Abstract
The capacity of risk prediction to guide management of CKD in underserved health settings is unknown. We conducted a retrospective cohort study of 28,779 adults with nondialysis-requiring CKD who received health care in two large safety net health systems during 1996–2009 and were followed for ESRD through September of 2011. We developed and evaluated the performance of ESRD risk prediction models using recently proposed criteria designed to inform population health approaches to disease management: proportion of cases followed and proportion that needs to be followed. Overall, 1730 persons progressed to ESRD during follow-up (median follow-up=6.6 years). ESRD risk for time frames up to 5 years was highly concentrated among relatively few individuals. A predictive model using five common variables (age, sex, race, eGFR, and dipstick proteinuria) performed similarly to more complex models incorporating extensive sociodemographic and clinical data. Using this model, 80% of individuals who eventually developed ESRD were among the 5% of cohort members at the highest estimated risk for ESRD at 1 year. Similarly, a program that followed 8% and 13% of individuals at the highest ESRD risk would have included 80% of those who eventually progressed to ESRD at 3 and 5 years, respectively. In this underserved health setting, a simple five-variable model accurately predicts most cases of ESRD that develop within 5 years. Applying risk prediction using a population health approach may improve CKD surveillance and management of vulnerable groups by directing resources to a small subpopulation at highest risk for progressing to ESRD.
Keywords: CKD, progression of chronic renal failure, ethnic, minority, ESRD
In the United States, CKD has been estimated to affect nearly 26 million Americans, is the ninth leading cause of death, and costs the federal government over $56 billion annually.1,2 Currently, over one third of incident patients with ESRD are enrolled in Medicaid (the United States joint federal and state health insurance program for the poor) or uninsured at ESRD onset.1 Despite the disproportionate burden of CKD among socially disadvantaged groups, relatively little progress has been made in reducing socioeconomic disparities in the incidence and treatment of ESRD over the past two decades.
In many instances, CKD can be slowed or prevented. Interventions, such as BP lowering, use of renin-angiotensin system inhibitors, and avoidance of nephrotoxins, are effective in slowing CKD progression if initiated in earlier stages.3–7 However, these interventions seem to be underutilized in patients at all stages of CKD from socially disadvantaged groups.8,9 Moreover, uninsured persons and Medicaid enrollees are suboptimally prepared for ESRD, as evidenced by worse biochemical abnormalities at ESRD onset, lower prevalence of permanent vascular access at dialysis initiation, and marked delays in accessing a kidney transplant compared with counterparts with Medicare or private health insurance.10–13
A major contributor to socioeconomic disparities in ESRD incidence and CKD care is the lack of a surveillance system for tracking the care of the poor or underinsured.1 Because of their relatively limited options for continuous ambulatory care, most of America’s poor and underinsured patients with CKD, when they do seek care, likely do so from public hospitals and safety net health systems. Although several tools are now available to assist health providers in estimating an individual patient’s risk of progressing to ESRD, these measures have yet to be evaluated in an underserved health setting.14–18 Moreover, the capacity of these tools to guide system-level decisions (for example, to determine the potential effectiveness or feasibility of surveillance, outreach, or risk factor intervention programs that target subgroups at high risk for ESRD) is unclear.19 To address these issues, we examined data from 28,779 patients with CKD who received ambulatory care in two large safety net health systems. The primary study objective was to assess the performance of models to estimate risk of CKD progression to ESRD in an underserved health setting using criteria designed to inform population health strategies to disease management. We hypothesized that ESRD risk would be highly concentrated among a small fraction of patients who could be identified using routinely available demographic and clinical data.
Results
Patient Characteristics
Compared with CKD cohorts from other health care settings,14,15,17,20 the study cohort was relatively young and racially/ethnically diverse. Over one half of the cohort either lacked health insurance or was enrolled in Medicaid (Table 1). On the basis of the distribution of eGFR, a slightly higher proportion of the Harborview Medical Center (HMC) subcohort had more advanced CKD compared with the Community Health Network (CHN) subcohort (Supplemental Table 1). The distribution of dipstick proteinuria was similar in the subcohorts, with the caveat that 42% of patients from the HMC subcohort were missing urinalysis assessment compared with 19% in the CHN subcohort.
Table 1.
Baseline characteristics of 28,779 persons with moderate to advanced CKD from the CHN and HMC health systems
| Demographics | |
| Age (yr), mean (SD) | 60.3 (14.2) |
| Men, n (%) | 13,903 (48.3) |
| Race or ethnicity, n (%) | |
| Non-Hispanic white | 11,757 (40.9) |
| Non-Hispanic black | 4930 (17.1) |
| Hispanic | 3466 (12.0) |
| Asian | 6247 (21.7) |
| Other race or ethnicity | 2379 (8.3) |
| Health insurance coverage, n (%) | |
| Uninsured | 8340 (29.0) |
| Medicaid | 6260 (21.8) |
| Medicare | 10,532 (36.6) |
| Private or commercial | 3647 (12.7) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 6569 (22.8) |
| Hypertension | 13,525 (47.0) |
| Cardiovascular disease | 7947 (27.6) |
| Substance abuse | 6594 (22.9) |
| Chronic viral diseasea | 5919 (20.6) |
| Laboratory data | |
| eGFR (ml/min per 1.73 m2), n (%) | |
| 45–59 | 21,942 (76.2) |
| 30–44 | 4729 (16.4) |
| 15–29 | 1574 (5.5) |
| <15 | 534 (1.9) |
| Dipstick proteinuria, n (%) | |
| None or trace | 12,709 (61.6) |
| 1+ | 3875 (18.8) |
| 2+ | 2483 (12.0) |
| ≥3+ | 1581 (7.7) |
| Serum albumin (g/dl), mean (SD) | 3.8 (0.7) |
| Serum calcium (mg/dl), mean (SD) | 9.2 (0.8) |
| Hemoglobin (g/dl), mean (SD) | 12.9 (2.0) |
| Serum creatinine (mg/dl), mean (SD) | 1.5 (1.1) |
| Serum cholesterol (mg/dl), mean (SD) | 195.8 (54.0) |
Missing values in the full cohort were distributed as follows: dipstick proteinuria, 28.3%; serum cholesterol, 15.1%; serum calcium, 5.9%; serum albumin, 1.9%; and hemoglobin, 1.8%.
HIV, hepatitis C virus, and/or hepatitis B virus infection.
Overall, 1730 (6%) individuals (959 individuals in the CHN subcohort and 771 individuals in the HMC subcohort) in the cohort progressed to ESRD over a median follow-up of 6.6 years. Persons ages 18–40 years experienced the highest incidence rates of ESRD. Men, black persons, and persons with hypertension, diabetes mellitus, chronic viral disease, substance abuse, and cardiovascular disease experienced higher incidence rates of ESRD compared with their respective reference group (Table 2). After covariate adjustment, younger age, men, nonwhite race/ethnicity, Medicaid, Medicare, hypertension, diabetes mellitus, lower eGFR, higher dipstick proteinuria, lower serum albumin, lower hemoglobin, and higher cholesterol were associated with progression to ESRD (Table 3). Similar associations were observed in the CHN and HMC cohorts when analyzed separately (data not shown).
Table 2.
Incidence rates of ESRD by patient characteristics (n=28,779)
| Variable | No. at Risk | ESRD Events | Time at Risk (×1000 person-yr) | Incidence Rate (×1000 person-yr) |
|---|---|---|---|---|
| All | 28,779 | 1730 | 198.8 | 8.7 |
| Age (yr) | ||||
| 18–39 | 2145 | 297 | 14.7 | 20.3 |
| 40–49 | 4961 | 442 | 34.4 | 12.9 |
| 50–59 | 7132 | 475 | 48.1 | 9.9 |
| ≥60 | 14,538 | 516 | 101.7 | 5.1 |
| Sex | ||||
| Women | 14,876 | 661 | 106.6 | 6.2 |
| Men | 13,903 | 1069 | 92.2 | 11.6 |
| Race or ethnicity | ||||
| White | 11,757 | 376 | 78.7 | 4.8 |
| Black | 4930 | 684 | 33.4 | 20.5 |
| Hispanic | 3466 | 239 | 25.4 | 9.4 |
| Asian | 6247 | 355 | 46.6 | 7.6 |
| Other race or ethnicity | 2379 | 76 | 14.7 | 5.2 |
| Comorbidities | ||||
| No hypertension | 15,254 | 674 | 107.7 | 6.3 |
| Hypertension | 13,525 | 1056 | 91.1 | 11.6 |
| No diabetes | 22,210 | 926 | 156.0 | 5.9 |
| Diabetes | 6569 | 804 | 42.8 | 18.8 |
| No viral disease | 22,860 | 1301 | 160.6 | 8.1 |
| HIV, HCV, or HBV | 5919 | 429 | 38.2 | 11.2 |
| No substance abuse | 22,185 | 1233 | 159.8 | 7.7 |
| Substance abuse | 6594 | 497 | 39.0 | 12.7 |
| No cardiovascular disease | 20,832 | 1108 | 148.1 | 7.5 |
| Cardiovascular disease | 7947 | 622 | 50.7 | 12.3 |
HBV, hepatitis B virus; HCV, hepatitis C virus.
Table 3.
Estimated hazard ratios and associated 95% confidence intervals of the association between each of the covariates and ESRD on the basis of the training subset of the full cohort (n=19,215)
| Variable | Hazard Ratio (95% Confidence Interval) | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Age (per 10 yr) | 0.78 (0.74 to 0.81) | 0.84 (0.81 to 0.88) | 0.76 (0.72 to 0.80) | 0.77 (0.73 to 0.80) |
| Men versus women | 1.35 (1.20 to 1.52) | 1.21 (1.08 to 1.37) | 1.25 (1.11 to 1.42) | 1.38 (1.22 to 1.57) |
| Black versus white | 3.09 (2.65 to 3.61) | 2.29 (1.96 to 2.68) | 2.14 (1.83 to 2.51) | 2.01 (1.71 to 2.35) |
| Hispanic versus white | 2.17 (1.79 to 2.63) | 1.65 (1.36 to 2.01) | 1.55 (1.27 to 1.89) | 1.52 (1.25 to 1.86) |
| Asian versus white | 2.16 (1.81 to 2.59) | 1.79 (1.50 to 2.14) | 1.63 (1.36 to 1.96) | 1.61 (1.34 to 1.94) |
| Other versus white | 1.27 (0.93 to 1.72) | 1.19 (0.87 to 1.61) | 1.39 (1.02 to 1.90) | 1.47 (1.08 to 2.00) |
| eGFR (ml/min per 1.73 m2; per 5 units) | 0.71 (0.70 to 0.72) | 0.70 (0.66 to 0.73) | 0.68 (0.64 to 0.72) | 0.70 (0.66 to 0.74) |
| Dipstick proteinuria=1+ versus none or trace | 0.98 (0.53 to 1.82) | 0.83 (0.45 to 1.53) | 0.80 (0.43 to 1.49) | |
| Dipstick proteinuria=2+ versus none or trace | 3.09 (1.84 to 5.18) | 2.98 (1.76 to 5.04) | 2.66 (1.57 to 4.51) | |
| Dipstick proteinuria≥3+ versus none or trace | 6.56 (3.93 to 10.94) | 6.13 (3.67 to 10.23) | 5.52 (3.31 to 9.21) | |
| Medicare versus private insurance | 1.41 (1.12 to 1.77) | 1.50 (1.19 to 1.89) | ||
| Medicaid versus private insurance | 1.30 (1.04 to 1.63) | 1.35 (1.08 to 1.69) | ||
| Uninsured versus private insurance | 0.73 (0.57 to 0.92) | 0.74 (0.59 to 0.95) | ||
| Diabetes mellitus | 2.49 (2.18 to 2.84) | 2.38 (2.09 to 2.72) | ||
| Cardiovascular disease | 1.12 (0.97 to 1.28) | 1.13 (0.98 to 1.31) | ||
| Hypertension | 1.27 (1.10 to 1.46) | 1.36 (1.18 to 1.57) | ||
| Substance abuse | 0.78 (0.67 to 0.90) | 0.79 (0.68 to 0.91) | ||
| Chronic viral diseasea | 0.94 (0.82 to 1.09) | 0.85 (0.74 to 0.98) | ||
| Serum albumin (per 1 g/dl) | 0.87 (0.80 to 0.95) | |||
| Serum calcium (per 1 mg/dl) | 1.00 (0.93 to 1.07) | |||
| Hemoglobin (per 1 g/dl) | 0.89 (0.86 to 0.91) | |||
| Serum cholesterol (per 10 mg/dl) | 1.02 (1.01 to 1.03) | |||
| eGFR×dipstick proteinuria 1+ | 1.12 (1.05 to 1.21) | 1.14 (1.06 to 1.23) | 1.14 (1.06 to 1.23) | |
| eGFR×dipstick proteinuria 2+ | 1.11 (1.05 to 1.18) | 1.11 (1.04 to 1.18) | 1.11 (1.05 to 1.19) | |
| eGFR×dipstick proteinuria≥3+ | 1.13 (1.07 to 1.20) | 1.12 (1.05 to 1.19) | 1.11 (1.05 to 1.18) | |
HIV, hepatitis C virus, and/or hepatitis B virus infection.
Model Performance
A simple model (model 1) incorporating age, sex, race/ethnicity, and eGFR performed well in discriminating between those who progressed and did not progress to ESRD at 1 year, which was evidenced by high area under the receiver operating characteristics (ROC) curve estimates (Table 4). Adding dipstick proteinuria to model 1 improved discrimination (model 2), especially at 3- and 5-year time frames, but little improvement was observed with the addition of health insurance coverage and comorbidities (model 3), and laboratory data (model 4) (Table 4, Supplemental Figure 1).
Table 4.
Estimates (SEMs) of prediction performance measures for models 1–4 on the basis of the full cohort
| Time frame/Measure | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Year 1 | ||||
| AUC | 0.94 (0.02) | 0.97 (0.01) | 0.96 (0.01) | 0.97 (0.01) |
| PE(×10) | 0.10 (<0.01) | 0.09 (0.01) | 0.09 (0.01) | 0.09 (0.01) |
| PCF(0.1) | 0.87 (0.03) | 0.91 (0.03) | 0.89 (0.03) | 0.90 (0.03) |
| PCF(0.2) | 0.92 (0.03) | 0.97 (0.02) | 0.95 (0.02) | 0.98 (0.02) |
| PNF(0.8) | 0.06 (0.04) | 0.05 (0.02) | 0.05 (0.02) | 0.04 (0.02) |
| PNF(0.9) | 0.14 (0.09) | 0.09 (0.05) | 0.11 (0.04) | 0.10 (0.04) |
| Year 3 | ||||
| AUC | 0.89 (0.01) | 0.94 (0.01) | 0.94 (0.01) | 0.95 (0.01) |
| PE(×10) | 0.26 (0.01) | 0.23 (0.02) | 0.22 (0.03) | 0.21 (0.03) |
| PCF(0.1) | 0.69 (0.02) | 0.83 (0.02) | 0.82 (0.02) | 0.86 (0.02) |
| PCF(0.2) | 0.83 (0.02) | 0.91 (0.02) | 0.92 (0.02) | 0.93 (0.02) |
| PNF(0.8) | 0.18 (0.04) | 0.08 (0.02) | 0.09 (0.02) | 0.08 (0.01) |
| PNF(0.9) | 0.35 (0.05) | 0.17 (0.04) | 0.18 (0.04) | 0.14 (0.04) |
| Year 5 | ||||
| AUC | 0.85 (0.01) | 0.92 (0.01) | 0.93 (0.01) | 0.94 (0.01) |
| PE(×10) | 0.46 (0.03) | 0.40 (0.04) | 0.39 (0.05) | 0.38 (0.05) |
| PCF(0.1) | 0.57 (0.02) | 0.74 (0.02) | 0.76 (0.02) | 0.77 (0.02) |
| PCF(0.2) | 0.74 (0.02) | 0.86 (0.02) | 0.87 (0.02) | 0.89 (0.02) |
| PNF(0.8) | 0.27 (0.03) | 0.13 (0.02) | 0.13 (0.02) | 0.12 (0.02) |
| PNF(0.9) | 0.51 (0.04) | 0.24 (0.04) | 0.23 (0.04) | 0.21 (0.03) |
Model 1: adjusted for age, sex, race/ethnicity, and eGFR. Model 2: model 1 covariates plus dipstick proteinuria and an interaction between eGFR and dipstick proteinuria. Model 3: model 2 covariates plus health insurance coverage and comorbidities (diabetes mellitus, cardiovascular disease, hypertension, substance abuse, and chronic viral disease). Model 4: model 3 covariates plus additional laboratory variables (serum albumin, calcium, hemoglobin, and cholesterol). AUC, area under the ROC curve; PE (×10), prediction error multiplied by a factor of 10.
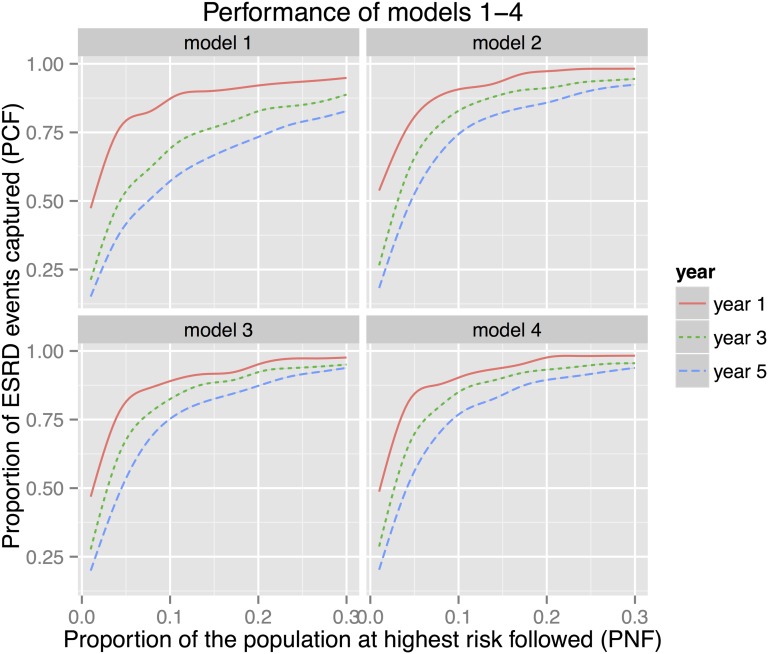
On the basis of model 2, an estimated 91% of ESRD events occurring within 1 year were included among 10% of subjects at highest estimated risk of ESRD; that value decreased to 83% and 74% for 3- and 5-year time frames, respectively (Figure 1). Similarly, following an estimated 5%, 8%, and 13% of the cohort would potentially include 80% of persons who progressed to ESRD at 1, 3, and 5 years, respectively (Table 4, Supplemental Figure 2). Nearly identical patterns resulted when the CHN and HMC cohorts were analyzed separately (Supplemental Table 2, A and B).
Figure 1.
The estimated proportion of ESRD events captured (PCF) among a given proportion of subjects at highest estimated risk of ESRD (PNF) for models 1–4 at 1-, 3-, and 5-year time frames. Model 2 outperformed model 1, especially at 3- and 5-year time frames, with models 3 and 4 providing modest improvement over model 2. The performances of all models were highest for the 1-year time frame and declined over time. On the basis of model 2, an estimated 91% (97%) of events occurring within 1 year were captured in 10% (20%) of subjects at highest estimated risk of ESRD; those values decreased to 83% (91%) and 74% (86%) for 3- and 5-year time frames, respectively.
Discussion
Recent federal initiatives, such as the Health Information Technology for Economic and Clinical Health Act and Health Data Initiative, prioritize health information technology and innovation in electronic health records use to promote health assessment, planning, and action.21,22 By leveraging the electronic health records data of two large public health systems, we observed that approximately one in 16 persons with moderate to advanced CKD receiving ambulatory care developed ESRD during a median follow-up of 6.6 years. Using criteria designed to inform population health approaches to disease management, we observed that a simple five-variable predictive model using common demographic and laboratory data accurately predicts most cases of ESRD for time frames up to 5 years. Moreover, we found that ESRD risk was highly concentrated among relatively few patients, which suggests that only a small proportion of individuals with CKD would need to be carefully followed to capture the majority of persons who eventually progress to ESRD. Our study approach may help to guide public health systems in identifying a high-risk subcohort that might, for example, undergo more intensive surveillance, risk factor management, and when necessary, preparation for ESRD care.19
The magnitude and persistence of socioeconomic disparities in the incidence and treatment of kidney disease have led the US Government to prioritize their elimination while also attempting to reduce the overall burden and costs of CKD.23 A major challenge in addressing these disparities is in the lack of information on processes and outcomes of CKD care among traditionally underserved populations. We previously reported that effective interventions to slow CKD progression and reduce mortality seemed to be highly underutilized in these populations.9 Additional studies support our observations, suggesting that disadvantaged groups likely receive less effective care in earlier stages of CKD.8,24,25 Fortunately, most patients with CKD will not progress to ESRD. However, a formidable challenge faced by municipal health systems and their lay and physician leadership is in identifying and managing those patients who will eventually develop progressive disease. Although prior studies have yielded several risk predictive models for ESRD, these models have been predominantly developed and evaluated in less diverse cohorts and were designed for use by individual clinicians in calculating patient-level risk.14–18 Moreover, these models depend on specialized laboratory data (e.g., urine albumin and creatinine concentrations), which are commonly missing in real-world underserved health settings.14–18 From the perspective of population health, few studies have examined the capacity of such models to help guide, for example, the planning and assessment of CKD care programs.19,26
In 2011, Pfeiffer and Gail27 proposed two pragmatic measures of risk concentration that were designed to guide population health approaches and help inform decision making at the level of the health system. In terms of ESRD risk, proportion of cases followed is useful for evaluating the effectiveness of an ongoing or proposed care program by estimating what proportion of future ESRD events would be observed within a specified subpopulation.27 Because of the concentration of ESRD risk in our study cohort, our results suggest that a high-risk prevention or preparation strategy might be effective in covering most persons with CKD who will eventually develop ESRD in a public or underserved health care setting. For example, by applying a simple risk predictive model that incorporates age, race, sex, eGFR, and dipstick urinalysis, a program that followed 10% of the population at highest risk for ESRD would potentially include 91% of persons who will eventually progress to ESRD by 1 year in our cohort. In addition to active surveillance (to identify out-of-care individuals) and heightened management of traditional risk factors for CKD morbidity, these individuals might receive additional care that specifically focuses on optimizing their transition to ESRD, such as timely dialysis education, kidney transplant referral, and placement of vascular or peritoneal dialysis access. Moreover, this high-risk cohort could be readily identified through systematic queries of electronic health records without relying solely on referrals from individual clinicians.
A measure complementary to the proportion of cases followed is the proportion that needs to be followed, which is useful in assessing the feasibility of a program required to cover a specific proportion of future ESRD cases. For example, if an ESRD preparation program aims to capture 90% of persons who will develop ESRD over the subsequent year, we estimated that approximately 9% of the CKD population at highest ESRD risk would need to be followed. In this manner, the proportion that needs to be followed provides important insight as to whether such a participation target (in this case, 9% of the cohort) is feasible.27
Using two CKD cohorts from Canada, Tangri et al.15 evaluated the discrimination of several risk predictive models using traditional criteria (C statistic and integrated discrimination improvement). Tangri et al.15 concluded that the most accurate model incorporated age, sex, eGFR, albuminuria, and serum concentrations of calcium, phosphate, bicarbonate, and albumin. When assessed using the above-described criteria (proportion of cases followed and proportion of cases that need to be followed), we observed that a complex model incorporating sociodemographic information, clinical comorbidities, and laboratory values provided little additional information over a more parsimonious one. The discriminatory ability of our simpler model was likely enhanced by the incorporation of race, and it also underscores the prognostic importance of dipstick proteinuria.28,29 We and others have previously described marked racial and ethnic differences in ESRD risk among adults receiving care in different United States health care settings.20,30–33 From a population health or health system perspective, a model that relies on commonly available variables is highly desirable, because detailed clinical comorbidity and laboratory data are often missing in a substantial proportion of patients treated in traditionally underserved health care settings.
Until recently, disadvantaged patients with nondialysis-requiring CKD in the United States were essentially invisible to much of the health care system until and if they reached ESRD.1 However, increasing use of electronic health records now allows health researchers, public health administrators, and local policymakers to potentially identify groups of individuals who may benefit from targeted programs that offer more intensive surveillance and risk factor management.19 Accordingly, our methodological approach provides a framework that a municipal (or any) health system might leverage to monitor care and outreach to the relatively small fraction of underserved patients who are at particularly high risk of experiencing progressive CKD, additional morbidity, and increased public costs.
Strengths and LimitationsOur study is strengthened by the inclusion of adults with moderate to advanced CKD from two large safety net health systems—populations rarely accessed in prior studies of CKD. In addition to providing detailed demographic and clinical data, we were able to link our cohort to national registries to obtain complete or nearly complete capture of treated ESRD and vital status. Our study also had several limitations. First, although diverse populations were well represented in our study, our cohort may not be fully reflective of persons receiving care from public hospitals or safety net health systems in other United States regions. However, the distributions of race/ethnicity and health insurance coverage in our study were consistent with estimates of populations generally served by members of the National Association of Public Hospitals.34 Second, our assessment of comorbid conditions was on the basis of diagnostic codes and thus, likely underestimates the prevalence of comorbidities, such as cardiovascular disease, diabetes mellitus, hypertension, viral illness, and drug or alcohol abuse in this population; moreover, although we incorporated laboratory measures that often reflect disease severity, we could not directly determine the severity or duration of most of the comorbid conditions. Third, we used multiple imputation to address missing data under the assumption of missing at random, a flexible assumption that allows missingness to be related to observed variables. However, we cannot rule out the possibility of bias in the event that this assumption does not hold.35 Fourth, although it is possible that we have misclassified some persons with AKI or near-normal kidney function as having CKD, we attempted to reduce this potential misclassification by requiring at least two ambulatory eGFR determinations and one additional ambulatory visit for study inclusion. Misclassification of CKD and its severity using population-based GFR-estimating equations may also be operative, because the Modification of Diet in Renal Disease (MDRD) study equation was derived in a population of largely white and black patients with moderate to advanced CKD, very few of whom had diabetes mellitus.36
Among persons with CKD receiving care in the American health care safety net, the risk of progressing to ESRD is markedly elevated and highly concentrated among relatively few individuals. A simple model using five commonly available variables adequately discriminates between most individuals with CKD who will and will not progress to ESRD. Application of risk prediction at the system level in the health care safety net may improve the effectiveness of CKD-related care delivery by allowing resources to be directed to a relatively small subcohort of patients who are at highest risk for developing progressive disease and disability.
Concise Methods
Design, Participants, and Setting
We conducted a cohort study of persons with nondialysis-requiring CKD stages 3–5 who received health care in the CHN and the HMC. The CHN is the health care delivery system of the Department of Public Health of the City and County of San Francisco. The CHN provides ambulatory and acute care to the majority of the estimated 130,000 uninsured residents of San Francisco.37 Services are available for free or on a sliding scale on the basis of income. Specific details of the cohort, including a description of the CHN, have been previously published.32,38 The HMC in Seattle, Washington is the Pacific Northwest’s largest provider of care to medically underserved populations, providing over 20% of all indigent (ambulatory and acute) care in the state of Washington. The HMC ambulatory clinics also provide care to a substantially higher proportion of patients from racial/ethnic minority backgrounds than other hospitals in the region.
Data Sources
The study cohort included 28,779 adults ages ≥18 years with nondialysis-requiring CKD stages 3–5 who received ambulatory care in either the CHN or the HMC from January 1, 1996 to December 31, 2009. We defined CKD on the basis of at least two ambulatory eGFR measurements <60 ml/min per 1.73 m2 (calculated by the re-expressed MDRD study equation on the basis of calibrated serum creatinine, age, race, and sex) that were separated by at least 3 months.36
Outcome Measures
The primary outcome measure was progression to ESRD, which was defined as having a first service date for maintenance dialysis or kidney transplantation. To ascertain ESRD, we performed linkage with the US Renal Data System (USRDS) files on the basis of patient name, date of birth, and Social Security number.1 To ascertain death, we performed identifier matching with the Social Security Administration Master Death files using the same patient identifiers described above. We assessed ESRD and death through September 29, 2011, the last date that data were available for both outcomes at the time of identifier linkage. We defined survival time as time from the first ambulatory eGFR date to ESRD, death, or the end of follow-up through September 29, 2011, whichever occurred first.
Independent Variables
We extracted data on important sociodemographic and clinical factors that we hypothesized might predict progression of established CKD to ESRD in the urban health care safety net on the basis of prior studies.14–19,29,32,33,38,39 Covariates were defined within the 2-year period closest to the index qualifying eGFR measurement. Individual-level sociodemographic covariates included patient age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander, or other), and health insurance coverage (uninsured, Medicaid, Medicare, or commercial insurance) on the basis of administrative data. We ascertained comorbid conditions on the basis of established algorithms using discharge diagnostic codes, ambulatory diagnostic codes, and procedural codes for diabetes mellitus, hypertension, cardiovascular disease (defined as coronary artery, cerebrovascular, or peripheral vascular disease), chronic viral disease (hepatitis B virus, hepatitis C virus, HIV, or AIDS), and drug or alcohol abuse.32 Laboratory covariates included eGFR, hemoglobin, serum calcium, serum albumin, and serum cholesterol concentrations as well as the presence and severity of proteinuria according to dipstick urinalysis.28,29
Statistical Analyses
We summarized the characteristics of the cohort using means (SDs) and proportions. We calculated unadjusted incidence rates of ESRD for the full cohort and subgroups on the basis of age, sex, race/ethnicity, hypertension, diabetes, chronic viral disease, substance abuse, and cardiovascular disease.
Model Development
We developed four proportional hazards models, each building on the previous model.14–16 Starting with age, sex, race/ethnicity, and eGFR (model 1), we added dipstick proteinuria plus an interaction between eGFR and dipstick proteinuria (model 2), health insurance coverage, comorbidities (diabetes mellitus, cardiovascular disease, hypertension, substance abuse, and chronic viral disease; model 3), and additional laboratory variables (serum albumin, calcium, cholesterol, and hemoglobin; model 4).
Model Validation
To evaluate the predictive capacity of the models, we used 2-fold cross-validation and trained and validated our models on separate subsets of the data.40 We divided the full cohort into training and validation sets (two thirds and one third of the full cohort, respectively) using stratified sampling stratifying on the level of eGFR.41 The final numbers of subjects in the training and validation sets were 19,215 and 9564, respectively.
Multiple Imputation
In the full cohort, 28% of patients were missing results for dipstick proteinuria, 15% of patients were missing results for serum cholesterol, 6% of patients were missing results for serum calcium, 2% of patients were missing results for serum albumin, and 2% of patients were missing results for hemoglobin. To reduce potential bias caused by excluding patients with missing data, we performed multiple imputation by chained equations with 10 imputations in the training and validation sets separately using the R package mice (version 2.18) on the basis of observed variables related to the missingness (i.e., missing at random).42,43
Model Fitting (and Validation)
We fitted each model to 10 training sets and estimated the hazard ratios and SEMs, taking into account the variability associated with the multiple imputation.42 The baseline hazard function and the estimated coefficients for each model fit to the training set were fixed and applied to the validation set to obtain the probability of ESRD-free survival beyond years 1, 3, and 5 for each subject in the validation set. We applied this procedure to each of 10 imputed training validation dataset pairs.
Model Performance
We used the following discrimination and calibration criteria to assess the predictive performance of each model: (1) ROC curve and the area under the ROC curve,44 (2) prediction error,45 (3) proportion of cases followed [PCF(q)], and (4) proportion of the population needed to be followed [PNF(p)].27 PCF and PNF are recently developed pragmatic measures of concentration of risk that are directly relevant to public health decision making. The PCF(q) represents the estimated proportion of cases (or events) that would be captured if we followed proportion q of the population at highest risk. PNF(p) represents the estimated proportion of the population at highest risk that we would need to follow to capture proportion p of the events. Larger values of PCF(q) and smaller values of PNF(p) indicate better performance. All measures were estimated nonparametrically, with inverse probability weighting used to account for censoring.46 The censoring weights were estimated using the Kaplan–Meier estimator of the censoring distribution. The SEMs within each validation set were estimated using perturbation, a resampling-based method for variance estimation, with 500 replications and weights distributed exp(1).47 The estimates from each of 10 imputed validation sets were combined to obtain the final estimates along with empirical SEMs accounting for variability within and between the imputed sets. In sensitivity analyses, we repeated the analyses in the CHN and HMC cohorts individually.
The USRDS and the institutional review boards at the University of Washington and the University of California San Francisco reviewed and approved the study protocol. We performed all statistical analyses using R 3.0.2 (http://cran.r-project.org).
Disclosures
J.H. served as a consultant for Biogen Idec and has ownership interest in Thrasos Innovations, Inc. G.M.C. serves on the Board of Directors of Satellite HealthCare and PuraCath; reports serving as a consultant for Amgen, Inc., Astra Zeneca, Gilead, Otsuka, and ZS Pharma; and has ownership interest in Ardelyx, Allocure, HD+, PuraCath, and Thrasos. Y.N.H. previously received research funding from Satellite HealthCare's Norman S. Coplon Extramural Grant Program.
Supplementary Material
Acknowledgments
We dedicate this manuscript to the friendship and memory of Dr. Andy Choi. Dr. Choi’s legacy of tireless work for vulnerable populations inspired and propelled this research. We thank Ms. Beth Forrest of US Renal Data System for her assistance with the identifier linkage and Dr. Andy Bindman for providing administrative support.
The study was funded by Grants K23-DK087900, R03-DK099487, and K24-DK085446 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH). The study was also supported by National Center for Advancing Translational Sciences of the NIH Grant UL1-TR000423.
The findings and conclusions in this report are solely the responsibility of the authors and do not necessarily represent the official views of the US Government or the NIH.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014060546/-/DCSupplemental.
References
- 1.US Renal Data System: USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014. Available at: http://www.usrds.org/adr.aspx. Accessed July 30, 2014
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Ruggenenti P, Perna A, Mosconi L, Matalone M, Garini G, Salvadori M, Zoccali C, Scolari F, Maggiore Q, Tognoni G, Remuzzi G, Migone L, Marubini E, DelFavero A, Ideo G, Geraci E, Loi U, Bracchi M, Costantino E, Scolari F, Maiorca R, Cofano F, Fellin G, The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) : Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 4.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS, AIPRD Study Group : Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: A patient-level meta-analysis. Ann Intern Med 139: 244–252, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS: The effect of a lower target blood pressure on the progression of kidney disease: Long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 142: 342–351, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Olomu AB, Gourineni V, Huang JL, Pandya N, Efeovbokhan N, Samaraweera J, Parashar K, Holmes-Rovner M: Rate and predictors of blood pressure control in a federal qualified health center in Michigan: A huge concern? J Clin Hypertens (Greenwich) 15: 254–263, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall YN, Rodriguez RA, Boyko EJ, Chertow GM, O’Hare AM: Characteristics of uninsured Americans with chronic kidney disease. J Gen Intern Med 24: 917–922, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thamer M, Richard C, Ray NF, Greer JW, Cotter DJ, Pearson BC: The effect of insurance status on use of recombinant erythropoietin therapy among end-stage renal disease patients in three states. Am J Kidney Dis 28: 235–249, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, Powe NR: The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 137: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Ward MM: Laboratory abnormalities at the onset of treatment of end-stage renal disease: Are there racial or socioeconomic disparities in care? Arch Intern Med 167: 1083–1091, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Johansen KL, Zhang R, Huang Y, Patzer RE, Kutner NG: Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clin J Am Soc Nephrol 7: 1490–1497, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR: Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol 20: 1069–1077, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Chronic Kidney Disease Prognosis Consortium : Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson ES, Thorp ML, Platt RW, Smith DH: Predicting the risk of dialysis and transplant among patients with CKD: A retrospective cohort study. Am J Kidney Dis 52: 653–660, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T, Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peeters MJ, van Zuilen AD, van den Brand JA, Bots ML, van Buren M, Ten Dam MA, Kaasjager KA, Ligtenberg G, Sijpkens YW, Sluiter HE, van de Ven PJ, Vervoort G, Vleming LJ, Blankestijn PJ, Wetzels JF: Nurse practitioner care improves renal outcome in patients with CKD. J Am Soc Nephrol 25: 390–398, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peralta CA, Shlipak MG, Fan D, Ordoñez J, Lash JP, Chertow GM, Go AS: Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol 17: 2892–2899, 2006 [DOI] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services: The Health Information Technology for Economic and Clinical Health (HITECH) Act. Available at: http://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/hitechact.pdf. Accessed July 30, 2014
- 22.US Department of Health and Human Services; Institute of Medicine: The Health Data Initiative (HDI). Available at: http://www.hhs.gov/digitalstrategy.html. Accessed July 30, 2014
- 23.US Department of Health and Human Services Office of Disease Prevention and Health Promotion: Healthy People 2020. Available at: http://healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=6. Accessed May 14, 2014
- 24.Wilper AP, Woolhandler S, Lasser KE, McCormick D, Bor DH, Himmelstein DU: A national study of chronic disease prevalence and access to care in uninsured U.S. adults. Ann Intern Med 149: 170–176, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Decker SL, Kostova D, Kenney GM, Long SK: Health status, risk factors, and medical conditions among persons enrolled in Medicaid vs uninsured low-income adults potentially eligible for Medicaid under the Affordable Care Act. JAMA 309: 2579–2586, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Maziarz M, Chertow GM, Himmelfarb J, Hall YN: Homelessness and risk of end-stage renal disease. J Health Care Poor Underserved 25: 1231–1244, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeiffer RM, Gail MH: Two criteria for evaluating risk prediction models. Biometrics 67: 1057–1065, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iseki K, Ikemiya Y, Iseki C, Takishita S: Proteinuria and the risk of developing end-stage renal disease. Kidney Int 63: 1468–1474, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS: Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med 169: 342–350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J: End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 277: 1293–1298, 1997 [PubMed] [Google Scholar]
- 31.Hall YN, Hsu CY, Iribarren C, Darbinian J, McCulloch CE, Go AS: The conundrum of increased burden of end-stage renal disease in Asians. Kidney Int 68: 2310–2316, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Hall YN, Choi AI, Chertow GM, Bindman AB: Chronic kidney disease in the urban poor. Clin J Am Soc Nephrol 5: 828–835, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipworth L, Mumma MT, Cavanaugh KL, Edwards TL, Ikizler TA, Tarone RE, McLaughlin JK, Blot WJ: Incidence and predictors of end stage renal disease among low-income blacks and whites. PLoS ONE 7: e48407, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.America's Public Hospitals and Health Systems: Results of the Annual NAPH Hospital Characteristics Survey, pages 2–17, 2010. Available at: http://www.naph.org/Publications/2009-Public-Hospital-Financial-Characteristics-.aspx?FT=.pdf. Accessed on July 21, 2014
- 35.Montez-Rath ME, Winkelmayer WC, Desai M: Addressing missing data in clinical studies of kidney diseases. Clin J Am Soc Nephrol 9: 1328–1335, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Schillinger D, Bibbins-Domingo K, Vranizan K, Bacchetti P, Luce JM, Bindman AB: Effects of primary care coordination on public hospital patients. J Gen Intern Med 15: 329–336, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall YN, Choi AI, Xu P, Smith NL, Boyko EJ: Predictors of end-stage renal disease in the urban poor. J Health Care Poor Underserved 24: 1686–1700, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turin TC, Tonelli M, Manns BJ, Ahmed SB, Ravani P, James M, Hemmelgarn BR: Lifetime risk of ESRD. J Am Soc Nephrol 23: 1569–1578, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Houweligen H, Putter H: Dynamic Prediction in Clinical Survival Analysis, Boca Raton, FL, CRC Press, Inc., 2011 [Google Scholar]
- 41.Lohr SL: Sampling: Design and Analysis, Boston, Brooks/Cole, 2010 [Google Scholar]
- 42.Little RJA, Rubin DB: Statistical Analysis with Missing Data, New York, J. Wiley & Sons, 1987 [Google Scholar]
- 43.van Buuren S, Groothuis-Oudshoom K: mice: Multivariate imputation by chained equations in R. J Stat Softw 45: 1–67, 2011 [Google Scholar]
- 44.Pencina MJ, D’Agostino RB, Sr., D’Agostino RB, Jr., Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Gerds TA, Schumacher M: Consistent estimation of the expected Brier score in general survival models with right-censored event times. Biom J 48: 1029–1040, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Schoop R, Graf E, Schumacher M: Quantifying the predictive performance of prognostic models for censored survival data with time-dependent covariates. Biometrics 64: 603–610, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Tian L, Cai T, Goetghebeur E, Wei LJ: Model evaluation based on the sampling distribution of estimated absolute prediction error. Biometrika 94: 297–311, 2007 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.