Abstract
The attrition rate of functioning allografts beyond the first year has not improved despite improved immunosuppression, suggesting that nonimmune mechanisms could be involved. Notably, glomerulopathies may account for about 40% of failed kidney allografts beyond the first year of engraftment, and glomerulosclerosis and progression to ESRD are caused by podocyte depletion. Model systems demonstrate that nephrectomy can precipitate hypertrophic podocyte stress that triggers progressive podocyte depletion leading to ESRD, and that this process is accompanied by accelerated podocyte detachment that can be measured in urine. Here, we show that kidney transplantation “reverse nephrectomy” is also associated with podocyte hypertrophy and increased podocyte detachment. Patients with stable normal allograft function and no proteinuria had levels of podocyte detachment similar to levels in two-kidney controls as measured by urine podocyte assay. By contrast, patients who developed transplant glomerulopathy had 10- to 20-fold increased levels of podocyte detachment. Morphometric studies showed that a subset of these patients developed reduced glomerular podocyte density within 2 years of transplantation due to reduced podocyte number per glomerulus. A second subset developed glomerulopathy by an average of 10 years after transplantation due to reduced glomerular podocyte number and glomerular tuft enlargement. Reduced podocyte density was associated with reduced eGFR, glomerulosclerosis, and proteinuria. These data are compatible with the hypothesis that podocyte depletion contributes to allograft failure and reduced allograft half-life. Mechanisms may include immune-driven processes affecting the podocyte or other cells and/or hypertrophy-induced podocyte stress causing accelerated podocyte detachment, which would be amenable to nonimmune therapeutic targeting.
Keywords: podocyte, transplantation, glomerulosclerosis, proteinuria
Podocytes are complex neuron-like postmitotic cells adherent to the underlying glomerular basement membrane via foot processes that must contiguously cover the filtration surface area to maintain the normal filtration barrier. Podocytes cannot divide in situ and have limited capacity for replacement.1 This means that when podocytes are lost, or the glomerular surface area increases due to glomerular growth, the major adaptive response is by hypertrophy. At the same time, the podocyte’s structural complexity means that its capacity to hypertrophy is limited. Inability to maintain contiguous coverage of the filtration surface by foot processes results in protein leak into the filtrate. If podocyte detachment exceeds hypertrophic capacity, other glomerular cells adapt by proliferating and laying down matrix resulting in glomerulosclerosis.2–6
In a transgenic (AA-4EBP1) rat model designed to selectively constrain podocyte hypertrophic capacity but to leave all other cells in the body able to divide and hypertrophy normally, glomerular growth triggered by nephrectomy causes proteinuria, glomerulosclerosis, and progression to ESRD within 12 weeks.6 In this model, prevention of glomerular growth by calorie restriction entirely prevents proteinuria and glomerulosclerosis after nephrectomy. Because susceptibility to glomerulosclerosis in this model is imposed by podocyte-specific transgene expression, this tells us that podocyte failure to hypertrophy is the key factor driving glomerulosclerosis and downstream interstitial fibrosis/tubular atrophy in this setting.
In all models of progression examined, including the 5/6 nephrectomy model, glomerular destabilization caused by podocyte stress resulted in accelerated podocyte detachment measurable in urine by urine podocyte markers.6–8 Over time, accelerated podocyte detachment causes progressive podocyte depletion from glomeruli. Reduced podocyte density beyond a critical threshold (about 30% in acute rat models) causes mesangial expansion and adhesions to Bowman’s capsule, and as >40%–50% podocyte depletion occurs, then glomerulosclerosis, interstitial fibrosis, and eventually ESRD supervene.6–9
These observations raise the specter that the one-kidney (1K) state could be on the borderline of being inherently unstable in susceptible patients, particularly in settings such as genetic susceptibilities, accelerated body growth, obesity, reduced nephron mass for any cause (e.g., CKD), immune attack aimed at endothelial cells or podocytes, circulating immune complexes, and/or hemodynamic stress. This concept is supported by clinical scenarios in which (1) kidney outcome is related to nephron mass,10 (2) postnephrectomy weight gain is associated with worse renal outcome,11 (3) a mismatch between allograft and recipient weight is related to worse outcome,12 (4) high body mass index is associated with worse allograft outcome in kidney transplantation,13 and (5) donating a kidney appears to be associated with an increased prevalence of ESRD.14,15 The fact that allograft outcome after the first year has not improved substantially in spite of improved immunosuppression must also be biologically explained.16
We recently adapted methodologies for quantitating podocytes in urine and kidney biopsies for use in humans.17,18 Therefore, tools are available to begin to understand what happens to podocytes in the transition from a two-kidney (2K) state to a 1K state as well as in glomerulopathies. Human kidney transplantation offers a scenario in which these questions can be examined.
Results
Approach
Protocol kidney biopsies are performed at the time of kidney implantation and 3 months later for surveillance. Thus, two or more biopsies are available for analysis from an individual kidney to compare the state of the kidney when it was one of a pair of kidneys serving the donor (the implantation “time 0” biopsy), and after the kidney has adapted for 3 months as it serves as a single kidney in the recipient (the 3- and 12-month biopsies). Demographics for the protocol biopsy group are shown in Table 1. All time-0 and 3-month biopsies were pathologically within normal limits.
Table 1.
Demographics for normal biopsy and transplant glomerulopathy patient groups
| Demographic | Protocol Biopsies (n=23) | Early TG (n=8) | Late TG (n=17) | Non-GN (n=6) |
|---|---|---|---|---|
| Donors | ||||
| Age (yr) | 41±10 | 45±14 | 39±12 | 39±10 |
| Men | 9 (39.1) | 2 (25.0) | 3 (17.6) | 3 (50.0) |
| Race | ||||
| Caucasian | 15 (65.2) | 4 (50.0) | 8 (47.1) | 1 (16.7) |
| African American | 3 (13.0) | 2 (25.0) | 1 (5.9) | 1 (16.7) |
| Other | 5 (21.7) | 2 (25.0) | 8 (47.1) | 4 (66.7) |
| Recipients | ||||
| Age (yr) | 51±15 | 52±15 | 46±13 | 54±7 |
| Men | 18 (78.3) | 4 (50.0) | 8 (47.1) | 3 (50.0) |
| Race | ||||
| Caucasian | 18 (78.4) | 6 (75.0) | 15 (88.2) | 4 (66.7) |
| African American | 3 (13.0) | 2 (25.0) | 2 (11.8) | 2 (33.3) |
| Others | 2 (8.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Urinary protein | 0.08 (0.05–0.14)b,c | 0.37 (0.20–1.75)a | 1.07 (0.20–5.57)a | 0.32 (0.25–ND) |
| eGFR (ml/min per 1.73 m2) | 59±11b,c,d | 45±15a | 34±11a | 36±13a |
| Transplant category | ||||
| Deceased donor | 0 (0.0)b,c | 6 (75.0)a,d | 5 (29.4)a,d | 0 (0.0)b,c |
| Living related | 15 (65.2)b | 0 (0.0)a | 8 (47.1) | 2 (33.3) |
| Living unrelated | 8 (34.8) | 2 (25.0) | 4 (23.5) | 4 (66.7) |
| Cause of ESRD | ||||
| Diabetic nephropathy | 8 (34.8) | 3 (37.5) | 2 (11.8) | 1 (16.7) |
| Hypertension | 2 (8.7) | 0 (0.0) | 0 (0.0) | 2 (33.3) |
| PKD | 2 (8.7) | 0 (0.0) | 1 (5.9) | 1 (16.7) |
| FSG | 1 (4.3) | 0 (0.0) | 1 (5.9) | 0 (0.0) |
| Other glomerulopathies | 4 (17.4) | 3 (37.5) | 8 (47.1) | 2 (33.3) |
| Other | 6 (26.1) | 2 (25.0) | 5 (29.4) | 0 (0.0) |
| Years to biopsy diagnosis | 0.8±0.4c,d | 0.7±0.7c,d | 9.6±3.6a,b | 11.4±0.6a,b |
| ESRD after transplant | 0 (0)b,c | 4 (50.0)a | 9 (52.9)a | 1 (16.7) |
| Years to ESRD | 2.6±0.8c | 12.4±3.9b | 14.3±0.0 | |
| History of rejection | ||||
| None | 12 (52.2) | 1 (12.5)a,d | 5 (29.4)a | 5 (83.3)b |
| Borderline ACR | 9 (39.1) | 1 (12.5) | 7 (41.2) | 0 (0.0) |
| Banff1A ACR or above | 1 (4.3)b | 5 (62.5)a,c | 2 (11.8)b | 1 (16.7) |
| ABMR | 0 (0.0) | 0 (0.0) | 1 (5.9) | 0 (0.0) |
| Mixed ABMR/ACR | 1 (4.3) | 1 (12.5) | 2 (11.8) | 0 (0.0) |
| DSA | ||||
| HLA class I | 0 (0.0) | 2 (25.0) | 2 (11.8) | 0 (0.0) |
| HLA class II | 1 (4.3)c | 4 (50.0) | 8 (47.1)a,d | 1 (16.7) |
| Column designation | a | b | c | d |
Data are presented as n (%), mean±1 SD, or median (interquartile range). Protocol biopsies are the time-0, 3-month, and 12-month protocol biopsy cohort. At time 0 and 3 months, all biopsies were pathologically within normal limits. At later time points, some of these patients developed ACR or borderline rejection (see the Concise Methods). All other biopsies were done for cause. ANOVA with Bonferroni correction was used for multiple comparisons. Statistical differences are shown by the superscript and the column designation at the bottom of the table. For superscripted letters regular font=P<0.05, italic font=P<0.01, and bold font=P<0.001. Absence of a footnote indicates no statistically significant difference. ABR, antibody-mediated rejection; ACR, acute cellular rejection.
Two independent methods for estimating podocyte density in a single histologic section were used (Figure 1). Podocyte nuclear density is estimated by imaging software using TLE4 antibodies to identify podocyte nuclei and measure their mean caliper diameter (D) and thereby estimate their density in the glomerular tuft.18 Podocyte cell area density is then estimated in the same section using Glepp1 immunoperoxidase histochemistry. Correlation between these parameters is shown in Figure 2.
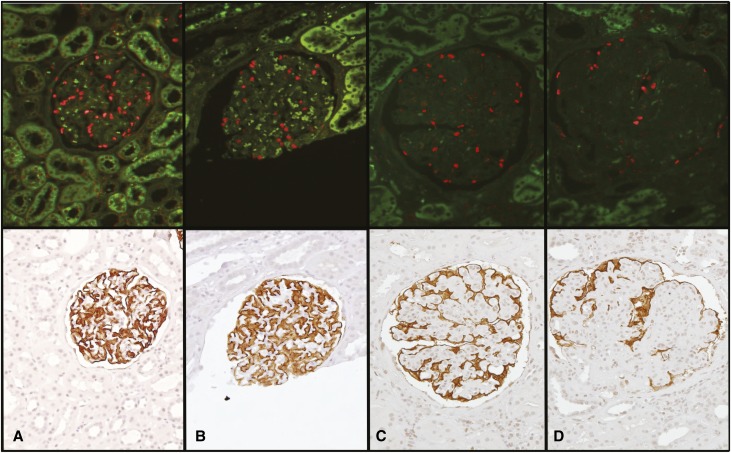
Figure 1.
Podocyte density estimation by two independent methods. The upper panels show TLE4-stained podocyte nuclei (red). The lower panels show Glepp1 peroxidase-stained podocyte cell bodies in the same sections. (A) Upper and lower panels illustrate a normal control glomerulus. (B) Upper and lower panels show a normal glomerulus from a 3-month protocol biopsy. (C and D) Upper and lower panels show glomeruli with moderate and severe transplant glomerulopathy, respectively. Note that the Glepp1 peroxidase-stained sections show an increasing proportion of the area that is Glepp1 negative (relative podocyte depletion and reduced podocyte density) in association with transplant glomerulopathy. These glomeruli have reduced podocyte nuclear density due to a combination of glomerular enlargement and reduced number of podocyte nuclei.
Figure 2.
Two independent methods of measuring podocyte density (podocyte nuclear density and Glepp1 area density). Linear regression analysis shows correlation with an R2=0.50. These data are derived from the time-0 implantation biopsies (n=20), the 3-month biopsies from the same kidneys (n=20), the 12-month biopsies (n=14), the early TG biopsies (n=16), the late TG biopsies (n=18), and the late non-GD biopsies (n=6) as shown in Table 1.
The 2K to 1K Transition
Table 2 shows morphometric data for paired time-0 and 3-month biopsies as follows. (1) Glomerular volume increased by 21%, as would be expected after kidney hypertrophy after uninephrectomy. (2) Podocyte nuclear number per glomerular tuft was not statistically significantly decreased. (3) The glomerular tuft volume served per podocyte therefore increased significantly by 23%. (4) Correspondingly, the podocyte nuclear density (number per volume) was significantly reduced by 20%. (5) By contrast, the podocyte Glepp1 area density was not significantly reduced (−5%) and the Glepp1-positive tuft volume tended to increase (+10%), although not statistically significantly. (6) The average individual podocyte volume increased significantly by 19%. This was accompanied by an almost statistically significant increase in podocyte nuclear volume (P=0.06). Therefore, transition from the 2K to the 1K state resulted in glomerular tuft enlargement with resulting reduction in podocyte density and compensatory podocyte hypertrophy of about 20% by 3 months.
Table 2.
The 2K to 1K transition is associated with reduced podocyte nuclear density due to glomerular enlargement with compensatory podocyte hypertrophy
| Parameter | At Implantation | 3 Mo Later | Change (%) | P Value |
|---|---|---|---|---|
| Tuft number per biopsy | 23.2±10.7 | 20.3±8.3 | 0.30 | |
| Glomerular volume (μm3×106) | 2.4±0.7 | 2.9±1.0 | +21 | 0.02 |
| Podocytes per glomerulus (n) | 492±117 | 475±129 | −4 | 0.50 |
| Glomerular volume per podocyte (μm3×106) | 5.1±1.5 | 6.2±2.3 | +23 | 0.02 |
| Podocyte nuclear density (n per 106 μm3) | 216±69 | 172±46 | −20 | <0.01 |
| Glepp1 area density (%) | 43.5±5.0 | 41.3±6.0 | −5 | 0.15 |
| Glepp1-positive glomerular volume (μm3×106) | 1.1±0.3 | 1.2±0.3 | +10 | 0.18 |
| Glepp1-negative glomerular volume (μm3×106) | 1.4±1.4 | 1.7±0.8 | +24 | 0.02 |
| Mean podocyte volume (μm3×103) | 2.2±0.7 | 2.6±1.0 | +19 | 0.03 |
| Mean nuclear caliper diameter (μm) | 7.1±0.7 | 7.4±0.8 | +4 | 0.06 |
Morphometric parameters are shown for paired sample analysis in which each implantation (time 0) biopsy was paired with its 3-month biopsy from the same kidney (n=18 pairs). For unpaired analysis shown in Table 4, additional samples were included that did not have pairs. Statistical and fold comparisons are shown.
On the basis of our previous studies in model systems, we would expect that this reduction in podocyte density in the 2K to 1K transition might be expected to trigger glomerular destabilization in susceptible individuals. If this were to occur, it should be detectable by an increased rate of podocyte detachment into the urine.
Urine Podocyte Detachment in Allograft Recipients
There were 737 urine samples collected and analyzed from 251 allograft recipients over a 3-year period. Demographic data are shown in Table 3. Where more than one sample was available from a patient, the averaged value for all available samples was used for comparison with the 2K control group. Podocin mRNA is specific to the podocyte. The urine pellet podocin mRNA/creatinine ratio (UPodCR) assay therefore provides an estimate of the amount of podocyte detachment from glomerular tufts measured in spot urine samples analogous to the urine protein/creatinine ratio, as previously described.17 Data are shown as a fold comparison with 2K age-matched control values (n=174). We previously reported that the UPodCR is detectable in normal urine and does not vary significantly with age, sex, or race.17
Table 3.
Demographics for control and transplant patient groups used for urine and biopsy analyses
| Demographic | Control (n=174) | Transplant (n=251) |
|---|---|---|
| Age (yr) | 40±12 | 40±19 |
| Men | 58 (33) | 162 (64.5) |
| Race | ||
| Caucasian | 135 (77.6) | 189 (75.3) |
| African American | 18 (10.3) | 43 (17.1) |
| Asian | 12 (6.9) | 8 (3.2) |
| Other | 9 (5.2) | 11 (4.4) |
| UPCR | 0.04 (0.03–0.06) | 0.13 (0.07–0.38) |
| eGFR (ml/min per 1.73 m2) | NA | 51±20 |
| Transplant category | ||
| Deceased donor | 111 (44.2) | |
| Living related | 65 (25.9) | |
| Living unrelated | 75 (29.9) | |
| Years post-transplant | ||
| <1 | 130 (51.8) | |
| 1–2 | 30 (12.0) | |
| 2–5 | 40 (15.9) | |
| >5 | 51 (20.3) | |
| Cause of ESRD | ||
| Diabetic nephropathy | 65 (25.9) | |
| Hypertension | 38 (15.1) | |
| ADPKD | 25 (10.0) | |
| FSGS | 15 (6.0) | |
| Other glomerulopathies | 53 (21.1) | |
| Other | 55 (21.9) |
Dat are presented as n (%), mean±1 SD, or median (interquartile range). UPCR, urine protein/creatinine ratio; NA, not available.
Figure 3A shows UPodCR data from allograft recipients (1K) compared with the 2K control. We had anticipated that because an allograft recipient has on average half the number of nephrons, they would excrete half the amount of podocytes. However, as shown in Figure 3A, allograft recipients excrete on average 6-fold more UPodCR than the 2K control (P<0.001). Furthermore, this level of podocyte excretion persisted for >5 years after transplantation.
Figure 3.
UPodCR is increased after transplantation. (A) UPodCR by time after transplantation. Average UPodCR is persistently increased approximately 6-fold above the 2K control for >60 months after transplantation. (B) UPodCR by transplant type. The UPodCR is significantly increased 6-fold above the 2K control for all transplant urine samples (All Tx). Similar UPodCR levels are present whether the recipient receives either a deceased donor kidney >4 years after reaching ESRD and therefore without residual urine (DDTx) or has a preemptive transplant that by definition has enough residual urine to survive (Preemptive Tx). Similar values are also present if the recipient has reached ESRD secondary to PKD even although patients with PKD do not have elevated UPodCR levels before transplantation (PreTx PKD). The boxes represent median and interquartile ranges, and bars represent 1.5-fold×the interquartile range below the 25th percentile and above the 75th percentile. Means are compared using ANOVA with post hoc Bonferroni correction for multiple comparisons. Outliers outside the mean±2 SD range of the biomarker are shown with dots, and those outside the mean±3 SD are shown with asterisks. LDTx, living donor kidney transplant; PKD, polycystic kidney disease.
Because allograft recipients have failing native kidneys in situ, it was possible that podocytes derived from native kidneys would contaminate allograft urine and thereby provide an increased urine podocin mRNA signal. The fact that the increased UPodCR persisted long term after allografting makes this possibility unlikely. However, to further address this question, Figure 3B shows data from deceased donor allograft recipients who were transplanted >4 years after starting dialysis when they would be expected to have minimal residual urine that could contaminate allograft urine. The level of UPodCR was similar in this group to all allografts and in particular to preemptively transplanted recipients, who by definition have residual urine that could contaminate allograft urine. In addition, allograft recipients who reached ESRD from autosomal dominant polycystic kidney disease (ADPKD) have the same increased UPodCR level after allografting as do other patient groups (Figure 3B). We previously reported that patients with ADPKD do not excrete increased UPodCR as they progress to ESRD, as is also confirmed in Figure 3B.17 Therefore, residual urine cannot be the source for the increased UPodCR signal in recipient urine. The major source must be from the allograft itself.
Subgroup analysis for UPodCR was performed according to either post-transplant eGFR or allograft biopsy diagnoses as shown in Figure 4A. Samples from long-term stable allografts with normal kidney function (eGFR≥60 ml/min) had close to normal UPodCR values (2-fold above the 2K value, P=0.02). Samples associated with a normal biopsy, rejection, tubulointerstitial injury, and interstitial fibrosis/tubular atrophy (IF/TA) were not significantly increased above the eGFR≥60 ml/min group. By contrast, transplant glomerulopathies (TGs) (whether occurring early or late after transplantation) or glomerular diseases (GDs) (including recurrent and de novo GDs) showed significantly higher levels 20- to 30-fold above the 2K control (P<0.001) and >8-fold above the eGFR≥60 ml/min control group (P<0.01). This result is similar to our previously published data in which all GD groups had increased UPodCR, whereas a tubulointerstitial disease (e.g., ADPKD) did not.17
Figure 4.
(A) UPodCR is increased in glomerulopathies while being close to normal in well functioning allografts. (B) The urine protein/creatinine ratio was increased with lower eGFR, and in glomerulopathies. Subgrouping is shown according to eGFR (left) and pathologic diagnosis (right). The box represents median and interquartile ranges, and error bars represent 1.5-fold×the interquartile range below the 25th percentile and above the 75th percentile. Means are compared using ANOVA with post hoc Bonferroni correction for multiple comparisons. Outliers outside the mean±2 SD range of the biomarker are shown with dots, and those outside the mean±3 SD are shown with asterisks. Statistical significance between the 2K control and each group (upper) the eGFR>60 allograft group (lower) is shown. s, P<0.05 (statistically significant); s, P<0.01; s=P<0.001; ns, not statistically significant.
We conclude that, on average, allografts have increased UPodCR signal after transplantation in spite of the fact that they have only half the number of podocytes. This is compatible with some level of podocyte stress occurring after transplantation and raises the question of whether some allograft recipients with high-level podocyte detachment could become depleted of podocytes over time.
Transplant Glomerulopathies
We therefore evaluated whether glomeruli of patients with TGs have reduced numbers of podocytes in their glomeruli compared with both time-0 kidneys and 3- and 12-month post-transplant protocol biopsies. Recurrent and de novo GDs in the allograft were excluded from analysis. To understand the time factor in relation to development of post-transplant glomerular injury (TG), we separated TG biopsies into those diagnosed within 2 years of transplantation (“early TG” average 0.9 years after transplantation) and those >2 years (average 10.6 years) after transplantation (“late TG”). Table 1 shows demographic data. Because long-lasting allografts with normal kidney function are not biopsied, we do not have this morphometric data. Instead, we analyzed biopsies at 10 years after allografting performed for reduced renal function in which the pathologic report did not note glomerulopathy as a significant element. Table 4 shows morphometric data comparing these groups. Results are summarized as follows.
Table 4.
Unpaired biopsy morphometry analysis showing that TG is associated with reduced podocyte density
| Parameter | Time 0 | 3 mo | 12 mo | Early TG | Late TG | Late Non-GD |
|---|---|---|---|---|---|---|
| Mean time after transplantation (yr) | 0 | 0.3±0.1 | 1.1±0.1 | 0.9±0.7 | 10.6±4.3 | 11.4±0.6 |
| Number of biopsies (n) | 20 | 20 | 14 | 16 | 18 | 6 |
| Tufts per biopsy | 23.1±10.3 | 21.0±7.7 | 19.6±6.8 | 20.0±6.6 | 17.9±7.5 | 12.7±4.9 |
| Glomeruli evaluated | 462 | 420 | 274 | 320 | 322 | 76 |
| Glomerular volume (μm3 × 106) | 2.5±0.7 e | 2.9±0.9 e | 3.2±1.4 e | 3.5±0.9 e | 5.4±3.1 a,b,c,d | 3.6±1.1 |
| Nuclear diameter (μm) | 7.2±0.7 | 7.4±0.8 | 7.5±0.6 | 7.2±0.9 | 7.6±0.9 | 7.6±0.7 |
| Podocyte nuclear density (n per 106 μm3) | 212±67 c,d,e,f | 169±45 d,e | 157±53 a,d,e | 99±40 a,b,c | 70±33 a,b,c | 110±26 a |
| Glomerular volume per podocyte (μm3 × 106) | 5.1±1.4 d,e | 6.4±2.1 d,e | 7.0±2.4 e | 11.6±4.2 a,b,e | 17.7±8.7 a,b,c,d,f | 9.6±2.6 e |
| Podocytes per glomerulus (n) | 498±111 d,e | 473±122 d,e | 463±85 d,e | 324±81 a,b,e | 315±117 a,b,c | 379±97 |
| GLEPP1 area (%) | 43.5±5.6 d,e,f | 41.3±5.8 d,e | 38.2±4.0 d,e | 26.7±5.8 a,b,c,d | 17.4±8.0 a,b,c,d,f | 33.2±5.1 a,e |
| GLP1-positive volume (μm3 × 106) | 1.0±0.3 | 1.2±0.3 | 1.2±0.4 | 0.9±0.3 | 0.9±0.5 | 1.2±0.3 |
| GLP1-negative volume (μm3 × 106) | 1.4±0.4 e | 1.7±0.7 e | 2.0±1.0 e | 2.6±0.8 e | 4.5±2.9 a,b,c,d,f | 2.4±0.9 e |
| Mean podocyte volume (μm3 × 103) | 2.2±0.7 | 2.6±1.0 | 2.6±0.7 | 3.0±1.1 | 2.7±0.9 | 3.1±0.4 |
| Column designation | a | b | c | d | e | f |
Morphometric parameters are shown for unpaired comparison of six biopsy groups including the implantation (time 0) biopsy, 3-month biopsy, 12-month biopsy, early TG (mean biopsy time after transplantation of 0.9 years), and late TG (mean time after transplantation 10.6 years) groups, as well as a heterogeneous late non-GD group (mean time after transplantation 11.4 years). Statistical comparisons are shown for each group with all other groups by letter identifying each column (see bottom row). For superscripted letters regular font=P<0.05, italic font=P<0.01, bold font=P<0.001, no letter=P>0.05.
Protocol Biopsies at Time 0, 3 Months, and 12 Months Compared by Unpaired Analyses
There was a trend (not statistically significant) for the podocyte number per tuft, podocyte nuclear density, and Glepp1 percent area to all decrease with time after transplantation, and for the glomerular volume and Glepp1-negative volume (primarily mesangial expansion) to increase over the 12-month period of observation. Only podocyte density decrease between time 0 and 12 months achieved statistical significance by unpaired analysis (P=0.02). The paired analysis shown in Table 2 confirms these same changes between time 0 and 3 months.
Early TG
Podocyte number per glomerulus, density, and Glepp1 percent area were all significantly decreased compared with the time-0, 3-month, and 12-month controls. The glomerular volume was not statistically increased. These data show that within 2 years after transplantation (average 0.9 years, range 3–24 months), a subset of allograft recipients with a poor outcome developed accelerated podocyte loss and significant reduction in podocyte density.
Late TG
Podocyte number per glomerulus, density, and Glepp1 percent area were all significantly decreased compared with the time-0, 3-month, and 12-month controls. The glomerular volume was also significantly increased above all other groups (2-fold above the time-0 control). Glepp1-negative (nonpodocyte) tuft volume was also significantly increased in association with increased glomerular volume. This result shows that by an average of 10 years after transplantation, a subset of allografts develops a marked reduction in podocyte density resulting from a combination of reduced podocyte number per tuft and increased glomerular tuft volume.
Late Non-GN
This heterogeneous group (arterial disease and/or IF/TA without prominent GD) also showed a trend toward reduction in podocyte density and number per tuft by 10 years after transplantation, although only the glomerular volume per podocyte and Glepp1 percent area were significantly higher than the late GN group.
Podocyte Hypertrophy
No further statistically significant increase in podocyte cell volume was noted between groups other than that noted in the paired analysis between time 0 and 3 months (Table 2). This is compatible with podocytes being maximally hypertrophied by 3 months after implantation.
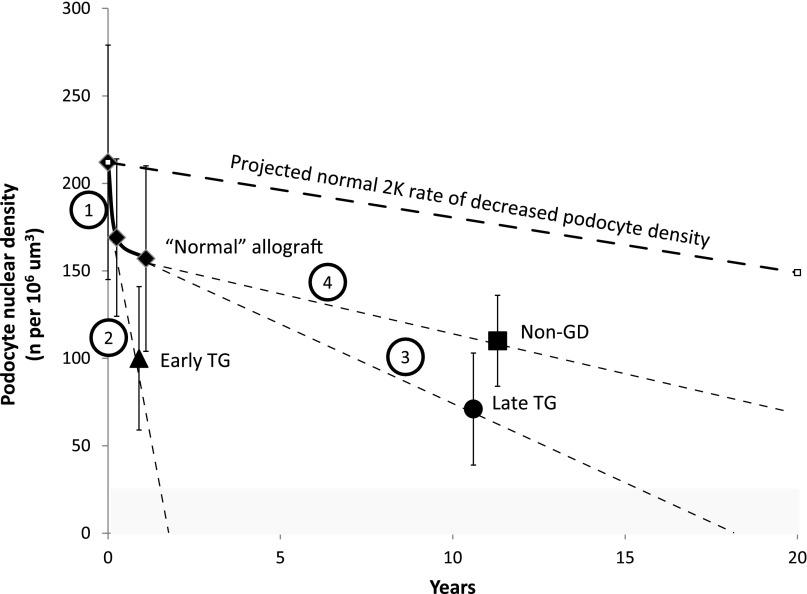
Figure 5 summarizes podocyte nuclear density changes in relation to time after transplantation and their implications for outcome. Potential sites for therapeutic intervention are indicated by numbers as outlined in the Figure 5 legend.
Figure 5.
Podocyte nuclear density is decreased after transplantation and further decreased in association with early and late TG, thereby setting the allograft up for failure. The normal 2K rate of podocyte density decline with time is shown by the dashed line above (unpublished data). The gray area shows the podocyte density below which ESRD is present. The 2K to 1K transition per se causes a 26% decrease in podocyte density over the first year (solid diamonds). The non-GD group is projected as running approximately parallel to the expected 2K rate of decline with time (solid square). By contrast, the early TG group (solid triangle) has a rapid decrease in podocyte density projected to reach ESRD within approximately 2 years (if untreated). The late TG group (solid circle) also has reduced podocyte density and is projected to reach ESRD by approximately 16 years. Prolongation of allograft half-life could potentially be achieved by targeting the following options: (1) the major reduction in podocyte density occurring at transplantation due to compensatory hypertrophy, (2) the rapid podocyte density reduction due to podocyte loss in the early TG group probably resulting from immune mechanisms directed at the podocyte and/or other cells, (3) accelerated long-term podocyte density reduction in the late TG group resulting from both reduction in podocyte number and increased glomerular volume that may in part be due to nonimmune mechanisms, and (4) the time-associated density decrease represented in the non-GD group that approximately parallels to the expected 2K rate of decreased podocyte density over time.
Relationship of Podocyte Density to eGFR
Model systems prove that podocyte depletion per se causes progressive glomerulosclerosis leading to ESRD. Figure 6, A and B, shows that podocyte density measured either by nuclear density or Glepp1 area also correlated strongly with eGFR in allograft recipients.
Figure 6.
Podocyte density (estimated by either nuclear density or Glepp1 area) in relation to clinical parameters (eGFR, glomerulosclerosis, and proteinuria). Clinical parameters in relation to podocyte density estimates measured by two independent methods (podocyte nuclear density [A, C, and E] and Glepp1 area density [B, D, and F]). Biopsy samples from all six groups (time 0, 3 months, 12 months, early TG, late TG, and non-GD samples [n=95]) are used for analysis. Podocyte nuclear density below approximately 100 per 106 μm3 and Glepp1 area density below 32% (shown by dotted lines) are associated with abnormal clinical parameters. (A and B) Relationship to eGFR. Podocyte nuclear density and Glepp1 area density correlate with eGFR with R2=0.43 and R2=0.47 (P<0.001), respectively. (C and D) Relationship to glomerulosclerosis score. Podocyte nuclear density and Glepp1 area density correlate with eGFR with R2=0.37 and R2=0.50 (P<0.001), respectively. (E and F) Relationship to proteinuria. Podocyte nuclear density and Glepp1 area density correlate with the urine protein/creatinine ratio (UPCR) with R2=0.25 and R2=0.38 (P<0.001), respectively.
Relationship of Podocyte Density to Glomerulosclerosis
Figure 6, C and D, shows that the glomerulosclerosis score increased in association with both reduced podocyte nuclear density and reduced Glepp1 area density. Figure 7 shows that Banff pathologic scores also correlated with podocyte density estimates.
Figure 7.
Podocyte density correlates with Banff 2005 criteria for TG. Podocyte density measured by two independent methods (podocyte nuclear density [n per 106 μm3] and Glepp1 area density [%]) correlate with Banff pathologic criteria for TG including % TG glomeruli per biopsy (upper panels), Banff TG score (middle panels), and Banff mesangial score (lower panels). P<0.001 for all correlations.
Relationship of Podocyte Density to Proteinuria
Figure 6, E and F, shows that high-level proteinuria also appears in association with podocyte nuclear density below about the 100 per 106 μm3 range or when the Glepp1 percent area falls below about 30%. Figure 8 shows that proteinuria correlated with the UPodCR assay consistent with the concept that podocyte detachment causes proteinuria.
Figure 8.
The UPodCR correlates with the urine protein/creatinine ratio for all samples.
Discussion
Kidney transplantation offers the opportunity to examine the 2K to 1K transition in human kidneys. Magnetic resonance imaging studies report that the remaining kidney hypertrophies by about 24% within a few days after uninephrectomy.19 We demonstrate that the compensatory glomerular hypertrophy in the 2K to 1K transition also averages about 20% within 3 months. The glomerular tuft gets 20% larger. Podocyte number does not increase. Podocytes therefore have to hypertrophy by about 20% to deal with the hypertrophic challenge. We know from the hDTR rat model of controlled podocyte depletion that a 30% reduction in podocyte density occurring over a short period of time triggers glomerular destabilization, leading to further long-term podocyte detachment that eventually causes glomerulosclerosis and ESRD by about 12 weeks.7–9 5/6 nephrectomy is a widely used model of progression to ESRD, and uninephrectomy triggers progression to ESRD in susceptible animals.6,8 This raised the possibility that nephrectomy or transplantation (“reverse nephrectomy”) per se might cause significant podocyte hypertrophic stress. From model systems, we know that podocyte stress results in an increased rate of podocyte detachment measurable in urine.6–8 We therefore examined the UPodCR in allograft recipients to determine whether similar events occur.
We found that allografts on average have a persistently increased amount of UPodCR about 6-fold above the 2K control, which is compatible with the concept that podocyte stress may indeed occur after kidney transplantation. This could not be explained by contamination from native kidney urine. In the subset of recipients that become stable long term with an eGFR≥60 ml/min and no proteinuria, the podocyte detachment rate is close to the normal 2K value. We speculate that this reflects successful adaptation by podocytes in these patients. However, in a subset of allograft recipients that develop glomerulopathies (either transplant glomerulopathy or recurrent GD), the rate of podocyte detachment was significantly increased 10- to 20-fold above the 2K median level. We would expect that this high level of podocyte detachment, if persistent, would be associated with progressive podocyte depletion and progression to ESRD over time, particularly because kidney allograft recipients have only one kidney and thus half the number of podocytes compared with the 2K state. In keeping with this expectation, biopsies from patients with late TG (average 10.6 years after grafting) showed a highly significant reduction in podocyte density caused by a combination of a reduction in podocyte number per glomerulus and increased glomerular volume. An early TG group (within 2 years of transplantation) also showed a reduction (53%) in podocyte density due to decreased podocyte number per glomerulus but without an associated increase in glomerular volume. A previous report similarly documented podocyte depletion in transplant glomerulopathy in humans,20 and podocyte injury/stress was previously reported in a rat model of kidney transplantation.21 Similar reductions in podocyte density have also been reported for both diabetic glomerulosclerosis and IgA nephropathy.22,23 Podocyte nuclear density was related to eGFR at the time of biopsy compatible with podocyte depletion causing progression to ESRD, as has been proven to occur in model systems.6–9 El-Zoghby and colleagues report that up to 40% of allografts fail from GDs.24
TG is thought to be associated with antibody-mediated immune attack on the kidney, although mechanisms by which this occurs have not been defined.25–30 Immune mechanisms directed either at the podocyte itself or at endothelial cells with secondary podocyte injury could drive podocyte depletion. On the basis of model systems, we speculate that the rate of podocyte depletion observed in the late TG group could be accounted for by hypertrophic podocyte stress causing accelerated podocyte detachment, although immune mechanisms could also contribute. Hypertrophic nonimmune processes would also amplify glomerular injury caused by other mechanisms, both by facilitating access of circulating antibody to podocytes and causing underlying podocyte stress rendering them more susceptible to immune injury directed primarily at endothelial cells.
Increased proteinuria has been demonstrated to be powerfully associated with allograft outcome.31–33 Proteinuria is a marker for podocyte detachment, depletion, and glomerulopathy, and may also independently play a role in driving progression to ESRD. Proteinuria correlated with amount of podocyte detachment compatible with this concept. Model systems demonstrate that proteinuria per se does not cause increased urine podocyte mRNA detectability.8
Figure 5 illustrates potential sites for therapeutic targeting to maintain podocyte density and thereby prolong allograft half-life. These include the average 25% podocyte density reduction occurring in the first year after transplantation (option 1), identifying and preventing putative immune mechanisms driving podocyte depletion (option 2), decreasing hypertrophy-induced accelerated podocyte detachment (option 3), and modulating the baseline rates of podocyte detachment and glomerular enlargement (option 4). Nonimmune mechanisms can already be targeted by available approaches. For example, weight gain would be expected to accelerate kidney and glomerular hypertrophy postimplantation and thereby amplify podocyte stress and detachment.6 The relationship between increased body mass index and shortened allograft half-life is well documented in humans.13 Angiotensin II blockade reduces the rate of podocyte detachment,8 although it would need to be commenced early after implantation to have an optimal protective effect. A recent randomized controlled trial of angiotensin II blockade after transplantation showed a borderline protective effect on IF/TA (P=0.08) even though treatment was started 6 weeks after implantation and the outcome parameter measured was not glomerulosclerosis.34 A nonimmune growth-dependent mechanism would also be expected to be ameliorated by mammalian target of rapamycin inhibition compatible with clinical reports of improved long-term allograft survival.35–37
The major strength of this study is that it is conceptually derived from prior work in model systems, although it is observational and cross-sectional and the sample size is relatively small. Nevertheless, the data show that a better understanding of podocyte biology in the kidney transplant setting could provide insights and tools to help prolong allograft half-life.
Concise Methods
Use of urine samples for mRNA analysis and archival kidney tissues was approved by the University of Michigan Institutional Review Board (HUM00055525, HUM00083116, and HUM00025707).
Urine Sample Collection
Samples were collected from allograft recipients in the University of Michigan kidney transplant program prospectively over a 3-year period. The samples used were the remaining urine left over from routine urine collections provided by patients at their regular clinic visits. All samples were assigned a study number that connected them to clinical information from the chart. Clinical information used for analysis was retrospectively collected for each sample from the clinical chart and used to populate a deidentified database.
Urine Assays
The methodology used was as previously reported.17
Normal Urine Samples
We collected 174 urine samples from unidentified people aged 18–73 years who had no known kidney disease or hypertension. Each sample was associated with a short consent form filled out by the urine donor that included information about age, sex, race, medical conditions, and medications. Exclusion criteria included any kidney-related disease, antihypertensive medication, urine dipstick positive for blood or protein, and UPodCR of >0.18. There were no statistical differences in mRNAs between the excluded samples on the basis of the above criteria (n=37) as a group and the samples included in the normal group for subsequent analysis.
Urine Processing
Urine (up to 50 ml in a sterile 50-ml plastic centrifuge tube) was centrifuged at 4°C for 15 minutes at 4000 rpm (3200×g) on a table-top centrifuge. Two 2-ml aliquots of the supernatant were removed and stored at −20°C for protein, creatinine, and other measurements. The urine pellet was suspended in 750 μl of cold DEPC-treated PBS, pH 7.4 (DEPC-PBS), at 4°C using a sterile disposable polystyrene transfer pipette and was then transferred to a labeled 1.7-ml plastic centrifuge tube. A second 750 μl of PBS was used to wash the bottom of the 50-ml centrifuge tube to recover remaining pellet material, and was added to the 1.7-ml tube. The transferred pellet material in 1.5 ml PBS was then centrifuged at 12,000 rpm in a minicentrifuge for 5 minutes at 4°C. The supernatant was discarded. To the centrifuged washed pellet, we added 350 μl of RLT buffer containing β-mercaptoethanol at 10 μl/ml of RLT buffer according to the RNeasy Qiagen protocol (Germantown, MD). The pellet was suspended in RLT/β-mercaptoethanol buffer and then frozen at −80°C for assay.
RNA Preparation and Quantitative RT-PCR Assay and Interpretation
The total urine pellet RNA was isolated using the protocol of the RNeasy Mini Kit (catalog no. 74106; Qiagen). Quantitation of the absolute podocin mRNA abundance was performed using the 7900 HT Fast Real-Time PCR System (Applied Biosystems) using TaqMan Fast Universal PCR Master Mix, with sample cDNA in a final volume of 25 μl per reaction. TaqMan probe for human NPHS2 (podocin) (catalog no. Hs00922492_m1; Applied Biosystems) was used. All data were from 2-μl samples measured in duplicate. Standard curves were constructed for each assay using serially diluted cDNA standards. Assays were accepted only if the r2 was >0.97 for standard curves using SDS 2.2.2 software (Applied Biosystems). Human podocin cDNAs of known sequence and concentration were used as standards for each assay so that the data could be calculated on a molar basis for each probe. We previously reported analysis of urine RNA quality, recovery, and stability. The coefficient of assay variation is 35%. In control samples, which contain low RNA levels, podocin mRNA was not detectable in 33% of samples starting with <20 ml urine and in 15% of samples starting with 30–50 ml urine.
Stability of Urine mRNAs and Sample Handling
For this study, we collected urine in clinics at any time of the day from 7 am until 4 pm and stored the samples at +4°C. At approximately 4 pm, they were then collected for transport to the laboratory for processing.
Urine Data Analyses and Expression
All assays were performed against cDNA standards. Because the urine sample volume varied from 5 to 50 ml, we first expressed data per milliliter of urine, and we then expressed data per gram of urine creatinine to compensate for urine concentration. All data are therefore expressed per gram of creatinine. Where more than one sample was available from a patient, the mean value of all available samples was used for analysis so that each patient is represented only once. Because these are mRNA assays, the possibility exists that the amount of mRNA in a cell could increase in relation to the amount of hypertrophy. Therefore, it might not be accurate to interpret these urine mRNA data in relation to cell number. We therefore use the term “amount” of urine mRNA under the concept that losing a hypertrophied cell could be equivalent to losing two nonhypertrophied cells in terms of glomerular basement membrane surface covered. The term “amount” deals with this concept in a reasonable way.
Medications
Triple medication including cyclosporin, mycophenolate mofetil, and prednisone was used as the standard immunosuppressive regimen at the University of Michigan until 2010, when cyclosporine was changed to tacrolimus. If the patient is considered as having high immunologic risk (e.g., panel-reactive antibody [PRA]>20%, African-American race, living unrelated donor kidney, extended criterion donor kidney, desensitization for high PRA, blood group ABO-incompatible kidney, positive cross-match, or high donor-specific antibody [DSA]), thymoglobulin is used perioperatively as induction therapy in addition to the standard immunosuppressive regimen.
Definition of Pathologic Cohorts and Background Information
All biopsy samples were from adults.
Control Time-0 and 3-Month Protocol Biopsy Cohorts
All time-0 and 3-month protocol biopsy samples used were pathologically within normal limits. Twenty-three pairs of biopsies performed at implantation and 3 months later for protocol surveillance were analyzed. In five cases, one or other of the pair could not be used for analysis because the number of glomerular profiles present was <8, which we have previously established as the lower limit for an adequate estimate of glomerular volume. Adequate profiles were present in 18 pairs of biopsies from both time-0 (implantation) and at 3 months as shown in Table 2. There were 20 biopsies with ≥8 glomerular profiles available for unpaired analysis for the time-0 and 3-month groups as shown in Table 4. Table 1 shows that some of these patients subsequently developed rejection.
Twelve-Month Protocol Biopsy Cohort
Seventeen protocol biopsies were available from the same group of patients as provided the time-0 and 3-month biopsies. Fourteen of these biopsies contained adequate glomerular profiles for analysis. Of the 17 patients in this group, 1 had biopsy evidence of a Banff1A acute cellular rejection level and 6 had borderline rejection at the 12-month biopsy.
Early TG Cohort
The term “early TG” is used as a descriptor for glomerulopathies identified at biopsy within the first 2 years after implantation which were not due to recurrent GD. Sixteen biopsies from eight patients were assigned to this group. Six had glomerulitis, of which two also had peritubular capillaritis meeting 2014 Banff criteria for acute antibody-mediated rejection.38 Seven patients were hepatitis C antibody negative, whereas one patient was hepatitis C antibody positive. One biopsy showed thrombotic microangiopathy. C4d immunoperoxidase was negative in 14 biopsies and was not done in 2 biopsies. Six patients were negative for DSA at transplantation, whereas two were positive (DRB3*0101, DPB1*0401, B70 MFI 1134, and DQ4 MFI 20,883) and two subsequently developed de novo DSA (Cw2 MFI 1,702, DQ4 2,149, and DQ8 MFI 4,580). Of the eight patients at transplantation, PRA was not available for one, was low in four (≤3% for both CI and CII), and was high (>71% in either or both CI and CII) in three.
Late TG Cohort
The term “late TG” is used as a descriptor for glomerulopathies identified after 2 years after implantation that were not recurrent GD. Eighteen biopsies from 17 patients were assigned to this group. These biopsies had duplicated basement membrane compatible with 2005 Banff TG criteria.39
Late Non-GD Cohort
Late non-GD was defined as biopsies done for cause (reduced eGFR) approximately 10 years after transplantation where the pathologic report did not include significant glomerulopathy. Six biopsies analyzed either did not have TG or recurrent GD (diabetes or membranous GN) and had an adequate number of profiles for analysis. Of these six biopsies all had arteriolopathy, one also had BK nephropathy and four had IF/TA.
Biopsies were scored by J.B.H., who was blinded to the morphometric data, according to the quantitative criteria described in the Banff 1997 classification40 for mesangial matrix increase (mm) and transplant glomerulopathy (cg). For mm, 0 means none, 1 means up to 25%, 2 means 26%–50%, and 3 means >50% of at least moderate matrix increase of nonsclerotic glomeruli affected. For cg, 0 means none, 1 means up to 25%, 2 means 26%–50%, and 3 means >50% of double contours affecting peripheral capillary loops in the most affected glomerulus. In addition, the number of glomeruli with transplant glomerulopathy, global sclerosis, segmental sclerosis, and adhesions was counted and presented as a percentage of total glomeruli. Because three pathologists were involved in reading the original biopsies with nonidentical thresholds for calling TG, all biopsies from a particular patient were assigned to a TG group if one or more biopsies from that patient had already been assigned a TG pathologic diagnosis.
Podocyte Density Methodology
The method used was as previously reported.18 Regular formalin-fixed paraffin-embedded histologic sections available as unstained slides from routine pathologic analysis were deparaffinized in fresh xylene, rehydrated, and permeabilized with 0.1% Triton-X100 in PBS for 10 minutes at room temperature. Slides were then incubated at 92°C for 2 hours in Retrieve-All 1 solution (Signet Laboratories, Dedham, MA) for antigen unmasking. After blocking with 1% BSA for 1.5 hours, slides were incubated overnight at 4°C with mouse mAb to TLE4 (SC-365406 monoclonal IgG1; Santa Cruz Biotechnology, Santa Cruz, CA) at 1:150 dilution in 1% BSA. After a wash step, they were then incubated for 1 hour at room temperature with Alexa Fluor 488 goat anti-mouse IgG (A11001; Invitrogen) diluted to 1:300 in 10% human serum diluted in PBS. A further amplification step used a third antibody against the secondary antibody by incubating slides with Alexa Fluor 488 donkey anti-goat IgG (A11055; Invitrogen) diluted at 1:500 in 10% human serum in PBS at room temperature for 1 hour. The second and third antibodies were absorbed using 10% human serum incubated overnight at 4°C followed by centrifugation at 4°C for 10 minutes at 16,000×g to remove immune aggregates immediately before use. Coverslips were mounted using SlowFade Gold antifade reagent with diamidino-2-phenylindole (S36939; Invitrogen Molecular Probes). Glomeruli were sequentially photographed using an Olympus DP70 Digital Microscope camera (Center Valley, PA) fitted onto a Leica DM IRB microscope (Deerfield, IL) using red, green, and blue filters and converting the green signal to red and vice versa, and then merging as RGB and RG files.
For image analysis using Image Pro Premier software, glomerular tufts are defined so as to exclude parietal podocytes from analysis. Podocyte nuclei are exactly masked using the software so that podocyte nuclear number and apparent nuclear mean caliper diameter (d) can be simultaneously measured and exported to an Excel file for each glomerular profile. The true podocyte nuclear mean caliper diameter (D) was then calculated for each section using the previously described spreadsheet incorporating a quadratic equation relating section thickness (T), apparent mean caliper diameter (d), and a podocyte nuclear shape coefficient (k=0.72).18 The appropriate correction factor (CF) required for each individual slide was then calculated using the equation CF=1/(D/T+1), where T is the section thickness (in micrometers) and D is the true mean podocyte nuclear caliper diameter (in micrometers). The observed podocyte nuclear number was then multiplied by the CF to obtain the true podocyte number present in each glomerular profile from the biopsy. The podocyte density is given by the sum of all podocyte nuclei counted in glomerular profiles×CF divided by the total volume of all glomerular tuft profiles assessed (sum of tuft area×thickness=volume) and expressed as podocytes per 106 μm3. Globally sclerotic glomeruli were excluded from analysis.
Glepp1-Positive Percent Area
This method is as previously reported for model systems.6–9 After imaging of the above sections, the coverslip is removed by incubation in xylene. After rehydration, sections were stained for immunoperoxidase using Vectastain Mouse IgG Kit (PK-6102; Vector Laboratories Inc, Burlingame, CA). After a blocking step, slides were incubated with mouse anti-human Glepp1 mAb (5C11) at 1:1000 dilution in 1% BSA for 2 hours at room temperature. The substrate diaminobenzidine (Sigma-Aldrich D4293) diluted according to the kit instructions was incubated on the section at room temperature. The development of brown peroxidase product was visually monitored under the microscope to avoid overdevelopment and high background. Slides were then washed and counterstained with hematoxylin, dehydrated, and mounted with permount. All glomerular tuft profiles were imaged using the Olympus DP70 Digital Microscope camera. Glomeruli tuft area and peroxidase Glepp1-positive area identified by masking the brown substrate were measured by using Meta Image series 6.1 software.
Glomerular profiles were imaged and numbered in the same order for both TLE4 immunofluorescence and Glepp1 immunoperoxidase so that the same tuft area could be used for both TLE4 and Glepp1 measurements. Globally sclerotic glomeruli were excluded from analysis. The lower limit of normal for Glepp1 calculated as the mean−2 SD is 32.4 (n=85) with no significant variation present by age or sex.
Glomerular Volume Estimation
The area of all glomerular tuft profiles present in the biopsy including all small profiles was measured as outlined above. The average radius (r) of all tuft profiles was then calculated assuming glomeruli are spherical. The average maximal radius (R) is then calculated as R=r×4/π as per Weibel.41 Glomerular tuft volume is given by 4/3πR3. If <8 tuft profiles were present in the biopsy, then glomerular volume estimates were not made because there are insufficient profiles to reliably make the estimate as previously described.18 Globally sclerotic glomeruli were excluded from analysis.
Podocyte Volume Estimation
The average Glepp1-positive percent area×glomerular volume gives an estimate of average total podocyte volume per glomerular tuft. Similarly, the Glepp1-negative glomerular tuft volume represents scarred tuft, mesangial matrix expansion, mesangial cells, endothelial cells, and open capillary loops containing blood products. The average total glomerular tuft podocyte volume divided by the average number of podocyte nuclei per tuft provides an estimate of average individual podocyte volume.
Statistical Analyses
For descriptive purposes, the mean±SD, or median and interquartile range (for skewed variables), was used to show the distributions of continuous variables. Count with relative frequency was applied as the descriptive statistic for categorical variables. All of the continuous skewed variables underwent base-10 log transformation before comparison. The paired t test was used to compare parameters of paired biopsies. Means of variables in two and more than two independent groups were compared by the independent t test and ANOVA, respectively. Bonferroni correction was applied in multiple comparisons. A linear regression model was utilized to quantify the relationship between the continuous variables. Best-fit curve estimation, including quadratic and cubic models, was applied to demonstrate the nonlinear associations between two continuous variables. Analyses were performed using SPSS version 21 (Armonk, NY).
Disclosures
None.
Acknowledgments
We thank the study coordinators who participated in this project, including Michael Kappler, Brittany Pannecouk, Cathrin Ring, Mary Maliarik, and Darlene McLean. We also thank the University of Michigan transplant patients, staff, and faculty for their support and for facilitating this work.
This study was funded by grants from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK46073, University of Michigan O’Brien Kidney Core Center P30-DK081943, K08-DK088944 to J.H., and 5T32-DK7378-34 to Y.Y. and F.A.). J.H. is also supported the NephCure-ASN Foundation. Funding from DBK through the Robert C. Kelsch Collegiate Professorship is also gratefully acknowledged.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Transplant Glomerulopathy: The View from the Other Side of the Basement Membrane,” on pages 1235–1237.
References
- 1.Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, Walz G, Moeller MJ, Grahammer F, Huber TB: Unraveling the role of podocyte turnover in glomerular aging and injury. J Am Soc Nephrol 25: 707–716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kriz W: Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech 57: 189–195, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC: Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: Prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC: Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol 23: 1351–1363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, Chang JW, Wiggins JE, Lienczewski C, Kretzler M, Wiggins RC: Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol 20: 1041–1052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC: Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int 81: 40–55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Luyckx VA, Brenner BM: The clinical importance of nephron mass. J Am Soc Nephrol 21: 898–910, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Praga M, Hernández E, Herrero JC, Morales E, Revilla Y, Díaz-González R, Rodicio JL: Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int 58: 2111–2118, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Giral M, Foucher Y, Karam G, Labrune Y, Kessler M, Hurault de Ligny B, Büchler M, Bayle F, Meyer C, Trehet N, Daguin P, Renaudin K, Moreau A, Soulillou JP: Kidney and recipient weight incompatibility reduces long-term graft survival. J Am Soc Nephrol 21: 1022–1029, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier-Kriesche H-U, Arndorfer JA, Kaplan B: The impact of body mass index on renal transplant outcomes: A significant independent risk factor for graft failure and patient death. Transplantation 73: 70–74, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Mjøen G, Hallan S, Hartmann A, Foss A, Midtvedt K, Øyen O, Reisæter A, Pfeffer P, Jenssen T, Leivestad T, Line P-D, Øvrehus M, Dale DO, Pihlstrøm H, Holme I, Dekker FW, Holdaas H: Long-term risks for kidney donors. Kidney Int 86: 162–167, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Muzaale AD, Massie AB, Wang M-C, Montgomery RA, McBride MA, Wainright JL, Segev DL: Risk of end-stage renal disease following live kidney donation. JAMA 311: 579–586, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR): OPTN/SRTR 2011 Annual Data Report, Rockville, MD, Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, 2012 [Google Scholar]
- 17.Wickman L, Afshinnia F, Wang SQ, Yang Y, Wang F, Chowdhury M, Graham D, Hawkins J, Nishizono R, Tanzer M, Wiggins J, Escobar GA, Rovin B, Song P, Gipson D, Kershaw D, Wiggins RC: Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J Am Soc Nephrol 24: 2081–2095, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkatareddy M, Wang SQ, Yang Y, Patel S, Wickman L, Nishizono R, Chowdhury M, Hodgin J, Wiggoins PA, Wiggins RC: Estimating podocyte number and density in histologic sections. J Am Soc Nephrol 25: 1118–1129, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song T, Fu L, Huang Z, He S, Zhau R, Lin T, Wei Q: Change in renal parenchymal volume in living transplant donors. Int Urol Nephrol 46: 743–747, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Agustian PA, Schiffer M, Gwinner W, Schäfer I, Theophile K, Modde F, Bockmeyer CL, Traeder J, Lehmann U, Grosshennig A, Kreipe HH, Bröcker V, Becker JU: Diminished met signaling in podocytes contributes to the development of podocytopenia in transplant glomerulopathy. Am J Pathol 178: 2007–2019, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pippin J, Kumar V, Stein A, Jablonski P, Shankland SJ, Davis CL: The contribution of podocytes to chronic allograft nephropathy. Nephron, Exp Nephrol 111: e1–e10, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD: Podocytopenia and disease severity in IgA nephropathy. Kidney Int 61: 1475–1485, 2002 [DOI] [PubMed] [Google Scholar]
- 24.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG: Identifying specific causes of kidney allograft loss. Am J Transplant 9: 527–535, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Gloor JM, Sethi S, Stegall MD, Park WD, Moore SB, DeGoey S, Griffin MD, Larson TS, Cosio FG: Transplant glomerulopathy: Subclinical incidence and association with alloantibody. Am J Transplant 7: 2124–2132, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Sis B, Campbell PM, Mueller T, Hunter C, Cockfield SM, Cruz J, Meng C, Wishart D, Solez K, Halloran PF: Transplant glomerulopathy, late antibody-mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. Am J Transplant 7: 1743–1752, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Cosio FG, Gloor JM, Sethi S, Stegall MD: Transplant glomerulopathy. Am J Transplant 8: 492–496, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Akalin E, Dinavahi R, Dikman S, de Boccardo G, Friedlander R, Schroppel B, Sehgal V, Bromberg JS, Heeger P, Murphy B: Transplant glomerulopathy may occur in the absence of donor-specific antibody and C4d staining. Clin J Am Soc Nephrol 2: 1261–1267, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Wavamunno MD, O’Connell PJ, Vitalone M, Fung CL, Allen RD, Chapman JR, Nankivell BJ: Transplant glomerulopathy: Ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant 7: 2757–2768, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Haas M, Mirocha J: Early ultrastructural changes in renal allografts: Correlation with antibody-mediated rejection and transplant glomerulopathy. Am J Transplant 11: 2123–2131, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Amer H, Cosio FG: Significance and management of proteinuria in kidney transplant recipients. J Am Soc Nephrol 20: 2490–2492, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Serón D, Burgos D, Alonso A: Histology and proteinuria after renal transplantation. Transplant Rev (Orlando) 26: 20–26, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Soler MJ, Riera M, Gutierrez A, Pascual J: New options and perspectives for proteinuria management after kidney transplantation. Transplant Rev (Orlando) 26: 44–52, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim HN, Jackson S, Connaire J, Matas A, Ney A, Najafian B, West A, Lentsch N, Ericksen J, Bodner J, Kasiske B, Mauer M: Angiotensin II blockade in kidney transplant recipients. J Am Soc Nephrol 24: 320–327, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rovira J, Arellano EM, Carreras J, Campos B, Vodenik B, Bañón-Maneus E, Ramírez-Bajo MJ, Moya-Rull D, Solé-González A, Hernández A, Revuelta I, Quintana LF, Howat WJ, Campistol JM, Diekmann F: Mammalian target of rapamycin inhibition prevents glomerular hypertrophy in a model of renal mass reduction. Transplantation 88: 646–652, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Paoletti E, Ratto E, Bellino D, Marsano L, Cassottana P, Cannella G: Effect of early conversion from CNI to sirolimus on outcomes in kidney transplant recipients with allograft dysfunction. J Nephrol 25: 709–718, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Hernández D, Martínez D, Gutiérrez E, López V, Gutiérrez C, García P, Cobelo C, Cabello M, Burgos D, Sola E, González-Molina M: Clinical evidence on the use of anti-mTOR drugs in renal transplantation. Nefrologia 31: 27–34, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Haas M, Sis B, Racusen L, Solez K, Glotz D, Colvin R, Castro M, David D, David-Neto E, Bagnasco S, Cendales L, Cornell L, Demetris A, Drachenberg C, Farver C, Farris A, 3rd, Gibson I, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez E, Rush D, Smith RN, Tan C, Wallace W, Mengel M, Banff Meeting Report Writing Committee : Banff 2013 Meeting Report: Inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transpl 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ: Banff ‘05 Meeting Report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7: 518–526, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Weibel ER: Stereologic Methods: Practical Methods for Biologic Morphometry, London, Academic Press Inc, 1979, pp 40–116, 415 [Google Scholar]