Abstract
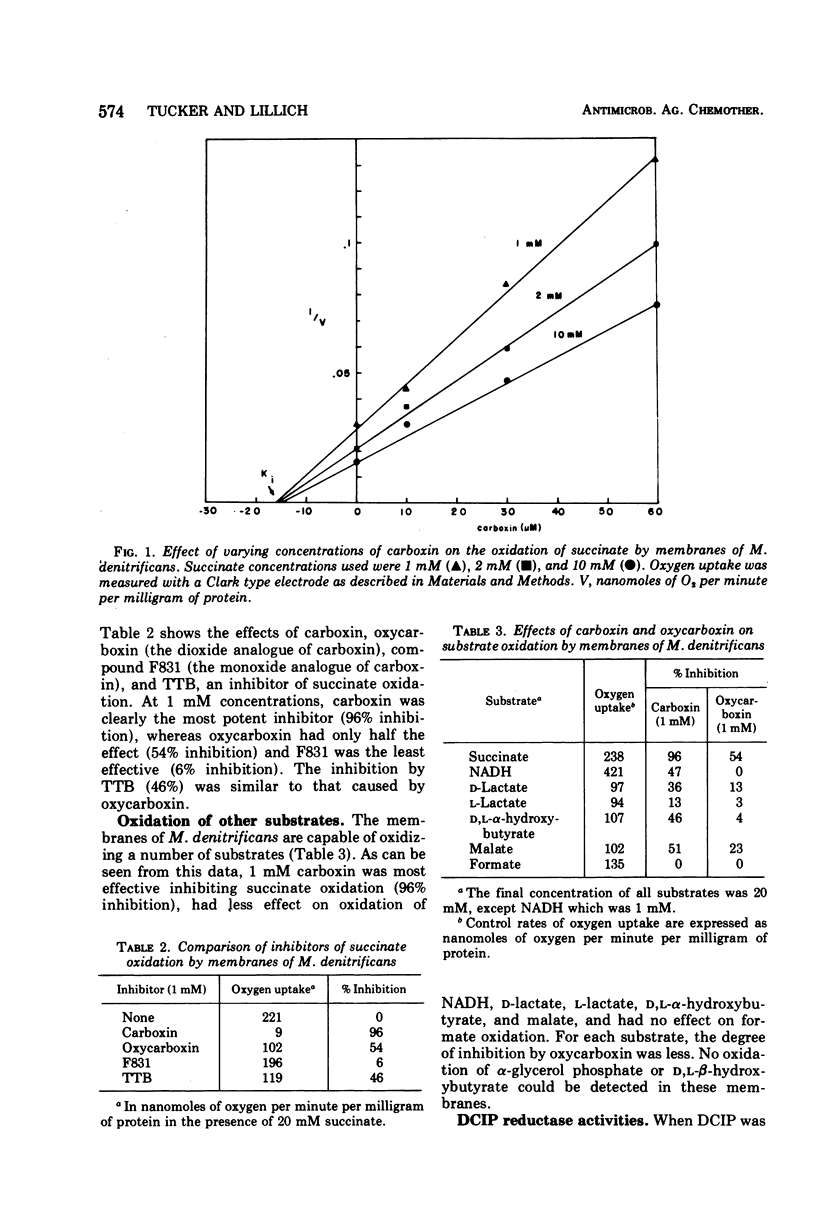
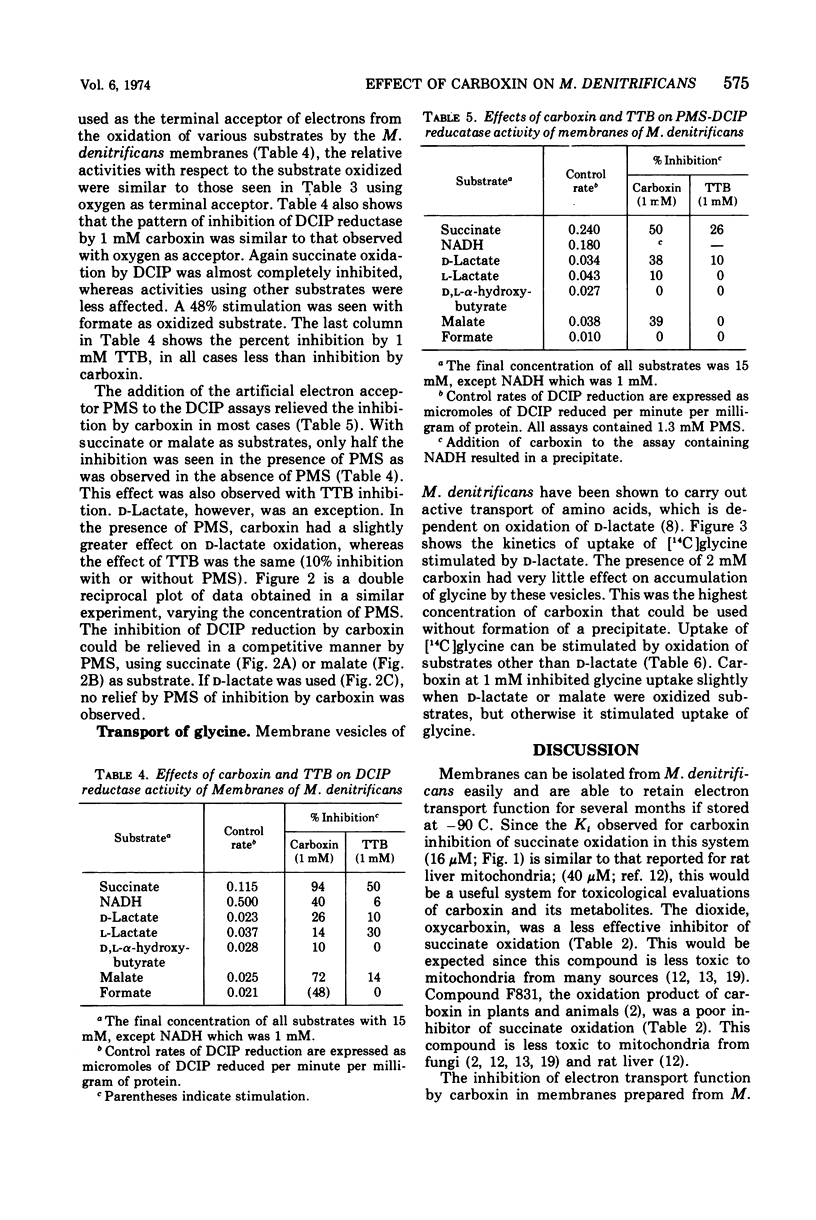
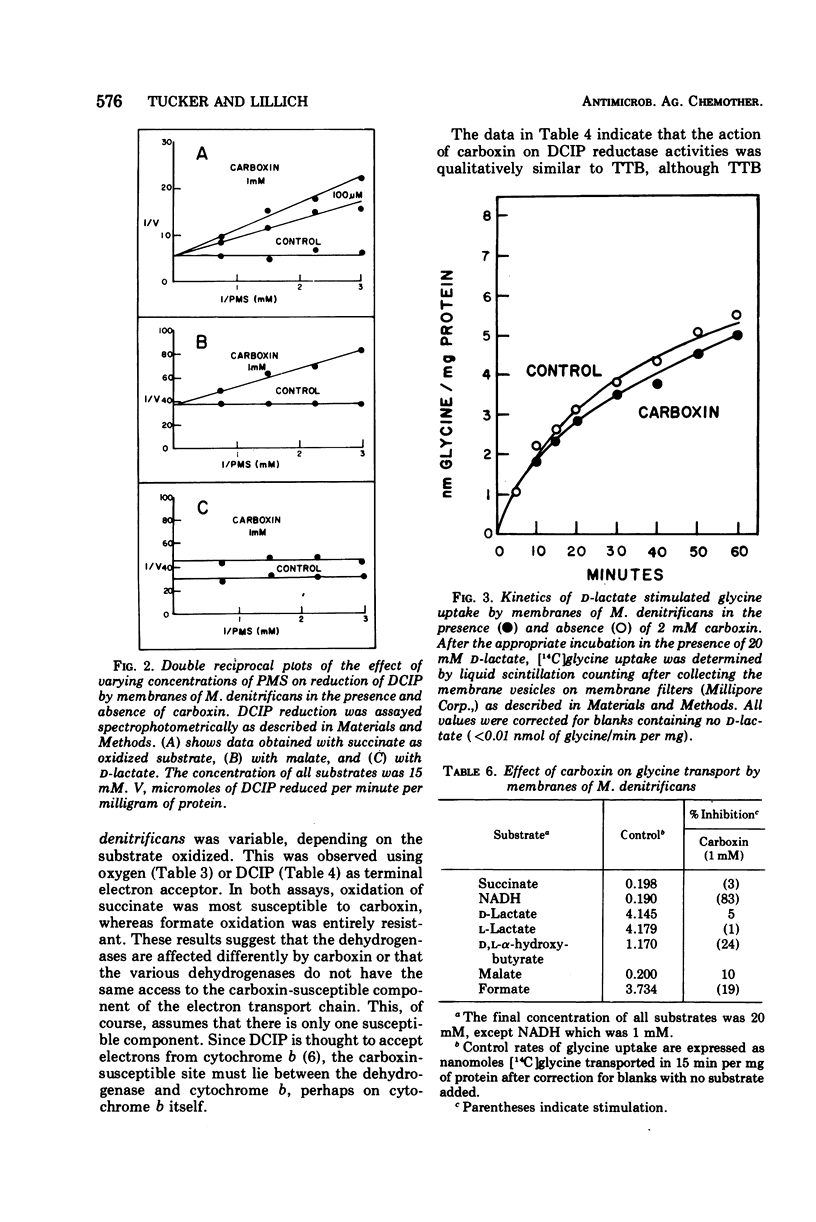
The systemic fungicide carboxin (5,6-dihydro-2-methyl-1,4-oxathiin-3-carboxanilide) inhibited oxidation of succinate by membranes prepared from Micrococcus denitrificans, the Ki being 16 μM. Oxycarboxin (5,6-dihydro-2-methyl-1,4-oxathiin-3-carboxanilide-4,4-dioxide), F831 (5,6-dihydro-2-methyl-1,4-oxathiin-3-carboxanilide-4-oxide), and another succinate oxidase inhibitor, 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione (TTB) were less effective inhibitors of succinate oxidation by membranes of M. denitrificans. Oxidation of other substrates (nicotinamide adenine dinucleotide, reduced form, d-lactate, l-lactate, malate, and d,l-α-hydroxybutyrate) was inhibited to a lesser degree by carboxin, and formate oxidation was entirely resistant. With all substrates tested, oxycarboxin, the dioxide analogue of carboxin, was less effective than carboxin. Carboxin also inhibited dichlorophenol indophenol (DCIP) reductase activities by these membranes in a manner both qualitatively and quantitatively similar to the inhibition of oxidation of the various substrates. The inhibition of DCIP reductase activities by TTB was qualitatively similar to carboxin, but TTB was a less effective inhibitor with all substrates tested. The inhibition of DCIP reductase by carboxin could be relieved by phenazine methosulfate with all substrates except d-lactate. Only slight inhibition of d-lactate-stimulated uptake of [14C]glycine by these membrane vesicles was seen with carboxin. Uptake of [14C]glycine could be stimulated to varying degrees with the other substrates tested, but in no case did carboxin cause significant inhibition. Membranes isolated from M. denitrificans are a useful system for investigating the mechanism of inhibition of electron transport function by carboxin, and the use of this system for evaluations of carboxin and its metabolites is suggested.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruni A., Racker E. Resolution and reconstitution of the mitochondrial electron transport system. I. Reconstitution of the succinate-ubiquinone reductase. J Biol Chem. 1968 Mar 10;243(5):962–971. [PubMed] [Google Scholar]
- CREMONA T., SINGER T. P. THE LACTIC DEHYDROGENASES OF YEAST. V. CHEMICAL PROPERTIES AND FUNCTION OF THE ZINC COMPONENT OF D-LACTIC CYTOCHROME REDUCTASE. J Biol Chem. 1964 May;239:1466–1473. [PubMed] [Google Scholar]
- Cohen N. S., Bogin E., Higashi T., Brodie A. F. Multiple cytochromes b in Mycobacterium phlei. Biochem Biophys Res Commun. 1973 Sep 18;54(2):800–807. doi: 10.1016/0006-291x(73)91495-2. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. E., WHARTON D. C. STOICHIOMETRY OF THE FIXED OXIDATION-REDUCTION COMPONENTS OF THE ELECTRON TRANSFER CHAIN OF BEEF HEART MITOCHONDRIA. Biochem Z. 1963;338:335–348. [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lombardi F. J., Reeves J. P., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. 8. Valinomycin-induced rubidium transport. J Biol Chem. 1973 May 25;248(10):3551–3565. [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeling B. V., Kulka M. Systemic fungicidal activity of 1,4-oxathiin derivatives. Science. 1966 Apr 29;152(3722):659–660. doi: 10.1126/science.152.3722.659. [DOI] [PubMed] [Google Scholar]
- Scholes P. B., Smith L. Composition and properties of the membrane-bound respiratory chain system of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):363–375. doi: 10.1016/0005-2728(68)90081-9. [DOI] [PubMed] [Google Scholar]
- Scholes P. B., Smith L. The isolation and properties of the cytoplasmic membrane of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):350–362. doi: 10.1016/0005-2728(68)90080-7. [DOI] [PubMed] [Google Scholar]
- Snel M., von Schmeling B., Edgington L. V. Fungitoxicity and structure-activity relationships of some oxathiin and thiazole derivatives. Phytopathology. 1970 Aug;60(8):1164–1169. doi: 10.1094/phyto-60-1164. [DOI] [PubMed] [Google Scholar]
- Ulrich J. T., Mathre D. E. Mode of action of oxathiin systemic fungicides. V. Effect on electron transport system of Ustilago maydis and Saccharomyces cerevisiae. J Bacteriol. 1972 May;110(2):628–632. doi: 10.1128/jb.110.2.628-632.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. DIFFERENTIAL SYNTHESIS OF FIVE PRIMARY ELECTRON TRANSPORT DEHYDROGENASES IN HEMOPHILUS PARAINFLUENZAE. J Biol Chem. 1964 Jun;239:2055–2060. [PubMed] [Google Scholar]
- WHITE D. C., SMITH L. LOCALIZATION OF THE ENZYMES THAT CATALYZE HYDROGEN AND ELECTRON TRANSPORT IN HEMOPHILUS PARAINFLUENZAE AND THE NATURE OF THE RESPIRATORY CHAIN SYSTEM. J Biol Chem. 1964 Nov;239:3956–3963. [PubMed] [Google Scholar]
- White G. A. A potent effect of 1,4-oxathiin systemic fungicides on succinate oxidation by a particulate preparation from Ustilago maydis. Biochem Biophys Res Commun. 1971 Sep;44(5):1212–1219. doi: 10.1016/s0006-291x(71)80215-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. J., Morman M. R., White D. C. Phospholipid composition and metabolism of Micrococcus denitrificans. J Bacteriol. 1972 Dec;112(3):1288–1294. doi: 10.1128/jb.112.3.1288-1294.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



