Abstract
Obesity can cause insulin resistance and type 2 diabetes. Moderate elevations in bilirubin levels have anti-diabetic effects. This study is aimed at determining the mechanisms by which bilirubin treatment reduces obesity and insulin resistance in a diet-induced obesity (DIO) mouse model. DIO mice were treated with bilirubin or vehicle for 14 days. Body weights, plasma glucose, and insulin tolerance tests were performed prior to, immediately, and 7 weeks post-treatment. Serum lipid, leptin, adiponectin, insulin, total and direct bilirubin levels were measured. Expression of factors involved in adipose metabolism including sterol regulatory element-binding protein (SREBP-1), insulin receptor (IR), and PPARγ in liver were measured by RT-PCR and Western blot. Compared to controls, bilirubin-treated mice exhibited reductions in body weight, blood glucose levels, total cholesterol (TC), leptin, total and direct bilirubin, and increases in adiponectin and expression of SREBP-1, IR, and PPARγ mRNA. The improved metabolic control achieved by bilirubin-treated mice was persistent: at two months after treatment termination, bilirubin-treated DIO mice remained insulin sensitive with lower leptin and higher adiponectin levels, together with increased PPARγ expression. These results indicate that bilirubin regulates cholesterol metabolism, adipokines and PPARγ levels, which likely contribute to increased insulin sensitivity and glucose tolerance in DIO mice.
Obesity has reached epidemic proportions globally, and at least 2.8 million people die each year as a result of being overweight or obese (WHO). Obesity also represents the most important risk factor for insulin resistance, cardiovascular diseases, and type 2 diabetes (T2D)1. Patients with T2D are commonly hyperglycemic, and suffer complications such as hypertension, stroke, atherosclerosis, and cancer2. Strategies that reduce obesity promise to reduce mortality and improve quality of life of affected individuals.
Adipose tissue plays a critical role in glucose homeostasis and lipid metabolism3,4 via production of various proteins known as adipokines or adipocytokines including tumor necrosis factor-α (TNF-α), interleukin-6, monocyte chemoattractant protein 1, leptin, and adiponectin5,6,7. Adipokines act as metabolic switches, connecting the body’s nutritional status to other energy consuming functions8. Dysregulation of adipokines leads to obesity related diseases9. Among known adipokines, leptin levels positively correspond with energy stored in the fat mass, and elevated leptin levels correlate with increased adiposity and inflammation resulting from an increased release of cytokines, such as TNF-α10. In contrast, adiponectin acts as an insulin-sensitizing agent and enhances fatty acid oxidation, liver insulin action, and glucose uptake5,11,12. Adiponectin deficient mice exhibit elevated plasma glucose and insulin levels after being fed a high-fat diet, but overexpression of adiponectin in these mice by adenoviral infection restores insulin sensitivity13. In addition, adiponectin also has anti-inflammatory and anti-atherogenic effects resulting from its capacity to suppress TNF-α-mediated NF-κB activation in endothelial cells14. Circulating adiponectin levels are increased following weight loss15, by induction of heme oxygenase-1 (HO-1)16, and by treatment with PPARγ ligands, including thiazolidinediones17,18.
HO-1 is a rate-limiting enzyme that degrades heme generating equal molar amounts of biliverdin, carbon monoxide, and ferrous iron19. Biliverdin can be rapidly converted into bilirubin by biliverdin reductase, and iron can up-regulate ferritin. HO-1 has been shown to play critical roles in reversing insulin resistance. For example, HO-1 induction sensitizes the insulin signaling pathway in Zucker rats20 and in leptin deficient (ob/ob) mice, in leptin-receptor deficient (db/db) mice, and in diet induced obese (DIO) mice16,21,22,23,24,25,26,27. Bilirubin mimics the effect of HO-1 induction and improves insulin sensitivity in obese mouse models28. Bilirubin is a strong anti-oxidant with anti-inflammatory and immune regulatory properties29. Although abnormally elevated bilirubin has been used as an indicator for hepatitis, hemolytic anemia, and cholestasis, mildly elevated unconjugated bilirubin in patients with Gilbert’s syndrome (1.1 mg/dl to 2.7 mg/dl) is associated with protection from coronary atherosclerosis, cardiovascular disease, as well as other diseases30,31,32,33. The positive association between unconjugated bilirubin and free plasma heme, iron, and carboxyl hemoglobin in these patients suggests a positive feedback loop in which HO-1 expression is induced by unconjugated bilirubin34.
We have shown that systemic administration of bilirubin increases insulin sensitivity via suppressing ER stress and inflammation in DIO mice35. We have now extended these observations and have investigated whether bilirubin regulates lipid metabolism as seen in patients with Gilbert’s disease34 and whether bilirubin regulates serum adipokine levels in DIO mice. We also assessed longer-term effects of bilirubin treatment on lipid metabolism and adipokine levels 7 weeks after completion of bilirubin treatment.
Results
Bilirubin administration reduces body weight and ameliorates insulin resistance in DIO mice
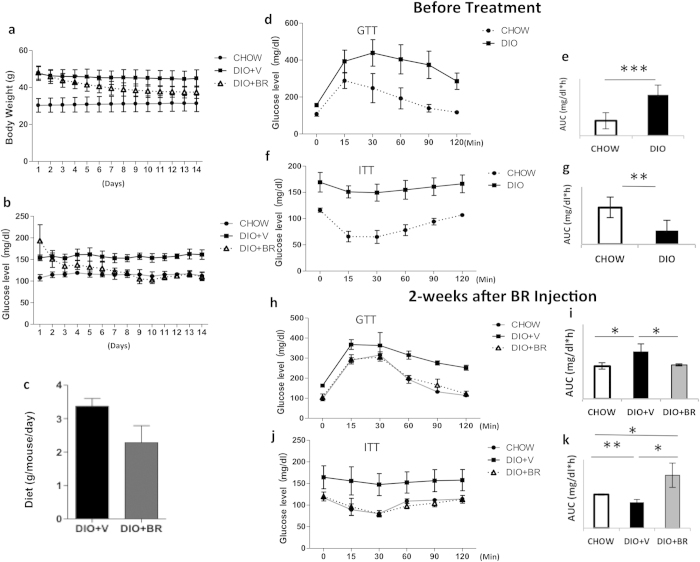
The effects of bilirubin administration on body weight, blood glucose level, and insulin sensitivity in DIO mice were determined. Animals were treated with bilirubin (20 μmol/kg) intraperitoneally twice per day for 14 days. This dose and dosing schedule has shown efficacy and was well tolerated in previously published islet transplantation and obese mouse models35,36. Feeding a high fat diet (Table 1) to C57BL/6 mice for 24 weeks resulted in significant increases in body weight (47.5 ± 3.7 g in DIO vs. 30.29 ± 3.5 in mice fed standard diet (CHOW), Fig. 1a). Bilirubin administration (DIO + BR) significantly reduced body weights in DIO mice (Fig. 1a). Blood glucose levels were reduced in bilirubin treated mice from 193.5 ± 37.1 mg/dl to 112.2 ± 8.9 mg/dl (Fig. 1b). Bilirubin treated mice ate less than DIO controls; however, this difference was not significant (p = 0.06, Fig. 1c). Bilirubin treatment resulted in improved glucose uptake and insulin utilization as evidenced by reduced blood glucose levels as well as reduced areas under the curve (GTT, Fig. 1h,j) or reverse areas above the curve (ITT, Fig. 1i,k), compared to GTT (Fig. 1d,e) and ITT (Fig. 1f,g) results before starting treatment. These data confirmed that bilirubin treatment reduced obesity and blood glucose levels and improved glucose tolerance and insulin sensitivity in DIO mice.
Table 1. Composition of the high fat diet.
| Ingredients | Percentage (%) |
|---|---|
| Corn starch | 27 |
| Bran | 19 |
| Rice | 16 |
| Soybean cake | 16 |
| Fish powder | 13 |
| Calcium powder | 3 |
| Bone powder | 3 |
| Yeast extract | 2.3 |
| Salt | 0.5 |
| Vitamin mix | 0.1 |
| Mineral mix | 0.1 |
Figure 1. Administration of bilirubin reduces body weight and increases insulin sensitivity in DIO mice.
(a) Changes in body weights of DIO mice treated with bilirubin (DIO + BR) or vehicle (DIO + V) compared to control mice fed standard diet (CHOW). (b) Daily non-fasting blood glucose levels in DIO + BR, DIO + V, and CHOW mice during bilirubin treatment. (c) Average food intake per mouse per 24-h period in DIO mice receiving BR or vehicle. (d) Intraperitoneal glucose tolerance test (GTT) of DIO mice and CHOW controls before bilirubin treatment; (e) area under the curve of GTT. (f) Insulin tolerance test (ITT) of DIO mice and CHOW mice before bilirubin treatment; (g) reverse area under the baseline above curve. (h) GTT of DIO + BR, DIO + V, and CHOW mice 14 days after the first bilirubin injection; (i) area under the curve. (j) ITT of DIO + BR, DIO + V, and CHOW mice at 14 days after the first bilirubin injection; (k) reverse area under the baseline above curve. At least 6-8 mice were included in each group; **p < 0.01, *p < 0.05, Student’s t test. DIO + BR: DIO mice treated with bilirubin; DIO + V: DIO mice treated with vehicle; CHOW: mice fed standard diet.
Bilirubin administration reduces liver and fat weights by reducing adiposity in those tissues
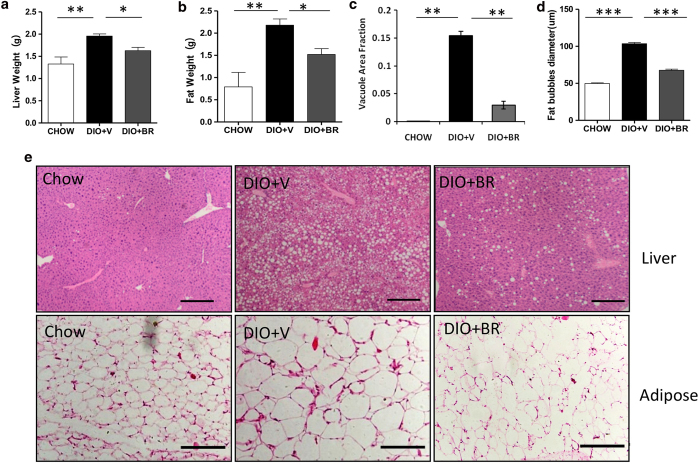
To determine the effects of bilirubin on adiposity, liver and epididymal fat tissue were obtained from half of the mice in each treatment arm (n = 3-4) and were sacrificed after 14-days of bilirubin treatment. Mice in the bilirubin group had significantly reduced liver and epididymal fat weights (Fig. 2). Using hematoxylin and eosin (H&E) staining, we observed that treatment with bilirubin improved hepatic steatosis in DIO mice (Fig. 2c,e, upper panel) and reduced the average size of adipocytes in epididymal fat (Fig. 2d,e, lower panel).
Figure 2. Bilirubin treatment reduces liver and fat weights by reducing adiposity 14 days after initiating bilirubin treatment.
(a) Liver weights of DIO + BR, DIO + V, and CHOW mice. (b) Epididymal fat weights of DIO + BR, DIO + V, and CHOW mice. (c) Vacuole area fractions in livers of DIO + BR, DIO + V, or CHOW mice. (d) Fat bubble diameters (μm) of epididymal adipocytes of DIO + BR, DIO + V, or CHOW. (e) Representative micrographs of H&E staining of liver and adipose tissue sections from DIO + BR, DIO + V, and CHOW mice. Scale bar = 200 μm. At least 3 mice were analyzed in each group; *p < 0.05, Student’s t test. Data are mean ± standard deviation.
Bilirubin therapy alters cholesterol and adipokine levels in DIO mice
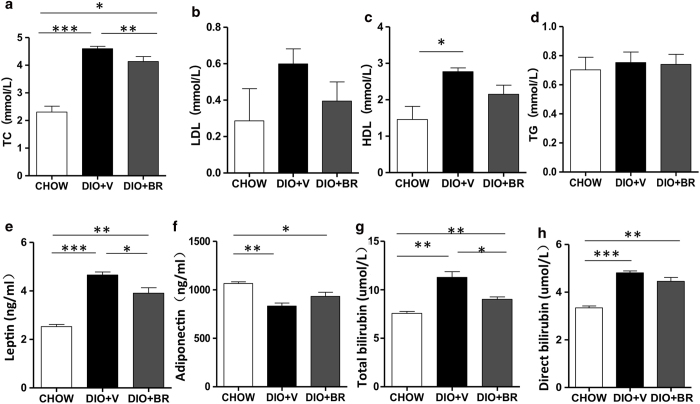
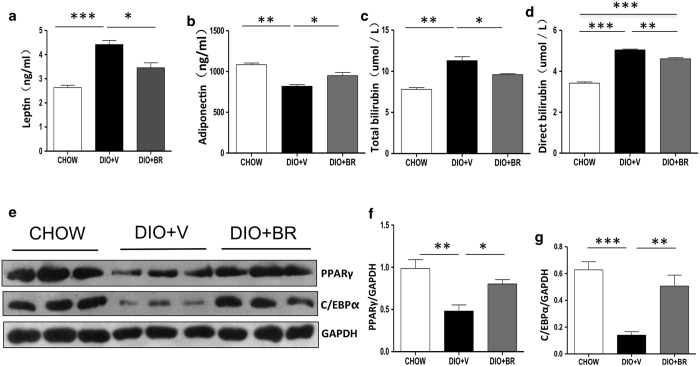
We measured TC, LDL, HDL, and TG levels in DIO mice treated with bilirubin or vehicle and in CHOW controls at 14 days post treatment. The data indicated that DIO mice had elevated TC, LDL, and HDL levels compared to CHOW controls (Fig. 3a–c). Bilirubin treatment significantly reduced TC levels in DIO mice (p < 0.01). Both LDL and HDL were also lower in bilirubin-treated DIO mice compared to vehicle-treated controls, although the differences were not significant. No changes in TG levels were observed among the groups (Fig. 3d). We evaluated whether bilirubin reversed changes in leptin and adiponectin levels caused by high fat diet in DIO mice. Bilirubin treatment significantly reduced leptin and slightly increased adiponectin levels 14 days post treatment (Fig. 3e,f), which supports the hypothesis that the effects of bilirubin on obesity and insulin resistance are, at least in part, mediated by leptin and adiponectin. Total serum bilirubin levels and direct bilirubin levels were also reduced in bilirubin-treated mice compared to DIO controls (Fig. 3g,h). Serum insulin levels were slightly reduced in bilirubin treated mice compared to DIO controls, but the differences were not significant (data not shown).
Figure 3. Bilirubin therapy alters cholesterol and adipokine levels in DIO mice at 14 days post treatment.
Serum levels of TC (a), LDL (b), HDL (c), TG (d), leptin (e), adiponectin (f), total bilirubin (g), and direct bilirubin (h) in DIO + BR, DIO + V, or CHOW mice were measured in serum immediately after completing bilirubin treatment. Each group contained 3-4 mice; Data are mean ± standard deviation; ***p < 0.001, **p < 0.01, and *p < 0.05, Student’s t test.
Bilirubin regulates the expression of genes related to obesity and insulin resistance in liver
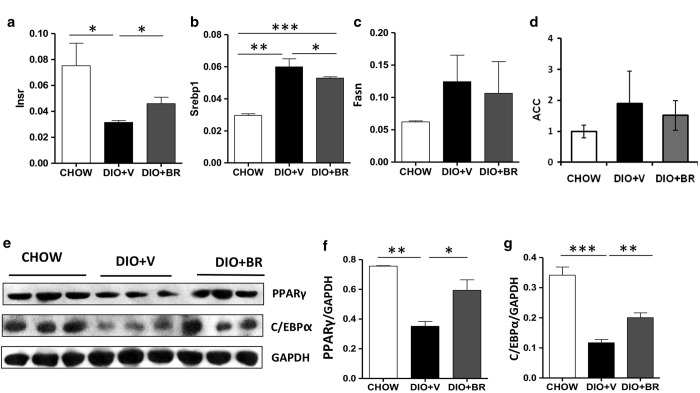
To examine the effect of bilirubin on hepatic lipid metabolism, we analyzed hepatic gene expression in DIO mice treated with vehicle or bilirubin and in CHOW controls. Bilirubin treatment reversed the decrease in insulin receptor (Insr) expression in liver caused by high fat diet (Fig. 4a). Moreover, bilirubin treatment significantly reduced expression of SREBP-1, a gene required for de novo lipogenesis (Fig. 4b). Expression levels of Fasn and acetyl-coA carboxylase (ACC) were not significantly different between DIO mice treated with bilirubin or vehicle (Fig. 4c,d). No differences in expression levels of the leptin receptor were observed among DIO mice treated with vehicle or bilirubin or CHOW controls (data not shown). Expression of PPARγ and CCAAT-enhancer-binding proteins (C/EBPs) were significantly reduced in DIO controls compared to CHOW but bilirubin treatment restored PPARγ levels and partially restored C/EBP levels (Fig. 4e–g), suggesting a possible role of PPARγ in the therapeutic effects of bilirubin.
Figure 4. Bilirubin promotes expression of genes related to obesity and insulin resistance in liver 14 days post treatment.
Relative mRNA expression of insulin receptor (Insr) (a), SREBP-1 (b), Fasn (c) and ACC (d) in livers of DIO + BR, DIO + V, and CHOW mice as measured by RT-PCR analysis. Values represent relative expression of target gene compared to expression of endogenous control. (e) Expression of PPARγ and C/EBPα protein determined in liver from DIO + BR, DIO + V, and CHOW mice by Western blot analysis. Gels had been run under same experimental conditions and cropped blots are shown. (f) Relative protein expression of PPARγ and (g) C/EBPα relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) control (NIH ImageJ software). Samples from 3 individual mice were analyzed. Data are mean ± standard deviation; ***p < 0.001, **p < 0.01 and *p < 0.05, Student’s t test.
The insulin sensitizing effects of bilirubin persist after completion of bilirubin treatment
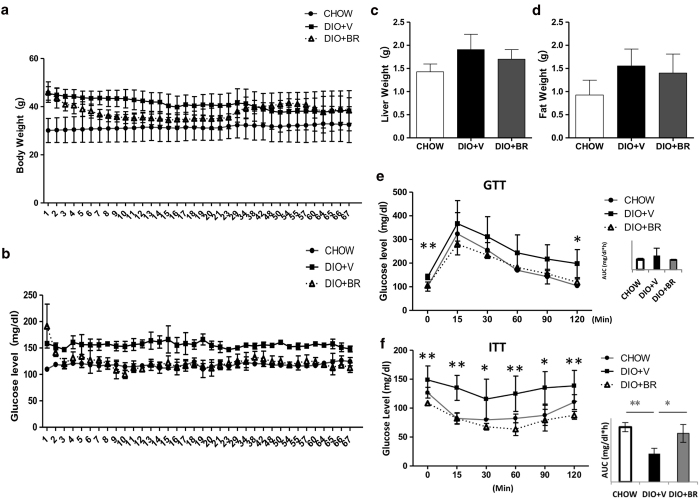
To determine whether the therapeutic effect of bilirubin on body weight and insulin sensitivity persists after treatment termination, DIO mice were treated with bilirubin or vehicle for 14 days (n = 3–4 per group) and were maintained of the high fat diet for a further 7 weeks following treatment. Body weight, glucose tolerance, insulin sensitivity, blood chemistry, and gene expression in liver were measured. Body weights in bilirubin-treated DIO mice remained reduced for a further two weeks and then slowly returned to a level comparable to DIO controls (Fig. 5a). Liver and fat weights showed trends similar to the total body-weight differences observed after discontinuing bilirubin treatment (Fig. 5c,d). The results of this experiment indicate that the effects of bilirubin on body weight are temporary. In contrast, bilirubin-treated mice maintained their insulin sensitivity and glucose tolerance, as manifested by their normoglycemia (Fig. 5b) and their lower blood glucose levels following GTT and ITT tests (Fig. 5e,f), 7 weeks after discontinuing bilirubin treatment. There were no differences in food intake between groups throughout the experiment (data not shown). At 7 weeks post treatment termination, mice treated with bilirubin retained slightly lower serum cholesterol (2.8 ± 1.0 mmol/L vs. 3.9 ± 1.7 mmol/L, P = 0.19), TG (0.7 ± 0.2 mmol/L vs. 0.9 ± 0.3 mmol/L, P = 0.14), leptin (3.5 ± 0.4 ng/ml vs. 4.4 ± 0.3 ng/ml, P = 0.01), total bilirubin (9.6 ± 0.2 μmol/L vs. 11.3 ± 0.28 μmol/L, P < 0.05), direct bilirubin (4.6 ± 0.1 μmol/L vs. 5.4 ± 0. 06 μmol/L, P < 0.01), and higher adiponectin (949.5 ng/ml ± 64.2 vs. 819.0 ± 34.2 ng/ml, p = 0.02) levels compared to DIO controls (Fig. 6.a–d). Expression of insulin receptor remained slightly higher compared to vehicle-treated controls (data not shown). Expression of PPARγ and C/EBPs remained significantly higher in bilirubin treated DIO mice compared to vehicle treated DIO mice (Fig. 6e–g), suggesting that the insulin-sensitizing effect of bilirubin is at least in part mediated by PPARγ. Taken together, these data suggest that short-term bilirubin treatment temporarily affects body weight but its insulin-sensitizing effects persist long after treatment ends.
Figure 5. The insulin sensitizing effects of bilirubin persists 7 weeks after completion of bilirubin treatment.
(a) Body weight of DIO + BR, DIO + V, and CHOW mice during the 9-week experiment. (b) Serum glucose levels in DIO + BR, DIO + V, and CHOW mice during the 9-week experiment. Liver (c) and epididymal fat weights (d) measured 7 weeks after treatment completion. (e) (left) GTT of DIO mice measured 7 weeks after treatment completion; (right) values of area under the curve above baseline; (f) (left) ITT of DIO mice measured 7 weeks after treatment completion; (right) values of reverse area above the curve under baseline. At least 3 mice were included in each group. Data are mean ± standard deviation; ***p < 0.001, **p < 0.01, *p < 0.05, Student’s t test.
Figure 6. Gene expression in liver 7 weeks after completion of bilirubin treatment.
Serum leptin (a), adiponectin (b), total bilirubin (c), and direct bilirubin (d) levels were measured in liver 7 weeks after completing bilirubin injection in DIO + BR, DIO + V, and CHOW mice. (e) Expression of PPARγ and C/EBPα protein determined in liver from DIO + BR, DIO + V, and CHOW mice by Western blot. (f) Relative protein expression of PPARγ and (g) C/EBPα relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) control. Samples from 3 individual mice were analyzed. Data are mean ± standard deviation; ***p < 0.001, **p < 0.01 and *p < 0.05, Student’s t test.
Discussion
We recently identified a novel function for bilirubin as an insulin sensitizer that reduces obesity and hyperglycemia in obese mouse models35. The current study assessed the mechanisms of this protection. We first confirmed that a 14-day administration of bilirubin reduced body weight and increased insulin sensitivity in DIO mice without detectable side effects. The action of bilirubin was paralleled by a decrease in liver and fat weights, by decreases in serum total cholesterol, insulin, and leptin levels, and by increases in adiponectin levels. Bilirubin treatment reduced expression of SREBP-1, a gene critical for fatty acid synthesis, and restored expression in liver of insulin receptor, PPARγ, and C/EBPα. Although bilirubin-treated mice gradually regained body weight after treatment termination, they remained sensitive to insulin stimulation 7 weeks post-treatment, and serum TC, leptin, and adiponectin levels and insulin receptor expression remained comparable to levels immediately after bilirubin treatment, and similar to levels in lean controls.
The most dramatic finding of this study is that treatment with bilirubin reduced total cholesterol levels in DIO mice. The reduction may be due to changes in both HDL and LDL levels, although reductions of these elements did not reach statistical significance. These findings are in agreement with observations in individuals with Gilbert’s syndrome in which mildly elevated bilirubin levels are associated with reduced total cholesterol, LDL, and triglyceride concentrations, and are associated with reduced pro-inflammatory status and reduced exposure to oxidative conditions31,34,37,38,39. Although we didn’t observe a change in triglyceride concentrations in bilirubin-treated mice 14 days after bilirubin treatment, a reduction was observed 7 weeks post treatment, indicating that the impact of bilirubin on triglyceride levels may be delayed. Our study provided evidence that exogenously administered bilirubin is associated with an altered lipid profile in obese mice.
We found that bilirubin administration significantly reduced leptin levels in DIO mice. Food intake by bilirubin-treated DIO mice did not appear to be a factor during bilirubin treatment, and no difference in food intake was observed after treatment ended. The reduction in leptin levels in bilirubin treated mice may be caused by a reduction in adipose tissue as supported by decreased expression of SREBP-1. Two other observations are relevant: leptin is known to augment inflammation through regulation of TNF-α40, and bilirubin treatment reduces TNF-α expression in DIO mice35. Therefore, it is reasonable to postulate that the effects of bilirubin on obesity are a consequence of altered lipid synthesis, reduction of leptin levels, and decreased TNF-α production. Of clinical relevance, bilirubin should be examined as a potential treatment to reduce lipid synthesis, as suggested by human studies34.
We found that bilirubin treatment resulted in increased PPARγ expression in liver. PPARγ is thought to be a “master” gene of adipocyte biology and differentiation17, and various PPARγ agonists including thiazolidinediones have been used as insulin sensitizers for the treatment of type 2 diabetes. Thiazolidinediones stimulate insulin sensitivity via upregulation of adiponectin, a critical adipokine that enhances fatty acid oxidation, liver insulin action, and glucose uptake 5,11,12. We observed increased adiponectin levels immediately after bilirubin treatment and 7 weeks after bilirubin treatment. HO-1, another PPARγ agonist, has been shown to increase insulin sensitivity through PPARγ upregulation and adiponectin production5. Bilirubin is known to upregulate HO-1 in humans41. We observed increased HO-1 induction in our bilirubin treated mice (Kim. et al. unpublished data). Therefore, it is possible that bilirubin exerts its protective effect on insulin sensitivity through upregulation of HO-1 and PPARγ. Of note, PPARγ levels were reported to be increased in mice fed with high fat diet for 2 weeks42. In contrast, we observed a decrease in PPARγ expression in vehicle-treated DIO mice. This disparity might be caused by the length of high fat-diet feeding since we fed our mice for 24 weeks to achieve obesity and insulin resistance. In addition, there are three PPARγ isotypes, and PPARγ2 has been shown to be required for adiponectin expression. The PPARγ antibody used in this study recognizes all three isotypes (PPARγ1, 2, and 3). Therefore, we did not distinguish specific isotypes of PPARγ that were upregulated by bilirubin treatment. Nevertheless, we did observe increased C/EBPα expression in mice treated with bilirubin.
As also reported by other investigators43,44, we found that DIO mice exhibited higher total and direct bilirubin levels than did Chow controls or DIO mice treated with exogenous bilirubin. The increased bilirubin in DIO animals may reflect blockage of bilirubin excretion due to impaired liver function in these mice. One possible explanation for the reduction in bilirubin levels in bilirubin treated DIO mice is that the reduction may be a consequence of the ability of exogenous bilirubin to regulate P450. P450s are a superfamily of heme-containing monooxygenase enzymes that metabolize a diverse range of compounds of both endogenous and exogenous origin. Studies have shown that the two different isoforms of P450, CTP1A1 and CYP1A2 mediate an alternate route for bilirubin degradation45,46,47. In addition, bilirubin can directly regulate CYP1A1 enzyme activity in an Aryl hydrocarbon receptor-dependent manner48, and thereby promote bilirubin excretion. Thus, it seems possible that this mechanism improves bilirubin excretion (and thus lower bilirubin levels compared to DIO alone) in our DIO mice treated with exogenous bilirubin. Further studies beyond the scope of the current manuscript will be needed to test this hypothesis.
In this study, we found that short-term bilirubin treatment was associated with decreased total cholesterol and increased PPARγ and adipokines. These findings provide mechanistic evidence that bilirubin or altered bilirubin metabolism (e.g., partial UGT1A1 inhibitors49) may be useful as a therapeutic approach to reduce obesity and improve insulin resistance and glucose tolerance.
Methods
Animals
Male C57BL/6 mice (6 weeks old) were purchased from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences (Beijing, China). Mice were fed either a high fat diet (Table 1) or standard CHOW-fat diet (10% of calories from fat) for 24 weeks before treatment. The Animal Care Committee at the Qingdao Agricultural University approved all animal experiments. The methods were carried out in accordance with the approved guidelines.
Bilirubin preparation and administration
Bilirubin (Frontier Scientific, Logan, UT) was dissolved in 0.1 N NaOH and the pH was adjusted to 7.4 using HCl. Bilirubin was administered intraperitoneally 20 μmol/kg (11.7 mg/kg) twice per day for 14 days. Control DIO or CHOW mice received vehicle on the same injection schedule.
Mouse monitoring after treatment
Non-fasting serum blood glucose levels and body weights of mice were measured daily at 9:00 am. A drop of whole blood (about 5–10 μl) was collected from a minimal tail incision from end of the mouse’s tail and analyzed using a Sannuo glucometer (San Nuo Inc., Changsha, China). Physical activities of mice (including behavior, food intake, drinking, and licking) were observed on a daily basis as described35. Food intake (excluding spillage) was measured during a 24 h period at 7 days post-treatment, and weekly for 7 weeks after bilirubin treatment.
Intraperitoneal glucose tolerance (IPGTT) and insulin tolerance test (ITT)
For IPGTT, mice were fasted overnight and then injected with 2 g/kg of glucose solution (i.p.). For ITT, mice were fasted for 5 hours and then injected with 0.75 U/kg insulin (i.p.; Eli Lili, Indianapolis, IN). Serum blood glucose levels were measured at 0, 15, 30, 60, 90, and 120 min after glucose or insulin injection.
Tissue harvesting, serum preparation, and blood biochemistry
At the end of each experiment, mice were anesthetized and blood was collected by retroorbital bleeding into a heparinized tube. Plasma was obtained by centrifugation and stored at -80 °C for further analysis. Mice were then euthanized and livers and epididymal fat tissues were separated, weighed, and snap frozen in liquid nitrogen for later analysis. Serum TC, TG, HDL, LDL, and bilirubin levels were measured using specific reagent kits (Sigma Aldrich, Saint Louis, MO). Leptin, insulin, and adiponectin levels were measured by ELISA (R&D Systems, Minneapolis, MN).
Real-Time (RT)-PCR analysis
Expression of the insulin receptor, leptin receptor, apolipoprotein A-IV (Apoa4), and SREBP-1 in livers were quantified by RT-PCR analysis as described previously50. β actin expression was quantified by RT-PCR in each sample and used as an endogenous control. Real time RT-PCR primers were purchased from Life Technologies (Invitrogen Trading Co., Ltd., Shanghai, China).
Western blot analysis
Protein content of whole cell lysates were quantified by BCA assay, and 30 μg protein from each sample was separated on a 4–12% Bis-Tris gel (Beyotime Institute of Biotechnology, Haimen, China). Proteins were transferred to a Hybond-P membrane (GE healthcare, Piscataway, NJ), blocked with 5% non-fat milk, and incubated with the following primary antibodies: PPARγ, C/EBPα (Abcam, USA), and GAPDH (Santa Cruz Biotechnology Inc., USA), overnight at 4 °C. Blots were then probed with horseradish peroxidase labeled secondary antibodies (Sangon Biotech Ltd., Shanghai, China) 45 minutes at room temperature and visualized by an ECL detection kit (Amersham Pharmacia Biotech, Little Chalfont, UK). The intensity of each signal was determined using Image J software (NIH).
Hematoxylin and eosin (H &E) staining
Immunohistochemistry of mouse liver and epididymal fat were performed as described35. Briefly, liver or adipose tissue sections were fixed in 10% buffered formalin overnight, embedded in paraffin, and sectioned. The slides were immersed in filtered hematoxylin for 6 min, rinsed with water, and stained with Eosin for another 1–2 min. Sections were rinsed with water and dehydrated in ascending alcohol solutions, cleared with xylene, and mounted with a cover slip onto a labeled glass slide. Slides were examined under a transflective dual-use Olympus BX51 microscope (Olympus, Tokyo, Japan), and images were captured using an Olympus DP72 digital camera. Vacuole area in total liver tissue area was determined in liver by the Image-Pro Plus analysis software. Diameters of individual fat cells (n = 75 in each group) were measured using CellSens Standard software (Olympus).
Statistical analysis
Data are expressed as mean ± SD. Differences between two groups were compared for statistical significance using unpaired Student’s t tests with Bonferroni correction. Differences was considered significant when p < 0.05.
Additional Information
How to cite this article: Liu, J. et al. Bilirubin Increases Insulin Sensitivity by Regulating Cholesterol Metabolism, Adipokines and PPARγ Levels. Sci. Rep. 5, 9886; doi: 10.1038/srep09886 (2015).
Acknowledgments
This work was made possible by the Taishan Scholar Construction Foundation of Shandong Province, P.R. China (631114), the Natural Science Foundation of Shandong Province, China (ZR2014CM011), and the National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases (EB015744, DK097544 and DK099696). We thank Ms. Yang Li and Zhen Sun for technical support.
Footnotes
Author Contributions J.L. and D.H. performed and analyzed all experiments. Y.Z, M.C. and L.S. performed some of experiments. Q.P., A.B., D.A. and X.D. contributed to scientific design and writing of the manuscript. H.W. designed the experiments and wrote the manuscript.
References
- Lee J. M., Okumura M. J., Davis M. M., Herman W. H. & Gurney J. G. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care 29, 2427–2432 (2006). [DOI] [PubMed] [Google Scholar]
- Masuo K., Rakugi H., Ogihara T., Esler M. D. & Lambert G. W. Cardiovascular and renal complications of type 2 diabetes in obesity: role of sympathetic nerve activity and insulin resistance. Curr Diabetes Rev 6, 58–67 (2010). [DOI] [PubMed] [Google Scholar]
- Chu N. F. et al. Plasma insulin, leptin, and soluble TNF receptors levels in relation to obesity-related atherogenic and thrombogenic cardiovascular disease risk factors among men. Atherosclerosis 157, 495–503 (2001). [DOI] [PubMed] [Google Scholar]
- Kahn B. B. & Flier J. S. Obesity and insulin resistance. J Clin Invest 106, 473–481 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes 57, 1526–1535 (2008). [DOI] [PubMed] [Google Scholar]
- Berg A. H. & Scherer P. E. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96, 939–949 (2005). [DOI] [PubMed] [Google Scholar]
- Kim J. Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117, 2621–2637 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel P. J. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol 13, 51–59 (2002). [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Alquier T., Carling D. & Hardie D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1, 15–25 (2005). [DOI] [PubMed] [Google Scholar]
- Frederich R. C. et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1, 1311–1314 (1995). [DOI] [PubMed] [Google Scholar]
- Berg A. H., Combs T. P., Du X., Brownlee M. & Scherer P. E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7, 947–953 (2001). [DOI] [PubMed] [Google Scholar]
- Basu R., Pajvani U. B., Rizza R. A. & Scherer P. E. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes 56, 2174–2177 (2007). [DOI] [PubMed] [Google Scholar]
- Maeda N. et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8, 731–737 (2002). [DOI] [PubMed] [Google Scholar]
- Ouchi N. et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 102, 1296–1301 (2000). [DOI] [PubMed] [Google Scholar]
- Gan S. K. et al. Changes in aerobic capacity and visceral fat but not myocyte lipid levels predict increased insulin action after exercise in overweight and obese men. Diabetes Care 26, 1706–1713 (2003). [DOI] [PubMed] [Google Scholar]
- Kim D. H. et al. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J Pharmacol Exp Ther 325, 833–840 (2008). [DOI] [PubMed] [Google Scholar]
- Choi J. H. et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature 466, 451–456 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki M. et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52, 1655–1663 (2003). [DOI] [PubMed] [Google Scholar]
- Otterbein L. E., Soares M. P., Yamashita K. & Bach F. H. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol 24, 449–455 (2003). [DOI] [PubMed] [Google Scholar]
- Ndisang J. F., Lane N. & Jadhav A. The heme oxygenase system abates hyperglycemia in Zucker diabetic fatty rats by potentiating insulin-sensitizing pathways. Endocrinology 150, 2098–2108 (2009). [DOI] [PubMed] [Google Scholar]
- Chen Y. H. et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet 111, 1–8 (2002). [DOI] [PubMed] [Google Scholar]
- Bruce C. R., Carey A. L., Hawley J. A. & Febbraio M. A. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 52, 2338–2345 (2003). [DOI] [PubMed] [Google Scholar]
- Arredondo M., Jorquera D., Carrasco E., Albala C. & Hertrampf E. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with iron status in persons with type 2 diabetes mellitus. Am J Clin Nutr 86, 1347–1353 (2007). [DOI] [PubMed] [Google Scholar]
- Shakeri-Manesch S. et al. Diminished upregulation of visceral adipose heme oxygenase-1 correlates with waist-to-hip ratio and insulin resistance. Int J Obes (Lond) 33, 1257–1264 (2009). [DOI] [PubMed] [Google Scholar]
- Li M. et al. Interdiction of the diabetic state in NOD mice by sustained induction of heme oxygenase: possible role of carbon monoxide and bilirubin. Antioxid Redox Signal 9, 855–863 (2007). [DOI] [PubMed] [Google Scholar]
- Nicolai A. et al. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension 53, 508–515 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S. J., Frishman W. H. & Abraham N. G. Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system. Cardiol Rev 17, 99–111 (2009). [DOI] [PubMed] [Google Scholar]
- Dong H. et al. Bilirubin increases insulin sensitivity in leptin-receptor deficient and diet-induced obese mice through suppression of ER stress and chronic inflammation. Endocrinology 155, 818–828 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal 6, 841–849 (2004). [DOI] [PubMed] [Google Scholar]
- Schwertner H. A., Jackson W. G. & Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem 40, 18–23 (1994). [PubMed] [Google Scholar]
- Wallner M. et al. Protection from age-related increase in lipid biomarkers and inflammation contributes to cardiovascular protection in Gilbert’s syndrome. Clin Sci (Lond) 125, 257–264 (2013). [DOI] [PubMed] [Google Scholar]
- Lin J. P., Vitek L. & Schwertner H. A. Serum bilirubin and genes controlling bilirubin concentrations as biomarkers for cardiovascular disease. Clin Chem 56, 1535–1543 (2010). [DOI] [PubMed] [Google Scholar]
- Breimer L. H., Wannamethee G., Ebrahim S. & Shaper A. G. Serum bilirubin and risk of ischemic heart disease in middle-aged British men. Clin Chem 41, 1504–1508 (1995). [PubMed] [Google Scholar]
- Bulmer A. C., Verkade H. J. & Wagner K. H. Bilirubin and beyond: a review of lipid status in Gilbert’s syndrome and its relevance to cardiovascular disease protection. Prog Lipid Res 52, 193–205 (2013). [DOI] [PubMed] [Google Scholar]
- Dong H. et al. Bilirubin Increases Insulin Sensitivity in Leptin-Receptor Deficient and Diet-Induced Obese Mice Through Suppression of ER Stress and Chronic Inflammation. Endocrinology , en20131667 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. Bilirubin can induce tolerance to islet allografts. Endocrinology 147, 762–768 (2006). [DOI] [PubMed] [Google Scholar]
- Bulmer A. C., Blanchfield J. T., Toth I., Fassett R. G. & Coombes J. S. Improved resistance to serum oxidation in Gilbert’s syndrome: a mechanism for cardiovascular protection. Atherosclerosis 199, 390–396 (2008). [DOI] [PubMed] [Google Scholar]
- Tapan S. et al. Decreased small dense LDL levels in Gilbert’s syndrome. Clin Biochem 44, 300–303 (2011). [DOI] [PubMed] [Google Scholar]
- Boon A. C. et al. Reduced circulating oxidized LDL is associated with hypocholesterolemia and enhanced thiol status in Gilbert syndrome. Free Radic Biol Med 52, 2120–2127 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 5, 836–847 (2004). [DOI] [PubMed] [Google Scholar]
- Wallner M. et al. Haem catabolism: a novel modulator of inflammation in Gilbert’s syndrome. Eur J Clin Invest 43, 912–919 (2013). [DOI] [PubMed] [Google Scholar]
- Inoue M. et al. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun 336, 215–222 (2005). [DOI] [PubMed] [Google Scholar]
- Karmakar S. et al. Black tea prevents high fat diet-induced non-alcoholic steatohepatitis. Phytother Res 25, 1073–1081 (2011). [DOI] [PubMed] [Google Scholar]
- Sindhu E. R., Firdous A. P., Preethi K. C. & Kuttan R. Carotenoid lutein protects rats from paracetamol-, carbon tetrachloride- and ethanol-induced hepatic damage. J Pharm Pharmacol 62, 1054–1060 (2010). [DOI] [PubMed] [Google Scholar]
- Kapitulnik J. & Ostrow J. D. Stimulation of bilirubin catabolism in jaundiced Gunn rats by an induced of microsomal mixed-function monooxygenases. Proc Natl Acad Sci U S A 75, 682–685 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Trenti T., Gibbs A. H. & Greig J. B. Inducible bilirubin-degrading system in the microsomal fraction of rat liver. Mol Pharmacol 35, 831–838 (1989). [PubMed] [Google Scholar]
- De Matteis F., Dawson S. J., Boobis A. R. & Comoglio A. Inducible bilirubin-degrading system of rat liver microsomes: role of cytochrome P450IA1. Mol Pharmacol 40, 686–691 (1991). [PubMed] [Google Scholar]
- Sinal C. J. & Bend J. R. Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells. Mol Pharmacol 52, 590–599 (1997). [DOI] [PubMed] [Google Scholar]
- McCarty M. F. Serum bilirubin may serve as a marker for increased heme oxygenase activity and inducibility in tissues–a rationale for the versatile health protection associated with elevated plasma bilirubin. Med Hypotheses 81, 607–610 (2013). [DOI] [PubMed] [Google Scholar]
- Wang H. et al. Donor treatment with carbon monoxide can yield islet allograft survival and tolerance. Diabetes 54, 1400–1406 (2005). [DOI] [PubMed] [Google Scholar]