Abstract
Cardiac dysfunction in CKD is characterized by aberrant cardiac remodeling with hypertrophy and fibrosis. CKD is a state of severe systemic Klotho deficiency, and restoration of Klotho attenuates vascular calcification associated with CKD. We examined the role of Klotho in cardiac remodeling in models of Klotho deficiency—genetic Klotho hypomorphism, high dietary phosphate intake, aging, and CKD. Klotho-deficient mice exhibited cardiac dysfunction and hypertrophy before 12 weeks of age followed by fibrosis. In wild-type mice, the induction of CKD led to severe cardiovascular changes not observed in control mice. Notably, non-CKD mice fed a high-phosphate diet had lower Klotho levels and greatly accelerated cardiac remodeling associated with normal aging compared with those on a normal diet. Chronic elevation of circulating Klotho because of global overexpression alleviated the cardiac remodeling induced by either high-phosphate diet or CKD. Regardless of the cause of Klotho deficiency, the extent of cardiac hypertrophy and fibrosis correlated tightly with plasma phosphate concentration and inversely with plasma Klotho concentration, even when adjusted for all other covariables. High-fibroblast growth factor–23 concentration positively correlated with cardiac remodeling in a Klotho-deficient state but not a Klotho-replete state. In vitro, Klotho inhibited TGF-β1–, angiotensin II–, or high phosphate–induced fibrosis and abolished TGF-β1– or angiotensin II–induced hypertrophy of cardiomyocytes. In conclusion, Klotho deficiency is a novel intermediate mediator of pathologic cardiac remodeling, and fibroblast growth factor–23 may contribute to cardiac remodeling in concert with Klotho deficiency in CKD, phosphotoxicity, and aging.
Keywords: CKD, heart disease, hypertrophy, ischemia-reperfusion, transgenic mouse, uremia
Cardiovascular disease in CKD is characterized by vascular calcification1 and uremic cardiomyopathy.2 Uremic cardiomyopathy was noted in 19433 as a state of pathologic cardiac remodeling histologically characterized by left ventricular hypertrophy (LVH) and extensive fibrosis.4,5 The prevalence of LVH is approximately 48% in children on chronic peritoneal dialysis.6 In adults, increase in left ventricular mass index is found in >60% of patients after 96 weeks of hemodialysis.7 After multivariable adjustment, patients with stage 3 or higher (<30 ml/min per 1.73 m2) CKD have 2-fold higher risk of LVH compared with patients with stage 2 or lower CKD (≥60 ml/min per 1.73 m2).8 Cardiac fibrosis is a prominent characteristic of uremic cardiomyopathy in biopsy and postmortem examinations in humans9,10 and animals,11 and the higher degree of fibrosis is associated with higher mortality.10 Although LVH in patients with CKD has been noted for a long time,12 the prevalence of cardiac fibrosis is not known. Furthermore, the origin and mechanisms of fibrosis in the heart in general are not well understood. Any improvement in understanding the pathogenesis of cardiac hypertrophy and fibrosis in CKD will help construct novel therapies for this dire complication.
Klotho was originally identified as an antiaging protein.13 Two paralogs were discovered subsequently,14–16 and these three members are termed α, β, and γ with diverse functions.17 The first member of the Klotho family (αKlotho, which is simply called Klotho here) has multiple actions.17 Transmembrane Klotho functions as a coreceptor for fibroblast growth factor–23 (FGF23) to regulate external phosphate balance.18–20 The extracellular domain of Klotho circulates as soluble Klotho21,22 and exerts myriad effects.17,23,24 We and others have shown that CKD is a state of severe Klotho deficiency25–27 and that Klotho restoration can ameliorate vascular calcification in CKD.26,28,29 It is not known whether Klotho plays a role in uremic cardiomyopathy, although genetic Klotho–deficient mice do have cardiac hypertrophy.30 Recently, Xie et al.31 found that Klotho can protect the heart by inhibiting TRPC6 calcium channels. In experimental animals, FGF23 seems to induce cardiac hypertrophy independent of CKD,30 but whether FGF23 contributes to the cardiac fibrosis in CKD and whether Klotho protects the heart from fibrosis are unknown. This study shows five points. (1) Cardiac hypertrophy and fibrosis are in primary genetic Klotho deficiency, and secondary Klotho deficiency from phosphate loading, aging, and CKD. (2) Cardiac hypertrophy precedes cardiac fibrosis and is associated with left ventricular dysfunction. (3) Higher phosphate and lower Klotho correlate with more cardiac hypertrophy and fibrosis in all models studied. (4) Higher FGF23 is associated with more dramatic cardiac hypertrophy and fibrosis only in concert with moderate or low plasma Klotho. (5) In vitro, Klotho blocks TGF-β1– and angiotensin II (Ang II)–induced hypertrophy in cardiomyocyte and attenuates TGF-β1–, Ang II–, and high phosphate–induced upregulation of fibrosis markers in cultured cardiac fibroblasts.
Results
Cardiac Dysfunction, Hypertrophy, and Fibrosis in Klotho-Deficient Mice
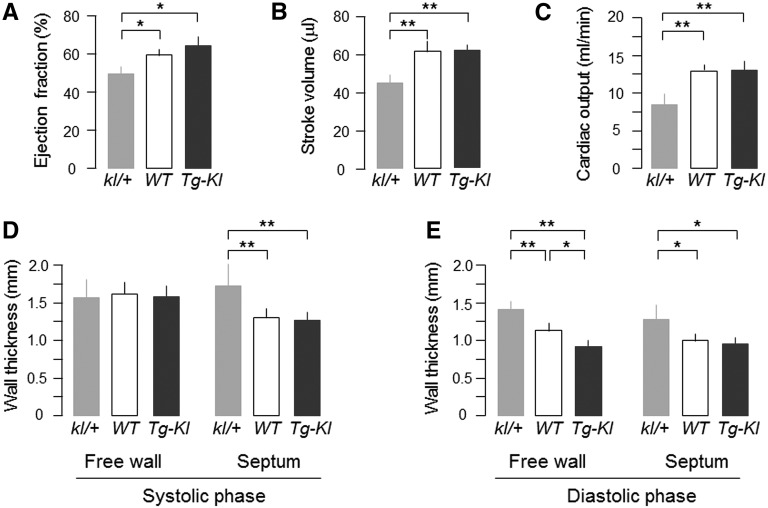
We first examined primary Klotho deficiency from silencing of the Klotho gene resulting in hypomorphic expression. Because of the universal mortality of kl/kl (silencing of both alleles) mice during stress,32 we assessed cardiac function only in heterozygous Klotho–deficient (kl/+) mice by magnetic resonance imaging (MRI). At 12 weeks, the body weights were similar in kl/+ (23.8±2.2 g; n=11), wild-type (WT) (24.1±1.9 g; n=12), and transgenic Klotho–overexpressing mice (Tg-Kl; 22.3±1.9 g; n=12), and kl/+ mice had lower ejection fraction (Supplemental Movie 1), stroke volume, and cardiac output (Figure 1, A–C) and thicker left ventricular wall compared with WT and Tg-Kl mice (Figure 1, D and E). The left ventricular free wall at diastole was slightly thicker in WT than Tg-Kl mice, but cardiac function was not different (Figure 1E). Klotho levels were clearly lower in kl/+ and higher in Tg-Kl mice compared with WT mice (Supplemental Figure 1).
Figure 1.
Klotho deficiency impairs cardiac function. kl/+, WT, and Tg-Kl mice at 12 weeks of age fed with normal diet were subjected to cardiac MRI under anesthesia. (A) Left ventricular ejection fraction, (B) left ventricular stroke volume, (C) cardiac output, (D) left ventricular wall thickness at systole, and (E) left ventricular wall thickness at diastole. Data expressed as means±SDs of eight mice from each group, and statistical significance was assessed by one-way ANOVA followed by Newman–Keuls test. Significant differences were accepted when *P<0.05 or **P<0.01 between groups.
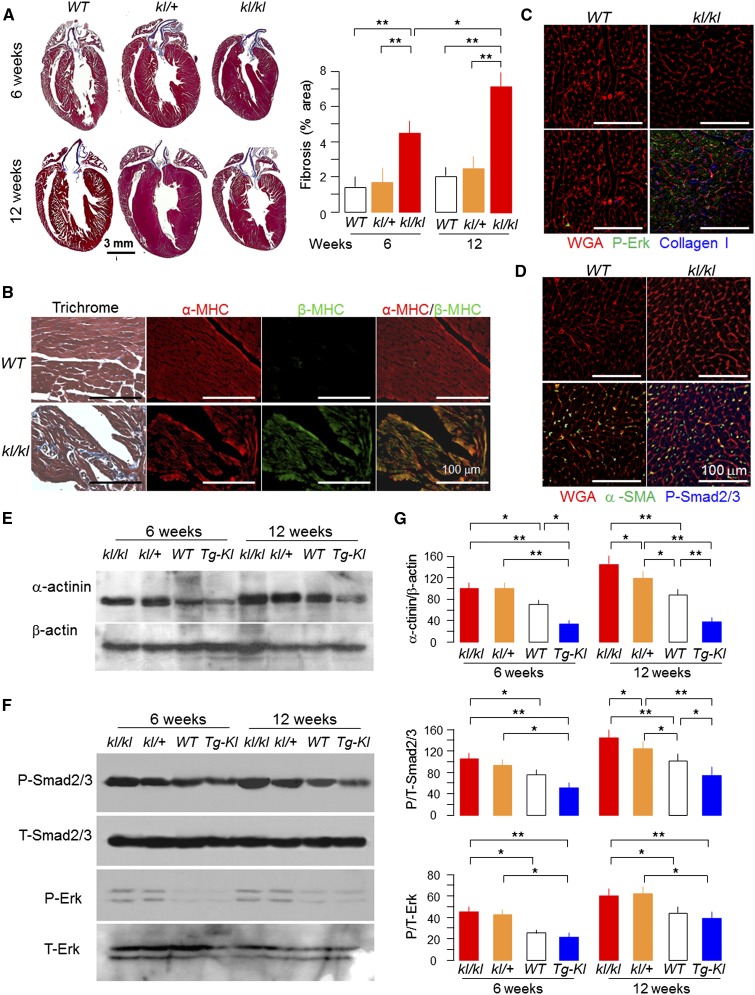
In addition to the cardiac hypertrophy, which was also shown previously,30 there was marked increase in the fibrotic area in kl/kl mice compared with WT mice reminiscent of hearts from human CKD.9,10 Note that young kl/+ mice (<12 weeks old) already had cardiac hypertrophy but mild or no fibrosis, indicating that cardiac hypertrophy precedes fibrosis and that fibrosis worsened with age (Figure 2, A and B). Cardiac function was already abnormal at this early stage with only modest Klotho deficiency (Supplemental Figure 1).
Figure 2.
Klotho-deficient mice with severe cardiac remodeling. (A, left panel) Representative macrograph of sagittal sections of the hearts (Trichrome) of kl/kl, kl/+, and WT mice at 6 and 12 weeks of age. (A, right panel) Shows semiquantification of the Trichrome-positive area over the whole-heart section (Image J). (B) Representative micrographs showing cardiac fibrosis by Trichrome staining and immunohistochemistry for α/β-MHC in the left ventricle of kl/kl and WT mice at 12 weeks of age. (C) Representative immunohistochemistry for P-Erk (green) and collagen I (blue). (D) α-SMA, P-Smad2/3, and Alexa-Fluor–WGA (red) in left ventricular sections of kl/kl and WT mice at 12 weeks old. (E) Representative immunoblots for α-actinin and β-actin. (F) P/T-Smad2/3 and P/T-Erk in left ventricular lysates from 6- and 12-week-old mice. (G) Summary of immunoblots in arbitrary units. Means±SDs (n=4 from each group). Statistical significance was assessed by one-way ANOVA followed by Newman–Keuls test. Significant differences were accepted when *P<0.05 or **P<0.01 between groups. α-SMA, α-smooth muscle actin; T, total; WGA, wheat germ agglutinin.
An increase in α-actinin and upregulation of β-MHC protein were accompanied by cardiomyocyte hypertrophy in kl/kl mice (Figure 2, B–E). Global Klotho deficiency increased, and ubiquitous Klotho overexpression suppressed phosphorylation of Smad2/3 and extracellular signal–regulated kinase (Erk), which are known to be involved in cardiac fibrosis (Figure 2B).33–35 These results suggest that Klotho levels affect cardiac remodeling.
Cardiac Hypertrophy and Fibrosis in CKD
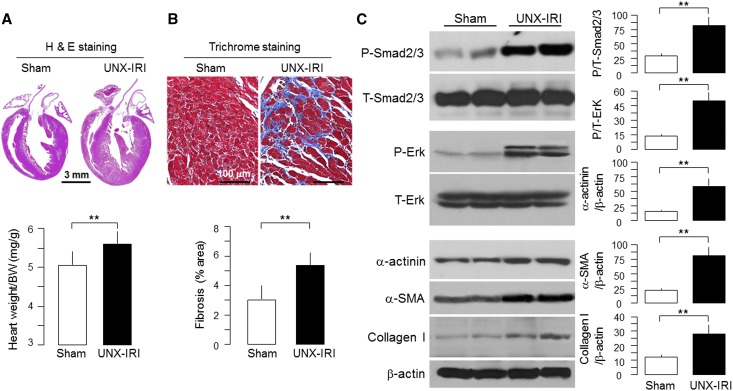
We next investigated whether secondary Klotho deficiency can lead to similar cardiac lesions. We studied two CKD models: (1) unilateral nephrectomy (UNX) and contralateral ischemia-reperfusion injury (IRI; UNX-IRI) followed by high-phosphate diet (2% phosphate)26 and (2) 5/6th nephrectomy. Features of CKD included increase in plasma creatinine, inorganic phosphate (Pi), FGF23, and parathyroid hormone (PTH), reduction in plasma 1,25-dihydroxyl-vitamin D3 [1,25(OH)2D3], and mild but statistically significant elevation of BP as described in our previous publications (data not shown).26,30 As expected,30 there was drastic reduction of plasma, urine, and renal Klotho in UNX-IRI (data not shown).
Both CKD models showed cardiac hypertrophy and left ventricular fibrosis (Figure 3, A and B, Supplemental Figure 2). There was significant increase in α-actinin (marker of cardiomyocyte hypertrophy), α-smooth muscle actin, and collagen I protein (marker of fibrosis) (Figure 3C). The increase in phospho-Smad2/3 (P-Smad2/3) and phospho-extracellular signal regulated kinase (P-Erk) in uremic heart (Figure 3C) suggests that activation of Smad and Erk signal pathways is involved.33–35
Figure 3.
Cardiac hypertrophy and fibrosis in CKD mice. WT mice at 12 weeks of age underwent CKD surgery (UNX-IRI) or laparotomy (sham). One month after surgery, mice were fed with high-phosphate diet for 12 weeks. (A, upper panel) Representative macrographs of sagittal sections (H&E). Scale bar, 3 mm. (A, lower panel) Summary of the ratio of HW to body weight of sham and CKD mice. (B, upper panel) Representative micrographs of left ventricular sections (Trichrome). Scale bar, 100 μm. (B, lower panel) Summary of semiquantification of the Trichrome-positive area over the whole-heart section by Image J. (C, left panel) Representative immunoblots for P/T-Smad2/3, P/T-Erk, and α-SMA. Collagen I and β-actin in left ventricular lysates from sham and CKD were induced by UNX-IRI. Summaries are shown in C, right panel. Data are means±SDs (n=4 from each group). Statistical significance was assessed by t test. Significance was accepted when **P<0.01 between groups. α-SMA, α-smooth muscle actin; H&E, hematoxylin and eosin; T, total.
Low Circulating and Renal Klotho, Aging, and High Dietary Phosphate Synergistically Induced Pathologic Cardiac Remodeling
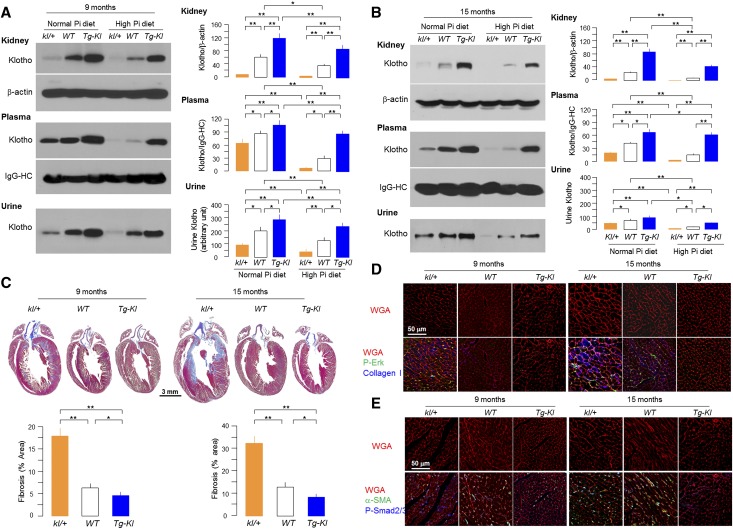
Phosphotoxicity is gathering attention as a potential risk factor for cardiovascular and renal disease.17,24,36 To better define the role of Klotho in cardiac remodeling induced by high-phosphate diet and aging, we challenged kl/+, WT, and Tg-Kl mice at 6 months of age with the same high-phosphate diet for 12 weeks. Klotho protein in kidney, plasma, and urine was decreased by phosphate loading (Figure 4A). The high plasma Pi and FGF23 levels were exaggerated in kl/+ mice and attenuated in Tg-Kl mice (Supplemental Table 1).
Figure 4.
Synergism of high-phosphate diet, Klotho deficiency, and aging on cardiac remodeling. kl/+, WT, and Tg-Kl mice at 6 or 12 months of age were fed normal or high-phosphate diet for 12 weeks and terminated at age 9 or 15 months, respectively. Plasma, urine, and kidney Klotho at (A) 9 and (B) 15 months old were determined by immunoprecipitation-immunoblotting or immunoblotting. Right panels summarize plasma, urine, and kidney Klotho protein levels. (C, upper panel) Representative macrographs of heart sections (Trichrome); (C, lower panel) Summarizes semiquantification of the Trichrome-positive area over the whole-heart section (Image J). (D) Representative immunohistochemistry for P-Erk, collagen I, and Alexa-Fluor–WGA in left ventricular sections of kl/+, WT, and Tg-Kl mice at 9 and 15 months old. (E) α-SMA, P-Smad2/3, and Alexa-Fluor–WGA in left ventricular sections of kl/+, WT, and Tg-Kl mice at 9 and 15 months. Data expressed as means±SDs (n=4 from each group). Statistical significance was assessed by one-way ANOVA followed by Newman–Keuls test. Significant differences were accepted when *P<0.05 or **P<0.01 between groups. α-SMA, α-smooth muscle actin; HC, heavy chain; WGA, wheat germ agglutinin.
To explore the contribution of aging, we fed a high-phosphate diet to older mice (12 months old) with low (kl/+), normal (WT), and high endogenous Klotho (Tg-Kl) for 12 weeks until they were 15 months old. Additional reductions in plasma and kidney Klotho were observed in older kl/+ mice compared with younger kl/+ mice. Renal Klotho was nearly undetectable in 12-month-old kl/+ mice fed with high phosphate (Figure 4B). Note that downregulation of Klotho in all three lines was more pronounced in older (Figure 4 B) compared with middle-aged (Figure 4A) mice. All mineral parameters and hormones were worse with aging and high-phosphate diet (Supplemental Table 1).
Cardiac hypertrophy and fibrosis were exaggerated in kl/+ mice and lessened in Tg-Kl mice compared with WT mice and more severe at age 15 months compared with 9 months (Figure 4, C–E). The histologic changes were compatible with alterations of α-actinin in the heart, and collagen I and α-smooth muscle actin were higher in hearts of kl/+ mice and lower in hearts of Tg-Kl mice compared with WT mice (Figure 4, D and E, Supplemental Figure 3). Expression of genes relevant to hypertrophy and fibrosis (data not shown) further supports the notions that low Klotho and high phosphate triggered pathologic cardiac remodeling, Klotho suppressed the fibrosis triggered by high dietary phosphate, and aging exacerbated phosphate or Klotho deficiency-induced pathologic cardiac remodeling.
Increased P-Smad2/3 and P-Erk (Figure 4, D and E) signifying Smad2/3 and ERK activation in left ventricle were found in kl/+ mice, and lower phosphorylation of these molecules was seen in Tg-Kl mice. Activation of Erk and Smad2/3 was more pronounced in kl/+ mice at 15 months of age compared with 9 months of age, indicating that aging amplifies pathologic cardiac remodeling with activation of Smad2/3 and Erk pathways, similar to the uremic heart (Figure 3C).
Direct Effects of Soluble Klotho Protein on Neonatal Cardiomyocytes and Cardiac Fibroblasts In Vitro
One drawback of in vivo studies is the inability to conclude whether there is a direct effect of Klotho on the heart. To address that question, we used primary culture of neonatal rat cardiac myocytes and fibroblasts to determine whether Klotho can directly confer protection against Ang II–, TGF-β1–, and phosphate-induced changes. Despite the inherent and unavoidable caveats of cultured cells, this is currently still the only way to examine direct effects of Klotho and phosphate.
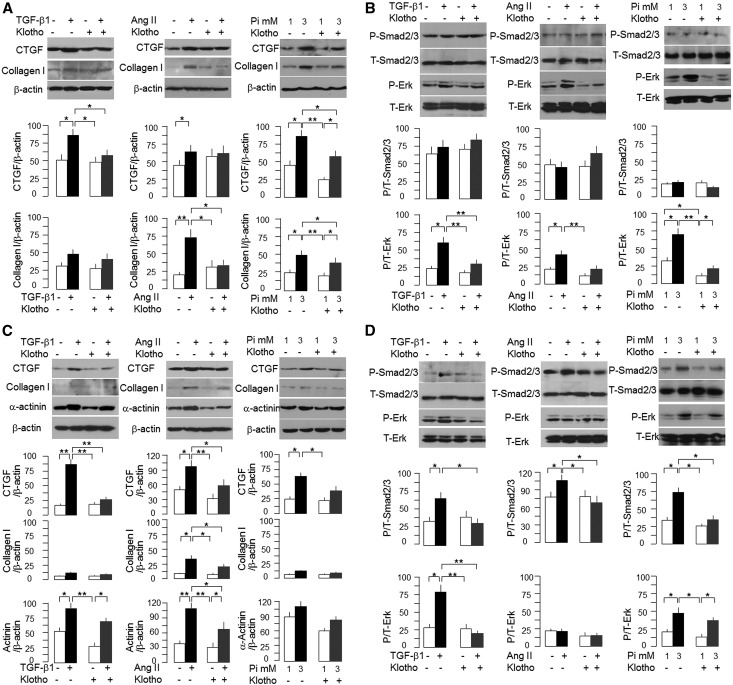
TGF-β1, Ang II, and high Pi media increased connective tissue growth factor and collagen I protein in cardiac fibroblasts, and the induction was attenuated by Klotho (Figure 5A). Erk phosphorylation induced by TGF-β1, Ang II, and high Pi in cardiac fibroblasts was attenuated by Klotho (Figure 5B). However, no significant activation of Smad2/3 was detectable in cultured neonatal cardiac fibroblasts.
Figure 5.
In vitro effect of soluble recombinant Klotho protein on neonatal cardiac fibroblasts and cardiomyocytes. (A and B) Cardiac fibroblasts were prepared from neonatal rat hearts and cultured in six-well plates. (A) After full confluence, TGF-β1, Ang II, and high Pi were added with Klotho or vehicle for 24 hours, and total cell lysate was immunoblotted for (left panel) TGF-β1, (center panel) Ang II, and (right panel) high Pi effect on CTGF and collagen I protein expression. (B) TGF-β1, Ang II, and high Pi were added with Klotho or vehicle for 30 minutes, and total cell lysate was prepared for immunoblot to examine (left panel) TGF-β1, (center panel) Ang II, and (right panel) high Pi on phosphorylation of Erk and Smad2/3 in cardiac fibroblasts. (Top panel) Representative immunoblots. (Middle and bottom panels) Summary of all experiments. (C and D) Cardiomyocytes were prepared from neonatal rat hearts and cultured in six-well plates. (C) On confluence, TGF-β1, Ang II, and high Pi were added with Klotho or vehicle for 24 hours, and total cell lysate was immunoblotted for (left panel) TGF-β1, (center panel) Ang II, and (right panel) high Pi effect on CTGF, collagen I, and α-actinin. (D) TGF-β1, Ang II, and high Pi were added with Klotho or vehicle for 30 minutes, and total cell protein lysate was immunoblotted for (left panel) TGF-β1, (center panel) Ang II, and (right panel) high Pi effect on phosphorylation of Erk and Smad2/3 in cardiomyocytes. (Top panel) Representative immunoblots. (Middle and bottom panels) Summary of all experiments. Data are expressed as means±SDs of three independent experiments for each group, and statistical significance was assessed by one-way ANOVA followed by Newman–Keuls test. Significant differences when *P<0.05 or **P<0.01 between groups. CTGF, connective tissue growth factor; T, total.
TGF-β1 or Ang II increased α-actinin protein (Figure 5C) in cardiomyocytes, which was blunted by soluble Klotho. However, high Pi only slightly increased α-actinin in cardiomyocytes (Figure 5C) over this short period. Connective tissue growth factor protein was appreciably increased by all Ang II, TGF-β1, or high Pi (Figure 5C). Collagen I protein was induced by Ang II but not TGF-β1 or high Pi (Figure 5D). Klotho abolished the stimulation of Smad2/3 phosphorylation by TGF-β1, Ang II, or high Pi (Figure 5D). Both TGF-β1 and high Pi but not Ang II induced activation of Erk1/2, suggesting differential signal pathways between cardiac fibroblasts and myocytes.
Correlation of Plasma Biochemistry, PTH, 1,25(OH)2D3, FGF23, and Klotho with Cardiac Hypertrophy and Fibrosis
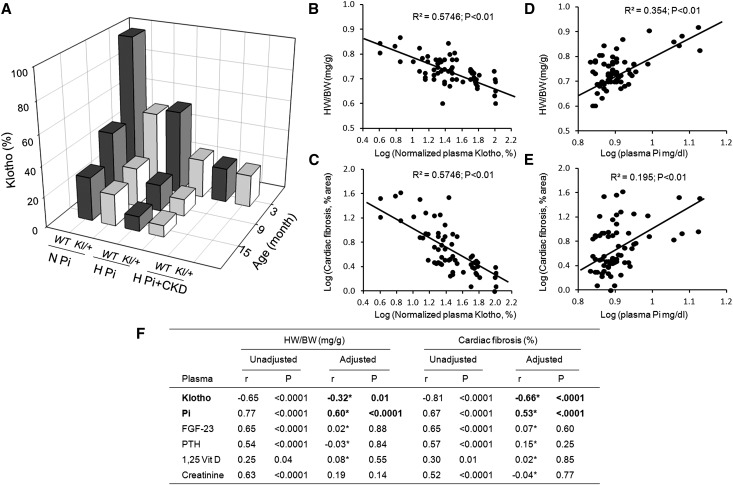
To quantitatively compare changes in plasma Klotho in aging, dietary phosphate manipulation, and CKD, we arbitrarily set normal plasma Klotho as that in young (3 months old) WT mice with normal kidney function fed normal phosphate diet as 100%. Aging, phosphate loading, and CKD seem to act in concert to lower plasma Klotho (Figure 6A). Cardiac hypertrophy and fibrosis negatively correlated with plasma Klotho (Figure 6, B and C) and positively correlated with plasma Pi (Figure 6, D and E). Univariable analysis showed positive correlation of heart weight (HW/body weight) and cardiac fibrosis index with plasma Klotho, FGF23, PTH, Pi, and creatinine and mild correlation with 1,25(OH)2D3 (Figure 6F). Importantly, plasma Klotho and Pi were significantly correlated with either HW/body weight or cardiac fibrosis (Figure 6F), even when adjusted for all other confounding factors supporting the notion that Klotho and phosphate are independent pathologic factors that induce cardiac remodeling.
Figure 6.
Summary of Klotho levels and cardiac phenotype: effect of age, high-phosphate diet, and CKD on plasma Klotho levels and pathologic cardiac remodeling. (A) Plasma Klotho levels normalized to that of 3-month-old WT mice with normal renal function on a normal Pi diet (set to 100%; z axis). Groups of animals: age (3, 9, and 15 months) and dietary phosphate (normal phosphate [N Pi], 0.9%; high phosphate [H Pi], 2.0%; high-phosphate diet plus CKD [H Pi+CKD]). (B) Double log10 plot of HW/body weight (BW) and (C) double log10 plot of percentage of cardiac fibrosis versus plasma Klotho concentration in all groups of animals. The relationships between log10 plasma Klotho concentration and log10 HW/BW or log10 cardiac fibrosis were determined by linear correlation analysis, and significant association was accepted when P<0.05. (D) Double log10 plot of HW/BW and (E) double log10 plot of percentage of cardiac fibrosis versus plasma phosphate concentration in all groups of animals. The relationships between log10 plasma phosphate concentration and log10 HW/BW or log10 cardiac fibrosis were determined by linear correlation analysis, and significant association was accepted when P<0.05. (F) Unadjusted and adjusted association of cardiac hypertrophy and fibrosis with plasma parameters. The association between cardiac variables (HW/BW and percentage of cardiac fibrosis) with each variable (Klotho, FGF23, PTH, 1,25 Vit D, Pi, and creatinine) was assessed with Pearson correlation coefficients with the SAS program (v9.3; SAS Institute, Cary, NC). For each plasma parameter, the partial correlation coefficients were computed to adjust for the potential confounding effects of the other five biomarkers in evaluating the association with cardiomyopathy. P≤0.05 was considered statistically significant. r, Pearson correlation coefficient. *Adjusted for the other five plasma parameters.
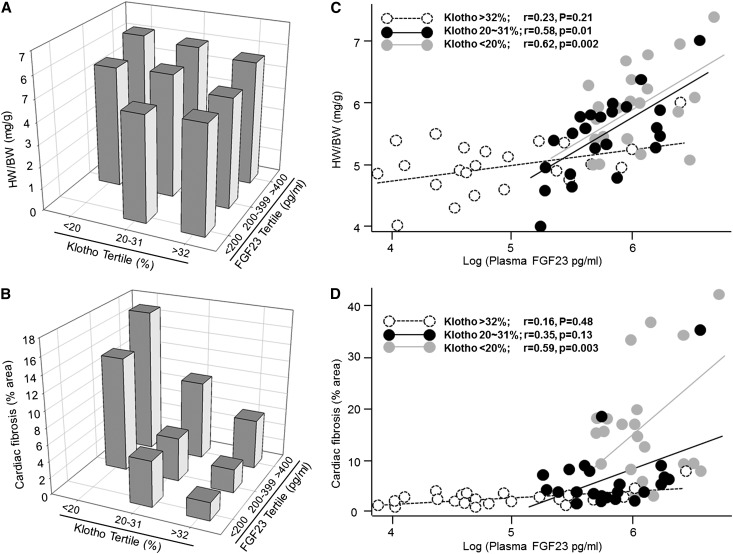
We did not find a relationship between FGF23, cardiac hypertrophy, and fibrosis by multivariable analysis. We then divided plasma Klotho and FGF23 levels into tertiles and analyzed correlation of plasma FGF23 with cardiac hypertrophy and fibrosis at three plasma Klotho levels. Animals with higher FGF23 and lower Klotho had more severe cardiac hypertrophy and fibrosis (Figure 7, A and B). There was a significant relationship of log10 plasma FGF23 concentration versus HW/body weight or fibrosis index (Figure 7, C and D) only in moderate or low plasma Klotho groups, suggesting that FGF23 acts as an important contributor to pathologic cardiac remodeling in concert with lower plasma Klotho.
Figure 7.
Association of plasma FGF23 levels with cardiac remodeling in three different plasma Klotho levels. (A and B) Plasma Klotho (x axis) and FGF23 (y axis) levels were divided into tertiles, and the effects on (A) cardiac hypertrophy and (B) cardiac fibrosis are depicted. (C and D) Associations of plasma FGF23 with (C) cardiac hypertrophy and (D) fibrosis were studied with three different plasma Klotho levels. The relationship between log10 plasma FGF23 concentration and (C) HW/body weight (BW) or (D) cardiac fibrosis was determined by Pearson correlation analysis, and significant association is accepted when P<0.05.
Discussion
Longitudinal observation from the Chronic Renal Insufficiency Cohort revealed that, although left ventricular mass index was relatively stable during transition from CKD to ESRD, ejection fraction declined.37 Reduced kidney function is associated with abnormal cardiac structure but not initially with overt abnormal systolic or diastolic function; kl/+ mice had lower cardiac hypertrophy, ejection fraction, stroke volume, and cardiac output compared with WT or Tg-Kl mice (Figures 1 and 2) but without florid heart failure. Frailty and sudden death precluded us from doing cardiac MRI in kl/kl mice, but one can fathom that cardiac function will only be worse with the worse histology in kl/kl mice. Whether the cardiac defect in kl/kl mice starts during embryonic development is a possibility that we cannot rule out at present.
The coexistence of cardiac hypertrophy, fibrosis, and low Klotho in CKD (Figure 3), dietary phosphate loading (Figure 4), and primary Klotho deficiency (Figure 2) strongly suggests that Klotho deficiency plays a causative role in pathologic cardiac remodeling. Another cardiac phenotype previously described in Klotho deficiency was sinoatrial node dysfunction, which is in the only part of the heart that expresses Klotho endogenously.32 Moreover, cardioprotection of Klotho was proposed to act through suppression of the TRPC6 signal pathway.31 Klotho mRNA was found in the sinoatrial node,32 but membrane Klotho protein has never been shown to be expressed in cardiomyocytes or cardiac fibroblasts. We are primarily examining a different aspect of Klotho biology, which is the systemic effects of soluble Klotho. Aging, phosphotoxicity, and CKD could individually and synergistically contribute to the decline in plasma Klotho (Figure 6A), and compound each other to induce cardiac remodeling. Plasma Klotho levels were negatively associated with severity of pathologic cardiac remodeling, even corrected for other variables. The in vitro experiments with neonatal rat cardiac fibrosis and myocytes provided additional evidence to support the notion that Klotho directly suppresses cardiac hypertrophy and fibrosis.
In addition to the indirect effect on cardiac remodeling through elevation of circulating FGF23, which induces cardiac hypertrophy,30 hyperphosphatemia is also independently associated with cardiac hypertrophy and fibrosis (Figure 6, D and E). In vitro studies only showed effects of high Pi on fibrosis but not on cardiomyocytes hypertrophy. High Pi alone may not be a sufficient trigger for hypertrophy in vitro but is able to initiate fibrosis. Another possibility is that hypertrophy is a chronic process and that 1 day of Pi treatment is not sufficient. Pathologic cardiac remodeling was appreciably attenuated in Tg-Kl mice, although they were fed a high-phosphate diet, supporting that Klotho and phosphate may independently affect the heart.
Although low Klotho is detrimental, extremely high circulating Klotho is not beneficial.38,39 In both humans39 and rodents38 with a >2-fold rise in plasma Klotho, one notes hypophosphatemia, elevated FGF23, osteomalacia, and pathologic fractures.38,39 In contrast, in Tg-Kl mice, the plasma Klotho is only increased by 50% or less compared with WT mice (Supplemental Figure 1A), and Tg-Kl mice do not have severe hypophosphatemia and only minimal derangement of FGF23.
Klotho suppresses renal fibrosis in an obstructive uropathy model.40,41 The mechanism can be partially caused by inhibition of Wnt-induced cell cycle arrest and the resultant decrease in fibrogenic cytokines,41,42 suppression of TGF-β1–induced Smad activation,40,43 or blockade of Ang II signaling.44 Klotho directly suppresses Ang II– and TGF-β1–induced hypertrophy and fibrosis in cardiomyocytes in vitro by inhibition of phosphorylation of Smad2/3 and/or Erk (Figure 6), which are key players in cardiac and renal fibrosis33–35,45
The heart is composed of cardiomyocytes and noncardiomyocytes; among them, cardiac fibroblast is the major cell type surrounding cardiomyocytes and contributing to cardiac development, structure, cell signaling, and electromechanic function.46 Fibroblasts can be transformed into myofibroblasts contributing to extracellular matrix accumulation. Fibrosis, originating from nonmyocytes47 and enhanced by cardiac myocytes,48 can lead to increased wall stiffness and diastolic dysfunction.49 Fibrosis also interrupts electrical signals,50 rendering the tissue more arrhythmogenic, which is highly prevalent in the CKD population.12,51,52
Phosphotoxicity is a risk factor for progression of kidney disease and cardiovascular disease in CKD/ESRD, but causality has not been established.24,53–55 In rodents with normal renal function, long-term high-phosphate intake induced cardiac remodeling and was associated with decreased renal and systemic Klotho (Figure 4) and increased plasma PTH and FGF23 (Supplemental Table 1). Epidemiologic studies showed that high plasma PTH and low plasma 1,25(OH)2D3 are associated with cardiac hypertrophy.56,57 In spontaneously hypertensive heart failure rats (hemizygous cp mutation), 1,25(OH)2D3 treatment prevents cardiac hypertrophy.58 The fact that high-dietary phosphate loading in normal animals induced moderate cardiac hypertrophy and fibrosis (Figure 4) with mild increase in vitamin D (Supplemental Table 1) does not support the pathogenic role of 1,25(OH)2D3 deficiency in phosphate-induced cardiac remodeling. Vitamin D, PTH, FGF23, phosphate, and Klotho plus other unknown uremic toxin(s) and aging itself may individually, additively, and synergistically contribute to pathologic cardiac remodeling, but their individual effects may not be of sufficient magnitude to emerge from a multivariate analysis in these complex models. Multivariate regression analysis only showed significant correlation with plasma Klotho and phosphate in cardiac remodeling in our collective experimental models (Figure 6).
FGF23 is increased in CKD,25,59 but the mechanism of the increase is not entirely known. Circulating and renal Klotho deficiency precedes the increase in plasma FGF2325,60 and is a potential mechanism of stimulating FGF23.17,36 The role of FGF23 in mediation of cardiac hypertrophy was suggested by epidemiologic data30,61–63 and strongly suggested by an animal study.30 There may be interactive effects between FGF23 and Klotho.38,39,64,65 Although plasma FGF23 is not independently associated with cardiac remodeling (Figure 6), a clear positive relation of plasma FGF23 levels with cardiac hypertrophy and fibrosis is present in moderate or severe Klotho deficiency (Figure 7), suggesting that FGF23 can still be pathogenic in conjunction with or perhaps, as a pathologic intermediate for Klotho deficiency.
We showed that circulating Klotho deficiency caused by genetic disruption, CKD, dietary phosphate loading, and aging was associated with cardiac hypertrophy and dysfunction. Low plasma Klotho and high plasma phosphate showed robust correlation with pathologic cardiac remodeling, even when corrected for other covariables. High FGF23 was also correlated with severity of cardiomyopathy but only in the presence of low plasma Klotho levels. A direct antifibrotic effect of Klotho was shown in cultured cells in vitro. This study shows the pathophysiologic importance of Klotho deficiency and high phosphate on the heart, which may be relevant for uremic cardiomyopathy, and opens up potential novel diagnostic and therapeutic potentials on the horizon.
Concise Methods
Animal Experiments
Rodent (mice and rats) models were approved by the Institutional Animal Care and Use Committees from the University of Texas Southwestern Medical Center and the University of Münster. Klotho hypomorphic 13 and Tg-Kl mice were described previously.26,66 Two CKD rodent models were used: UNX-IRI26 and 5/6th nephrectomy.30,67 Sham animals underwent laparotomy and manual manipulation of the kidneys. For high-phosphate diet, 2 weeks after surgery, animals were switched from normal rodent chow to 2.0% phosphate diet (Teklad 08020; Harlan, Madison, WI) for 18 weeks.
Cardiac MRI
Cardiac function was evaluated by cardiac MRI using a 7T Small Animal MR Scanner (Varian, Inc., Palo Alto, CA) with a 38-mm birdcage RF coil as previously described.68
Primary Culture of Neonatal Rat Ventricular Myocytes and Cardiac Fibroblasts
Cells were isolated from hearts of neonatal SD rats with established methods.69,70 For immunofluorescence, 1×106 cells were seeded per well on glass coverslips in six-well plates. For protein and RNA isolation, 2×106 cells were seeded in 6-cm culture dishes.
Static Heart Morphology and Morphometry
Sections were stained with Trichrome for fibrosis and imaged with a 5× objective. To measure surface area of cardiomyocytes, paraffin-embedded sections were labeled with wheat germ agglutinin conjugated to Alexa Fluor 555 (Invitrogen, Carlsbad, CA) with standard methods.30
Immunohistochemistry in the Heart and the Kidney
For immunofluorescence study, monoclonal rat antibody (KM2076) against human Klotho (1:250)71 was used. Rhodamine-phalloidin (1:50; Molecular Probes, Eugene, OR) for staining β-actin filaments was applied for double staining.
Quantifying Klotho Protein and Transcript in the Kidney, Urine, and Blood of Rodents
Immunoblots in the kidney and urine were performed as described.26 Heparinized plasma was subjected to immunoprecipitation with rabbit anti-human Klotho and immunoblotted with anti-Klotho antibody (KM2076; 0.5 μg/ml). Lysates from cardiac myocytes, fibroblasts, and tissues were prepared for immunoblot as described.26,30 Total RNA was extracted followed by generation of cDNA. Primers used for quantitative PCR are in Supplemental Table 2, and the methods are described.26 Data were expressed at amplification number of 2−ΔΔCt by normalization of cyclophilin and comparison of controls.
Additional detailed experimental methods are presented in Supplemental Material.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants R01-DK091392, R01-DK092461, R01-DK20543, R01-DK081374, R01-DK07611, P41-RR0022584, R01-HL080144, R01-HL0980842, and R01-HL100401, and George M. O’Brien Kidney Research Core Center NIH Grant P30DK-07938. Additional support was from American Heart Association (AHA) Grant 0865235F, AHA DeHaan Foundation Grant 0970518N, an AHA Young Investigator Award, the Gottschalk Award of the American Society of Nephrology, Stifterverband für die Deutsche Wissenschaft and Simon-Claussen-Stiftung Grant H1405409999915626, Cancer Prevention and Research Institute of Texas Grant RP110486P3, Fondation Leducq Grant 11CVD04, the Simmons Family Foundation, and the Endowed Professor Collaborative Grant Program of the Pak Center of Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Klotho Deficiency and the Cardiomyopathy of Advanced CKD,” on pages 1229–1231.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014050465/-/DCSupplemental.
References
- 1.Mizobuchi M, Towler D, Slatopolsky E: Vascular calcification: The killer of patients with chronic kidney disease. J Am Soc Nephrol 20: 1453–1464, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Stack AG, Bloembergen WE: Prevalence and clinical correlates of coronary artery disease among new dialysis patients in the United States: A cross-sectional study. J Am Soc Nephrol 12: 1516–1523, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Rössle R: Über die serösen Entzündugen der Organe. Virchows Arch 311: 252–284, 1943 [Google Scholar]
- 4.Tyralla K, Amann K: Morphology of the heart and arteries in renal failure. Kidney Int Suppl 84: S80–S83, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE: Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 47: 186–192, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Bakkaloglu SA, Borzych D, Soo Ha I, Serdaroglu E, Büscher R, Salas P, Patel H, Drozdz D, Vondrak K, Watanabe A, Villagra J, Yavascan O, Valenzuela M, Gipson D, Ng KH, Warady BA, Schaefer F, International Pediatric Peritoneal Dialysis Network : Cardiac geometry in children receiving chronic peritoneal dialysis: Findings from the International Pediatric Peritoneal Dialysis Network (IPPN) registry. Clin J Am Soc Nephrol 6: 1926–1933, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley RN, Curtis BM, Randell EW, Parfrey PS: Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol 5: 805–813, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 23: 1725–1734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mall G, Huther W, Schneider J, Lundin P, Ritz E: Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol Dial Transplant 5: 39–44, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Aoki J, Ikari Y, Nakajima H, Mori M, Sugimoto T, Hatori M, Tanimoto S, Amiya E, Hara K: Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int 67: 333–340, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Martin FL, McKie PM, Cataliotti A, Sangaralingham SJ, Korinek J, Huntley BK, Oehler EA, Harders GE, Ichiki T, Mangiafico S, Nath KA, Redfield MM, Chen HH, Burnett JC, Jr.: Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: A kidney-heart connection. Am J Physiol Regul Integr Comp Physiol 302: R292–R299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M: Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282: 26687–26695, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Eskiocak U, Stadler G, Lou Z, Kuro-o M, Shay JW, Wright WE: Short hairpin RNA screen indicates that Klotho beta/FGF19 protein overcomes stasis in human colonic epithelial cells. J Biol Chem 286: 43294–43300, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y: Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta 1576: 341–345, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Hu MC, Shiizaki K, Kuro-o M, Moe OW: Fibroblast growth factor 23 and Klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75: 503–533, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW, Kuro-o M, Mohammadi M: Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci U S A 107: 407–412, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M: Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120–6123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razzaque MS, Lanske B: The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol 194: 1–10, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR: Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 104: 19796–19801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloch L, Sineshchekova O, Reichenbach D, Reiss K, Saftig P, Kuro-o M, Kaether C: Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 583: 3221–3224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massy ZA, Drüeke TB: Vascular calcification. Curr Opin Nephrol Hypertens 22: 405–412, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Kuro-o M: Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol 9: 650–660, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Pavik I, Jaeger P, Ebner L, Wagner CA, Petzold K, Spichtig D, Poster D, Wüthrich RP, Russmann S, Serra AL: Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: A sequence suggested from a cross-sectional study. Nephrol Dial Transplant 28: 352–359, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, Yamanari T, Kikumoto Y, Uchida HA, Kitamura S, Maeshima Y, Nakamura K, Ito H, Makino H: A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS ONE 8: e56695, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM: Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int 82: 1261–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL: Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation 125: 2243–2255, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie J, Cha SK, An SW, Kuro-O M, Birnbaumer L, Huang CL: Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun 3: 1238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, Yasui K, Lee JK, Kamiya K, Kitaichi K, Yamamoto K, Ito M, Kondo T, Iino S, Inden Y, Hirai M, Murohara T, Kodama I, Nabeshima Y: Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation 109: 1776–1782, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, Lee HB: Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol 16: 667–675, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Olson ER, Naugle JE, Zhang X, Bomser JA, Meszaros JG: Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am J Physiol Heart Circ Physiol 288: H1131–H1138, 2005 [DOI] [PubMed] [Google Scholar]
- 35.House SL, House BE, Glascock B, Kimball T, Nusayr E, Schultz JE, Doetschman T: Fibroblast growth factor 2 mediates isoproterenol-induced cardiac hypertrophy through activation of the extracellular regulated kinase. Mol Cell Pharmacol 2: 143–154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu MC, Kuro-o M, Moe OW: Klotho and chronic kidney disease. Contrib Nephrol 180: 47–63, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bansal N, Keane M, Delafontaine P, Dries D, Foster E, Gadegbeku CA, Go AS, Hamm LL, Kusek JW, Ojo AO, Rahman M, Tao K, Wright JT, Xie D, Hsu CY, CRIC Study Investigators : A longitudinal study of left ventricular function and structure from CKD to ESRD: The CRIC study. Clin J Am Soc Nephrol 8: 355–362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith RC, O’Bryan LM, Farrow EG, Summers LJ, Clinkenbeard EL, Roberts JL, Cass TA, Saha J, Broderick C, Ma YL, Zeng QQ, Kharitonenkov A, Wilson JM, Guo Q, Sun H, Allen MR, Burr DB, Breyer MD, White KE: Circulating αKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest 122: 4710–4715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP: A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci U S A 105: 3455–3460, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M: Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286: 8655–8665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satoh M, Nagasu H, Morita Y, Yamaguchi TP, Kanwar YS, Kashihara N: Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol 303: F1641–F1651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y: Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 24: 771–785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugiura H, Yoshida T, Shiohira S, Kohei J, Mitobe M, Kurosu H, Kuro-o M, Nitta K, Tsuchiya K: Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am J Physiol Renal Physiol 302: F1252–F1264, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R: In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 39: 838–843, 2002 [DOI] [PubMed] [Google Scholar]
- 45.de Boer RA, Pokharel S, Flesch M, van Kampen DA, Suurmeijer AJ, Boomsma F, van Gilst WH, van Veldhuisen DJ, Pinto YM: Extracellular signal regulated kinase and SMAD signaling both mediate the angiotensin II driven progression towards overt heart failure in homozygous TGR(mRen2)27. J Mol Med (Berl) 82: 678–687, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Bowers SL, Borg TK, Baudino TA: The dynamics of fibroblast-myocyte-capillary interactions in the heart. Ann N Y Acad Sci 1188: 143–152, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, Kim JB, Schmitt JP, Molkentin JD, Norris RA, Tager AM, Hoffman SR, Markwald RR, Seidman CE, Seidman JG: Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-β. J Clin Invest 120: 3520–3529, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fredj S, Bescond J, Louault C, Potreau D: Interactions between cardiac cells enhance cardiomyocyte hypertrophy and increase fibroblast proliferation. J Cell Physiol 202: 891–899, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Brown RD, Ambler SK, Mitchell MD, Long CS: The cardiac fibroblast: Therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol 45: 657–687, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Vasquez C, Benamer N, Morley GE: The cardiac fibroblast: Functional and electrophysiological considerations in healthy and diseased hearts. J Cardiovasc Pharmacol 57: 380–388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts PR, Green D: Arrhythmias in chronic kidney disease. Heart 97: 766–773, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Shamseddin MK, Parfrey PS: Sudden cardiac death in chronic kidney disease: Epidemiology and prevention. Nat Rev Nephrol 7: 145–154, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Razzaque MS: Phosphate toxicity and vascular mineralization. Contrib Nephrol 180: 74–85, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Martin KJ, González EA: Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: What is normal, when to start, and how to treat? Clin J Am Soc Nephrol 6: 440–446, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Zoccali C, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, Mallamaci F, Remuzzi G, REIN Study Group : Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J Am Soc Nephrol 22: 1923–1930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayashi SY, Rohani M, Lindholm B, Brodin LA, Lind B, Barany P, Alvestrand A, Seeberger A: Left ventricular function in patients with chronic kidney disease evaluated by colour tissue Doppler velocity imaging. Nephrol Dial Transplant 21: 125–132, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Levin A, Li YC: Vitamin D and its analogues: Do they protect against cardiovascular disease in patients with kidney disease? Kidney Int 68: 1973–1981, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Mancuso P, Rahman A, Hershey SD, Dandu L, Nibbelink KA, Simpson RU: 1,25-Dihydroxyvitamin-D3 treatment reduces cardiac hypertrophy and left ventricular diameter in spontaneously hypertensive heart failure-prone (cp/+) rats independent of changes in serum leptin. J Cardiovasc Pharmacol 51: 559–564, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Wan M, Smith C, Shah V, Gullet A, Wells D, Rees L, Shroff R: Fibroblast growth factor 23 and soluble klotho in children with chronic kidney disease. Nephrol Dial Transplant 28: 153–161, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto S, Ikebe M, Yuasa K, Yamanaka S, Sugiura T, Terada Y: Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol 16: 722–729, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Ky B, Shults J, Keane MG, Sutton MS, Wolf M, Feldman HI, Reese PP, Anderson CA, Townsend RR, Deo R, Lo J, Gadegbeku C, Carlow D, Sulik MJ, Leonard MB, CRIC Study Investigators : FGF23 modifies the relationship between vitamin D and cardiac remodeling. Circ Heart Fail 6: 817–824, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE: Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 207: 546–551, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Shibata K, Fujita S, Morita H, Okamoto Y, Sohmiya K, Hoshiga M, Ishizaka N: Association between circulating fibroblast growth factor 23, α-Klotho, and the left ventricular ejection fraction and left ventricular mass in cardiology inpatients. PLoS ONE 8: e73184, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS: In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23) -mediated regulation of systemic phosphate homeostasis. FASEB J 23: 433–441, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakatani T, Ohnishi M, Razzaque MS: Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. FASEB J 23: 3702–3711, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M: Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu MC, Bankir L, Michelet S, Rousselet G, Trinh-Trang-Tan MM: Massive reduction of urea transporters in remnant kidney and brain of uremic rats. Kidney Int 58: 1202–1210, 2000 [DOI] [PubMed] [Google Scholar]
- 68.Aoyagi T, Kusakari Y, Xiao CY, Inouye BT, Takahashi M, Scherrer-Crosbie M, Rosenzweig A, Hara K, Matsui T: Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 303: H75–H85, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yund EE, Hill JA, Keller RS: Hic-5 is required for fetal gene expression and cytoskeletal organization of neonatal cardiac myocytes. J Mol Cell Cardiol 47: 520–527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN: Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A 105: 13027–13032, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, Nakamura K, Iida A, Anazawa H, Koh N, Iwano A, Imura A, Fujimori T, Kuro-o M, Hanai N, Takeshige K, Nabeshima Y: Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun 267: 597–602, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.