Abstract
The effect of preexisting hypertension on living donor nephron number has not been established. In this study, we determined the association between preexisting donor hypertension and glomerular number and volume and assessed the effect of predonation hypertension on postdonation BP, adaptive hyperfiltration, and compensatory glomerular hypertrophy. We enrolled 51 living donors to undergo physiologic, morphometric, and radiologic evaluations before and after kidney donation. To estimate the number of functioning glomeruli (NFG), we divided the whole-kidney ultrafiltration coefficient (Kf) by the single-nephron ultrafiltration coefficient (SNKf). Ten donors were hypertensive before donation. We found that, in donors ages >50 years old, preexisting hypertension was associated with a reduction in NFG. In a comparison of 10 age- and sex-matched hypertensive and normotensive donors, we observed more marked glomerulopenia in hypertensive donors (NFG per kidney, 359,499±128,929 versus 558,239±205,152; P=0.02). Glomerulopenia was associated with a nonsignificant reduction in GFR in the hypertensive group (89±12 versus 95±16 ml/min per 1.73 m2). We observed no difference in the corresponding magnitude of postdonation BP, hyperfiltration capacity, or compensatory renocortical hypertrophy between hypertensive and normotensive donors. Nevertheless, we propose that the greater magnitude of glomerulopenia in living kidney donors with preexisting hypertension justifies the need for long-term follow-up studies.
Keywords: GFR, kidney donation, nephron
Judged by survival and quality of life, kidney transplantation is the most successful treatment for ESRD.1,2 Unfortunately, the gap between available donors and waitlisted candidates in the United States is growing wider each year.3 The presence of well controlled primary or essential hypertension in an otherwise low-risk candidate over 50 years of age is not a contraindication to kidney donation.4 Nevertheless, the proportion of hypertensive donors over the past 40 years has remained static at 5%–8%,5 suggesting persistent reservations about potentially adverse consequences of predonation hypertension on donor outcomes.
Much of our understanding about loss of nephron mass and development of progressive failure of the remaining kidney stems from observations in the 5/6 nephrectomized rat. In this model, the remnant nephrons undergo a maladaptive, compensatory increase in glomerular pressure and flow that, ultimately, results in the development of hypertension, proteinuria, and glomerulosclerosis.6 Previous work published by our group showed an association between increasing kidney donor age and decreased nephron number.7 Hypertension per se has also been associated with a reduction in nephron number. In an autopsy study, 10 men with left ventricular hypertrophy or hypertensive renal arteriolar changes had substantially fewer glomeruli along with glomerular hypertrophy compared with normotensive controls.8 These findings raise concern that the cumulative effect of aging and hypertension may result in living kidney donors having a level of postdonation glomerulopenia sufficient to lead to long-term renal failure.
To address this issue, we compared GFR, glomerular volumes, and calculated number of functioning glomeruli (NFG) in living donors with predonation hypertension with those in normotensive donors. We also examined the consequences of predonation hypertension on the magnitude of adaptive hyperfiltration and compensatory hypertrophy in the wake of the donation.
The study cohort (n=51) was stratified into three groups according to age and predonation BP: normotensive youthful donors (<50 years old and normotensive), normotensive aging donors (>50 years old and normotensive), and hypertensive aging donors (>50 years old and hypertensive).
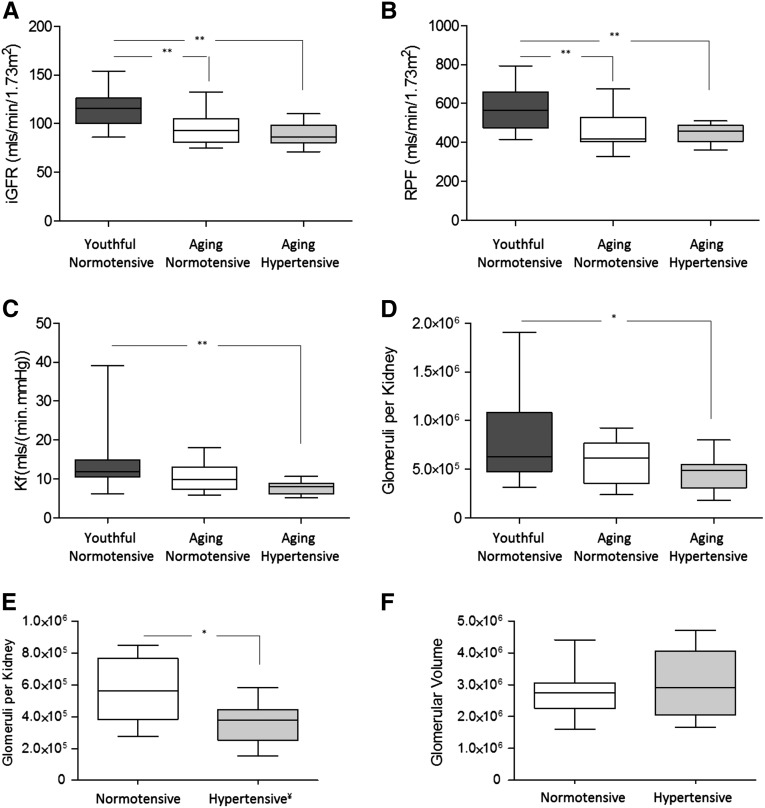
Predonation physiologic, morphometric, and radiologic evaluations of the three groups are summarized in Table 1. Figure 1, A–D shows GFR, renal plasma flow (RPF), whole-kidney ultrafiltration coefficient (Kf), and NFG for the three study groups assuming ΔP=40 mmHg across all groups for the calculation of Kf and NFG. NFG values for normotensive youthful donors, normotensive aging donors, and hypertensive aging donors were 797,250±443,668, 561,774±225,774, and 457,752±176,140, respectively (P<0.05 for hypertensive aging versus youthful normotensive).
Table 1.
Predonation demographic, physiologic, and morphometric data
| Parameter | Normotensive Youthful Donors (n=24) | Normotensive Aging Donors (n=17) | Hypertensive Aging Donors (n=10) |
|---|---|---|---|
| Women (%) | 11 (46) | 8 (47) | 6 (60) |
| Age (yr) | 33.5 (29–42) | 56 (55–59.6) | 58 (55.75–61.5) |
| MAP (mmHg) | 88 (84–92) | 93 (83–101) | 97 (91–109) |
| Iothalamate GFR (ml/min per 1.73 m2) | 115±18 | 95±16a | 89±12a |
| RPF (ml/min per 1.73 m2) | 574±110 | 466±103a | 448±52a |
| Filtration fraction | 0.20±0.03 | 0.21±0.02 | 0.2±0.03 |
| πA (mmHg) | 26.7±2.7 | 26.5±1.9 | 24.8±1.9 |
| Whole-kidney Kf (ml/[min/mmHg]) | 11.9 (10.5–14.9) | 9.8 (7.3–13) | 8 (6.1–8.8)a |
| Global sclerosis (%) | 0 (0–3) | 4.3 (0–7.5) | 5.6 (0–10.5) |
| Glomerular volume (µm3×106) | 2.71±0.69 | 2.67±0.76 | 3.03±1.03 |
| Basement membrane thickness (nm) | 342 (318–378) | 328 (291–357) | 331 (314–391) |
| Filtration slit frequency (per mm) | 1650±192 | 1642±143 | 1553±159 |
| Hydraulic permeability (m/s per Pa×10−9) | 2.7±0.3 | 2.7±0.3 | 2.5±0.2 |
| SNKf (nl/[min/mmHg]) | 9.27 (7.63–11.45) | 10.94 (7.8–11.9) | 9.21 (6.99–11.74) |
| Renocortical volume (cm3) | 115±21b | 105±15c | 98±26 |
P<0.01 versus normotensive youthful donors.
n=23.
n=15.
Figure 1.
Predonation filtration dynamics, glomerular number, and glomerular volume in living kidney donors. (A) Iothalamate GFR (iGFR) measured predonation in youthful normotensive (n=24), aging normotensive (n=17), and aging hypertensive (n=10) groups. (B) RPF estimated by para-aminohippuric acid clearance in subjects before donation. (C) Predonation ultrafiltration coefficient (two kidneys) before donation. (D) Estimated number of glomeruli per kidney in youthful normotensive, aging normotensive, and aging hypertensive groups. (E) Estimated number of glomeruli per kidney in 10 age- and sex-matched normotensive and hypertensive subjects. ¥Glomerular number computed using ΔP=43 mmHg for hypertensive versus ΔP=40 mmHg for normotensive to allow for glomerular transmission of one third of the 9-mmHg difference in MAP between the groups. (F) Glomerular volume in 10 age- and sex-matched normotensive and hypertensive subjects. *P<0.05; ** P<0.01.
To better assess the association between BP and glomerular number, we matched hypertensive donors with normotensive controls (1:1) by age and sex (Table 2). On average, mean arterial pressure (MAP) was 9.2 mmHg higher in the hypertensive group (P=0.02). We accordingly assumed that 33% of the excess was transmitted into glomerular capillaries and used ΔP=43 mmHg to estimate NFG in the hypertensive donors versus 40 mmHg in normotensive controls. Figure 1E shows the distribution of NFG for matched normotensive and hypertensive donors. For normotensive and hypertensive donors, NFG was 558,239±205,152 and 359,499±128,929, respectively (P=0.02). In the less likely event that ΔP remained the same in hypertensive donors as in normotensive donors (i.e., ΔP=40 mmHg), NFG in the former would have been 457,752±176,140 (P=0.25), a value no longer significantly lower than normotensive controls. Figure 1F shows that the glomerular volume was not significantly different in the matched normotensive and hypertensive donors: 2,763,000±795,546 µm3 versus 3,034,000±1,034,821 µm3, respectively (P=0.55).
Table 2.
Matched hypertensive and normotensive donors
| Subject | Donor Sex | Donor Age (yr) | Race | MAP (mmHg) | GFR (ml/min per 1.73 m2) | Glomerular Volume (µm3×106) | Glomeruli per Kidney (×105) |
|---|---|---|---|---|---|---|---|
| H1 | Woman | 55 | Asian | 96 | 82 | 2.77 | 2.78 |
| H2 | Woman | 55 | Asian | 108 | 84 | 4.29 | 3.77 |
| H3 | Woman | 56 | Caucasian | 77 | 96 | 1.88 | 4.30 |
| H4 | Woman | 58 | Caucasian | 96 | 86 | 2.6 | 2.57 |
| H5 | Woman | 58 | Caucasian | 112 | 71 | 1.67 | 5.81 |
| H6 | Woman | 63 | Hispanic | 81 | 86 | 4.71 | 1.51 |
| H7 | Man | 56 | Caucasian | 101 | 75 | 3.97 | 2.38 |
| H8 | Man | 59 | African American | 95 | 105 | 3.05 | 4.88 |
| H9 | Man | 61 | Asian | 98 | 91 | 2.12 | 4.17 |
| H10 | Man | 68 | Caucasian | 114 | 110 | 3.29 | 3.77 |
| Mean/median | 58 (55.8–61.5) | 98±12 | 89±12 | 3.03±1.04 | 3.59±1.29 | ||
| C1 | Woman | 55 | Caucasian | 79 | 98 | 2.88 | 2.75 |
| C2 | Woman | 55 | Hispanic | 90 | 132 | 2.89 | 7.66 |
| C3 | Woman | 56 | Caucasian | 74 | 105 | 1.60 | 8.47 |
| C4 | Woman | 56 | Caucasian | 76 | 105 | 4.41 | 6.36 |
| C5 | Woman | 57 | Caucasian | 99 | 93 | 2.63 | 4.34 |
| C6 | Woman | 62 | Caucasian | 86 | 87 | 2.47 | 6.64 |
| C7 | Man | 56 | Caucasian | 92 | 78 | 2.37 | 2.92 |
| C8 | Man | 59 | Caucasian | 89 | 84 | 1.93 | 4.88 |
| C9 | Man | 60 | Asian | 106 | 78 | 3.58 | 7.65 |
| C10 | Man | 67 | Caucasian | 95 | 93 | 2.86 | 4.13 |
| Mean/median | 56.5 (55.8–60.5) | 89±10 | 95±16 | 2.76±0.8 | 5.58±2.05 | ||
| P value | 0.05 | 0.11 | 0.02 | 0.34 | 0.55 | 0.02 |
We collected follow-up data 6 months postkidney donation on BP, GFR, and RPF for 49 patients (normotensive youthful, n=23; normotensive aging, n=16; and hypertensive aging, n=10). We also determined the postdonation change in renocortical volume for 42 subjects (normotensive youthful, n=19; normotensive aging, n=13; and hypertensive aging, n=10) (Figure 2). No subjects in the young or normotensive groups were using antihypertensive medications at 6 months postdonation. Postdonation antihypertensive medication was initiated in one subject and continued in seven subjects of the hypertensive group (Supplemental Table 1). We also compared change in GFR, RPF, and MAP (n=9 per group) as well as renocortical volume (n=8 per group) before and 6 months postdonation in the matched normotensive and hypertensive aging groups; we found no significant differences in percentage increase in GFR (35±9% versus 36±9%, respectively), RPF (34±6% versus 31±18%%, respectively), or renocortical volume (27±7% versus 26±11%%, respectively), and mean percentage change in MAP was −4±10% versus −4±7%%, respectively. No subjects in the matched hypertensive and normotensive aging groups had evidence of micro- or macroalbuminuria at baseline or follow-up. The median urine albumin-to-creatinine ratios in the matched hypertensive and control groups were 3.8 (nondetected: −5.9) versus 1.4 mg/g (nondetected: −9.7) and 4.1 (nondetected: −7.9) versus 1.1 mg/g (nondetected: −4.8) for predonation versus postdonation, respectively.
Figure 2.
Changes in GFR, RPF, renocortical volume, and MAP before and approximately 6 months after kidney donation. (A) Pre- and postdonation single-kidney GFR in youthful normotensive (n=23), aging normotensive (n=16), and aging hypertensive (n=10) groups. (B) Pre- and postdonation single-kidney RPF. (C) Pre- and postdonation single-kidney renocortical volume measurements in youthful normotensive (n=19), aging normotensive (n=13), and aging hypertensive (n=10) groups. (D) Change in MAP from pre- to post-donation. *P<0.05.
In our detailed study of structure and function in living donor kidneys, we found predonation hypertension to be associated with modest reduction below age- and sex-matched normotensive controls in glomerular number. We identified no difference in predonation glomerular volume or postdonation capacity for adaptive hyperfiltration or compensatory renocortical hypertrophy between hypertensive and normotensive donors. Our findings contrast with the autopsy findings in the work by Keller et al.,8 which found that subjects with hypertension had an almost 50% reduction in glomerular number and doubling of glomerular volume compared with age-matched controls. However, the subjects in that study had long-standing hypertension complicated by end organ damage. The wide variation in derived NFG, particularly in our younger subjects, is consistent with the autopsy observations in the work by Nyengaard and Bendtsen9 of glomerular number in 37 subjects with normal antemortem renal function.
Long-term studies of the risk of ESRD and mortality in living kidney donors have been broadly reassuring.10,11 Newer data suggest that, although the risk of mortality, cardiovascular disease, and ESRD in living donors is similar to the population at large, it is significantly higher than that of stringently matched healthy controls.12,13 However, the absolute risk of ESRD after kidney donation remains relatively low, with an estimated increase in lifetime absolute risk of 76 cases of ESRD per 10,000 United States kidney donors compared with healthy nondonors.13
Studies that have focused on the outcome of donors with preexisting hypertension have been small; a Dutch study that followed 29 and 13 donors with preexisting hypertension to 1 and 5 years postdonation, respectively, found no difference in postdonation BP or GFR compared with normotensive controls.14 Textor et al.15 reported no difference in GFR between 24 living donors with preexisting hypertension and normotensive controls followed in the first 1 year postdonation, and BP remained easy to control in the hypertensive group postdonation.15 In a recent study, 14 Japanese living donors with hypertension and high-normal albumin-to-creatinine ratios (15–30 mg/g) had similar outcomes to normotensive donors in terms of kidney function over 2 years of follow-up.16
A question that is still unanswered is how many nephrons does a human need after kidney donation to avoid the maladaptive compensatory response observed in the experimental remnant kidney? Our findings suggest that otherwise healthy kidney donors with essential hypertension have modest glomerulopenia. In the short term, the hypertensive donor group adapted to uninephrectomy with increments in GFR and renocortical volume comparable with those in age- and sex-matched normotensive controls; however, long-term follow-up is undoubtedly required to better address this issue.
To our knowledge, this study represents the most comprehensive assessment of renal structure and function in living donors with preexisting hypertension to date. Our hypertensive subjects had mild, well controlled hypertension and are representative of the hypertensive donor population currently used in the United States.4 Shortcomings of the study include the small sample of hypertensive subjects and consequent limitations of statistical power as well as the short period of follow-up. All hypertensive subjects were >50 years of age (because of selection policy), which limits the applicability of our findings to younger subjects. In addition, our derivation of glomerular number has certain limitations, most notably in the estimation of ΔP. In the analysis of age- and sex-matched normotensive and hypertensive donors, we calculated that the mean 9-mmHg increase in arterial pressure would translate into a 3-mmHg rise in glomerular capillary pressure and hence, ΔP=43 mmHg in the hypertensive group.17–19 Using a sensitivity analysis, we estimate that NFG in the hypertensive group would remain depressed at 42 mmHg but would no longer be significantly depressed if ΔP=40 or 41 mmHg in this population. Another confounding factor was the use of angiotensin-converting enzyme inhibitors or angiotensin-converting receptor blockers in one half of the hypertensive donors, which could have resulted in a lowering of ΔP.20 Finally, we lack data on birth weight, which is a well established correlate of glomerular number.21
In summary, we estimate that older kidney donors with preexisting hypertension have modestly fewer glomeruli than in those who are normotensive. In particular, those hypertensive donors in the lower quartile of glomerular number seem to be left with only 150,000–250,000 remaining glomeruli, a number that may be sufficiently low to result in the remnant kidney phenomenon. However, we observed no difference in glomerular volume or 6-month postdonation hyperfiltration capacity or compensatory hypertrophy in those donors with hypertension compared with their normotensive controls. Our findings raise concern about the potential for postdonation glomerulopenia in donors with preexisting hypertension. However, larger studies with longer follow-up of living donors in this category are required before additional conclusions can be drawn.
Concise Methods
Study Population
The study population consisted of 51 adult living kidney donors recruited before donation. All donors underwent standard medical and psychosocial predonation assessment. At our center, candidates under the age of 50 years old with hypertension are routinely excluded because of concern regarding increased lifetime risk of end organ damage. Candidates who were over 50 years of age and had well controlled hypertension on up to two medications but no evidence of end organ damage were considered eligible for donation. Hypertension was defined as antihypertensive medication use or in untreated subjects, hypertension on 24-hour ambulatory monitoring (average≥140/90 mmHg was considered abnormal). Exclusion criteria included body mass index>30 kg/m2, abnormal glucose tolerance test, urinary creatinine clearance <80 ml/min per 1.73 m2, and proteinuria. The entire cohort was divided into three groups according to age and BP: (1) youthful normotensive group: <50 years old and normotensive, (2) aging normotensive group: >50 years old and normotensive, and (3) aging hypertensive group: >50 years old and hypertensive. For direct comparison of normotensive and hypertensive groups, 10 hypertensive subjects were age- and sex-matched with 10 normotensive controls.
Assessment of Kidney Function
Before and approximately 6 months after kidney donation, subjects underwent assessment of GFR and RPF as determined by urinary clearances of iothalamate and para-aminohippuric acid, respectively. RPF was corrected for an assumed para-aminohippuric acid extraction ratio of 0.9, which has been described previously.22 BP was measured using Dynamap. Plasma oncotic pressure was assayed by membrane osmometry.23 For each subject, we calculated Kf using a modification of the formula described by Deen et al.24: Kf = QA/ΔP [FF+Aln(1−A/1−A−FF)], where ΔP is the difference in hydraulic pressure across the glomerular capillary wall, A is arteriolar oncotic pressure/ΔP, QA is RPF, and FF is the filtration fraction (GFR/RPF).25 We have estimated that ΔP in our healthy human controls approximates 40 mmHg. Because humans are in filtration pressure disequilibrium,26–29 ΔP must be higher than the oncotic pressure in the efferent arteriole (πea); πea can be calculated by multiplying measured plasma oncotic pressure by 1+FF and ranges in value between 30 and 35 mmHg. Our group has previously shown that assigning ΔP values of 3–10 mmHg above πea (36–43 mmHg) yields a realistic value for expected Kf in humans.26 To allow for transmission of elevated MAP into glomerular capillaries, we assigned a ΔP=43 mmHg to the hypertensive group.
Estimation of Kidney Volumes
Donors underwent either magnetic resonance imaging (MRI) or computed tomography (CT) renal angiography pre- and postdonation. Cortical volume at 6 months postnephrectomy was compared with the corresponding prenephrectomy values. Whole-kidney and cortical volumes were estimated using three-dimensional imaging with either CT or MRI according to the Cavalieri principle.7,30 Because of concerns about gadolinium toxicity,31 we replaced MRI with contrast CT imaging during the course of our study. In six subjects, direct comparison between the two techniques showed excellent concordance (within 7 cm3) for estimating kidney volume.
Structural Evaluation of Glomeruli
A cortical wedge biopsy was taken from the donated kidney before transplantation, fixed in paraformaldehyde/gluteraldehyde, and embedded in LX-112 epoxy resin (Ladd Research, Williston, VT). Samples were prepared for light and electron microscopy as described previously.25 Briefly, the percentage of global glomerular sclerosis was determined from (×50) light microscopic images using an equation that takes into account the smaller diameter of sclerotic glomeruli and the consequent difference in the probability of encountering a glomerulus of either type in a random cross-section.32,33 The fractional interstitial area and glomerular volume were measured using light microscopy. Montages of three whole glomerular profiles from each subject were prepared from the transmission electron microscopy images for determination of filtration surface density (Sv) of the peripheral glomerular capillary wall by line intercept methods.34 Filtration surface area provided by the peripheral capillary wall per glomerulus (S) was calculated from the product of Sv and glomerular volume35; we used resin-embedded sections to circumvent the substantial glomerular shrinkage associated with paraffin embedding.36 The assessment of hydraulic permeability (k) of the glomerular capillary walls was performed at the ultrastructural level (×12,000). Dimensions of the podocyte layer, which accounts for 50% resistance to water flow, included the width and the frequency of the filtration slits, in which the latter is determined by counting the total number of epithelial filtration slits and dividing it by the total length of the peripheral glomerular capillary wall.32,33 The glomerular basement membrane accounts for the remaining resistance to water flow, and its thickness, an important determinant of k, was calculated from the harmonic mean glomerular basement membrane thickness as measured by the orthogonal intercept method.34 The single-nephron ultrafiltration coefficient (SNKf) was calculated as the product of filtration surface area per glomerulus and hydraulic permeability of the glomerular capillary wall: SNKf=S×k.32,33 NFG was then calculated by dividing the Kf by SNKf: NFG=Kf/SNKf.
Statistical Analyses
Results are reported as means±1 SD or medians and interquartile ranges, where distributions were Gaussian or non-Gaussian, respectively. Statistical analyses were performed using the chi-squared test, t test, one-way ANOVA, or Kruskal–Wallis test and where appropriate, followed by Bonferroni or Dunne multiple comparison tests.
Disclosures
None.
Supplementary Material
Acknowledgments
Funding was provided by the Satellite Extramural Grant Foundation and the John M. Sobrato Foundation. C.R.L. is supported by an American Society of Nephrology Research Fellowship. J.C.T. is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant K23-DK087937.
The study was presented in abstract form at the National Kidney Foundation Young Investigator’s Forum (April 22, 2014) in Las Vegas, NV and the World Transplant Congress (July 27, 2014) in San Francisco, CA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014030304/-/DCSupplemental.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N: A study of the quality of life and cost-utility of renal transplantation. Kidney Int 50: 235–242, 1996 [DOI] [PubMed] [Google Scholar]
- 3.OPTN/SRTR: OPTN/SRTR 2011 Annual Data Report: Kidney. Available at: http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/01_kidney_12.pdf. Accessed March 25, 2014
- 4.OPTN: OPTN Guidelines for the Medical Evaluation of Living Kidney Donors (Living Donor Committee): 2007. Available at: http://optn.transplant.hrsa.gov/PublicComment/pubcommentPropSub_208.pdf. Accessed March 24, 2014
- 5.Taler SJ, Messersmith EE, Leichtman AB, Gillespie BW, Kew CE, Stegall MD, Merion RM, Matas AJ, Ibrahim HN, RELIVE Study Group : Demographic, metabolic, and blood pressure characteristics of living kidney donors spanning five decades. Am J Transplant 13: 390–398, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson JL, Hostetter TH, Rennke HG, Brenner BM, Venkatachalam MA: Altered glomerular permselectivity and progressive sclerosis following extreme ablation of renal mass. Kidney Int 22: 112–126, 1982 [DOI] [PubMed] [Google Scholar]
- 7.Tan JC, Busque S, Workeneh B, Ho B, Derby G, Blouch KL, Sommer FG, Edwards B, Myers BD: Effects of aging on glomerular function and number in living kidney donors. Kidney Int 78: 686–692, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller G, Zimmer G, Mall G, Ritz E, Amann K: Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Nyengaard JR, Bendtsen TF: Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, Gross CR, Matas AJ: Long-term consequences of kidney donation. N Engl J Med 360: 459–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehrman-Ekholm I, Elinder CG, Stenbeck M, Tydén G, Groth CG: Kidney donors live longer. Transplantation 64: 976–978, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Mjøen G, Hallan S, Hartmann A, Foss A, Midtvedt K, Oyen O, Reisæter A, Pfeffer P, Jenssen T, Leivestad T, Line PD, Ovrehus M, Dale DO, Pihlstrøm H, Holme I, Dekker FW, Holdaas H: Long-term risks for kidney donors. Kidney Int 86: 162–167, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, Segev DL: Risk of end-stage renal disease following live kidney donation. JAMA 311: 579–586, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tent H, Sanders JS, Rook M, Hofker HS, Ploeg RJ, Navis G, van der Heide JJ: Effects of preexistent hypertension on blood pressure and residual renal function after donor nephrectomy. Transplantation 93: 412–417, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Textor SC, Taler SJ, Driscoll N, Larson TS, Gloor J, Griffin M, Cosio F, Schwab T, Prieto M, Nyberg S, Ishitani M, Stegall M: Blood pressure and renal function after kidney donation from hypertensive living donors. Transplantation 78: 276–282, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Sofue T, Inui M, Hara T, Moriwaki K, Kushida Y, Kakehi Y, Nishiyama A, Kohno M: Short-term prognosis of living-donor kidney transplantation from hypertensive donors with high-normal albuminuria. Transplantation 97: 104–110, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Steiner RW, Tucker BJ, Gushwa LC, Gifford J, Wilson CB, Blantz RC: Glomerular hemodynamics in moderate Goldblatt hypertension in the rat. Hypertension 4: 51–57, 1982 [DOI] [PubMed] [Google Scholar]
- 18.Dworkin LD, Hostetter TH, Rennke HG, Brenner BM: Hemodynamic basis for glomerular injury in rats with desoxycorticosterone-salt hypertension. J Clin Invest 73: 1448–1461, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baer PG, Bianchi G, Liliana D: Renal micropuncture study of normotensive and Milan hypertensive rats before and after development of hypertension. Kidney Int 13: 452–466, 1978 [DOI] [PubMed] [Google Scholar]
- 20.Anderson S, Meyer TW, Rennke HG, Brenner BM: Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest 76: 612–619, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughson M, Farris AB, 3rd, Douglas-Denton R, Hoy WE, Bertram JF: Glomerular number and size in autopsy kidneys: The relationship to birth weight. Kidney Int 63: 2113–2122, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Battilana C, Zhang HP, Olshen RA, Wexler L, Myers BD: PAH extraction and estimation of plasma flow in diseased human kidneys. Am J Physiol 261: F726–F733, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Canaan-Kühl S, Venkatraman ES, Ernst SI, Olshen RA, Myers BD: Relationships among protein and albumin concentrations and oncotic pressure in nephrotic plasma. Am J Physiol 264: F1052–F1059, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Deen WM, Robertson CR, Brenner BM: A model of glomerular ultrafiltration in the rat. Am J Physiol 223: 1178–1183, 1972 [DOI] [PubMed] [Google Scholar]
- 25.Tan JC, Workeneh B, Busque S, Blouch K, Derby G, Myers BD: Glomerular function, structure, and number in renal allografts from older deceased donors. J Am Soc Nephrol 20: 181–188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers BD, Hilberman M, Carrie BJ, Spencer RJ, Stinson EB, Robertson CR: Dynamics of glomerular ultrafiltration following open-heart surgery. Kidney Int 20: 366–374, 1981 [DOI] [PubMed] [Google Scholar]
- 27.Shemesh O, Deen WM, Brenner BM, McNeely E, Myers BD: Effect of colloid volume expansion on glomerular barrier size-selectivity in humans. Kidney Int 29: 916–923, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Myers BD, Peterson C, Molina C, Tomlanovich SJ, Newton LD, Nitkin R, Sandler H, Murad F: Role of cardiac atria in the human renal response to changing plasma volume. Am J Physiol 254: F562–F573, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Coruzzi P, Biggi A, Musiari L, Ravanetti C, Novarini A: Renal hemodynamics and natriuresis during water immersion in normal humans. Pflugers Arch 407: 638–642, 1986 [DOI] [PubMed] [Google Scholar]
- 30.Christiansen T, Rasch R, Stødkilde-Jørgensen H, Flyvbjerg A: Relationship between MRI and morphometric kidney measurements in diabetic and non-diabetic rats. Kidney Int 51: 50–56, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, Djamali A: Nephrogenic systemic fibrosis: Risk factors and incidence estimation. Radiology 243: 148–157, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Drumond MC, Kristal B, Myers BD, Deen WM: Structural basis for reduced glomerular filtration capacity in nephrotic humans. J Clin Invest 94: 1187–1195, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drumond MC, Deen WM: Structural determinants of glomerular hydraulic permeability. Am J Physiol 266: F1–F12, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Jensen EB, Gundersen HJ, Osterby R: Determination of membrane thickness distribution from orthogonal intercepts. J Microsc 115: 19–33, 1979 [DOI] [PubMed] [Google Scholar]
- 35.Weibel ER: Practical Methods of Biological Morphometry, London, Academic Press, 1979, pp 1–415 [Google Scholar]
- 36.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.