Abstract
Arginine vasopressin (AVP) has a key role in osmoregulation by facilitating water transport in the collecting duct. Recent evidence suggests that AVP may have additional effects on renal function and favor cyst growth in polycystic kidney disease. Whether AVP also affects kidney structure in the general population is unknown. We analyzed the association of copeptin, an established surrogate for AVP, with parameters of renal function and morphology in a multicentric population-based cohort. Participants from families of European ancestry were randomly selected in three Swiss cities. We used linear multilevel regression analysis to explore the association of copeptin with renal function parameters as well as kidney length and the presence of simple renal cysts assessed by ultrasound examination. Copeptin levels were log-transformed. The 529 women and 481 men had median copeptin levels of 3.0 and 5.2 pmol/L, respectively (P<0.001). In multivariable analyses, the copeptin level was associated inversely with eGFR (β=−2.1; 95% confidence interval [95% CI], −3.3 to −0.8; P=0.002) and kidney length (β=−1.2; 95% CI, −1.9 to −0.4; P=0.003) but positively with 24-hour urinary albumin excretion (β=0.11; 95% CI, 0.01 to 0.20; P=0.03) and urine osmolality (β=0.08; 95% CI, 0.05 to 0.10; P<0.001). A positive association was found between the copeptin level and the presence of renal cysts (odds ratio, 1.6; 95% CI, 1.1 to 2.4; P=0.02). These results suggest that AVP has a pleiotropic role in renal function and may favor the development of simple renal cysts.
Keywords: vasopressin, renal morphology, renal function, albuminuria, cystic disease
The antidiuretic hormone arginine vasopressin (AVP) plays an essential role in osmoregulation by facilitating water transport in the collecting duct.1 This effect is mediated by the stimulation of the G protein–coupled vasopressin V2 receptor (V2R), increasing cAMP levels and leading to a transient increase in osmotic water transport across the principal cells.2 Furthermore, it was recently demonstrated that V2Rs are also expressed in the thick ascending limb of the human kidney.3,4 In addition to the V2R, AVP acts on the V1a receptor (Ca2+ as second messenger), which is distributed in the endothelial cells lining systemic and renal blood vessels and capillaries (including vasa recta), as well as the glomerulus, the macula densa, the medullary interstitial cells, and the cortical collecting duct.5–7 Of note, V1a receptors seem to antagonize V2 effects on urinary concentration by activating prostaglandin synthesis, but higher levels of AVP are required to activate those receptors.7 The functional relevance of the complex expression pattern of V1a receptors and V2Rs remains debated. Experimental studies using the Brattleboro rat, which is genetically deficient in AVP, as well as pharmacologic inhibition based on V2R-specific or V1a/V2R antagonists showed a decrease in kidney disease progression, kidney hypertrophy, and proteinuria.8–10 Abnormal AVP signaling and increased cAMP levels are also involved in renal cyst formation in various rodent models of autosomal dominant polycystic kidney disease (ADPKD), as evidenced by the protective effect of high water intake, selective V2R antagonists, and genetic deletion of AVP in such models.9,11,12 The role of AVP/V2R signaling on cystogenesis was recently confirmed by a large randomized, double-blind placebo-controlled trial testing the effect of a V2R antagonist in patients with ADPKD.13 Taken together, these studies suggest that AVP exerts pleiotropic effects on the kidney, beyond regulation of water balance. Whether such effects can be detected in the general population remains unknown.
Thus far, population-based studies addressing the role of AVP were limited by technical issues related to AVP measurement,14–16 such as high preanalytical instability,16,17 low sensitivity linked to competitive assay,18 and a long turn-around time (4–10 days). AVP is a small nonapeptide that must be isolated from plasma by chromatography and further concentrated before the assay, which notoriously complicates the analytical procedure.14,19 Even with RIAs, AVP remains difficult to measure: Its plasma t1/2 is short, it is not stable ex vivo, and it interferes with plasma components or factors such as heparin.15,20,21 In addition, storage at −20°C and freeze-thaw cycles result in a decline of plasma AVP levels over time. These issues have limited the use of AVP measurement in clinical practice, explaining the small number of cohort studies. Copeptin (also named CT-proAVP) is a peptide derived from the cleavage of the precursor of AVP (provasopressin), produced in an equimolar ratio.17 Copeptin is strongly correlated to AVP over a large range of plasma levels and has the same kinetics in response to changes in plasma osmolality or during hemorrhagic shock.18,22–26 Furthermore, copeptin has a longer t1/2 than AVP, is stable at room temperature, and does not need extraction nor special sample handling procedures. For these reasons, copeptin is now considered as a robust surrogate for AVP, overcoming the technical problems related to AVP dosage.17
In this study, we investigated the potential link of copeptin with renal function parameters, including eGFR, urine albumin excretion and urine osmolality, the morphology of the kidney, and the presence of simple renal cysts, in a large, multicentric population-based cohort.
Results
From December 2009 to March 2013, 1128 participants were included in the Swiss Kidney Project on Genes in Hypertension (SKIPOGH) study. Renal ultrasonography measurements were obtained in 1125 participants (99.7%). We excluded 6 (0.5%) participants because of missing copeptin measures and 111 (9.8%) with incomplete data for the outcomes of interest or covariables, mainly urinary ones. Another participant was excluded because of a fortuitous diagnosis of typical ADPKD on ultrasonography. Finally, we included 1010 participants in these analyses.
The characteristics of the 529 women and 481 men are presented in Table 1. Men had higher plasma copeptin levels than women. They also had higher body mass index (BMI), higher BP, eGFR, triglycerides, urinary osmolality values, and 24-hour osmolar excretion, as well as a higher prevalence of other cardiovascular risk factors such as smoking, treated hypertension, and diabetes.
Table 1.
Characteristics of the participants according to sex (N=1010)
| Characteristic | Women (n=529) | Men (n=481) | P Value |
|---|---|---|---|
| Age (yr) | 47.9±17.3 | 46.5±17.8 | 0.20 |
| BMI (kg body wt/m2) | 24.2±4.7 | 25.9±4.2 | <0.001 |
| Height (cm) | 164.8±6.5 | 177.6±6.9 | <0.001 |
| Systolic BP (mmHg) | 115.3±17.5 | 120.9±15.5 | <0.001 |
| Diastolic (mmHg) | 73.6±9.6 | 77.9±9.1 | <0.001 |
| Copeptin (pmol/L) | 3.0 (2.2–4.2) | 5.2 (3.7–7.8) | <0.001 |
| eGFR (ml/min per 1.73 m2) | 94.7±17.2 | 98.5±18.4 | 0.001 |
| Plasma sodium (mmol/L) | 140.3±2.6 | 140.3±2.5 | 0.88 |
| Estimated plasma osmolarity (mOsm/L) | 288.7±5.2 | 288.8±5.0 | 0.68 |
| Plasma triglycerides (mmol/L) | 0.8 (0.6–1.1) | 1.0 (0.7–1.4) | <0.001 |
| 24-h urine flow rate (ml/min) | 1.2 (0.8–1.5) | 1.1 (0.8–1.5) | 0.17 |
| 24-h urine sodium excretion (mmol) | 117.5(88.3–150.5) | 158.2(121.6–198.4) | <0.001 |
| 24-h urine osmolality (mOsm/kg of H2O) | 417 (314–563) | 570 (430–773) | <0.001 |
| 24-h urine creatinine excretion (mg/kg of body wt) | 18.1 (14.7–20.8) | 22.1 (19.0–25.0) | <0.001 |
| 24-h urine albumin excretion (mg) | 5.8 (3.8–10.1) | 6.0 (3.7–10.6) | 0.52 |
| 24-h osmolar excretion (mOsm) | 669.4 (560.1–800.7) | 888.9 (735.7–1047.6) | <0.001 |
| Hypertension treatment (yes) | 66 (12.5) | 90 (18.7) | 0.01 |
| Diabetes (yes) | 15 (2.8) | 34 (7.1) | 0.002 |
| Smoking (yes) | 103 (19.5) | 137 (28.5) | 0.001 |
| CKD (yes) | 16 (3.0) | 16 (3.3) | 0.78 |
| Albuminuria (yes)a | 22 (4.2) | 38 (7.9) | 0.01 |
| Kidney length (mm) | 107.0±7.9 | 113.7±8.2 | <0.001 |
| Cysts | 48 (9.1) | 73 (15.2) | 0.003 |
| Number of cysts | |||
| None | 481 (90.6) | 408 (85.0) | 0.003 |
| 1 | 38 (7.2) | 47 (9.8) | |
| ≥2 | 10(1.9) | 26 (5.6) |
Continuous variables are expressed as the mean±SD or median and IQR (25th–75th percentiles) as appropriate. Categorical variables are expressed as n (%). CKD was defined as an eGFR<60 ml/min per 1.73 m2. eGFR was calculated with the CKD-EPI equation. Plasma osmolarity was calculated as 2×(Na+K). The 24-hour osmolar excretion was calculated as urine osmolality×24-hour urine volume.
Presence of albuminuria was defined as urinary ACR≥25 µg/mg in women and ≥17 µg/mg in men.
Ultrasonography examination revealed that men had significantly larger kidneys and more cysts than women. Only one participant had missing information on kidney length because of a horseshoe kidney. The prevalence of renal simple cysts was 12% (n=121) in the studied population, and increased with age: 2.4%, 12.3%, and 24.6% for those aged <40, 40–59, and ≥60 years, respectively. A significantly higher prevalence of cysts was observed in men than in women in each age category: 3.1% versus 1.7% before 40 years (P=0.39), 16.7% versus 9.1% at 40–59 (P=0.03), and 31.5% versus 18.3% after 60 years (P=0.01). The median of the largest diameter measured in the biggest cyst was 17.0 mm (interquartile range [IQR], 10.5–24.8).
In addition to cysts, the presence of kidney stones was suspected by ultrasonography in 65 participants (6.4%), without significant differences between men and women (7.5% versus 5.5%; P=0.20).
Association Analyses
In univariate analyses, participants aged >60 years had higher median copeptin levels than younger participants (4.4 pmol/L [IQR, 3.0–6.8] versus 3.8 pmol/L [2.6–5.7]; P<0.001), and copeptin was higher in participants with higher BMI (BMI ≥30 kg/m2=5.1 pmol/L [3.4–7.9] versus BMI 25–29.9 kg/m2=4.4 pmol/L [3.2–6.5] versus BMI <25 kg/m2=3.4 pmol/L [2.4–5.2]; P for trend, P<0.001). Copeptin levels were also significantly higher in smokers than in nonsmokers (4.4 pmol/L [IQR, 2.9–6.6] versus 3.8 pmol/L [2.6–5.7]; P=0.003) and in patients with diabetes than in patients without diabetes (6.1 pmol/L [4.0–9.7] versus 3.9 pmol/L [2.7–5.7]; P<0.001) or CKD (5.1 pmol/L [3.8–10.4] versus 3.9 pmol/L [2.7–5.9]; P<0.001). Participants with a pathologic 24-hour albumin-to-creatinine ratio (ACR) also presented higher copeptin levels (5.0 pmol/L [IQR, 3.2–8.7] versus 3.9 pmol/L [2.7–5.8]; P<0.001). Copeptin levels were higher in individuals taking diuretics than in those not taking them (4.5 pmol/L [IQR, 3.3–7.9] p versus 3.9 pmol/L [2.7–5.9]; P=0.03) but not when all hypertension treatment were considered (4.4 [2.8–6.6] versus 3.9 [2.7–5.8]; P=0.10).
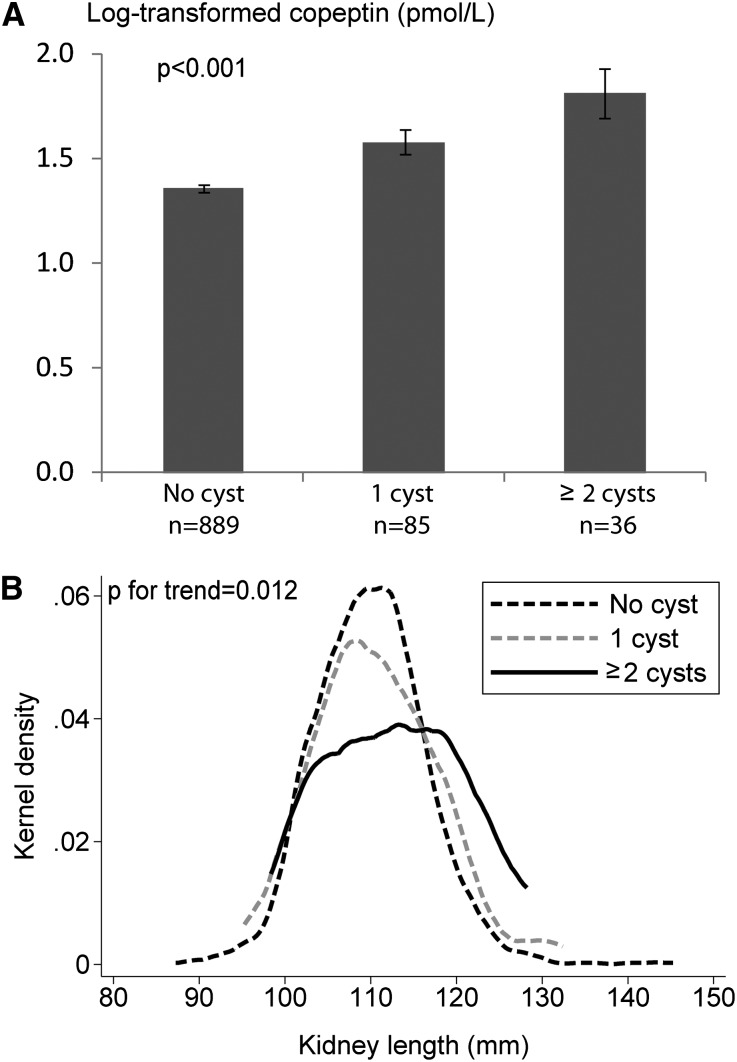
Copeptin levels were positively associated with both the presence of simple cysts (4.8 pmol/L [IQR, 3.6–7.9] versus 3.8 pmol/L [2.6–5.7]; P<0.001) and the number of cysts in the kidneys (Figure 1A). Kidney size increased with the number of cysts (Figure 1B). Participants with numerous kidney cysts were significantly older and presented higher systolic BP and 24-hour urinary albumin excretion and lower eGFR compared with those with only one cyst or without cysts (Table 2). The association of cysts with copeptin was stronger in younger participants (<61 years; odds ratio [OR], 2.27; 95% confidence interval [95% CI], 1.29 to 3.00; P=0.01) than in older participants (≥61 years; OR, 1.26; 95% CI, 0.70 to 2.15; P=0.40). These associations remained similar upon adjustment for eGFR. A higher urinary osmolality was observed in participants with numerous kidney cysts (P=0.03).
Figure 1.
Association of the number of renal cyst with copeptin levels and kidney length. (A) Unadjusted analysis. (B) Analysis adjusted for age, sex, center, and body height. Bars represent the SEM.
Table 2.
Characteristics of participants according to the number of cysts
| Variable | No Cysts (n=889) | 1 Cyst (n=85) | ≥2 Cysts (n=36) | P for Trend |
|---|---|---|---|---|
| Age (yr) | 45.4±17.1 | 58.0±14.6 | 67.5±11.0 | <0.001 |
| Systolic BP (mmHg) | 116.8±16.4 | 125.5±17.3 | 130.8±16.8 | <0.001 |
| eGFR (ml/min per 1.73 m2) | 98.0±17.3 | 88.8±18.8 | 79.8±18.7 | <0.001 |
| 24-h urine osmolality (mOsm/kg of H2O) | 486 (357–647) | 527 (338–740) | 602 (419–723) | 0.03 |
| Urine albumin excretion (mg/24 h) | 5.7 (3.6–9.7) | 8.4 (3.9–14.5) | 10.0 (5.5–14.9) | <0.001 |
| Kidney length (mm) | 109.9±8.5 | 110.8±9.0 | 114.5±11.5 | 0.01 |
Data are expressed as the mean±SD or the median and IQR (25th–75th percentiles).
Compared with participants without evidence of kidney stones, those with suspected stones had higher median 24-hour urine osmolality (569.0 mOsm/kg H2O [IQR, 385.7–795.2] versus 489.3 mOsm/kg H2O [355.5–646.5]; P=0.02), a lower urinary flow rate (0.9 ml/min [0.7–1.3] versus 1.1 ml/min [0.8–1.5]; P=0.02), and higher copeptin levels (4.9 pmol/L [3.0–8.4] versus 3.9 pmol/L [2.7–5.8]; P=0.01).
We found a linear association between copeptin and most of the continuous variables in Table 1. Because sex was a major confounder for many of the associations, we presented univariate correlations separately for men and women (Table 3). Only BMI, height, eGFR, 24-hour urinary flow rate, and urinary osmolality had a significant association with copeptin in men and women.
Table 3.
Associations between copeptin and continuous variables according to sex
| Variable | Women (n=529) | Men (n=481) | ||
|---|---|---|---|---|
| Correlation | P Value | Correlation | P Value | |
| Age (yr) | 0.12 | 0.01 | 0.07 | 0.13 |
| BMI (kg of body wt/m2) | 0.24 | <0.001 | 0.10 | 0.02 |
| Height (cm) | −0.13 | 0.002 | −0.19 | <0.001 |
| Systolic BP (mmHg) | 0.16 | <0.001 | 0.07 | 0.14 |
| Diastolic BP (mmHg) | 0.10 | 0.02 | −0.02 | 0.66 |
| eGFR (ml/min per 1.73 m2) | −0.12 | 0.01 | −0.12 | 0.01 |
| Plasma sodium (mmol/L) | 0.01 | 0.79 | 0.08 | 0.08 |
| Estimated plasma osmolarity (mOsm/L) | 0.02 | 0.73 | 0.08 | 0.09 |
| Plasma triglycerides (mmol/L) | 0.03 | 0.53 | −0.01 | 0.88 |
| 24-h urine flow rate (ml/min) | −0.38 | <0.001 | −0.41 | <0.001 |
| 24-h urine sodium excretion (mmol) | 0.001 | 0.98 | −0.02 | 0.62 |
| 24-h urine osmolality (mOsm/kg H2O) | 0.37 | <0.001 | 0.44 | <0.001 |
| 24-h urine creatinine excretion (mg/kg of body wt) | −0.16 | <0.001 | −0.02 | 0.67 |
| 24-h urine albumin excretion (mg) | 0.04 | 0.38 | 0.13 | 0.004 |
| 24-h osmolar excretion (mOsm) | −0.03 | 0.54 | −0.01 | 0.81 |
| Kidney length (mm) | −0.06 | 0.20 | −0.12 | 0.01 |
Data are Pearson’s correlation coefficients and corresponding P values. Copeptin, triglycerides, urine flow rate, 24-hour urine osmolality, and albumin excretion were log-transformed. Urine sodium and creatinine excretion were square-root transformed. Plasma osmolarity was calculated as 2×(Na+K). The 24-hour osmolar excretion was calculated as urine osmolality×24-hour urine volume.
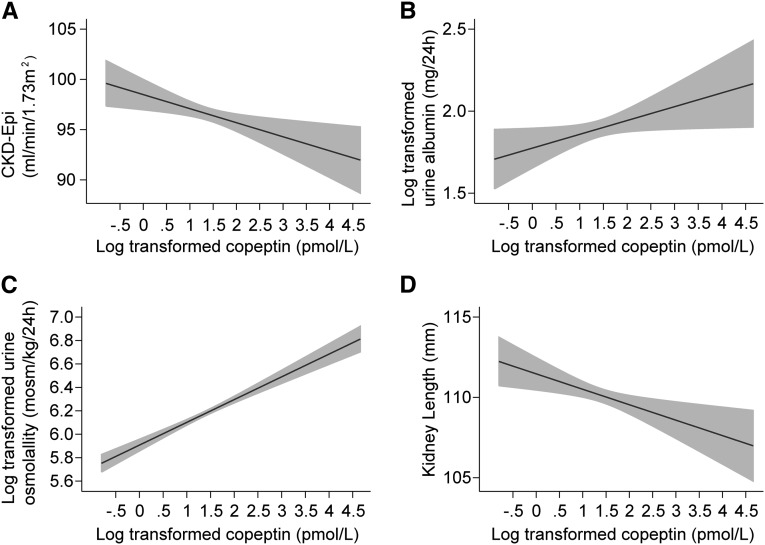
The association of copeptin with eGFR, 24-hour urine albumin excretion, 24-hour urine osmolality, and kidney length adjusted for age, sex, and center is presented graphically in Figure 2. Table 4 describes the β coefficients, ORs, and 95% CIs for the associations between copeptin levels and these variables of interest (dependent variables) in different multivariate models progressively integrating other covariates. Because all of the models are adjusted for sex, results are presented altogether for men and women. In all models, urinary albumin excretion and urinary osmolality were positively associated with adjusted copeptin levels. On the contrary, eGFR and kidney length presented an inverse association with adjusted copeptin levels. Adding mean BP as a covariate to the last model did not change these associations. The inverse association with kidney length was maintained even when the model was adjusted for body height instead of BMI, although the β coefficients were smaller. Of note, we observed that kidney length remained associated with eGFR (β=0.16; 95% CI, 0.12 to 0.20; P<0.001), urine osmolality (β=0.004; 95% CI, 0.001 to 0.01; P=0.03), and 24-hour osmolar excretion (β=0.01; 95% CI, 0.003 to 0.01; P<0.001) beyond the fully adjusted model 3. These associations were linear.
Figure 2.
Association of copeptin with CKD-EPI, 24-hour urine albumin excretion, 24-hour measured osmolality, and kidney length. A–D are adjusted for age, sex, and center. Shaded areas represent the 95% CI.
Table 4.
Multilevel multivariable regression analyses showing associations of GFR, 24-hour urine albumin excretion, urine osmolality, kidney length, and the presence of renal cysts with log-transformed copeptin levels
| Dependent Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| eGFR (ml/min per 1.73 m2) | −2.06 (−3.33 to −0.79) | 0.001 | −2.12 (−3.41 to −0.84) | 0.001 | −2.05 (−3.33 to −0.76) | 0.002 |
| Urine albumin excretion (mg/24 h)a | 0.12 (0.03 to 0.22) | 0.01 | 0.10 (0.001 to 0.19) | 0.05 | 0.11 (0.01 to 0.20) | 0.03 |
| 24-h urine osmolality (mOsm/kg)b | 0.28 (0.24 to 0.32) | <0.001 | 0.28 (0.24 to 0.32) | <0.001 | 0.08 (0.05 to 0.10) | <0.001 |
| Kidney length (mm) | ||||||
| Adjusted for BMI | −1.42 (−2.26 to −0.57) | 0.001 | −1.70 (−2.52 to −0.89) | <0.001 | −1.62 (−2.43 to −0.81) | <0.001 |
| Adjusted for body height and body wt | −1.01 (−1.83 to −0.19) | 0.02 | −1.22 (−1.99 to −0.45) | 0.002 | −1.16 (−1.93 to −0.39) | 0.003 |
| Renal cysts (present, n=121)c | 1.62 (1.11 to 2.38) | 0.01 | 1.55 (1.05 to 2.31) | 0.03 | 1.61 (1.08 to 2.41) | 0.02 |
Model 1 is adjusted for age, sex, and center. Model 2 is adjusted for model 1 plus diabetes, hypertension treatment, triglycerides, smoking, BMI, and eGFR. Model 3 is adjusted for model 2 plus estimated plasma osmolarity, 24-hour urine creatinine excretion, and 24-hour osmolar excretion.
For 24-hour urine albumin excretion analyses, all models were adjusted for 24-hour urine creatinine excretion. Models 2 and 3 were adjusted for fasting glucose instead of diabetes. Urinary albumin was log-transformed.
The 24-hour urinary osmolality was log-transformed and model 3 included urine flow rate alone instead of osmolar excretion.
ORs are presented for renal cysts.
We also looked at the association between copeptin and the presence of CKD (n=32) or albuminuria (n=60) using dichotomized eGFR and sex-specific cutoffs for ACR. In univariate analyses, the odds of having CKD or pathologic ACR with higher levels of copeptin were 2.86 (95% CI, 1.65 to 4.98; P<0.001) and 2.08 (95% CI, 1.39 to 3.11; P<0.001), respectively. In the models adjusting for sex, age, and center, copeptin remained associated with CKD (OR, 2.82; 95% CI, 1.45 to 5.50; P=0.002) and ACR (OR, 1.70; 95% CI, 1.08 to 2.68; P=0.02).
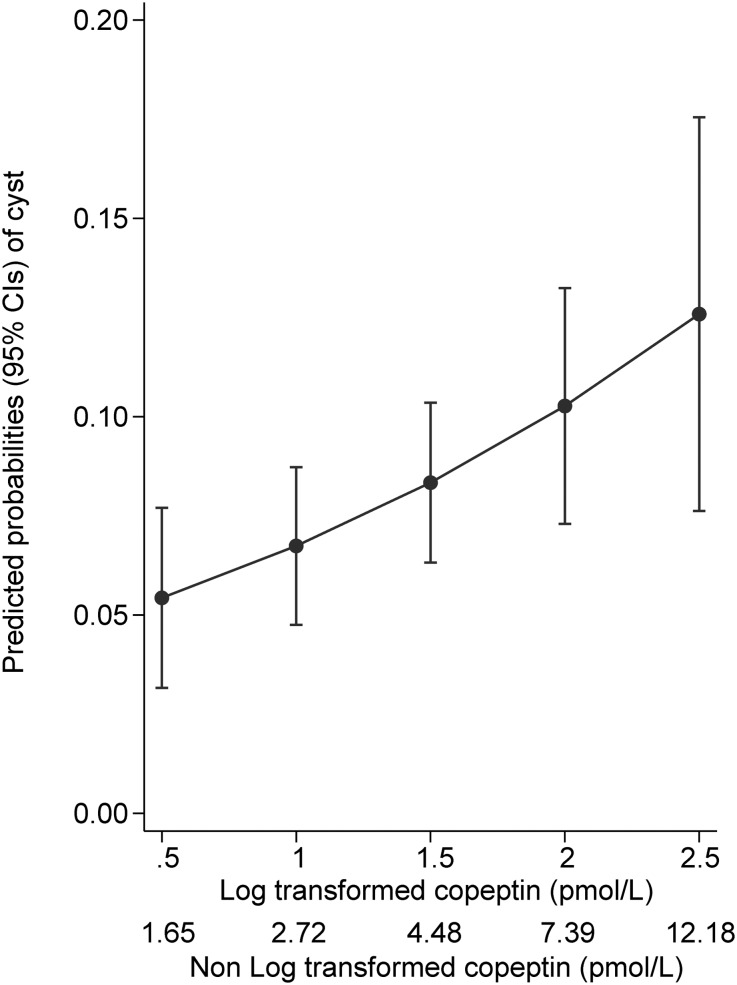
Copeptin was positively associated with the presence of cysts (Table 4). Even after adjusting for age, sex, and center, participants with higher copeptin levels had a higher probability of having renal cysts (Figure 3). The association remained significant when adjusting for kidney size and urine concentrating ability. As described in the univariate analyses, the association was stronger in younger participants than in older ones, even when adjusting for age, sex, center, and eGFR. Of note, the largest diameter of the biggest cyst measured, used as a surrogate of cyst size, was not associated with copeptin levels neither in univariate nor in any of the multivariable models (data not shown).
Figure 3.
Higher copeptin levels are associated with higher probability of having cysts adjusted for age, sex, and center.
The association of kidney stones with copeptin observed in univariate analyses was less significant when adjusting for age, sex, and center (OR, 1.62; 95% CI, 1.02 to 2.58; P=0.04) and was no longer statistically significant upon further adjustments.
Sensitivity Analyses
Analyses repeated after winsorizing copeptin values led to similar results. We also obtained similar results after excluding participants with suspected inaccurate urine collection (n=6), as defined in the Concise Methods. We obtained similar results for the association of renal size with copeptin after excluding five participants with a single kidney. Sensitivity analyses including only participants having a kidney length within the normal range of 90–120 mm (n=868) led to similar significant associations, although the β coefficients were smaller, as expected. Excluding participants with renal cysts did not change the association of eGFR, urinary osmolality, and kidney length with copeptin, but the association with albuminuria was no longer significant in any of the models.
Heritability estimates (±SEM) for copeptin levels were 34.7% (6.1%; P<0.001) without a sibship component and 32.7% (5.5%; P<0.001) with a sibship component.
Discussion
Several lines of evidence have demonstrated a link between AVP, renal cyst development, and total kidney volume in rodent models and patients with ADPKD.27 However, to the best of our knowledge, the potential association of AVP with the presence of simple renal cysts, or with kidney size, has not been investigated in normal individuals without ADPKD. For the first time, this cross-sectional study describes the association of plasma copeptin levels with renal function, kidney length, and renal simple cysts in a large, multicentric population-based cohort. We demonstrate that higher copeptin levels are associated with lower eGFR, higher urinary albumin excretion, higher urinary osmolality, smaller kidney length, and the presence of simple renal cysts even after multiple adjustments.
Kidney length measured by ultrasonography is an easy and reproducible way of estimating kidney size, and is generally considered as a proxy of renal mass.28,29 In previous studies, kidney length correlated positively with eGFR in living kidney donors and the general population.28,30 Conversely, a shorter kidney length has been associated with higher BP and albuminuria in an Australian community.31 Accordingly, it is tempting to hypothesize that the negative association between copeptin levels and kidney length, persisting even after adjusting for osmolar excretion, may reflect an influence of the later parameter on urinary concentrating ability. The association of a shorter kidney length with lower urinary concentrating ability is in fact supported by our results showing a positive correlation between kidney length and eGFR and urine osmolality even after adjustment for several covariates. It must be noted that the negative correlation between copeptin and kidney length is observed in participants without kidney cysts and in participants with kidney length within the normal range (90–120 mm). Taken together, these data suggest that the urinary concentrating ability determined by kidney length is probably the main factor driving AVP/copeptin levels in a normal population and support the hypothesis that kidney length might somehow reflect kidney function.
The association between copeptin levels and the presence and number of simple cysts in a general population is a novel finding. We show that this association is independent of age, renal function, kidney length, and urinary concentrating ability. Thus far, AVP/copeptin levels have only been considered in patients with ADPKD. In ADPKD, increased levels of AVP contribute to the abnormal, cAMP-mediated signaling in collecting duct cells, promoting cyst development by several mechanisms.27 The positive association of copeptin levels with total kidney volume in ADPKD also reflects the cystic disruption of the medulla, leading to the defective urinary concentrating ability that is consistently observed early in the disease.19,27 By contrast, our results suggest that AVP may also play a role in the formation of simple renal cysts in the general population.
The prevalence of simple cysts tends to increase with age and may reflect kidney aging.32,33 The 12% prevalence of cysts detected by ultrasonography in our series, as well as the increased risk with age and the sex difference are in line with previous reports.32,34,35 The mechanisms leading to the formation of simple cysts in the kidney remain largely unknown. By definition, these cysts are acquired and fundamentally distinct from the ADPKD cysts that appear in utero as the result of complex genetic mechanisms.36 Simple cysts likely originate from the distal convoluted or collecting duct and are thought to arise from renal tubular diverticula, or from tubular cell hyperplasia secondary to nephron loss.37–39 Previous studies in healthy subjects described associations between hypertension and benign renal cysts.40–42 In a cross-sectional analysis of hospitalized patients without overt renal disease undergoing an abdominal computed tomography scan, the presence of cysts was associated with a reduced renal function estimated by creatinine clearance.33 In our cohort, the association between copeptin and cysts was independent of age, hypertension, kidney function, and even urinary concentration, suggesting a distinct pathway. If elevated copeptin was simply a consequence of kidney damage rather than being causally involved in cyst formation, the association of copeptin with cysts should be stronger in older people who have accumulated kidney damage over time and have lower eGFR, and would decrease upon adjustment for eGFR. By contrast, stratified analyses revealed that the association of copeptin with renal cysts was stronger in younger participants than in older participants in this study. The mechanisms linking the potential association of AVP with simple cyst formation, as well as the clinical relevance of such association, will require further investigations.
Our results confirm the previously reported negative association between copeptin levels and eGFR. This association has been observed in prospective studies involving patients with ADPKD as well as patients with a renal transplant and patients with diabetes.43–48 Only two studies have used a population-based sample to test the hypothesis that AVP might be involved in the development of albuminuria. In the Prevention of Renal and Vascular Endstage Disease (PREVEND) study, which includes 7593 nondiabetic participants with an urinary albumin concentration ≥10 mg/L, circulating copeptin was positively associated with albuminuria and negatively with renal function in a cross-sectional analysis.49 In 2064 participants from the Malmö Diet and Cancer study, baseline circulating copeptin was associated with the incidence of diabetes, obesity, and albuminuria after 16 years of follow-up.50 In healthy volunteers, eGFR was also found to be an independent predictor of copeptin levels.51 Some studies have measured GFR using iothalamate, 99mTc-DTPA, or inulin clearance.43–46 Other studies estimated eGFR using the Modification of Diet in Renal Disease formula or cystatin C but not the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, which is known to be the most reliable formula.47,49–51 In our study, we observe an inverse linear association with copeptin levels even in participants with normal eGFR and confirm an almost 3 times increased odds of CKD in patients with higher copeptin levels.
Several hypotheses could explain the association of AVP/copeptin levels with renal function. It could be that copeptin (molecular mass, 5 kD) is cleared by the kidneys and accumulates in the circulation when kidney function decreases. However, longitudinal studies in humans have shown that plasma copeptin levels increase before eGFR decreases.44,45,47,48 A decreased urinary concentrating capacity precedes the decrease of GFR in numerous kidney diseases. Increased copeptin levels might be a marker of this early phase of renal dysfunction. From an adaptive response, increased AVP/copeptin levels might become maladaptive and enhance CKD progression. Water ingestion, which readily decreases circulating AVP/copeptin levels, may modify CKD progression.52 Studies in the 5/6 nephrectomized rat model suggested that increased water intake decreases circulating AVP levels and slows down the progression of kidney disease.53 Taken together, these data suggest that high levels of AVP may be deleterious for kidney function, either by a direct hemodynamic effect through V1a vascular receptors or through V2 tubular receptors.
Our results also suggest an association between copeptin levels and albuminuria (24-hour collection) in the general population. These data support earlier reports in ADPKD,43 and the general population, although the significance levels were lower in our study.49,50 This can be due to the higher number of patients in those studies, the fact that the PREVEND study used a cutoff of 10 mg/L albuminuria for inclusion, and the Malmo study analyzed ACR in spot urine instead of 24-hour urine collection. Previous studies have shown an association between albuminuria and urinary concentrating ability in diabetic rats,54 as well as in healthy rats and humans,55 suggesting that increased AVP has a proalbuminuric effect. Because only V2 antagonists decrease proteinuria in animal models, one can hypothesize that albuminuria is somehow related to tubular V2Rs.9,54 Whether AVP antagonists can reduce albuminuria in humans is actually unknown and should be assessed in clinical studies. Post hoc analysis from the TEMPO 3.4 trial, presented at the 2013 American Society of Nephrology Annual Meeting by Gansevoort et al. shows that tolvaptan reduces albuminuria in patients with ADPKD.56 Our results raise the question of whether targeting AVP to decrease kidney disease progression could be an option in patients with kidney diseases other than ADPKD.
Because kidney stones are associated with concentrated urines and lower urinary flow rate, we explored their potential association with copeptin in our sample. The observed positive association was no longer statistically significant upon multivariable adjustment. Although we found a prevalence of stones similar to a previous study in a very selected population of kidney donors,57 assessment by ultrasonography is associated with underdetection of stones compared with computed tomography.57,58 The administration of vasopressin antagonist in order to reduce cystine stone formation was described in a case report.59 To the best of our knowledge, there is no evidence for the association of AVP or copeptin with kidney stones. Thus, further studies are needed to determine whether AVP or copeptin are associated with kidney stones.
This study has some limitations. Its cross-sectional nature limits causal inferences: We see associations but we cannot confirm the direction of these associations or the causality. However, because the heritability integrates the genetic pattern that occurs before the disease, this suggests causality. We only included participants of European ancestry and therefore our findings might not be valid for other populations. There was no difference in terms of age and sex between the participants and the sample from the general population from which it was drawn, and it is unlikely that this would induce spurious associations as strong and consistent as the ones we detected. Although renal ultrasonography is a reliable tool for estimating kidney length, it is not as sensitive as computed tomography or magnetic resonance imaging to detect renal cysts, and very small cysts might have been missed.60 The significance of very small cysts is unknown but we may have underestimated the association between copeptin and renal cysts. Given the cross-sectional design of our study, whether high copeptin levels are the consequence rather than the cause of simple renal cysts remains uncertain. Only long-term follow-up studies will be able to confirm whether individuals with higher baseline copeptin levels are at risk of having shorter kidneys and more cysts or the reverse. Finally, the positive association of copeptin with urinary osmolality is similar to that reported for AVP. Although the physiologic regulation of copeptin release seems to be similar than that of AVP,26 we cannot exclude that other regulatory factors are involved. In addition, it is unknown whether copeptin acts on the same receptors as AVP. Copeptin is currently the most widely accepted surrogate marker for AVP despite the fact that these peptides are different entities.
In conclusion, we show a novel association of circulating copeptin with kidney length and the prevalence of simple renal cysts in a large multicentric population-based study. We also confirm that higher copeptin is associated with increasing urine osmolality, similar to AVP, and with lower renal function and higher 24-hour urine albumin excretion.
These results suggest that copeptin is a marker of renal morphology and function in the general population. By extension, they give new insights into the pleiotropic role of AVP, including the formation of simple renal cysts.
Concise Methods
Study Population
SKIPOGH is a family-based cross-sectional study exploring the role of genes and kidney hemodynamics in BP regulation and kidney function in the general population. It is nested in the European Project on Genes in Hypertension study and shares the same validated protocol. A detailed description of the methods was provided elsewhere and is briefly described here.61
From December 2009 to March 2013, adult participants were recruited in two regions (Berne and Geneva) and one city (Lausanne) of Switzerland. A random sample of the inhabitants was drawn using different strategies. Inclusion criteria were as follows: (1) having a minimum age of 18 years; (2) being of European ancestry; (3) having at least one and ideally three first-degree family members willing to participate; and (4) providing a written informed consent. Pregnant or breastfeeding women were not included. The SKIPOGH study adhered to the Declaration of Helsinki and was approved by the institutional ethical committees of each participating university hospital.
Measurement and Definitions
Participants were seen in the morning after an overnight fast. In each center, the same experienced operator performed renal grayscale and Doppler ultrasonography according to a standardized procedure.28 Briefly, the renal length of each kidney was measured in the supine position or left/right decubitus if needed. The mean of three optimal measures was reported. Because there was no difference between the left and right kidneys, the mean length of both kidneys was used for the analysis. Benign renal cysts were diagnosed based on known criteria: echolucent structure, round or oval in shape with a thin wall, well defined and smooth contours with a sharp demarcated posterior wall, no calcification, no septa, and no Doppler signal within the cyst.62 The number of cysts and the maximal diameter of the biggest cyst were reported. We also reported the presence of kidney stones suspected on the same ultrasound examination.
Fasting blood venous samples were drawn after the participants had been lying in a supine position for at least 30 minutes. A 24-hour urine sample was collected separately for day and night, and oral and written instructions were given on how to perform the urinary collection. Blood glucose, lipid profile, and renal function tests as well as serum and urinary electrolytes were analyzed by standard clinical laboratory methods in each center. Creatinine was measured using IDMS-traceable Jaffe kinetic compensated methods. Urine albumin was measured quantitatively by immunonephelometry on a BN Prospec automated analyzer (Siemens Healthcare Diagnostics, Marburg, Germany) or immunoturbidimetry (Roche/Hitachi Modular P). In addition to the continuous urinary albumin excretion in mg per 24 hours, we used urinary creatinine and albumin to calculate the ACR in milligrams per millimole. The correlation between albumin excretion and ACR was strongly positive (r=0.93; P<0.001). Urinary osmolality was measured centrally using an Advanced Osmometer (Advanced Instruments, Norwood, MA) based on the freezing-point depression. A control (Clinitrol 290) and a set of calibration standards (50, 850, and 2000 mOsm/kg) were used before running each sample batch. The coefficient of variability was 0.19% in urine. The 24-hour osmolar excretion was calculated as the product of 24-hour urine osmolality and 24-hour urine volume. Copeptin was measured on −80° frozen EDTA-plasma samples in a batch using a previously described sandwich immunoluminometric assay (Thermo Fisher Scientific CT-proAVP KRYPTOR; BRAHMS GmbH, Hennigsdorf, Germany).17 The lower detection limit was 0.9 pmol/L and the functional assay sensitivity (20% interassay coefficient of variation) was <2 pmol/L. Estimated plasma osmolarity was calculated as 2×(plasma sodium+plasma potassium).
Participants filled out a questionnaire at home, which requested information on current and past medical history, medication, and habits (nutrition, smoking, and alcohol).
Diabetes was defined as reported, treated, or fasting glycemia≥7 mmol/L. In this analysis, antihypertensive treatment was used as a covariate to account for hypertension. The CKD-EPI formula was used to estimate the eGFR.63 CKD was defined as present for eGFR<60 ml/min per 1.73 m2. The presence of albuminuria was defined using published sex-specific cut-points: whenever ACR (multiplied by 8.84) was ≥25 µg/mg in women and ≥17 µg/mg in men.64
Statistical Analyses
All of the continuous variables with normal distribution are expressed as the mean±SD and as the median (IQR) in case of skewed distribution. Categorical variables are expressed as numbers and frequencies. The normality of the distribution of each continuous variable was assessed graphically. Serum triglycerides, copeptin, 24-hour urinary albumin excretion, and urine osmolality levels were log-transformed for statistical analyses. Finally, t tests and chi-squared tests were performed to compare baseline characteristics for continuous and categorical variables, respectively.
Association Analyses
We first conducted univariate analyses to look for associations between copeptin and the outcomes of interest (CKD-EPI, 24-hour urinary albumin excretion, 24-hour urinary osmolality, renal length, and presence of CKD, cysts, or albuminuria) and the other covariates. We calculated Pearson’s correlation coefficients for continuous variables (transformed whenever needed) and used the t test or trend test for categorical variables.
We applied different multivariable mixed linear regression models to determine the independent association of copeptin with CKD-EPI, albuminuria, urinary osmolality, and renal length, while taking familial correlation into account. In the first model, we adjusted for sex, age, and center. In the second model, we additionally adjusted for cardiovascular risk factors (diabetes, hypertension treatment, triglycerides, smoking, BMI, and CKD-EPI). In the third model, we adjusted for estimated plasma osmolarity, 24-hour osmolar excretion, and urinary creatinine excretion. For 24-hour urine osmolality, we adjusted for urinary flow rate instead of osmolar excretion. Because hypertension treatment was included as a covariate in the models, we did not adjust for BP. Although the results are not shown, we systematically checked that adding BP did not change the associations.
For the continuous albuminuria analysis, 24-hour urinary creatinine excretion (in milligrams per kilogram) was added as a covariate in all models to account for the quality of urine collection. For kidney length, we first generated a model including BMI as for the other outcomes of interest. Because body height is a major determinant of kidney length,28 we then repeated the models adjusting for body height and weight instead of BMI. Results of all linear regression analyses are presented as β coefficients and their 95% CIs.
Linearity between log-transformed copeptin and the outcomes of interest was tested in three ways: (1) graphically, (2) using sex-specific tertiles of copeptin, and (3) including the square of the log-transformed copeptin as a covariate in the models to confirm the absence of a quadratic association.
We also looked into the association of copeptin with the presence of cysts, CKD, or albuminuria (ACR) using mixed logistic regression taking familial correlations into account. Sex, age, and center were used as covariates. For CKD and dichotomized albuminuria, we did not conduct additional adjustment models because of the small number of events. For the association of copeptin with cysts, we also stratified the analyses by age, using a cutoff of 61 years to obtain two groups with the same number of cysts.
Interaction of copeptin with sex, diabetes, smoking, and center was tested for their effects on continuous outcomes of interest (CKD-EPI, 24-hour urine albumin, 24-hour urine osmolality, and renal length) but not for the categorical ones (presence of albuminuria and CKD), because of the small number of events. Interaction was found between diabetes and copeptin when looking at the association with continuous albuminuria (P<0.001), but stratified multivariate analyses could not be conducted because of the small number of participants with diabetes (n=49). In that case, models 2 and 3 were adjusted for fasting glucose instead of diabetes, as done in previous studies.49,50
To generate the panels in Figure 2, for each dependent variable separately, we first generated adjusted residuals from mixed linear models, including age, sex, and center as covariates, and then scaled them by adding the mean of the dependent variable. In Figure 3, we generated the adjusted predicted probability of cyst at selected levels of log-transformed copeptin, including age, sex, and center as covariates in the model, using the margin function in Stata.
Statistical significance was considered for a two-sided P<0.05. Statistical analyses were conducted using STATA 12.0 software (StataCorp, College Station, TX).
Sensitivity Analyses
We repeated the analyses replacing extreme values of copeptin by percentiles 99 (18.77) and 1 (1.161) in order to determine the influence of outliers (winzorization).
We repeated the analyses excluding participants that could have inaccurate urine collections defined as a 24-hour volume <300 ml or a 24-hour creatinine excretion of <4 mmol or >25 mmol in women and <6 mmol or >30 mmol in men.65
The same multivariate analyses were conducted in participants without cysts and with kidney length within the normal range (90–120 mm).
Heritability Analyses
Heritability is a measure of familial resemblance. It is useful in family-based studies to estimate the role of genes in a specific phenotype. Because heritability takes into account the genetic pattern that occurs before the disease, it gives an argument for causality.
To estimate heritability, we applied a maximum likelihood approach, as implemented in the ASSOC procedure of the S.A.G.E. package, as previously described.66 We also estimated heritability in the presence of an additional sibship component of variance, which captures both the common environment and a dominance genetic variance. Heritability estimates are expressed as h2 values with SEM.
Disclosures
None.
Acknowledgments
We thank the study nurses Marie-Odile Levy, Guler Gök-Sogüt, Ulla Spüchbach, and Dominique Siminski for their involvement and help with recruitment. We also thank Sandrine Estoppey and Julien Weber for their help in logistic and database management.
B.P., M.P., D.A., I.G., and G.E. and this study were supported by a grant from the Swiss National Science Foundation (FN 33CM30-124087). B.P. was also supported by the Tremplin funding and research institutional funds of the University Hospital of Geneva (departmental and Projects & Research Development funds). M.B. was supported by the Swiss School of Public Health Plus. O.D. is supported by grants from the European Community’s Seventh Framework Programme (305608 EURenOmics), Action de Recherche Concertée (ARC10/15-029, Communauté Française de Belgique), Inter-University Attraction Pole (Belgium Federal Government), the Swiss National Centre of Competence in Research Kidney Control of Homeostasis program, and the Swiss National Science Foundation (310030-146490).
We thank the study nurses involved in the study and the recruitment: Marie-Odile Levy, Guler Gök-Sogüt, Ulla Spüchbach, Dominique Siminski. We thank Sandrine Estoppey and Julien Weber for her help in the logistic and database management.
Part of these results were presented orally at the 2014 European Renal Association–European Dialysis and Transplant Association Congress held May 31–June 3, 2014, in Amsterdam, The Netherlands.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Hasler U, Leroy V, Martin PY, Féraille E: Aquaporin-2 abundance in the renal collecting duct: New insights from cultured cell models. Am J Physiol Renal Physiol 297: F10–F18, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Wilson JL, Miranda CA, Knepper MA: Vasopressin and the regulation of aquaporin-2. Clin Exp Nephrol 17: 751–764, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paliege A, Roeschel T, Neymeyer H, Seidel S, Kahl T, Daigeler AL, Mutig K, Mrowka R, Ferreri NR, Wilson BS, Himmerkus N, Bleich M, Bachmann S: Group VIA phospholipase A2 is a target for vasopressin signaling in the thick ascending limb. Am J Physiol Renal Physiol 302: F865–F874, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Mutig K, Paliege A, Kahl T, Jöns T, Müller-Esterl W, Bachmann S: Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am J Physiol Renal Physiol 293: F1166–F1177, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Terada Y, Tomita K, Nonoguchi H, Yang T, Marumo F: Different localization and regulation of two types of vasopressin receptor messenger RNA in microdissected rat nephron segments using reverse transcription polymerase chain reaction. J Clin Invest 92: 2339–2345, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park F, Mattson DL, Skelton MM, Cowley AW, Jr: Localization of the vasopressin V1a and V2 receptors within the renal cortical and medullary circulation. Am J Physiol 273: R243–R251, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Bankir L: Antidiuretic action of vasopressin: Quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc Res 51: 372–390, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Miranda CA, Lee JW, Chou CL, Knepper MA: Tolvaptan as a tool in renal physiology. Am J Physiol Renal Physiol 306: F359–F366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bankir L, Bouby N, Ritz E: Vasopressin: A novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol 9: 223–239, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Bouby N, Hassler C, Bankir L: Contribution of vasopressin to progression of chronic renal failure: Study in Brattleboro rats. Life Sci 65: 991–1004, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH, 2nd: Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE: Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 14.El-Farhan N, Hampton D, Penney M: Measurement of arginine vasopressin. Methods Mol Biol 1065: 129–139, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Baumann G, Dingman JF: Distribution, blood transport, and degradation of antidiuretic hormone in man. J Clin Invest 57: 1109–1116, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kluge M, Riedl S, Erhart-Hofmann B, Hartmann J, Waldhauser F: Improved extraction procedure and RIA for determination of arginine8-vasopressin in plasma: Role of premeasurement sample treatment and reference values in children. Clin Chem 45: 98–103, 1999 [PubMed] [Google Scholar]
- 17.Morgenthaler NG, Struck J, Alonso C, Bergmann A: Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52: 112–119, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Szinnai G, Morgenthaler NG, Berneis K, Struck J, Müller B, Keller U, Christ-Crain M: Changes in plasma copeptin, the c-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab 92: 3973–3978, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Ho TA, Godefroid N, Gruzon D, Haymann JP, Maréchal C, Wang X, Serra A, Pirson Y, Devuyst O: Autosomal dominant polycystic kidney disease is associated with central and nephrogenic defects in osmoregulation. Kidney Int 82: 1121–1129, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Robertson GL, Mahr EA, Athar S, Sinha T: Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest 52: 2340–2352, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preibisz JJ, Sealey JE, Laragh JH, Cody RJ, Weksler BB: Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension 5: I129–I138, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Balanescu S, Kopp P, Gaskill MB, Morgenthaler NG, Schindler C, Rutishauser J: Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar States. J Clin Endocrinol Metab 96: 1046–1052, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Morgenthaler NG, Müller B, Struck J, Bergmann A, Redl H, Christ-Crain M: Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock 28: 219–226, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Westermann I, Dünser MW, Haas T, Jochberger S, Luckner G, Mayr VD, Wenzel V, Stadlbauer KH, Innerhofer P, Morgenthaler N, Hasibeder WR, Voelckel WG: Endogenous vasopressin and copeptin response in multiple trauma patients. Shock 28: 644–649, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Jochberger S, Zitt M, Luckner G, Mayr VD, Wenzel V, Ulmer H, Morgenthaler NG, Hasibeder WR, Dünser MW: Postoperative vasopressin and copeptin levels in noncardiac surgery patients: A prospective controlled trial. Shock 31: 132–138, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Fenske WK, Christ-Crain M, Hörning A, Simet J, Szinnai G, Fassnacht M, Rutishauser J, Bichet DG, Störk S, Allolio B: A copeptin-based classification of the osmoregulatory defects in the syndrome of inappropriate antidiuresis [published online ahead of print April 10, 2014]. J Am Soc Nephrol 10.1681/ASN.2013080895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devuyst O, Torres VE: Osmoregulation, vasopressin, and cAMP signaling in autosomal dominant polycystic kidney disease. Curr Opin Nephrol Hypertens 22: 459–470, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Pruijm M, Ponte B, Ackermann D, Vuistiner P, Paccaud F, Guessous I, Ehret G, Eisenberger U, Mohaupt M, Burnier M, Martin PY, Bochud M: Heritability, determinants and reference values of renal length: A family-based population study. Eur Radiol 23: 2899–2905, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF: A stereological study of glomerular number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl (83, Suppl): S31–S37, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Yano M, Lin MF, Hoffman KA, Vijayan A, Pilgram TK, Narra VR: Renal measurements on CT angiograms: Correlation with graft function at living donor renal transplantation. Radiology 265: 151–157, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Singh GR, Hoy WE: Kidney volume, blood pressure, and albuminuria: Findings in an Australian aboriginal community. Am J Kidney Dis 43: 254–259, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Terada N, Ichioka K, Matsuta Y, Okubo K, Yoshimura K, Arai Y: The natural history of simple renal cysts. J Urol 167: 21–23, 2002 [PubMed] [Google Scholar]
- 33.Al-Said J, Brumback MA, Moghazi S, Baumgarten DA, O’Neill WC: Reduced renal function in patients with simple renal cysts. Kidney Int 65: 2303–2308, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Ravine D, Gibson RN, Donlan J, Sheffield LJ: An ultrasound renal cyst prevalence survey: Specificity data for inherited renal cystic diseases. Am J Kidney Dis 22: 803–807, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Rule AD, Sasiwimonphan K, Lieske JC, Keddis MT, Torres VE, Vrtiska TJ: Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. Am J Kidney Dis 59: 611–618, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giapros V, Tsoni C, Challa A, Cholevas V, Argyropoulou M, Papadopoulou F, Siomou E, Drougia A, Andronikou S: Renal function and kidney length in preterm infants with nephrocalcinosis: A longitudinal study. Pediatr Nephrol 26: 1873–1880, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Grantham JJ: Acquired cystic kidney disease. Kidney Int 40: 143–152, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Baert L, Steg A: Is the diverticulum of the distal and collecting tubules a preliminary stage of the simple cyst in the adult? J Urol 118: 707–710, 1977 [DOI] [PubMed] [Google Scholar]
- 39.Bisceglia M, Galliani CA, Senger C, Stallone C, Sessa A: Renal cystic diseases: A review. Adv Anat Pathol 13: 26–56, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Chin HJ, Ro H, Lee HJ, Na KY, Chae DW: The clinical significances of simple renal cyst: Is it related to hypertension or renal dysfunction? Kidney Int 70: 1468–1473, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Lee CT, Yang YC, Wu JS, Chang YF, Huang YH, Lu FH, Chang CJ: Multiple and large simple renal cysts are associated with prehypertension and hypertension. Kidney Int 83: 924–930, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Hong S, Lim JH, Jeong IG, Choe J, Kim CS, Hong JH: What association exists between hypertension and simple renal cyst in a screened population? J Hum Hypertens 27: 539–544, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Meijer E, Bakker SJ, van der Jagt EJ, Navis G, de Jong PE, Struck J, Gansevoort RT: Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6: 361–368, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boertien WE, Meijer E, Li J, Bost JE, Struck J, Flessner MF, Gansevoort RT, Torres VE, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease CRISP : Relationship of copeptin, a surrogate marker for arginine vasopressin, with change in total kidney volume and GFR decline in autosomal dominant polycystic kidney disease: Results from the CRISP cohort. Am J Kidney Dis 61: 420–429, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boertien WE, Meijer E, Zittema D, van Dijk MA, Rabelink TJ, Breuning MH, Struck J, Bakker SJ, Peters DJ, de Jong PE, Gansevoort RT: Copeptin, a surrogate marker for vasopressin, is associated with kidney function decline in subjects with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 27: 4131–4137, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Lacquaniti A, Chirico V, Lupica R, Buemi A, Loddo S, Caccamo C, Salis P, Bertani T, Buemi M: Apelin and copeptin: Two opposite biomarkers associated with kidney function decline and cyst growth in autosomal dominant polycystic kidney disease. Peptides 49: 1–8, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Meijer E, Bakker SJ, de Jong PE, Homan van der Heide JJ, van Son WJ, Struck J, Lems SP, Gansevoort RT: Copeptin, a surrogate marker of vasopressin, is associated with accelerated renal function decline in renal transplant recipients. Transplantation 88: 561–567, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Velho G, Bouby N, Hadjadj S, Matallah N, Mohammedi K, Fumeron F, Potier L, Bellili-Munoz N, Taveau C, Alhenc-Gelas F, Bankir L, Marre M, Roussel R: Plasma copeptin and renal outcomes in patients with type 2 diabetes and albuminuria. Diabetes Care 36: 3639–3645, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meijer E, Bakker SJ, Halbesma N, de Jong PE, Struck J, Gansevoort RT: Copeptin, a surrogate marker of vasopressin, is associated with microalbuminuria in a large population cohort. Kidney Int 77: 29–36, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Enhörning S, Bankir L, Bouby N, Struck J, Hedblad B, Persson M, Morgenthaler NG, Nilsson PM, Melander O: Copeptin, a marker of vasopressin, in abdominal obesity, diabetes and microalbuminuria: The prospective Malmö Diet and Cancer Study cardiovascular cohort. Int J Obes (Lond) 37: 598–603, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Bhandari SS, Loke I, Davies JE, Squire IB, Struck J, Ng LL: Gender and renal function influence plasma levels of copeptin in healthy individuals. Clin Sci (Lond) 116: 257–263, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Clark WF, Sontrop JM, Macnab JJ, Suri RS, Moist L, Salvadori M, Garg AX: Urine volume and change in estimated GFR in a community-based cohort study. Clin J Am Soc Nephrol 6: 2634–2641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bouby N, Bachmann S, Bichet D, Bankir L: Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am J Physiol 258: F973–F979, 1990 [DOI] [PubMed] [Google Scholar]
- 54.Bardoux P, Bruneval P, Heudes D, Bouby N, Bankir L: Diabetes-induced albuminuria: Role of antidiuretic hormone as revealed by chronic V2 receptor antagonism in rats. Nephrol Dial Transplant 18: 1755–1763, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Bardoux P, Bichet DG, Martin H, Gallois Y, Marre M, Arthus MF, Lonergan M, Ruel N, Bouby N, Bankir L: Vasopressin increases urinary albumin excretion in rats and humans: Involvement of V2 receptors and the renin-angiotensin system. Nephrol Dial Transplant 18: 497–506, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Gansevoort RT, Chapman AB, Czerwiec FS, Devuyst O, Grantham JJ, Higashihara E, Krasa H, Ouyang J, Perrone RD, Torres VE: The effect of tolvaptan on albuminuria in ADPKD. Results of the TEMPO 3:4 trial [Abstract]. J Am Soc Nephrol 24: 688A, 2013. 23110544 [Google Scholar]
- 57.Olsburgh J, Thomas K, Wong K, Bultitude M, Glass J, Rottenberg G, Silas L, Hilton R, Koffman G: Incidental renal stones in potential live kidney donors: Prevalence, assessment and donation, including role of ex vivo ureteroscopy. BJU Int 111: 784–792, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Kanno T, Kubota M, Sakamoto H, Nishiyama R, Okada T, Higashi Y, Yamada H: The efficacy of ultrasonography for the detection of renal stone. Urology 84: 285–288, 2014 [DOI] [PubMed] [Google Scholar]
- 59.de Boer H, Roelofsen A, Janssens PM: Antidiuretic hormone antagonist to reduce cystine stone formation. Ann Intern Med 157: 459–460, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Al-Said J, O’Neill WC: Reduced kidney size in patients with simple renal cysts. Kidney Int 64: 1059–1064, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Ponte B, Pruijm M, Ackermann D, Vuistiner P, Eisenberger U, Guessous I, Rousson V, Mohaupt MG, Alwan H, Ehret G, Pechere-Bertschi A, Paccaud F, Staessen JA, Vogt B, Burnier M, Martin PY, Bochud M: Reference values and factors associated with renal resistive index in a family-based population study. Hypertension 63: 136–142, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Nahm AM, Ritz E: The simple renal cyst. Nephrol Dial Transplant 15: 1702–1704, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mattix HJ, Hsu CY, Shaykevich S, Curhan G: Use of the albumin/creatinine ratio to detect microalbuminuria: Implications of sex and race. J Am Soc Nephrol 13: 1034–1039, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerová J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovský J, Kawecka-Jaszcz K, Nikitin Y, Staessen JA, European Project on Genes in Hypertension (EPOGH) Investigators : Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 305: 1777–1785, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Bochud M, Elston RC, Maillard M, Bovet P, Schild L, Shamlaye C, Burnier M: Heritability of renal function in hypertensive families of African descent in the Seychelles (Indian Ocean). Kidney Int 67: 61–69, 2005 [DOI] [PubMed] [Google Scholar]