Sen S, Marcus RL, Joy E, Brixner DI: Development of a practical screening tool to predict low muscle mass using NHANES 1999-2004. J Cachexia Sarcopenia Muscle 4: 187–197, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thibault R, Pichard C: The evaluation of body composition: A useful tool for clinical practice. Ann Nutr Metab 60: 6–16, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Toda Y, Segal N, Toda T, Morimoto T, Ogawa R: Lean body mass and body fat distribution in participants with chronic low back pain. Arch Intern Med 160: 3265–3269, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Boneva-Asiova Z, Boyanov MA: Body composition analysis by leg-to-leg bioelectrical impedance and dual-energy X-ray absorptiometry in non-obese and obese individuals. Diabetes Obes Metab 10: 1012–1018, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW: Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat Rev Nephrol 8: 293–300, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Sasson AN, Cherney DZI: Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes 3: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rüster C, Wolf G: The role of the renin-angiotensin-aldosterone system in obesity-related renal diseases. Semin Nephrol 33: 44–53, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Phung DT, Wang Z, Rutherford S, Huang C, Chu C: Body mass index and risk of pneumonia: A systematic review and meta-analysis. Obes Rev 14: 839–857, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Bhala N, Jouness RI, Bugianesi E: Epidemiology and natural history of patients with NAFLD. Curr Pharm Des 19: 5169–5176, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Foley RN: Infections and cardiovascular disease in patients with chronic kidney disease. Adv Chronic Kidney Dis 13: 205–208, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Bonora E, Targher G: Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat Rev Gastroenterol Hepatol 9: 372–381, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Vazquez G, Duval S, Jacobs DR, Jr., Silventoinen K: Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: A meta-analysis. Epidemiol Rev 29: 115–128, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meira-Machado L, Cadarso-Suárez C, Gude F, Araújo A: smoothHR: An R package for pointwise nonparametric estimation of hazard ratio curves of continuous predictors. Comput Math Methods Med 2013: 745742, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benítez-Parejo N, Rodríguez del Águila MM, Pérez-Vicente S: Survival analysis and Cox regression. Allergol Immunopathol (Madr) 39: 362–373, 2011 [DOI] [PubMed] [Google Scholar]