Abstract
Parietal epithelial cells have been identified as potential progenitor cells in glomerular regeneration, but the molecular mechanisms underlying this process are not fully defined. Here, we established an immortalized polyclonal human parietal epithelial cell (hPEC) line from naive human Bowman’s capsule cells isolated by mechanical microdissection. These hPECs expressed high levels of PEC-specific proteins and microRNA-193a (miR-193a), a suppressor of podocyte differentiation through downregulation of Wilms’ tumor 1 in mice. We then investigated the function of miR-193a in the establishment of podocyte and PEC identity and determined whether inhibition of miR-193a influences the behavior of PECs in glomerular disease. After stable knockdown of miR-193a, hPECs adopted a podocyte-like morphology and marker expression, with decreased expression levels of PEC markers. In mice, inhibition of miR-193a by complementary locked nucleic acids resulted in an upregulation of the podocyte proteins synaptopodin and Wilms’ tumor 1. Conversely, overexpression of miR-193a in vivo resulted in the upregulation of PEC markers and the loss of podocyte markers in isolated glomeruli. Inhibition of miR-193a in a mouse model of nephrotoxic nephritis resulted in reduced crescent formation and decreased proteinuria. Together, these results show the establishment of a human PEC line and suggest that miR-193a functions as a master switch, such that glomerular epithelial cells with high levels of miR-193a adopt a PEC phenotype and cells with low levels of miR-193a adopt a podocyte phenotype. miR-193a–mediated maintenance of PECs in an undifferentiated reactive state might be a prerequisite for PEC proliferation and migration in crescent formation.
Keywords: podocyte, renal epithelial cell, expression
Parietal epithelial cells (PECs) are squamous cells that line the Bowman’s capsule (BC) of the renal glomerulus. PECs have a thin cell body and a simple cytoskeleton. They are interconnected by tight junctions, which prevent the leakage of urine from the urinary space into the periglomerular compartment.1 Currently, PECs have taken the center stage of attention for their contribution to glomerular diseases and as potential podocyte progenitor cells in glomerular regeneration.2 PECs are derived from the metanephric mesenchyme. During the vesicle and comma stages, PECs share a common phenotype with the other epithelial cells of the later glomerulus, namely podocytes and proximal tubular cells. With the formation of the Bowman’s space in the S-shaped body, the phenotypes of PECs, podocytes, and proximal tubular cells diverge. Some PECs constitute the BC, whereas others upregulate podocyte-specific genes, such as the transcription factor Wilms' tumor-1 (WT1) protein. Upregulation of WT1 plays an important role in the differentiation of PECs toward podocytes during the S-shaped body phase. WT1 downregulates Pax-23 and inhibits the β-Catenin/Wnt signaling pathway,4 both considered prerequisites for the differentiation of PECs to podocytes. Deficiency in β-Catenin signaling in PECs during nephrogenesis was shown to result in the development of podocytes but not PECs along the BC5 in mice. In normal development, podocytes form foot processes along the glomerular basement membrane, lose their mitotic activity,6 and express proteins specific to the mature podocyte, such as Nephrin, Podocalyxin, Podocin, Synaptopodin, and α-Actinin-4. However, mature PECs have their own limited set of marker proteins, including Pax-8, Claudin-1, -2, and -16, and UCH-L1.7 Under physiologic conditions in mature glomeruli, PECs do not express podocyte proteins. However, they have been found to respond to injury with changes in marker expression.8–12 In our recent work, we found low levels of podocyte-specific transcripts in naïve PECs of rats,13 suggesting that PECs had the transcriptional prerequisite to express podocyte markers. We could, furthermore, show that expression of podocyte proteins was negatively regulated through UCH-L1/the ubiquitin proteasome and the autophagosomal/lysosomal degradation system in an immortalized rat PEC cell line13 and cultured human podocytes.14 However, inhibition of neither UCH-L1 nor the proteasome was sufficient to induce a full PEC-to-podocyte conversion13 (C. Meyer-Schwesinger, unpublished observation). This suggests the existence of an additional layer of regulation, which could be performed by a microRNA (miR). To this end, Gebeshuber et al.15 recently showed that inducible overexpression of miR-193a in mice downregulated the expression levels of WT1 and additional markers of podocytes and resulted in FSGS. Of note, a preferential expression of miR-193a in PECs but not podocytes was observed by in situ hybridization in normal human renal tissue.15 Therefore, miR-193a might be an additional switch to regulate PEC transdifferentiation toward a podocyte phenotype during normal development and under pathologic conditions.
In this study, we established and characterized a novel polyclonal human PEC (hPEC) line and showed the potential of PECs to adopt a podocyte-like phenotype on inhibition of miR-193a.
Results
Generation of a Human Immortalized PEC Line
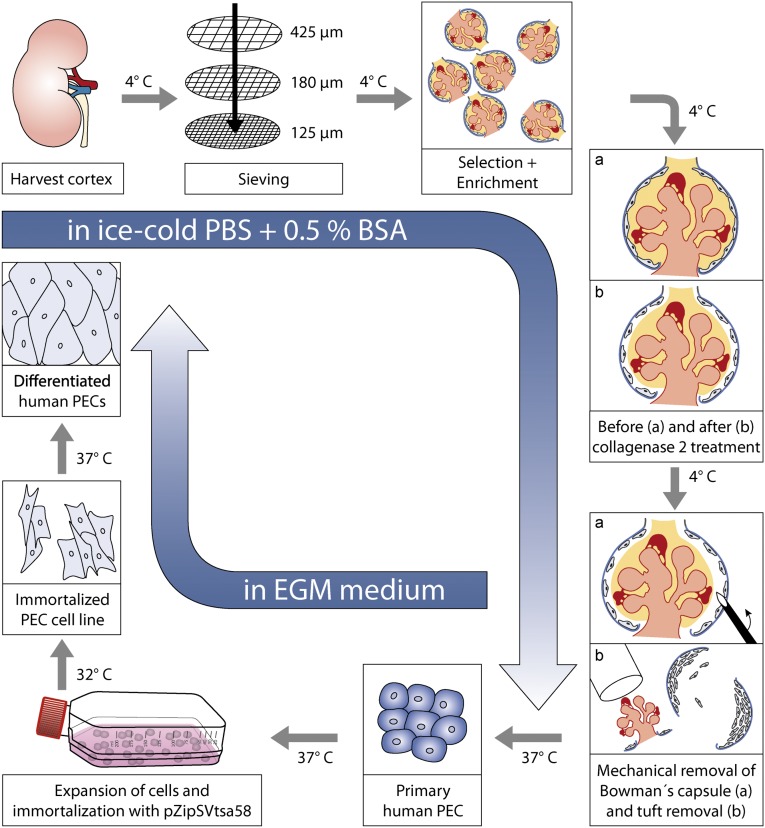
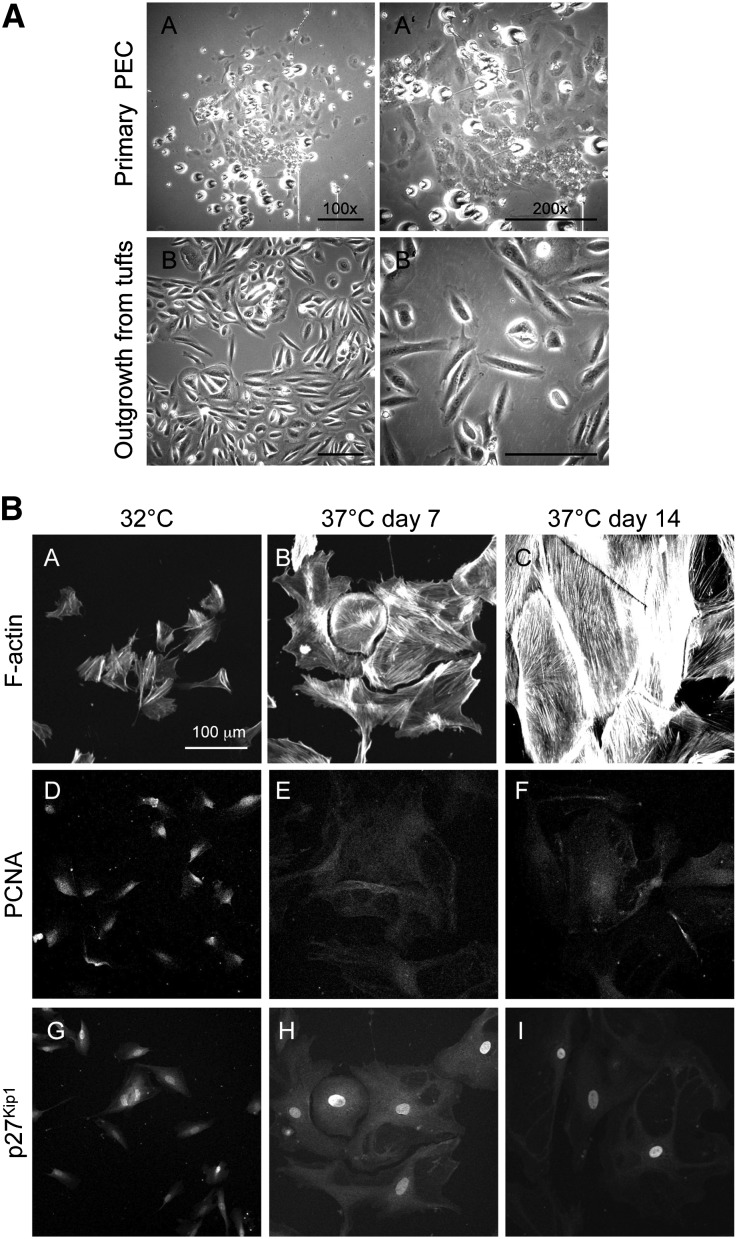
Glomeruli were isolated from a kidney biopsy of a 2.5-year-old child by conventional sieving.16 As described in detail in Concise Methods, BCs were gently dissected off the glomerular tufts with minutia pins under visual control.13 hPECs were allowed to adhere to and proliferate on collagen-1–coated dishes (Scheme 1). The adhering primary hPECs exhibited a clonal regular cobblestone morphology (Figure 1A, A). This morphology contrasted the outgrowth from decapsulated glomeruli, where cells exhibited an irregular morphology (Figure 1A, B). For immortalization, primary hPECs were cultured with the retrovirus encoding vector pZipSVtsa5817 as described previously for the establishment of an immortalized human podocyte cell line.16 When cultured under growth-permissive conditions, immortalized hPECs were small and exhibited an irregular triangular morphology (Figure 1B, A). On culture under growth-restrictive conditions, hPECs flattened and enlarged considerably, and cell borders acquired a smooth and regular appearance. After 14 days of differentiation under growth-restrictive conditions, hPECs had grown to a monolayer of large, flat, and regular-bordered cells (Figure 1B, C). The actin cytoskeleton showed extensive actin stress fibers throughout the cell. When evaluating the proliferation status, we found that nuclear expression of the proliferation marker PCNA (Figure 1B, C–F) decreased, whereas nuclear expression of the inhibitor of cell cycle progression p27Kip1 (Figure 1B, G–I) increased in hPECs during differentiation. This suggests that switching hPECs from growth-permissive to growth-restrictive conditions resulted in proper reduction of pZipSVtsa58-driven hPEC proliferation.
Scheme 1.
Isolation and immortalization scheme of human parietal cells.
Figure 1.
Generation of a hPEC line. Human BCs were isolated as described in Concise Methods. (A) BCs or isolated tufts were cultured in EGM medium at 37°C. (A, A and A′) The first primary hPEC cell clusters appeared 9 days after isolation of BCs. (A, B and B′) Outgrowths from isolated glomeruli were also visible 9 days after isolation. Note (A′) the cobblestone appearance of primary culture hPECs compared with (B′) the multiform appearance of tuft outgrowths. (B) After immortalization of primary hPECs, immortalized hPECs were propagated at 32°C. For differentiation, cells were cultured at 37°C for 14 days. (B, A–C) F-Actin staining to visualize the actin cytoskeleton. Note the increase of cell size toward large polygonal cells on day 14. (D–F) Immunofluorescence against the proliferation marker PCNA. Note the decreased expression of PCNA in hPECs after 7 days of differentiation. (G–I) Immunofluorescence against the cyclin-dependent kinase inhibitor p27Kip1 to visualize increased proliferation arrest. Note the strong nuclear staining for p27Kip1 upon differentiation.
Immortalized hPECs Highly Express PEC-Specific Markers
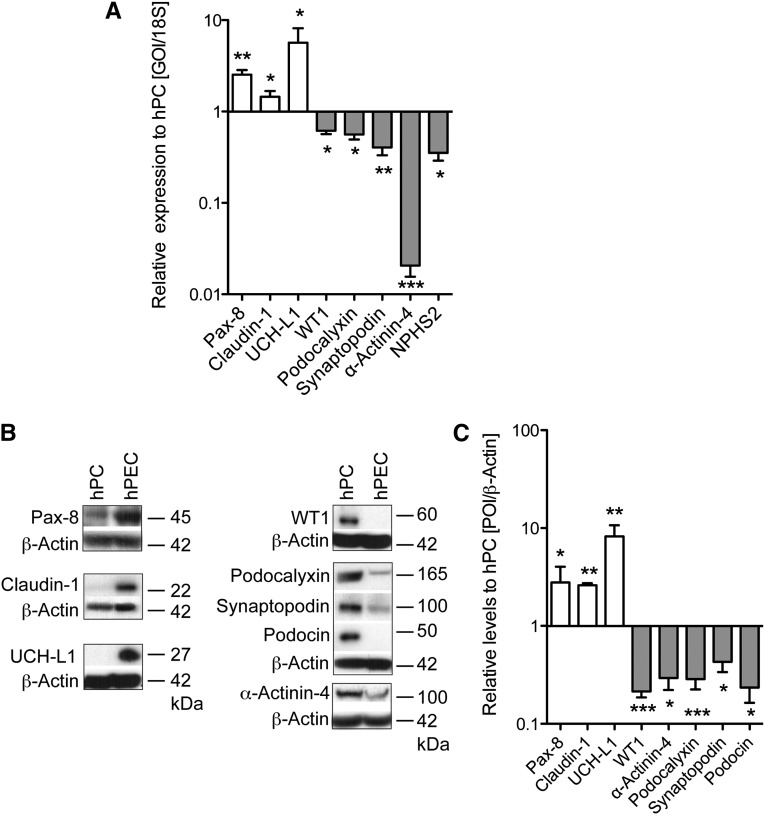
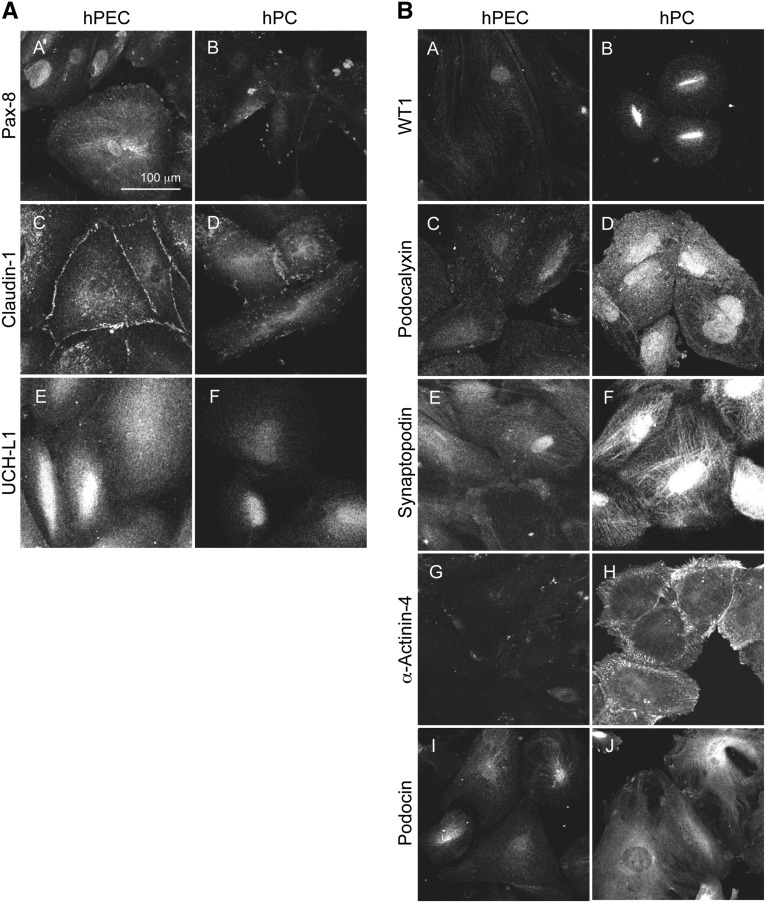
Differentiated immortalized hPECs were analyzed for the expression of PEC and podocyte markers compared with an established human podocyte cell line.16 Quantitative PCR (qPCR) and Western blot analyses (Figure 2, A and B) showed that hPECs expressed the PEC markers Pax-8, Claudin-1, and UCH-L1 at much higher levels than human podocytes. However, the podocyte transcripts for WT1, Podocalyxin, Synaptopodin, α-Actinin-4, and NPHS2 were expressed at high levels in human podocytes but not or only weakly in hPECs (Figure 2, A and B). These data show distinct marker protein expression in hPECs and podocytes. Immunolocalization studies confirmed that hPECs and human podocytes expressed PEC and podocyte proteins at different signal intensities and/or subcellular localizations (Figure 3). Pax-8 was highly expressed in nuclei of hPECs but not in podocytes (Figure 3A). Claudin-1 (at the cell–cell border) and UCH-L1 (cytoplasmic and partly nuclear) also had higher signal intensities in hPECs (Figure 3A). The podocyte-specific proteins WT1, α-Actinin-4, Podocalyxin, Synaptopodin, and Podocin expressions were only detected at background levels in hPECs (Figure 3B). The most striking differences were observed for WT1 and α-Actinin-4. WT1 was strongly expressed in nuclei of human podocytes but not in hPECs (Figure 3B). Expression of α-Actinin-4 was concentrated at the cortical actin ring of human podocytes and centripetal F-actin filaments (Figure 3B). In contrast, α-Actinin-4 expression was delicate and punctate in hPECs and failed to accentuate at a cortical actin ring or centripetal F-actin filaments (Figure 3B). This represents the first establishment of an immortalized polyclonal hPEC line from isolated BCs.
Figure 2.
Immortalized hPECs highly expressed PEC-specific markers and express podocyte-specific markers at low levels. hPECs were differentiated for 14 days at 37°C, and the mRNA and protein expression levels of PEC-specific and podocyte-specific proteins were analyzed compared with an established immortalized human podocyte cell line (hPC). (A) Measurement of relative expression of PEC (white bars) and podocyte genes of interest (GOIs; gray bars) by qPCR in hPECs compared with hPC. Normalization occurred to 18S mRNA levels in each individual sample. n=3–4 per transcript. Values are expressed as mean±SEM. hPC=1. (B) Representative Western blots against (left panel) PEC- and (right panel) podocyte-specific proteins. (C) Densitometric quantification of WB experiments. β-Actin of the same membrane was used to control for equal loading. White bars represent the relative expression of PEC-specific proteins; gray bars represent the relative expression of podocyte-specific proteins to hPC (hPC=1). n=3–7. Values are expressed as mean±SEM. POI, protein of interest. *P<0.05; **P<0.01; ***P<0.001 to control (=1).
Figure 3.
Expression pattern of PEC- and podocyte-specific markers in immortalized hPECs. hPECs were differentiated for 14 days at 37°C, and the expressions of (A) PEC- and (B) podocyte-specific proteins were analyzed by immunofluorescence compared with an established immortalized human podocyte cell line (hPC). The PEC markers Pax-8 (A, A and B), Claudin-1 (A, C and D), and UCH-L1 (A, E and F) were strongly expressed in hPECs and only expressed a little in hPCs. The podocyte markers WT1 (B, A and B), Podocalyxin (B, C and D), Synaptopodin (B, E and F), α-Actinin-4 (B, G and H), and Podocin (B, I and J) were only weakly expressed in hPECs compared with hPC.
Knockdown of miR-193a in hPECs Results in Transdifferentiation toward a Podocyte Phenotype
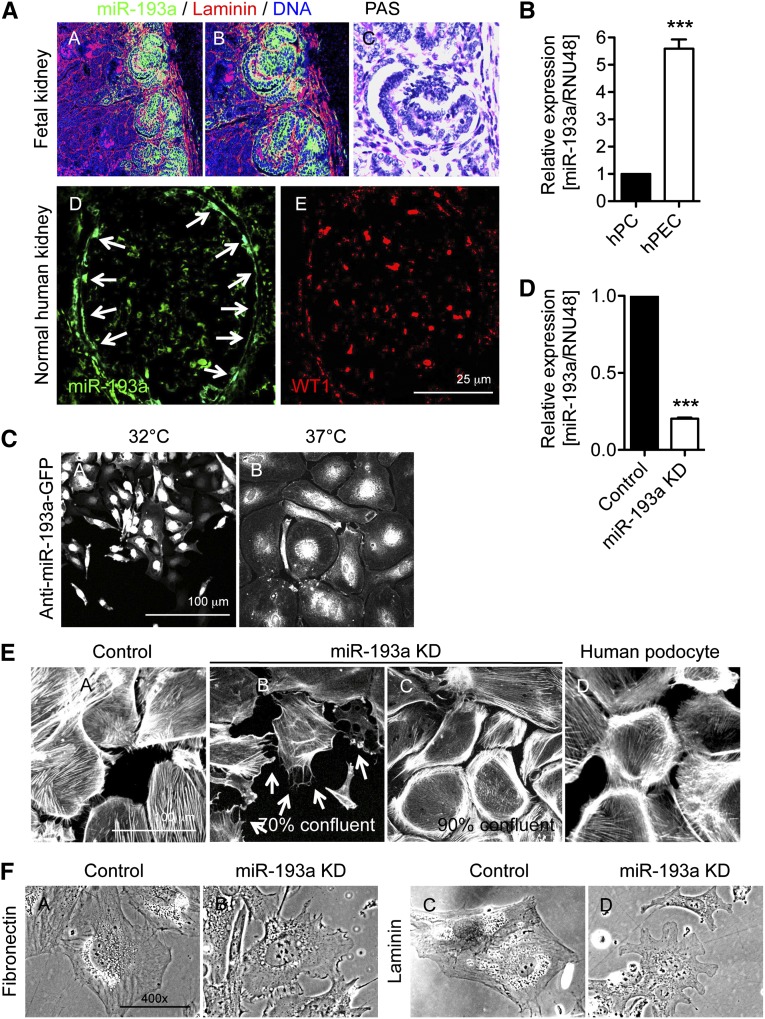
Inducible overexpression of miR-193a in mice resulted in suppression of WT1, loss of WT1-dependent transcripts, including Podocalyxin, Nephrin, and Sulf1, and podocyte dedifferentiation.15 We, thus, hypothesized that miR-193a could represent an important switch in the establishment of podocyte and PEC identity and differentiation and that high miR-193a levels might promote a PEC-like phenotype. Fluorescent in situ hybridizations for miR-193a exhibited a pronounced expression of miR-193a in immature PECs and podocytes in developing glomeruli of a fetal human kidney (Figure 4A). In mature glomeruli of an adult kidney, however, mature PECs maintained a marked miR-193a expression, whereas this was lost in WT1-positive podocytes (Figure 4A). By qPCR, we observed a >5-fold higher expression of miR-193a in hPECs compared with human podocytes (Figure 4B). We, therefore, transduced undifferentiated hPECs with a plasmid harboring an anti–miR-193a–GFP construct. Immunofluorescence analysis for GFP expression showed a transduction efficiency of 90%–100% (Figure 4C). After cell selection on the basis of puromycin resistance and differentiation of transduced hPECs, qPCR analysis revealed a strong reduction of miR-193a levels in differentiated hPECs compared with mock control cells (Figure 4D).
Figure 4.
hPECs express miR-193a, and KD of miR-193a results in hPEC transdifferentiation toward a podocyte phenotype. (A) Fluorescent in situ hybridization for miR-193a (green) in (A, A and B) a glomerulus of a fetal kidney (gestation week 27) and (A, D) a normal human kidney. (A, C) Corresponding light micrograph of a Periodic acid–Schiff (PAS) -stained section of the fetal kidney. (A, E) Immunofluorescence for WT1 (red) in a consecutive section of the same glomerulus exhibited in A, D. (B) hPECs were differentiated for 14 days at 37°C, and the relative expressions of miR-193a levels were measured by qPCR compared with differentiated human podocytes (hPCs; black bar; n=3). ***P<0.001 to hPC (=1). (C) Immunofluorescence image of GFP expression 24 hours after viral transduction of undifferentiated hPECs with anti–miR-193a–GFP plasmid (undifferentiated hPECs [C; A] and following differentiation [C; B]). Note the 90%–100% transduction efficiency before selection with puromycin. (D) miR-193a was stably knocked down in immortalized hPECs. qPCR analysis of relative miR-193a levels in differentiated hPECs with miR-193a KD compared with mock transfection (control; black bar). RNU48 was used as a home keeper, and values are expressed as mean±SEM (n=3). ***P<0.001 to control (=1). (E) Representative immunofluorescence against F-Actin (phalloidin) in (E, A) mock-transfected (control), miR-193 KD-differentiated hPECs (E, B) 70% confluent and (E, C) 90% confluent, and (E, D) differentiated human podocytes on day 14. Note the cortical actin ring in miR-193a KD hPECs and the processes (arrows). (F) Representative phase-contrast micrographs show arborization of hPECs with miR-193a KD when VRADD is added to the culture medium and the cells are grown on (F, B) fibronectin- or (F, D) laminin-coated dishes. Note the smooth cell border of control hPECs (mock-transfected hPECs) under the same culture conditions (F, A and C).
Next, we evaluated whether miR-193a knockdown (KD) in hPECs resulted in phenotypic changes toward podocyte-like characteristics. Visualization of the actin cytoskeleton showed that hPECs with decreased miR-193a levels developed a prominent cortical F-actin ring with centripetal F-actin fibers characteristic for cultured human podocytes (Figure 4E). hPECs with miR-193 KD located at the rim of cell assemblies developed processes. Strikingly, culture of hPECs with miR-193a KD on fibronectin- or laminin-coated plates at low density in VRADD medium18 resulted in morphologic changes toward an arborized phenotype typical for podocytes. Control hPECs maintained their polygonal nonarborized morphology under the same culture conditions (Figure 4F).
Knockdown of miR-193a in hPECs Results in Increased Levels of Podocyte Markers
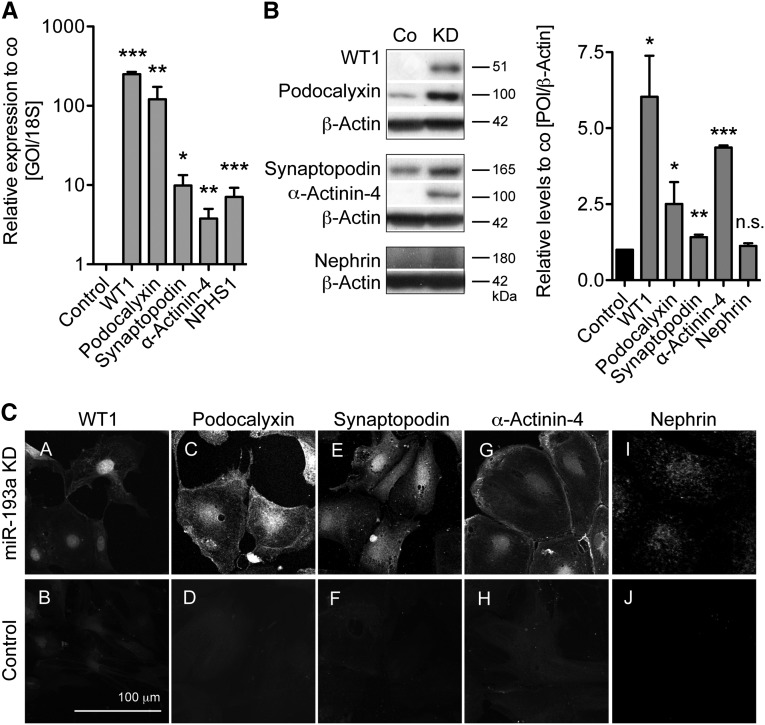
We evaluated whether KD of miR-193a in hPECs increased the expression of podocyte-specific markers. qPCR analysis showed a significant increase in the levels of WT1, Podocalyxin, Synaptopodin, α-Actinin-4, and NPHS1 compared with hPECs with mock GFP expression (Figure 5A). The protein levels of WT1, Podocalyxin, Synaptopodin, and α-Actinin-4 were also significantly elevated in miR-193a KD hPECs (Figure 5B). Immunolocalization studies supported the above data. KD of miR-193a resulted in a strong nuclear expression of WT1 in hPECs (Figure 5C). Furthermore, Podocalyxin was expressed in a punctate pattern on hPECs, and the F-actin–associated cytoskeletal proteins Synaptopodin and α-Actinin-4 were expressed at the cortical actin ring and centripetal F-actin fibers as observed in human podocytes (Figure 5C). Nephrin was expressed in a faint punctate pattern in hPECs with miR-193 KD (Figure 5C). In comparison, staining of these proteins was very weak (WT1, Podocalyxin, Synaptopodin, and α-Actinin-4) to absent (Nephrin) in mock GFP-transduced hPECs (Figure 5C). The changes in protein expression were observed in all hPECs (data not shown). Of note, KD of miR-193a in hPECs resulted in a decreased expression of the progenitor markers CD24 and CD13319 and the PEC activation marker CD4420 (Supplemental Figure 1).
Figure 5.
KD of miR-193a in hPECs results in increased transcript and protein levels of podocyte proteins. miR-193a was knocked down in hPECs differentiated for 14 days at 37°C, and the mRNA and protein expression levels of podocyte-specific proteins were analyzed compared with mock-transduced hPEC control cells. (A) Relative expression levels of podocyte-specific transcripts in hPECs with miR-193a KD compared with mock-transfected hPEC cells (control; n=3–6). 18S mRNA was used as a home keeper, and values are expressed as mean±SEM. GOI, gene of interest. *P<0.05; **P<0.01; ***P<0.001 to control (=1). (B, left panel) Western blotting for podocyte-specific proteins in mock-transduced hPECs or miR-193a KD hPECs after differentiation for 14 days. (B, Right panel) Densitometric quantification of n=3 WB experiments. β-Actin of the same membrane was used to control for equal loading. Values are expressed as mean±SEM. Co, control; POI, protein of interest. *P<0.05; **P<0.01; ***P<0.001 to control (=1). (C) Representative confocal micrographs of immunofluorescence staining for podocyte-specific proteins WT1 (C, A and B), Podocalyxin (C, C and D), Synaptopodin (C, E and F), α-Actinin-4 (C, G and H), Nephrin (C, I and J) in differentiated hPECs with miR-193a KD (C; A, C, E, G and I) or mock-GFP plasmid transduction (control) (C; B, D, F, H and J).
Knockdown and Inhibition of miR-193a in hPECs Result in Decreased Transcript and Protein Levels of PEC Proteins
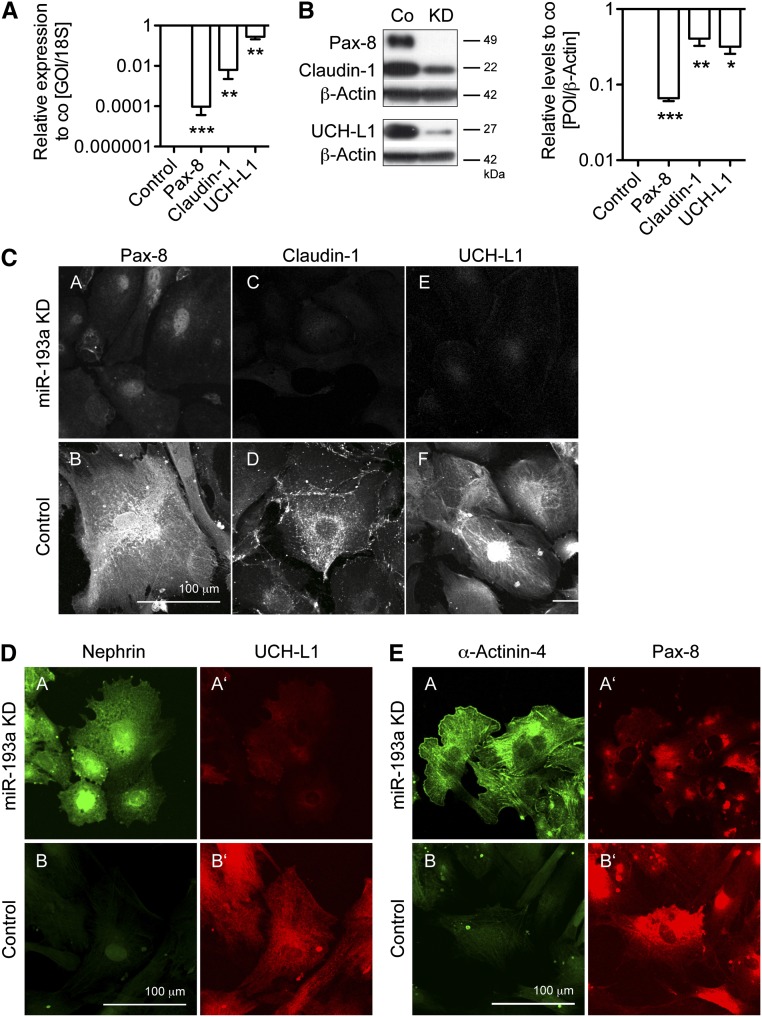
We investigated if the expression of PEC-specific makers was also altered on miR-193a KD. qPCR analyses showed a significant decrease of Pax-8, Claudin-1, and UCH-L1 levels in hPECs with miR-193a KD compared with control hPECs (Figure 6A). A similar downregulation was observed at the protein level (Figure 6B). Immunolocalization studies (Figure 6C) confirmed that Claudin-1 and UCH-L1 expressions were strongly reduced in hPECs with miR-193 KD. For Pax-8, the cytoplasmic expression was strongly reduced on miR-193a KD, whereas a residual expression was found in the nuclei. Double immunofluorescence analyses showed that KD of miR-193a reduced the expression of PEC proteins and increased podocyte proteins within individual cells, revealing an identity change (Figure 6, D and E). Because hPECs were derived from the healthy pole of a kidney from a patient with a Wilms' tumor, eventual WT1 mutations or other individual genetic alterations could potentially confound our results. To circumvent these limitations, we confirmed our results obtained in hPECs with experiments in immortalized rat PECs.13 On inhibition of miR-193a with complementary locked nucleic acids (LNAs),15 rat PECs arborized and expressed high levels of podocyte proteins compared with control rat PECs treated with scrambled LNA (Supplemental Figure 2). In summary, we could successfully show a conversion of PECs to podocytes in vitro on KD or inhibition of miR-193a.
Figure 6.
KD of miR-193a in hPECs results in decreased transcript and protein levels of PEC proteins. miR-193a was knocked down in hPECs differentiated for 14 days at 37°C, and the protein expression levels of PEC-specific proteins were analyzed compared with mock-transduced hPEC control cells. (A) Relative expression levels of PEC-specific transcripts in hPECs with miR-193a KD compared with mock-transfected hPEC (control; n=3–6). 18S mRNA was used as a home keeper, and values are expressed as mean±SEM. GOI, gene of interest. **P<0.01 to control (=1). (B, left panel) Western blotting for PEC-specific proteins in mock-transduced hPECs or miR-193a KD hPECs after differentiation for 14 days. (B, right panel) Densitometric quantification of n=3 WB experiments. β-Actin of the same membrane was used to control for equal loading. Values are expressed as mean±SEM. Co, control; POI, protein of interest. *P<0.05; **P<0.01; ***P<0.001 to control (=1). (C) Representative micrographs of immunofluorescence staining against PEC-specific proteins Pax-8 (C; A and B), Claudin-1 (C; C and D), UCH-L1 (C; E and F) in differentiated hPECs with miR-193a KD (C; A, C and E) or mock-GFP plasmid transduction (control) (C; B, D and F). (D and E) Representative micrographs of double immunofluorescence staining in hPECs differentiated on laminin-coated dishes and cultured with VRADD-supplemented medium show strong expression of the podocyte proteins Nephrin and α-Actinin-4 with a concomitant low expression of the PEC proteins UCH-L1 and Pax-8 in hPECs with (D, A, A′ and E, A, A′) miR-193a KD compared with (D, B and B′ and E, B and B′) control cells (mock-transfected hPECs) under the same culture conditions.
miR-193a Regulates PEC and Podocyte Marker Expression In Vivo
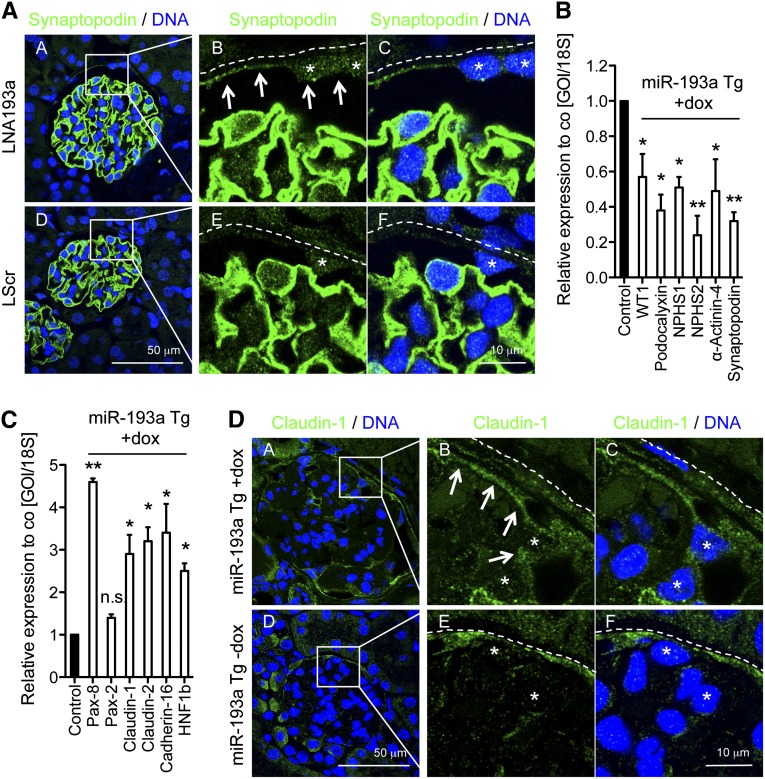
Finally, we evaluated if our findings in the established hPEC line were also relevant in vivo. To this end, we blocked miR-193a in the kidney cortex of adult wild-type mice by intraperitoneal injection of complementary LNA. By immunofluorescence, we could show that the podocyte marker protein Synaptopodin was upregulated in PECs on inhibition of miR-193a compared with a scrambled control (Figure 7A). Furthermore, the amount of PECs expressing nuclear WT1 was significantly increased on inhibition of miR-193a (11±3% in controls versus 30±4% on LNA-193a treatment; P<0.001). However, doxycycline-induced overexpression of miR-193a in transgenic mice15 led to a strong decrease of the podocyte markers WT1, Podocalyxin, NPHS1, NPHS2, α-Actinin-4, and Synaptopodin, whereas the PEC markers Pax-8, Claudin-1, Claudin-2, Cadherin-16, and HNF1b and the precursor marker CD133 exhibited a strong increase in isolated glomeruli as shown by qPCR (Figure 7, B and C and Supplemental Figure 1). By immunofluorescence, we confirmed the upregulation of Claudin-1 in podocytes on induced overexpression of miR-193a in vivo (Figure 7D). These data suggest that miR-193a might also be a master switch between PEC and podocyte identity in vivo.
Figure 7.
miR-193a regulates PEC and podocyte marker expression in vivo. (A) Mice were treated with intraperitoneal injections of LNA193a-5p to miR-193a or scrambled LNA as control, and the expression of Synaptopodin (green) in PECs was analyzed by immunohistochemistry of paraffin-embedded tissue. BC is highlighted with the dotted line (A; B; C, E and F), asterisks indicate PEC nuclei, and arrows point toward Synaptopodin-expressing PECs in the LNA193a-treated mouse (A; B). (B and C) miR-193a expression was induced in transgenic mice (miR-193a Tg) by administration of doxycycline (dox). Glomeruli were isolated and analyzed for the expression of (B) podocyte-specific mRNA or (C) PEC-specific mRNA expression compared with dox-treated wild-type littermate controls (co). Values are expressed as mean±SD. *P<0.05; **P<0.01 compared with wild type+dox. (D) Paraffin sections were analyzed for Claudin-1 green expression in podocytes after the induction of miR-193a overexpression in mice for 10 weeks. The dotted line indicates the BCs (D; B; C, E and F), asterisks indicate podocyte nuclei, and arrows point toward Claudin-1–expressing podocytes on overexpression of miR-193a (D; B).
miR-193a Is Expressed in Human and Mouse Crescents, and Inhibition of miR-193a in a Mouse Model of Nephrotoxic Nephritis Decreases Crescent Formation and Proteinuria
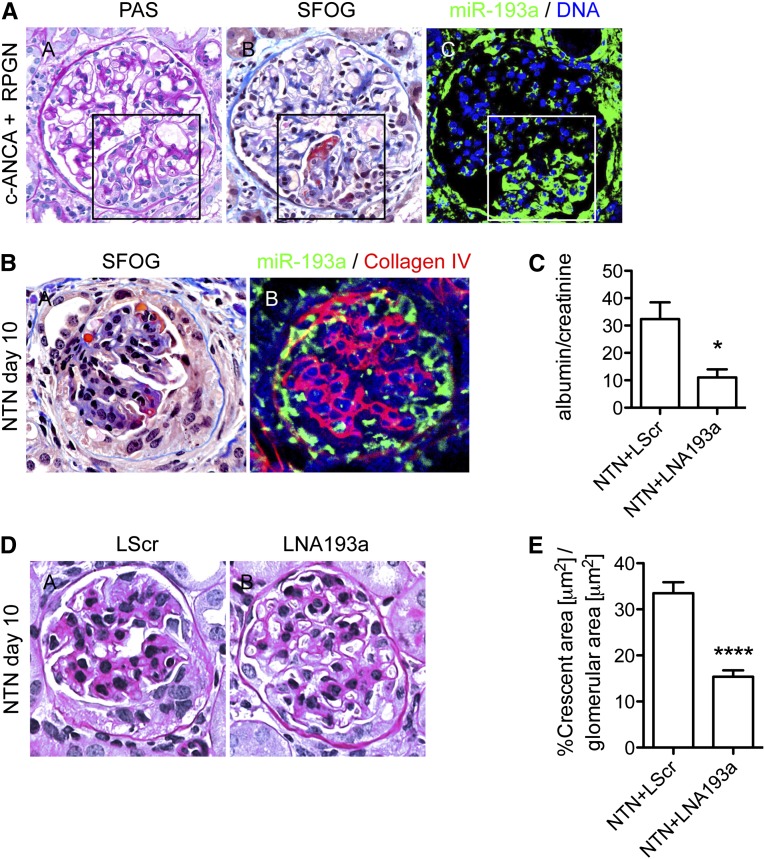
The role of miR-193a expression in PECs in glomerular diseases is unknown to date. In human rapid progressive glomerulosclerosis (RPGN) and murine models of RPGN, PECs are the main cellular component of extracapillary lesions called crescents.20 Fluorescent in situ hybridizations for miR-193a in human RPGN and a mouse model of RPGN, the nephrotoxic nephritis (NTN), showed an enhanced expression of miR-193a in cells populating the crescents (Figure 8, A and B). To investigate if miR-193a influenced crescent formation and disease progression, we induced NTN in C75BL/6 wild-type mice and inhibited miR-193a by intraperitoneal injection of LNA-193a.15 Inhibition of miR-193a resulted in decreased proteinuria (Figure 8C) and decreased formation of crescents in NTN mice compared with NTN mice treated with control LNA (Figure 8, D and E).
Figure 8.
miR-193a is expressed in human and murine crescents, and inhibition of miR-193a in murine NTN decreases crescent formation and proteinuria. (A) Representative micrographs of consecutive sections of a patient with c-ANCA–positive RPGN show miR-193a expression in PECs and crescents. (A, A) Periodic acid–Schiff staining (PAS). (A, B) Sour Fuchsin Orange G (SFOG) staining. (A, C) Fluorescent in situ hybridization (FISH) to miR-193a. The box in A, B and C highlights a crescent. (B, A) NTN was induced in male C57BL/6 mice. Mice were treated with intraperitoneal injections of LNA193a-5p-3p to miR-193a or scrambled LNA as control, and kidneys were analyzed 10 days after induction of NTN. (B, B) Representative micrographs of consecutive sections of a glomerulus of a mouse with NTN show miR-193a expression by FISH (green) in crescents and collagen type IV (red), and nuclei were visualized with DRAQ5 (blue). (C) Quantification of proteinuria by ELISA. Values were normalized to creatinine and are expressed as mean (milligrams/milligram) albumin/creatinine±SEM of n=5 mice per group. *P<0.05. (D) Representative micrographs of PAS staining in (D, A) LNA-scrambled (LScr) or (D, B) LNA193a-treated NTN mice. (E) Quantification of crescent size in 40 glomeruli per mouse in n=5 mice using axiovision computation. Crescent size in an individual glomerulus was normalized to glomerular area of the same glomerulus. Values are expressed as mean±SEM of n=5 mice per group. ****P<0.001.
Discussion
In this study, we established a polyclonal hPEC line and showed that hPECs adopt a podocyte-like phenotype in culture on KD of miR-193a. The hPEC line was established with the help of a manual dissection technique of BCs that had previously led to the successful generation of a rat polyclonal PEC line.13 The hPECs resembled cultured mouse and rat PECs13 with respect to cellular morphology, growth characteristics, and gene expression profiles.
How is the expression of podocyte proteins in PECs regulated?
We have shown before that proteolysis by the UCH-L1/ubiquitin proteasomal and the autophagosomal/lysosomal systems regulated the expression levels of podocyte-specific proteins in cultured rat PECs13 and cultured human podocytes.14 Others have shown that isolated glomerular cells, referred to as PECs or renal progenitor cells, have the potential to increase the expression of podocyte markers when cultured in a specific induction medium containing vitamin D3 and retinoic acid (VRAD medium). By culturing sieved rat glomeruli and selecting for Claudin-1, Benigni et al.21 isolated rat PECs, which expressed Synaptopodin when cultured in VRAD medium. These findings were supported by work from Ronconi et al.,22 which showed the potential of CD24/CD133-positive and Podocalyxin-negative cells from sieved human glomeruli to acquire a podocyte-like phenotype and adopt the expressions of Nephrin, WT1, and Podocin in VRAD medium. This potential of PECs to change marker profiles led to a highly debated concept that PECs could represent podocyte progenitors. The origin of this debate leads back to a finding in human kidney sections that PECs in the BC were heterogeneous. Some PECs coexpressed the two human stem or progenitor cell markers CD24 and CD133.19
We showed that hPECs expressed high levels of miR-193a compared with human podocytes in culture. This finding was in concordance with the original description of a significant miR-193a expression in PECs but no profound expression of miR-193a in podocytes of normal individuals.15 The expression of miR-193a was, however, strongly enhanced in affected podocytes of individuals with the diagnosis of FSGS,15 suggesting that miR-193a might be responsible for podocyte dedifferentiation and the acquisition of PEC traits. Here, we could support this hypothesis by three observations. First, miR-193a was highly expressed in mature podocytes and PECs of the developing fetal glomerulus and mature PECs of an adult kidney, whereas mature podocytes lost their miR-193a expression. Second, miR-193a overexpression in mice increased the expression of PEC markers in podocytes and decreased the expression of podocyte markers. Third, KD of miR-193a in cultured hPECs resulted in a strong increase of podocyte marker expression in PECs. This included increased levels of WT1 targets, such as Nephrin and Podocalyxin, and also, levels of α-Actinin-4 and Synaptopodin. We could also show a significant decrease of PEC-specific transcripts and proteins on KD of miR-193a in hPECs, supporting the molecular rapprochement of PECs toward podocytes by inhibition of miR-193a. Furthermore, the in vivo inhibition of miR-193a in mice resulted in a strong upregulation of several podocyte markers in isolated glomeruli, suggesting that this process might also be relevant in vivo.
The observed adaption of podocyte or PEC markers on inhibition or overexpression of miR-193a, respectively, might also be relevant in disease. To this end, it has been shown that overexpression of miR-193a induces FSGS concomitant with a loss of podocyte identity.15 A similar loss of podocyte markers has been observed in samples from patients with FSGS, whereas several PEC markers, including Claudin-1, Pax-2, and Pax-8, were upregulated. In line with this finding, it has been shown recently that podocytes transdifferentiate into a PEC-like phenotype in active lesions of FSGS.23
Furthermore, our data also suggest a role for miR-193a in PECs in glomerular diseases. We could show a marked expression of miR-193a in cells populating human and mouse glomerular crescents and a decreased crescent formation on inhibition of miR-193a in a mouse model of crescentic GN, the NTN model. Genetic fate tracings have shown that the majority of cells populating crescents in mouse NTN is derived from PECs.20 Therefore, miR-193a might play a role in maintaining PECs in an undifferentiated reactive state. This might be a prerequisite for PEC proliferation and migration in the phase of crescent formation in the course of NTN. Inhibition of miR-193a in PECs in the course of NTN, however, could switch PECs to a more differentiated podocyte-like phenotype, which precludes proliferation, migration, and crescent formation.
Taken together, this study shows the first successful establishment of a polyclonal hPEC line. Furthermore, our data show that miR-193a represents a master switch that regulates the expression of PEC and podocyte markers in glomerular epithelial cells in vitro that might also be relevant in vivo.
Concise Methods
Antibodies
Primary antibodies used for the study were guinea pig antinephrin (IF, 1:100; WB, 1:500; Acris), rabbit α-Actinin-4 (IF, 1:200; WB, 1:1000; ImmunoGlobe), rabbit α-Podocin (IF, 1:100; Sigma-Aldrich), goat α-Podocin (WB, 1:1000; Santa Cruz Biotechnology), rat α-Podocalyxin (IF, 1:100; WB, 1:1000; R&D Systems), rabbit α-Synaptopodin (IF, 1:200; WB, 1:1000; Santa Cruz Biotechnology), goat α-Pax-8 (IF, 1:50; WB, 1:500), rabbit α-UCH-L1 (IF, 1:50; Abcam, Inc.), rat α-UCH-L1 (IF, 1:50; WB, 1:250; Biochemistry CAU Kiel, J. Grötzinger), rabbit α-Claudin-1 (IF, 1:50; WB, 1:1000; Invitrogen), mouse α-β-Actin (WB, 1:40,000; Sigma-Aldrich), goat α-CD24 (IF, 1:50; WB, 1:1000; Santa Cruz Biotechnology), mouse α-CD133 (IF, 1:50; Miltenyi), rabbit α-CD44 (IF, 1:100; WB, 1:1000; Abcam, Inc.). All secondary antibodies were either fluorochrome or horseradish peroxidase (HRP) -conjugated affinity purified donkey antibodies (Jackson ImmunoResearch Laboratories, Hamburg, Germany).
Isolation of Human Parietal Cells
The normal pole of a nephrectomy specimen was obtained from a 2.5-year-old child who suffered from a WT. Ethical permission for human kidney usage was obtained from SouthWest Multicenter Research Ethics Committee (MR£C 00/6/02). The tissue was quickly transported to the laboratory in cool sodium chloride. All additional steps were performed in ice-cold PBS supplemented with 0.5% BSA (Sigma-Aldrich). Healthy pieces of the tissue were cut into small slices. Capsule and medulla were removed on a precooled glass dish in sterile PBS. The homogenized tissue was then pushed through a stainless steel sieve with a pore size of 425 µm by applying gentle pressure with a plunger from a 50-ml syringe. The sieve was rinsed several times with PBS. The tissue below the sieve was pushed again through a sieve with a pore opening of 180 µm as mentioned above. The tissue below the sieve contained an enrichment of glomeruli and was collected and transferred to a sieve with a pore size of 125 µm. After several washings with 50 ml PBS, the material remaining on the top of the sieve contained the glomeruli, which were collected in 50 ml PBS and centrifuged for 10 minutes at 1000 rpm. The supernatant was discarded, whereas the pellet containing the glomeruli was resuspended in PBS. The washing step was repeated three times until the filtrate was clear. Glomeruli were enriched under a dissecting microscope, taking care to select solely glomeruli with a contiguous capsule without adhering proximal tubules or arterioles. Enriched encapsulated glomeruli (1% of all glomeruli) were removed from the culture plate with a 20-µm Gilson pipette and then snap digested with collagenase 2 (Qiagen) for up to 40 seconds to loosen BC adherence. Under microscopic control, capsules were microdissected off the glomerular tuft as described previously by Guhr et al.13 for rat PEC isolation. Decapsulated tufts were removed by suction from the culture plates with 10-µl pipettes; 20–30 BCs were cultured at 37°C and 5% CO2 in endothelial growth medium supplemented with 5% FCS, 0.4% bovine brain extract, 0.1% hEGF, 0.1% hydrocortisone, 0.1% gentamicin and amphotericin B, 100 units/ml penicillin, and 100 mg/ml streptomycin (Lonza, Basel, Switzerland). Naïve BCs adhered to the culture dish, and primary PECs started to grow out 9 days after preparation.
Human Parietal Cell Immortalization
Cells were transfected with the retrovirus-encoding vector pZipSVtsa58 as described previously by Jat and Sharp17 and Saleem et al.16 to establish an immortalized cell line. Freshly thawed supernatant was mixed 1:1 with EGM medium, and 8 µg/ml polybrene was added. Medium was transferred to 35-mm plates containing target cells (primary human parietal cells 9 days after isolation). The virus supernatant was left for 48 hours on the cells. At day 11, the supernatant was removed, and EGM medium was added. On day 14, cells were trypsinized and transferred into a TC25 flask. An additional transduction with virus supernatant was carried out two more times. After the third infection cycle, the target cells were grown and selected for integration of the expression plasmids using G418 at 400 µg/ml.
Cell Culture
Human podocytes were provided by M. Saleem (Bristol, United Kingdom) and cultured under permissive conditions (32°C and 5% CO2 in RPMI 1640 supplemented with 10% FCS and 1× ITS) in uncoated 75-mm2 tissue culture flasks (Sarstedt, Nümbrecht, Germany) as described.16 For differentiation, podocytes were cultured for 14 days under nonpermissive conditions (37°C and 5% CO2 in RPMI 1640 supplemented with 10% FCS and 1× ITS) on either uncoated or coated (collagen 1; Becton Dickinson GmbH, Heidelberg, Germany) 75-mm2 tissue culture flasks or collagen-1–coated plasticware. Cell density was kept below 90% to allow foot process development.
hPEC primary cells were kept on Biocoat Collagen I Cellware 35-mm culture plates (Becton Dickinson Labware) in EGM endothelial cell medium supplemented as described above at 38°C and 5% CO2.
Immortalized PECs were grown under permissive conditions (32°C and 5% CO2) in 1:1 EGM endothelial growth medium supplemented with 5% FCS, 0.4% bovine brain extract, 0.1% hEGF, 0.1% hydrocortisone, 0.1% gentamicin and amphotericin B, 100 units/ml penicillin, 100 mg/ml streptomycin (Lonza, Basel, Switzerland), and RPMI 1640 supplemented with 10% FCS and 1× ITS. For differentiation, hPECs were cultured for 14 days under nonpermissive conditions (37°C and 5% CO2) in EGM endothelial growth medium and RPMI 1640 with supplement mentioned above. Cell density was kept at 90% to allow cell contact formation. For the formation of processes, hPECs were differentiated on laminin- or fibronectin-coated dishes (BD Biocoat Cellware; BD Biosciences), and the medium was supplemented with a specific induction medium, termed VRADD medium, containing vitamin D3 (100 nM; Sigma-Aldrich), retinoic acid (100 µM; Sigma-Aldrich), and dexamethasone (0.1 µM; Sigma-Aldrich)18 for 5 days.
Fluorescent In Situ Hybridization on Paraffin Sections
We followed a protocol established previously.24 In short, 5-μm-thick paraffin sections were prepared RNAse-free. Paraffin was removed by treatment with xylol (three times for 10 minutes), ethanol (100%, 75%, 50%, and 25%), and d(DEPC)-treated water and then washed two times for 5 minutes with PBS. Consecutive blocks with avidin and biotin for 10 minutes were performed followed by two PBS washes for 5 minutes. Sections were treated with proteinase K at 37°C for 20 minutes followed by a 30-second glycine in PBS wash and three PBS washes for 5 minutes. Postfixation was performed in 4% formaldehyde for 10 minutes followed by two PBS washes for 5 minutes. Sections were acetylated followed by two PBS washes for 5 minutes. Prehybridization in hybridization solution was performed for 2 hours followed by hybridization at 55°C with modified LNA oligonucleotides. Washing was performed in 2× SSC for 15 minutes, and three washes were performed in 50% formamide in 2× SSC for 30 minutes at hybridization temperature. Blocking was done for 60 minutes in 0.5% blocking reagent in TNB at room temperature. Incubation with streptavidin-HRP in TNB buffer was performed for 30 minutes followed by three washes in PBS and Tween 20 for 5 minutes. TSA Plus Fluorescence Amplification solution was used to enhance the signals.
Animal Experiments
miR-193a was induced by doxycycline (1 mg/ml in 5% sucrose) in transgenic mice as described before.15 PEC-specific markers were measured by qPCR from RNA of isolated glomeruli 8 days postinduction, podocyte-specific markers were measured 2 weeks postinduction, and PPIA levels were used for normalization. IF staining for Claudin-1 was performed 10 weeks postinduction. For inhibition of endogenous miR-193a in the kidney cortex, complementary LNA was injected intraperitoneally as described before.15 Expression of Synaptopodin and WT1 was assessed 6 weeks post-inhibition. For induction of NTN, 0.75 ml polyclonal sheep serum directed against a crude murine glomerular basement extract was injected intraperitoneally in 10-week-old male C57BL/6 mice purchased from Jackson Laboratories as described before.25,26 Inhibition of miR-193a was commenced 1 day after induction of NTN by intraperitoneal injection of LNA-193a-3p and 5p at half the concentration described by Gebeshuber et al.15 and repeated on days 4 and 8. Mice were euthanized on day 10. Albuminuria was measured by a commercial ELISA kit (Bethyl), and values were normalized to creatinine values assessed by the Department of Clinical Chemistry (University Clinic Hamburg-Eppendorf, Hamburg, Germany) as described before.27 Periodic acid–Schiff staining to detect polysaccharides/glycoproteins and to evaluate the glomerulus and Sour Fuchsin Orange G staining to visualize immune deposits were performed according to routine protocols. Crescent formation was measured in 40 glomeruli per mouse and five mice per condition. Briefly, the total glomerular area (excluding the urinary and tubular glomerular poles) and the area of BC including crescents (if present) were measured using the Axiovision program (Carl Zeiss). The BC with or without crescent area was normalized to the glomerular area of the respective glomerulus.
KD of miR-193a
Immortalized hPECs cultured under permissive conditions were transduced with virus containing supernatants (LentimiRa-Off-hsa-193a-3p virus or Lenti.III-mir.Off Control virus; Applied Biologic Materials Inc.; virus titer=107pfu/ml: 50 μl/5×105 cells) and incubated 1:1 with EGM endothelial growth medium and RPMI 1640 for 12 hours. Integration efficiency was assessed by immunofluorescence against GFP after 24 hours. Positively transduced cells were selected with puromycin (2.5 μm/ml) for 7 days. For subsequent experiments, miR-193a KD and mock-transduced hPECs were cultured for 14 days under nonpermissive conditions.
Immunofluorescence
For immunofluorescence, cells were differentiated on collagen-1–coated plasticware and fixed with 4% paraformaldehyde (EM Sciences) for 8 minutes at room temperature or ice cold 100% methanol for 5 minutes at −20°C, rinsed with PBS, and permeabilized with 0.05% Triton X-100 in PBS; 2-µm paraffin sections were deparaffinized, and antigen retrieval was obtained by boiling in citrate buffer (pH 6.1). Unspecific binding was blocked with 5% horse serum (hs) for 30 minutes at room temperature before incubation for 2 hours at room temperature or o/n at 4°C with primary antibodies in 5% hs. Staining was visualized with fluorochrome (Cy2 or AF488 for hPECs and human podocytes and Cy3 for miR-193a or mock-transduced hPECs) -conjugated secondary antibodies (1:200, 30 minutes at room temperature in 5% hs; Jackson ImmunoResearch Laboratories). Nuclei were visualized using DRAQ5 (Molecular Probes). Negative controls were performed by omitting primary antibodies. Stainings were evaluated with a confocal LSM 510 Meta microscope using the LSM software (Carl Zeiss). For quantification of WT1-positive PECs, the percentage of WT1-expressing PECs was assessed and normalized to the total amount of PECs of the respective glomerulus (20 glomeruli per mouse were counted; n=2–3 mice per group).
qPCR
Total RNA was isolated from hPECs and human podocytes with the Nucleospin RNA II Kit (Macherey und Nagel) according to the manufacturer’s instructions, and 20 μl mRNA was reverse-transcribed with random hexamer primers (Invitrogen) and revert aid reverse transcription (Fermentas). For miR quantification, RNA was prepared with the miRVana miRNA Isolation Kit and TaqMan-based detection primers (Life Technologies). Real-time PCR was performed on a Step One Plus Real-Time PCR System (Applied Biosystems). The mRNA levels were quantified with an AbiPrism NN8860 using SYBR green as recently described.28 Exon junction spanning or intron including primer pairs was used when possible: 18S fw 5′ TTC GAA CGT CTG CCC TAT CAA; rev 5′ CTG CCT TCC TTG GAT GTG GTA ′3, WT1 fw 5′ TTG TGT GGTTAT CGC TCT CG ′3; rev 5′ CAA ATG ACA TCC CAG CTT GA ′3, Synaptopodin fw 5′ CTT CTC CGT GAG GCT AGT GC ′3; rev 5′ TGA GAA AGG CTT GAAA GG ′3, Podocalyxin fw 5′ TGT TTT GTT AGA TGA GTC CGT AGT A ′3; rev 5′ CGC TGC TAC TGT CA ′3, α-Actinin-4 fw 5′ GTT CTC GAT CTG TGT GCC TG ′3; rev 5′ GAC CTG GAC CC ′3, NPHS1 fw 5′ GGC CAC CTG GTC ATA GAT TC ′3; rev 5′ ACC CCT CTA CGA TGA AGT GC ′3, NPHS2 fw 5′ TGA AGA GCA GGG AAA TG AGG ′3; rev 5′ CTG TTG GAG AGC GAG CG ′3, Claudin-1 fw 5′ TCA CTC CCA GGA TGC ′3; rev 5′ GGC AGA TCC AGT GCA AAG TC ′3, Pax-8 fw 5′ CAG GTC TAC GAT GCG CTG ′3; rev 5′ TGC CTC ACA ACT CCA TCA GA ′3, Pax-2 fw 5′ CGG ATG GGG CAG GGA CAG GA ′3; rev 5′ GGC GCT GGA AAC AGG GG ′3, HNF1b fw 5′ ATA GCT CCA ACC AGA CGC AC ′3; rev 5′ GTT GTA GCG CAC TCC TGA CA ′3, UCH-L1 fw 5′ AGC TGG AAT TTGA GGA TGG A ′3; rev 5′ GGC CTC GTT CTT CTC GAA A ′3, miR-193a 5p fw 5′ TGG GTC TTT GCG GGC GAG ATG A ′3; rev 5′ ACC CAG AAA CGC CCG CTC TAC T ′3. Mouse Claudin-2 and Cadherin 16 primer pairs were obtained from Qiagen; 18S (RNA) and RNU48 (miR) were used as an internal control to correct for small variations in RNA quality and cDNA synthesis essentially as described by AbiPrism. Amplicons of random samples for each primer pair were determined by automatic PCR sequencing to show the specificity of the PCR reaction. Relative quantification of gene expression was calculated using the ΔΔCT method.
Immunoblot Analyses
Immunoblots were performed as described previously.29 Briefly, samples were lysed (T-PER [Pierce] containing phosphatase inhibitors, 1 mM sodium vanadate, 1 mM sodium fluorid, and 1 mM calyculin A) and denatured with 4× lithium dodecyl sulfate. Samples were separated on a 4%–12% Bis-Tris NuPage Gel (Invitrogen) in NuPage running buffer. Protein transfer was performed in transfer buffer (50 mM Tris base and 0.192 M glycine in ddH2O) in a Novex Mini Cell (Invitrogen). PVDF membranes (EMD Millipore) were blocked (3% nonfat milk) before incubation with primary antibodies diluted in Superblock blocking reagent (Pierce). Binding was detected by incubation with HRP-coupled secondary antibodies (1:10,000; 3%–5% nonfat milk). Proteins were visualized with ECL SuperSignal (Pierce) according to the manufacturers’ instructions on a Biomax Light Film (Kodak). Immunoblots were analyzed using ImageJ software.30
Statistical Analyses
Values are expressed as means±SEMs or ±SDs; n refers to the number of animals or individual measurements in separate samples. Statistical comparisons were performed with the program PRISM using the Mann–Whitney U test for nonparametric data and the unpaired t test for parametric data. A P value<0.05 was accepted as statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Sabine Wuttke (Graphic Department, University Clinic Hamburg-Eppendorf, Hamburg, Germany) for graphical assistance.
S.G. was supported by the Integrated Research Training Group of the Sonder Forschungsbereich 877 (Christian Albrechts University, Kiel, Germany). D.K. was supported by European Research Projects on Rare Diseases Project I923-B13. C.M.-S. is supported by Deutsche Forschungsgemeinschaft Grant KFO288.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014020190/-/DCSupplemental.
References
- 1.Ohse T, Chang AM, Pippin JW, Jarad G, Hudkins KL, Alpers CE, Miner JH, Shankland SJ: A new function for parietal epithelial cells: A second glomerular barrier. Am J Physiol Renal Physiol 297: F1566–F1574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shankland SJ, Smeets B, Pippin JW, Moeller MJ: The emergence of the glomerular parietal epithelial cell. Nat Rev Nephrol 10: 158–173, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Discenza MT, He S, Lee TH, Chu LL, Bolon B, Goodyer P, Eccles M, Pelletier J: WT1 is a modifier of the Pax2 mutant phenotype: Cooperation and interaction between WT1 and Pax2. Oncogene 22: 8145–8155, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Kim MK, McGarry TJ, O Broin P, Flatow JM, Golden AA, Licht JD: An integrated genome screen identifies the Wnt signaling pathway as a major target of WT1. Proc Natl Acad Sci U S A 106: 11154–11159, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grouls S, Iglesias DM, Wentzensen N, Moeller MJ, Bouchard M, Kemler R, Goodyer P, Niggli F, Gröne HJ, Kriz W, Koesters R: Lineage specification of parietal epithelial cells requires β-catenin/Wnt signaling. J Am Soc Nephrol 23: 63–72, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreidberg JA: Podocyte differentiation and glomerulogenesis. J Am Soc Nephrol 14: 806–814, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Ohse T, Pippin JW, Chang AM, Krofft RD, Miner JH, Vaughan MR, Shankland SJ: The enigmatic parietal epithelial cell is finally getting noticed: A review. Kidney Int 76: 1225–1238, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bariéty J, Bruneval P, Meyrier A, Mandet C, Hill G, Jacquot C: Podocyte involvement in human immune crescentic glomerulonephritis. Kidney Int 68: 1109–1119, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bariety J, Hill GS, Mandet C, Irinopoulou T, Jacquot C, Meyrier A, Bruneval P: Glomerular epithelial-mesenchymal transdifferentiation in pauci-immune crescentic glomerulonephritis. Nephrol Dial Transplant 18: 1777–1784, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Achenbach J, Mengel M, Tossidou I, Peters I, Park JK, Haubitz M, Ehrich JH, Haller H, Schiffer M: Parietal epithelia cells in the urine as a marker of disease activity in glomerular diseases. Nephrol Dial Transplant 23: 3138–3145, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, Hudkins KL, Alpers CE, Pippin JW, Shankland SJ: De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol 298: F702–F711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Hansen KM, Pippin JW, Chang AM, Taniguchi Y, Krofft RD, Pickering SG, Liu ZH, Abrass CK, Shankland SJ: De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol 302: F571–F580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guhr SS, Sachs M, Wegner A, Becker JU, Meyer TN, Kietzmann L, Schlossarek S, Carrier L, Braig M, Jat PS, Stahl RA, Meyer-Schwesinger C: The expression of podocyte-specific proteins in parietal epithelial cells is regulated by protein degradation. Kidney Int 84: 532–544, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Meyer-Schwesinger C, Meyer TN, Münster S, Klug P, Saleem M, Helmchen U, Stahl RA: A new role for the neuronal ubiquitin C-terminal hydrolase-L1 (UCH-L1) in podocyte process formation and podocyte injury in human glomerulopathies. J Pathol 217: 452–464, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Gebeshuber CA, Kornauth C, Dong L, Sierig R, Seibler J, Reiss M, Tauber S, Bilban M, Wang S, Kain R, Böhmig GA, Moeller MJ, Gröne HJ, Englert C, Martinez J, Kerjaschki D: Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat Med 19: 481–487, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Jat PS, Sharp PA: Cell lines established by a temperature-sensitive simian virus 40 large-T-antigen gene are growth restricted at the nonpermissive temperature. Mol Cell Biol 9: 1672–1681, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takano Y, Yamauchi K, Hiramatsu N, Kasai A, Hayakawa K, Yokouchi M, Yao J, Kitamura M: Recovery and maintenance of nephrin expression in cultured podocytes and identification of HGF as a repressor of nephrin. Am J Physiol Renal Physiol 292: F1573–F1582, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P: Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ: Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 20: 2604–2615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benigni A, Morigi M, Rizzo P, Gagliardini E, Rota C, Abbate M, Ghezzi S, Remuzzi A, Remuzzi G: Inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am J Pathol 179: 628–638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P: Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamoto K, Ueno T, Kobayashi N, Hara S, Takashima Y, Pastan I, Matsusaka T, Nagata M: The direction and role of phenotypic transition between podocytes and parietal epithelial cells in focal segmental glomerulosclerosis. Am J Physiol Renal Physiol 306: F98–F104, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerjaschki D: Lymphatic neoangiogenesis in renal transplants: A driving force of chronic rejection? J Nephrol 19: 403–406, 2006 [PubMed] [Google Scholar]
- 25.Krebs CF, Kapffer S, Paust HJ, Schmidt T, Bennstein SB, Peters A, Stege G, Brix SR, Meyer-Schwesinger C, Müller RU, Turner JE, Steinmetz OM, Wolf G, Stahl RA, Panzer U: MicroRNA-155 drives TH17 immune response and tissue injury in experimental crescentic GN. J Am Soc Nephrol 24: 1955–1965, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner JE, Krebs C, Tittel AP, Paust HJ, Meyer-Schwesinger C, Bennstein SB, Steinmetz OM, Prinz I, Magnus T, Korn T, Stahl RA, Kurts C, Panzer U: IL-17A production by renal γδ T cells promotes kidney injury in crescentic GN. J Am Soc Nephrol 23: 1486–1495, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer-Schwesinger C, Dehde S, Klug P, Becker JU, Mathey S, Arefi K, Balabanov S, Venz S, Endlich KH, Pekna M, Gessner JE, Thaiss F, Meyer TN: Nephrotic syndrome and subepithelial deposits in a mouse model of immune-mediated anti-podocyte glomerulonephritis. J Immunol 187: 3218–3229, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Panzer U, Steinmetz OM, Reinking RR, Meyer TN, Fehr S, Schneider A, Zahner G, Wolf G, Helmchen U, Schaerli P, Stahl RA, Thaiss F: Compartment-specific expression and function of the chemokine IP-10/CXCL10 in a model of renal endothelial microvascular injury. J Am Soc Nephrol 17: 454–464, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Meyer TN, Schwesinger C, Wahlefeld J, Dehde S, Kerjaschki D, Becker JU, Stahl RA, Thaiss F: A new mouse model of immune-mediated podocyte injury. Kidney Int 72: 841–852, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Abramoff MP, Ram SJ: Image processing with ImageJ. Biophotonics Int 11: 36–42, 2004 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.