Abstract
Steatotic livers are more sensitive to ischemia/reperfusion (I/R) and are thus routinely rejected for transplantation because of their increased rate of primary nonfunction (PNF). Lean livers have less I/R-induced damage and inflammation due to Kupffer cells (KC), which are protective after total, warm, hepatic I/R with associated bowel congestion. This protection has been linked to KC-dependent expression of the potent anti-inflammatory cytokine interleukin-10 (IL-10). We hypothesized that pretreatment with exogenous IL-10 would protect the steatotic livers of genetically obese (ob/ob) mice from inflammation and injury induced by I/R. Lean and ob/ob mice were pretreated with either IL-10 or liposomally-encapsulated bisphosphonate clodronate (shown to deplete KC) prior to total, warm, hepatic I/R. IL-10 pretreatment increased survival of ob/ob animals at 24 hrs post-I/R from 30% to 100%, and significantly decreased serum ALT levels. At six hrs post-I/R, IL-10 pretreatment increased IL-10 mRNA expression, but suppressed up-regulation of the pro-inflammatory cytokine IL-1β mRNA. However, ALT levels were elevated at six hrs post-I/R in KC-depleted animals. These data reveal that pretreatment with IL-10 protects steatotic livers undergoing I/R, and that phagocytically active KC retain a hepatoprotective role in the steatotic environment.
Keywords: liver, transplantation, obesity, inflammation, cytokine, IL10
Liver transplant remains the only curative treatment for acute and chronic irreversible liver failure [1]. Primary nonfunction/dysfunction (PNF), defined as irreversible graft failure necessitating retransplantation or death not stemming from technical error or acute immunologic rejection, has significant detrimental effects on patient morbidity and mortality [2, 3]. Retransplantation necessitates prolonged and expensive stays in intensive care units while exerting further demands on an already inadequate donor pool [4].
Over 25% of donated livers are steatotic (hepatocyte lipid accumulation), with clear differences in survival following liver transplantation and ischemia/reperfusion (I/R) injury between lean and fatty livers [5]. Donor livers with greater than 30% fat content are considered to have a prohibitive rate of PNF and a higher susceptibility for I/R injury, but the underlying molecular and cellular mechanisms of fatty liver transplant failure are not well understood [6, 7]. Furthermore, while the damage that occurs in steatotic livers is linked to a decreased ability to regenerate ATP after I/R stress, and an increased sensitivity to proinflammatory factors, the mechanisms of these effects remain unresolved. During the anhepatic phase of a liver transplant procedure; the portal vein is divided, followed by anastomosis of the native portion to the donor organ. Despite techniques for decompression of the splanchnic circulation, bowel congestion secondary to reduced portal outflow remains a feature of clinical transplantation [8]. Bacterial products, in particular lipopolysaccharide (LPS, endotoxin), a component of Gram-negative bacterial cell walls, are thought to translocate across the bowel wall during portal venous stasis. LPS levels have been shown to rise in the portal vein after the anhepatic phase of clinical liver transplantation, presenting as a bolus to the liver upon reperfusion [9].
There exists a differential inflammatory response between fatty and lean livers subjected to total, warm, hepatic ischemia with bowel congestion, where bacterial products (specifically LPS) translocate across the gut wall during the ischemic phase and incite toll-like receptor 4 (TLR4) ligands [10, 11]. LPS binds to TLR4 to stimulate activation of liver sinusoidal endothelial cells (LSEC), with subsequent increases in inflammatory injury and hepatocellular necrosis [12]. Kupffer cells (KC), the resident hepatic macrophages, may maintain a homeostatic level of inflammation within lean livers through production of IL-10, thus suppressing LSEC activation and the immunopathologies of I/R within lean mice [13]. In lean organs, pathological inflammation is limited except under severe insults. This is not the case in steatotic livers where TLR4-dependent pathological levels of inflammation in reperfused steatotic livers may be due to a failure of these organs to properly regulate inflammatory responses.
IL-10 is a potent anti-inflammatory cytokine that limits the production of proinflammatory cytokines and chemokines by multiple cell types [14]. IL-10 production is key to the regulation of leukocyte-endothelial cell interactions in the setting of endotoxemia [15, 16]. IL-10 has also been shown to reduce the incidence of hepatic injury after various insults, in particular those mediated through TLR4 activation [17, 18]. Importantly, it has been shown that steatotic livers fail to significantly upregulate IL-10 expression in response to I/R [19–21]. This effect contrasts lean livers in which IL-10 expression increases dramatically after reperfusion [22].
Traditionally, KC were thought to be involved in the hepatic I/R injury process via production of proinflammatory cytokines and the production of reactive oxygen species [23, 24]. Many studies that have suggested a pathogenic role for KC in liver I/R injury utilized gadolinium chloride (GdCl3) to deplete or deactivate KC. Recently, it has been proposed that GdCl3 impairs surface expression of KC markers such as F4/80, and promotes a phenotypic shift within the KC population toward a less inflammatory phenotype [25, 26]. GdCl3 has also been shown to selectively deplete large, highly active KC from the liver [27], a population that produces more superoxide and tumor necrosis factor alpha (TNF-α) in response to LPS than other populations.
Recent work in our laboratory found that KC are required for protection of lean livers after hepatic I/R. Lean mice subjected to total, warm, hepatic I/R with bowel congestion suffered significantly more liver injury when depleted of KC beforehand by administration of liposome-encapsulated clodronate (LC) [28]. The liver injury was associated with increased expression of proinflammatory cytokines and adhesion molecules (i.e. interleukin 1 beta (IL-1β) and intercellular adhesion molecule 1 (ICAM-1)) within the liver, and a failure to increase IL-10 expression. KC depletion has also been shown to impair hepatic IL-10 production after partial hepatectomy [29]. Additionally, the IL-10 secreted by KC controls proinflammatory mediators released from LSEC in response to LPS challenge [30]. Studies have indicated that macrophages in genetically ob/ob mice possess an altered, pro-inflammatory, phenotype [31]. Furthermore, there is increasing evidence that the KC phenotype is altered in the setting of hepatic steatosis [32]. Thus, we hypothesized that impaired KC-production of IL-10 sensitizes steatotic livers to I/R injury.
EXPERIMENTAL PROCEDURES
Animal studies
Five-week-old, inbred C57BL/6J (lean) and B6.V-Lepob/J (obese) male mice were purchased from Jackson Laboratories (Jackson Laboratory, USA). The mice were allowed one week to acclimatize during which they were fed a standard diet ad libitum. At six weeks of age the mice were used for the I/R experiments. Obese mice of this age display approximately 60% hepatic steatosis [33, 34].
All mice were housed three-to-four per cage in a temperature-controlled room (22–25°C), with a 12-hour light-dark cycle, and provided with water and food available ad libitum. All experiments were performed under sterile conditions, were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee (MUSC IACUC), and were in accordance with National Institute of Health guidelines for laboratory animal usage.
Collection of tissue and sera
Blood samples were collected under isoflurane inhalation anesthesia by sterile cardiac puncture. Mice were euthanized by isoflurane followed by cervical dislocation. Livers were promptly dissected from the animal. The median, right, and caudate lobes were immediately snap-frozen in liquid nitrogen and stored at −80°C until analysis. The left hepatic lobe was divided into several sections, and one of these was stored in RNAlater (Qiagen, USA) for Quantitative Real Time RT-PCR analysis. All sections were obtained in a similar fashion, by the same operator.
Warm I/R
Prior to surgery, mice were anesthetized with a single, intraperitoneal injection of pentobarbital at 50 mg/kg body weight. The abdomen was shaved and prepped with beta-dine and 70% ethanol, and a small vertical incision through the skin and peritoneum was made slightly to the right of the animal’s midline, below the costal margin. A sterile cotton swab was used to expose and delineate the porta-hepatis and a pediatric vessel loop was drawn under and around the portal triad. The vessel loop was then tightened to induce total hepatic ischemia for 15 min, a time shown to produce significant hepatic injury in ob/ob mice [35]. At the end of the ischemia period, the vessel loop was removed, and the portal vein, hepatic artery, and liver were observed for restoration of blood flow (reperfusion). Sham operations were performed on obese mice to control for the possibility of increased sensitivity to mechanical manipulation. Sham animals underwent all elements of the procedure except ischemia. Warm, isotonic saline (0.5 mL) was administered into the peritoneal cavity to compensate for intraoperative fluid loss. Both layers of the incision, skin and peritoneum, were closed with a running 5-0 proline suture. The animal was immediately placed in a temperature-controlled recovery cage, and allowed free access to food and water upon waking. Surviving animals were monitored hourly following surgery, and animals were sacrificed at six and 24 hrs following reperfusion.
Kupffer cell depletion
When applicable, 48 hrs prior to ischemia, animals were injected with 0.2 ml of the indicated doses of liposomal clodronate (dichloromethylene biphosphonate, LC) intraperitoneally. The LC was provided by Nico van Rooijen (www.clodronateliposomes.org) and was produced as described elsewhere [36]. It was previously shown by our lab that this dose and time course results in complete ablation of F4/80+ cells in the liver [28].
IL-10 supplementation
When applicable, animals were pre-treated with recombinant murine IL-10 (BD Biosciences, USA). Animals received 1 μg of IL-10 diluted in 0.5 mL saline, approximately 30 min prior to the I/R via intravenous injection of the dorsal tail vein. This dose significantly reduced I/R injury in lean models [28].
Serum transaminase measurement
Whole blood was allowed to clot at room temperature for 20 min followed by centrifugation at 3,500 g for five min at room temperature to separate the serum. Serum alanine aminotransferase (ALT) concentrations were measured with a Synchron LX20 system (Beckman Coulter, USA) and expressed as international units per liter (Clinical Laboratory Services, Medical University of South Carolina, Charleston, SC, USA).
Quantitative real time RT-PCR
The mRNA coding for IL-10, IL-1β, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was quantified by quantitative PCR. Total RNA was isolated by phenol chloroform extraction, followed by silica membrane purification and DNAse digest (Qiagen, USA). Thereafter, 1 mg of total RNA from each sample was used for reverse transcription using the Transcriptor first-strand cDNA synthesis kit (Roche) to generate first-strand complementary DNA. A polymerase chain reaction mixture was prepared with the use of SYBR Green I PCR Master Mix (Roche, USA) and followed by amplification on a Roche LightCycler 480 instrument. The primer sequences used were as follows: GAPDH forward 5′-TTCACCACCATGGAGAAGGC-3′ and reverse 5′-GGCATGGACTGTGGTCATGA-3′, IL-1β forward 5′-CAACCAACAAGTGATATTCTCC-3′ and reverse 5′-GATCCACACTCTCCAGCTGCA-3′, IL-10 forward 5′-GGTTGCCAAGCCTTATCGGA-3′ and reverse 5′-ACCTGCTCCACTGCCTTGCT-3′, and TNF-α forward 5′-GCACCACCATCAAGGACTCA-3′ and reverse 5′-TCGGAGGCTCCAGTGAATTCG-3′. Thermal cycling conditions were 10 min at 95°C followed by 45 cycles at 95°C for 10 seconds, 60°C for 20 seconds and 72°C for 20 seconds. Cm values ranged from 25–35 for the cytokine gene transcripts. The expression of each gene was normalized to GAPDH mRNA, and calculated with respect to the baseline control using the comparative cycle threshold method (ΔΔCp).
Statistical analysis
All values here are expressed as mean ± standard error of the mean. Statistical significance was chosen a priori as α ≤0.05. For single, pairwise comparisons of normally distributed data sets, Student’s t-test was used. For multiple comparisons of means, a one-way analysis of variance with Tukey-Kramer post hoc tests was used. For non-normally distributed data, complimentary non-parametric methods were applied: the Mann-Whitney U test and Kruskal Wallis’s one-way analysis of variance with Dunn’s post hoc test. Hypothesis testing was performed using GraphPad PRISM version 5 for Windows (GraphPad Software, USA).
RESULTS
IL-10 pretreatment improves survival and decreases liver damage after I/R
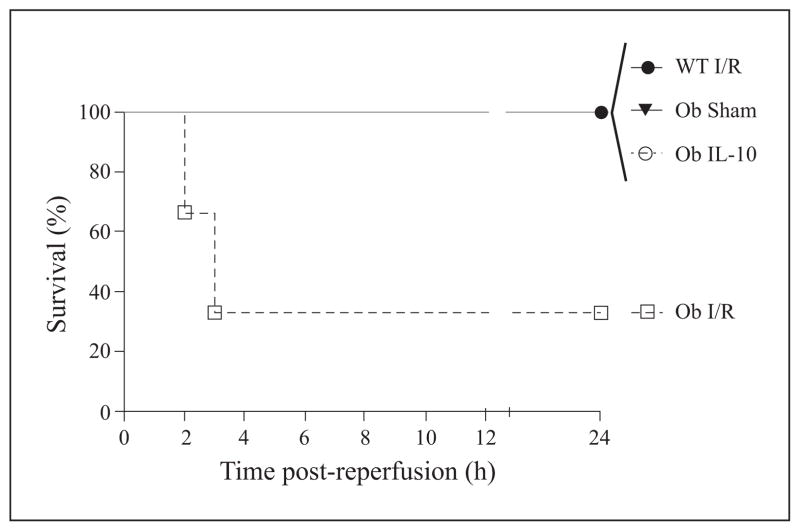
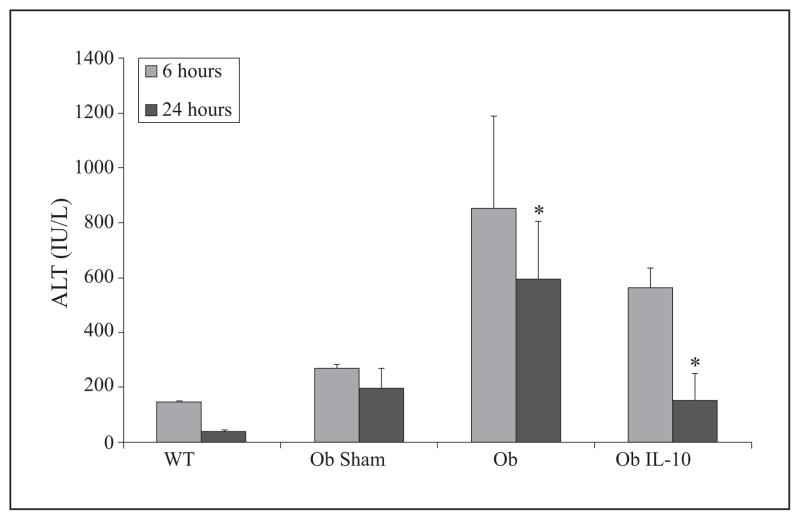
To determine if supplementation with IL-10 would protect ob/ob mouse livers from I/R-induced hepatic injury, we pretreated (30 min) animals with murine recombinant IL-10 (1 μg) or diluent via tail vein injection. Animals were then subjected to 15 min, total, warm, hepatic ischemia followed by reperfusion, which has been previously shown to recapitulate the clinical phenomenon of bowel congestion during the anhepatic phase [35]. The steatotic livers of ob/ob mice are more sensitive to I/R than lean organs, and 15 minutes of total, warm, ischemia time leads to significant injury in these animals as others, as we have previously reported [37–39]. All sham-operated and lean animals survived in this study (figure 1). Ob/ob animals had a 24-hr survival rate of approximately 30%, but IL-10 treatment increased survival of ob/ob animals to 100% (figure 1). Additionally, IL-10 pretreatment of ob/ob animals resulted in significantly decreased ALT levels at 24 hrs as compared to control-treated ob/ob mice (figure 2). This change indicates that at 24 hrs, hepatocellular injury was significantly reduced in IL-10 supplemented ob/ob animals. However, the decrease in ALT was not present at six hrs. Lean (wild type, Wt) animals suffered very little injury secondary to this modest period of ischemia. As previously shown, ALT levels of lean animals after reperfusion were in fact lower than sham-operated, obese animals [40].
Figure 1. IL-10 supplementation increases animal survival.
Obese (Ob) or lean (wild type, WT) mice were subjected to 15 min, warm I/R or sham surgery and monitored for 24 hrs. Some Ob mice were given 1 μg IL-10 (Ob IL-10) 30 min prior to I/R. IL-10 pretreatment restored survival in ob/ob mice (p<0.05, log rank). Data are expressed as mean ± SEM; n = 3–6/group.
Figure 2. IL-10 decreases hepatic injury following I/R.
Mice subjected to I/R as described in Figure 1 were evaluated for ALT, an indicator of liver damage, at six and 24 hrs post-reperfusion. Hepatic injury was significantly reduced in IL-10-supplemented ob/ob (Ob IL-10) mice compared to ob/ob controls (Ob) at 24 hrs (*p<0.05). Data are expressed as mean ± SEM; n = 3–6/group.
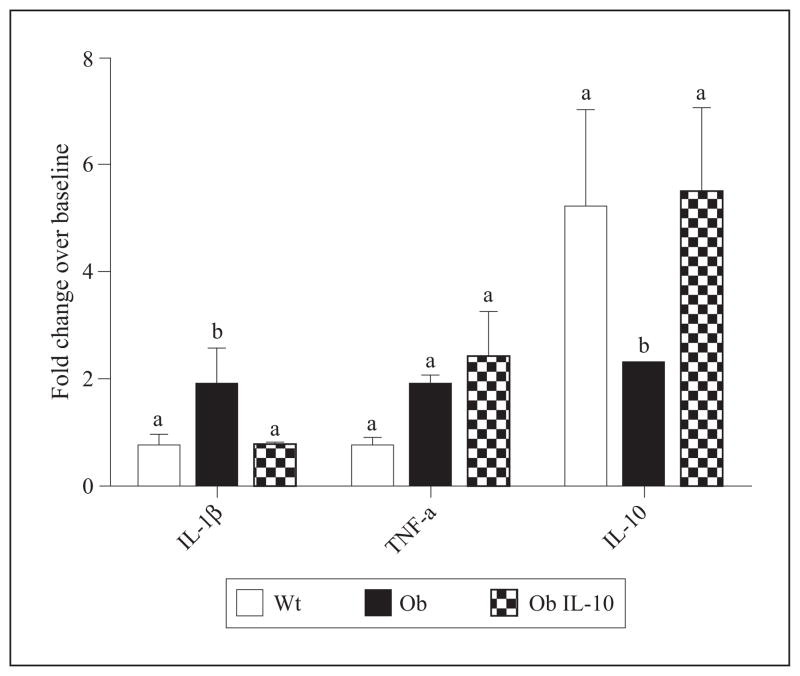
To investigate further the effect of exogenous IL-10 on the inflammatory milieu in the livers of ob/ob mice following I/R, we used real-time qRT-PCR to examine transcription levels of key cytokines in the process of inflammation-mediated hepatic cytotoxicity, i.e. TNF-α and IL-1β, at six hrs post-I/R [41]. This time point was chosen based upon previous data demonstrating cytokine expression to be maximally upregulated around this point post-insult [28]. There was a marked increase in IL-1β mRNA levels in diluent-treated ob/ob animals (figure 3). In contrast, IL-1β mRNA levels in Wt and IL-10 pretreated ob/ob animals did not increase following I/R. IL-10 pretreatment however, did not abolish TNF-α upregulation. This suggests that IL-10 pretreatment may prevent upregulation in expression of the inflammatory cytokine IL-1β after I/R in steatotic livers.
Figure 3. IL-10 pretreatment modulates post-I/R cytokine expression.
Mice were evaluated for hepatic cytokines IL-1β, TNF-α, and IL-10 mRNA at six hrs post-reperfusion by qRT-PCR. IL-1β mRNA levels were significantly upregulated in the livers of ob/ob (Ob) animals as compared to lean control (Wt) and IL-10-pretreated ob/ob (Ob IL-10) animals. No significant changes in TNF-α expression were noted. Elevation of IL-10 transcripts in livers of Ob animals after I/R was significantly less than that seen in Wt and Ob IL-10 mice. Fold-change is a comparison to mice not subjected to I/R. Means with different lettered subscripts within each group are significantly different from each other (a is significantly different from b), p<0.05. Data are expressed as mean ± SD; n = 3–6.
We also investigated if expression of IL-10 is altered in steatotic mice or by IL-10 administration. In Wt animals, IL-10 mRNA levels increased five-fold, six hrs post-I/R. In diluent-treated ob/ob animals, there was only a two-fold increase in IL-10 mRNA levels, but exogenous IL-10-augmented ob/ob mice had similar IL-10 mRNA levels as seen in Wt mice. This experiment might suggest that the presence of IL-10 increases IL-10 transcription.
KC depletion increases injury in ob/ob mice after I/R
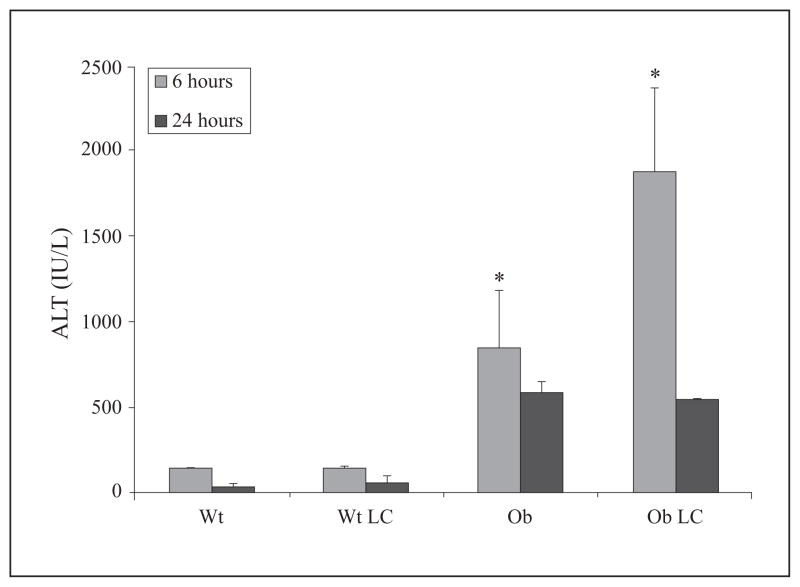
To investigate the role of KC in the development of hepatic I/R injury and expression of IL-10 within the steatotic environment, we removed KC from mice prior to ischemia by pretreating mice with LC 48 hours prior to 15 min, total, warm, hepatic I/R. This LC dose and time course has previously been shown by our lab to deplete KC from the liver completely, while not inducing detectable hepatic injury [28]. Liver damage in LC-treated Wt mice, as assessed by ALT levels, was no greater than diluent-treated Wt animals at six and 24 hrs post-reperfusion (figure 4). This suggests that KC depletion by LC does not significantly exacerbate I/R injury in lean mice subjected to only 15 min of hepatic ischemia. ALT levels in ob/ob mice were elevated compared to Wt mice, and LC treatment significantly exacerbated this increase in ALT at six hrs but not 24 hrs. The peaking of serum aminotransferase levels at six hours after reperfusion marks the acute phase of hepatocellular necrosis [42]. Serum aminotransferase proteins are subsequently cleared by sinusoidal endothelial cells and other non-parenchymal cells [43]. These data indicate that KC depletion prior to hepatic I/R increases injury in ob/ob, but not lean mice.
Figure 4. LC-treated ob/ob animals have increased injury.
Obese (Ob) or lean (wild type, WT) mice were subjected to 15 min, warm, I/R or sham surgery and monitored for 24 hrs. Some Ob mice were given LC (Ob LC), 48 hrs prior to I/R to depleted KC. Hepatic injury was significantly increased in OB LC mice at six hrs when assessed by measuring circulating levels of ALT (*p<0.05). Data are expressed as mean ± SEM; n = 3–6.
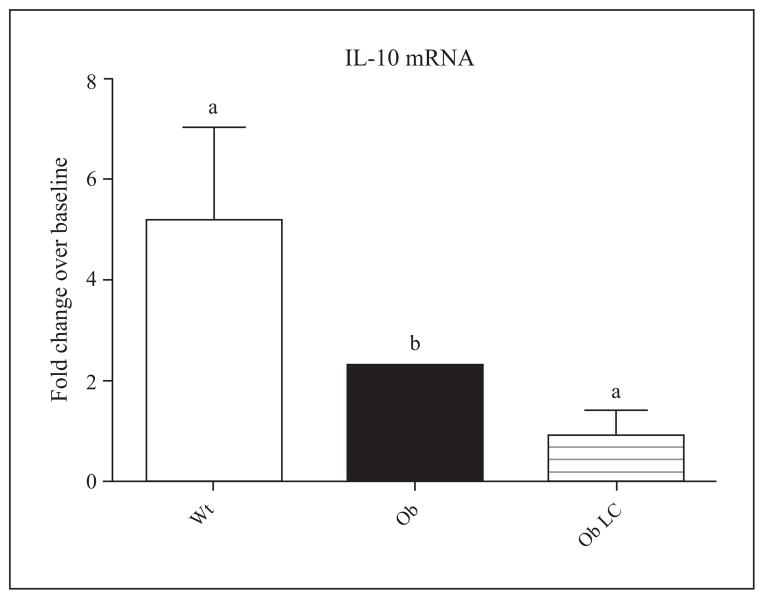
To investigate further the role of KC in hepatic inflammatory activation of ob/ob mice after I/R, we used real-time qRT-PCR to examine transcription levels of IL-10. Ob/ob mice showed a significant reduction in IL-10 mRNA expression compared to lean mice (figure 5). LC-treated ob/ob mice failed to show significant changes in IL-10 mRNA expression after I/R, as compared to diluent-treated, control ob/ob mice (figure 5).
Figure 5. LC-treated ob/ob animals do not show upregulated hepatic IL-10 mRNA expression in response to I/R.
Mice from figure 4 were evaluated for hepatic IL-10 mRNA levels six hrs post-reperfusion as assessed by qRT-PCR. Both ob/ob (Ob) and LC-treated ob/ob (Ob LC) mice expressed fewer hepatic IL-10 mRNA transcripts compared to lean (Wt) counterparts. Fold-change is a comparison to mice not subjected to I/R. Means with different lettered subscripts within each group are significantly different from each other (a is significantly different from b), p<0.05. Data are expressed as mean ± SD; n = 3–6.
DISCUSSION
This study addressed two main questions: whether exogenous recombinant IL-10 can be used to prevent the increased injury occurring in steatotic livers, and whether KC play a different role in I/R within the steatotic versus lean liver. Dysregulated inflammatory responses are known to be of central importance to the development of steatotic liver I/R injury. The failure of steatotic livers to significantly upregulate expression of the anti-inflammatory cytokine IL-10 was implicated in this process. Thus, the development of interventions capable of modifying the hepatic inflammatory milieu is important to the future, safe use of fatty liver grafts in transplantation.
In our IL-10 studies, exogenous IL-10 administration increased survival and attenuated one aspect of the injury that occurs during I/R in the setting of steatosis as indicated by lower ALT values at 24 hours (figures 1–2). This may occur via various mechanisms such as downregulation of inflammatory cytokine expression, prevention of endothelial cell activation, or recruitment of regulatory T cells [44]. Endothelial cells have been shown to play a role in the pathogenesis of sepsis, with changes in permeability and protein expression after exposure to danger signals [45]. Adenoviral IL-10 can prevent expression of adhesion molecule such as ICAM-1 and vascular cell adhesion protein 1 induced on endothelial cells after hypoxia/reoxygenation in the cerebral vasculature [46]. Similarly, the chronic colitis present in IL-10-deficient mice has been linked to increased expression of adhesion molecules, and endogenous IL-10 production is key to leukocyte-endothelial cell interactions in the setting of endotoxemia [15, 16].
ALT levels were significantly higher in IL-10-pretreated ob/ob animals at six hours compared to Wt lean mice, but similar to Wt mice at 24 hours. Previous investigations have defined a critical role for ATP depletion and mitochondrial dysfunction in steatotic liver I/R injury. It is probable that the failure of IL-10 to significantly reduce liver injury at the earlier six-hour time point is due to a relative independence of this initial injury from inflammation. However, hepatocyte death and dysfunction during this early phase of reperfusion due to oncotic mechanisms would also be associated with the release of endogenous, innate, immune-activating ligands such as high-mobility group protein B1 and heat shock proteins. Thus, in the later stages of injury, IL-10 may prevent the endogenous damage-associated molecular patterns (DAMPs) from activating inflammatory programs of cytokine expression, thus slowing the continued injury past the initial metabolic insult.
It has also been shown that gut-derived LPS, and its receptor TLR4, are critical in determining the increased injury and mortality observed after reperfusion of steatotic livers. In our experiments, all of the mice pretreated with IL-10 survived to 24 hours, despite significant liver injury as indicated by ALT levels at six hours (figures 1–2). Furthermore, the majority of the mortalities within the control ob/ob group occurred quite early, between two-four hours after reperfusion (figure 1). This implies that different mechanisms may be at play between the survival benefit of IL-10 pretreatment and the associated decreased injury observed at 24 hours. IL-10 pretreatment may prevent the initial shock occurring at the time of reperfusion when an endotoxin bolus hits the liver through the portal system. This initial inflammatory insult results in the activation of endothelial cells, expression of proinflammatory cytokines, and mitochondrial dysfunction [47]. As discussed earlier, IL-10 suppresses cytokine expression and endothelial cell activation. IL-10 can also attenuate responses to endothelin, which is involved in the early microcirculatory disturbances of hepatic reperfusion injury [48, 49]. Prevention of early pathological changes in endothelial function may explain IL-10’s effects on survival at these time points. We showed a decrease in the upregulation of the cytokine IL-1β in ob/ob mice after I/R when animals were pretreated with IL-10 (figure 3). Hepatic I/R injury is mediated, in part, by neutrophil recruitment to the liver during reperfusion, and IL-1β receptor knockouts have been shown to decrease neutrophil recruitment after hepatic I/R [50]. Also, overexpression of IL-1β receptor antagonist within the liver reduces I/R injury [51, 52].
We observed no effect of IL-10 treatment on TNF-α transcript levels. This was rather unexpected given the critical role of TNF-α in reperfusion injury and response to LPS via TLR4. The fact that this does not exclude the null hypothesis does not mean that TNF-α levels were not changed by IL-10 administration, only that we were unable to identify such an effect. This may also be the result of the ‘snapshot’ nature of our analysis in a dynamic system. Finally, is also known that IL-10 functions to down-regulate TNF-α production by destabilization of its mRNA transcript. It is not known if our PCR primers could detect this specific degradation of the mRNA transcript or at what time point.
Upregulation of IL-10 mRNA expression in IL-10-pre-treated ob/ob mice after I/R is an unusual finding (figure 3). IL-10 is known to be a suppressor of pan-cytokine expression [53]. Thus one would expect decreased IL-10 expression levels in animals receiving recombinant IL-10. It may be the case the IL-10 pre-treatment polarizes immune cells within the liver toward an anti-inflammatory phenotype, or promotes the formation of regulatory T-cells [54]. For example, the addition of recombinant human IL-10 to T-cells derived from the intestinal mucosa of celiac disease patients increases the frequency of T-cells that produce IL-10 in response to stimulation [55]. However, few studies are available which investigate IL-10 expression after IL-10 pretreatment and acute injury.
This study was performed to investigate the hypothesis that KC play a different role within the steatotic liver subjected to I/R compared to lean livers. Decreased IL-10 expression in both steatotic livers and KC-depleted lean livers subjected to I/R suggests that KC within the fatty liver may be skewed toward an M1 or pro-inflammatory phenotype. If this were the case, depletion of KC should protect ob/ob mice from hepatic I/R injury, rather than exacerbate it as seen in this study (figure 4). Numerous studies have defined a pathological role for KC within the reperfused liver [56, 57]. As discussed earlier, KC are also implicated in the protection of lean livers from I/R injury through IL-10 production, thus fulfilling an anti-inflammatory function. However, in the steatotic liver, the absence of sufficient IL-10 upregulation at reperfusion leaves the function KC in this environment in doubt. Thus, to assess whether KC may, in the absence of broad anti-inflammatory IL-10 production, be pro-inflammatory or deleterious, we depleted ob/ob mice of KC with liposomal clodronate prior to I/R.
KC did retain a similar functional role within lean and steatotic livers subjected to I/R, as KC-depleted ob/ob mice suffered increased injury as indicated by elevated serum ALT levels at six hrs post-reperfusion (figure 4). This suggests a continued beneficial role for KC in the liver subjected to I/R, despite the presence of steatosis. The fact that IL-10 expression in LC-treated mice was not significantly attenuated also calls into question whether KC protect the steatotic livers through IL-10 production (figure 5). Since there was no significant difference in IL-10 mRNA between control ob/ob animals and those depleted of KC, the depletion of KC sensitizes steatotic livers to I/R injury through non-IL-10-dependent mechanisms.
KC are an integral component of the reticuloendothelial system, and are primarily responsible for the clearance and detoxification of gut-derived LPS [58]. KC detoxify endotoxin derived from the portal system via acyloxyacyl hydrolase, which removes the fatty acyl chains decorating lipid-A that are necessary for LPS recognition by MD2-TLR4 [59]. In the absence of KC, endotoxin would not be effectively metabolized within the liver and could continue to induce injury. It was previously shown in our lab that in the absence of KC, LPS is present after I/R within the liver, where it binds to sinusoidal endothelial cells. Endothelial cell activation by LPS leads to secretion of vasoactive mediators and proinflammatory cytokines, and the upregulation of adhesion molecules, enabling neutrophil and monocyte adhesion. Ultimately, this inflammatory excess leads to increased hepatic damage following LPS challenge and death. Thus, the absence of KC may promote injury through cytokine-independent mechanisms. These results suggest that indiscriminant depletion of KC would not be an effective strategy for reducing steatotic liver I/R injury.
In spite of significant improvements in the field of liver transplantation, there are wide gaps in our knowledge that prevent the effective use of all potential donor organs. All current pharmacological interventions dealing with obesity and steatosis are long-term treatments, requiring weeks to months. Consequently, in the setting of a steatotic donor liver, these interventions are ineffective. A functional window of approximately 18–24 hrs exists between the identification of non-living donors and organ procurement where intervention is possible. Herein lies the need for fast-acting pharmacological manipulations that will stabilize and protect the steatotic liver from the insults that render these organs more susceptible to PNF. An interventional protocol for increasing the safe utilization of steatotic livers in transplantation should target both the initial, hyper-acute injury, as well as the later, inflammation-associated damage. Pretreatment with IL-10 is a promising strategy for the prevention inflammatory injury occurring within steatotic livers after I/R. Unlike most disease processes where therapies must be curative rather than preventative, liver transplantation provides the opportunity for pretreatment of organs or patients before acute insult. IL-10 may provide a means to reduce steatotic I/R injury in the setting of transplantation, and thus drastically increase the number of useable livers.
Footnotes
Disclosure. Financial support: These studies were supported by a RO1 grant from NIH (5RO1DK069369-05). Conflict of interest: none.
References
- 1.Rai R, Liver R. Transplantatation- an Overview. Indian J Surg. 2013;75:185–91. doi: 10.1007/s12262-012-0643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almond N, Kahwati L, Kinsinger L, Porterfield D. Prevalence of overweight and obesity among U.S. military veterans. Mil Med. 2008;173:544–9. doi: 10.7205/milmed.173.6.544. [DOI] [PubMed] [Google Scholar]
- 3.Hermos JA, Cohen SA, Hall R, et al. Association of elevated alanine aminotransferase with BMI and diabetes in older veteran outpatients. Diabetes Res Clin Pract. 2008;80:153–8. doi: 10.1016/j.diabres.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 4.D’Alessandro AM, Kalayoglu M, Sollinger HW, et al. The predictive value of donor liver biopsies for the development of primary nonfunction after orthotopic liver transplantation. Transplantation. 1991;51:157–63. doi: 10.1097/00007890-199101000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–13. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 6.Fukumori T, Ohkohchi N, Tsukamoto S, Satomi S. The mechanism of injury in a steatotic liver graft during cold preservation. Transplantation. 1999;67:195–200. doi: 10.1097/00007890-199901270-00002. [DOI] [PubMed] [Google Scholar]
- 7.Seifalian AM, Piasecki C, Agarwal A, Davidson BR. The effect of graded steatosis on flow in the hepatic parenchymal microcirculation. Transplantation. 1999;68:780–4. doi: 10.1097/00007890-199909270-00009. [DOI] [PubMed] [Google Scholar]
- 8.Isern MR, Massarollo PC, de Carvalho EM, et al. Randomized trial comparing pulmonary alterations after conventional with venovenous bypass versus piggyback liver transplantation. Liver Transpl. 2004;10:425–33. doi: 10.1002/lt.20067. [DOI] [PubMed] [Google Scholar]
- 9.Abdala E, Baia CE, Mies S, et al. Bacterial translocation during liver transplantation: a randomized trial comparing conventional with venovenous bypass vs. piggyback methods. Liver Transpl. 2007;13:488–96. doi: 10.1002/lt.21085. [DOI] [PubMed] [Google Scholar]
- 10.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–87. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 11.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita M, Uchida T, Sato A, et al. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J Hepatol. 2010;53:903–10. doi: 10.1016/j.jhep.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 13.Movita D, Kreefft K, Biesta P, et al. Kupffer cells express a unique combination of phenotypic and functional characteristics compared with splenic and peritoneal macrophages. J Leukoc Biol. 2012;92:723–33. doi: 10.1189/jlb.1111566. [DOI] [PubMed] [Google Scholar]
- 14.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–7. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 15.Hickey MJ, Issekutz AC, Reinhardt PH, Fedorak RN, Kubes P. Endogenous interleukin-10 regulates hemodynamic parameters, leukocyte-endothelial cell interactions, and microvascular permeability during endotoxemia. Circulation research. 1998;83:1124–31. doi: 10.1161/01.res.83.11.1124. [DOI] [PubMed] [Google Scholar]
- 16.Kawachi S, Jennings S, Panes J, et al. Cytokine and endothelial cell adhesion molecule expression in interleukin-10-deficient mice. American journal of physiology Gastrointestinal and liver physiology. 2000;278:G734–43. doi: 10.1152/ajpgi.2000.278.5.G734. [DOI] [PubMed] [Google Scholar]
- 17.Louis H, Le Moine O, Peny MO, et al. Hepatoprotective role of interleukin 10 in galactosamine/lipopolysaccharide mouse liver injury. Gastroenterology. 1997;112:935–42. doi: 10.1053/gast.1997.v112.pm9041256. [DOI] [PubMed] [Google Scholar]
- 18.Nagaki M, Tanaka M, Sugiyama A, Ohnishi H, Moriwaki H. Interleukin-10 inhibits hepatic injury and tumor necrosis factor-alpha and interferon-gamma mRNA expression induced by staphylococcal enterotoxin B or lipopolysaccharide in galactosamine-sensitized mice. J Hepatol. 1999;31:815–24. doi: 10.1016/s0168-8278(99)80282-7. [DOI] [PubMed] [Google Scholar]
- 19.Urata K, Nguyen B, Brault A, et al. Decreased survival in rat liver transplantation with extended cold preservation: role of portal vein clamping time. Hepatology. 1998;28:366–73. doi: 10.1002/hep.510280211. [DOI] [PubMed] [Google Scholar]
- 20.Arai M, Mochida S, Ohno A, Arai S, Fujiwara K. Selective bowel decontamination of recipients for prevention against liver injury following orthotopic liver transplantation: evaluation with rat models. Hepatology. 1998;27:123–7. doi: 10.1002/hep.510270120. [DOI] [PubMed] [Google Scholar]
- 21.Vetelainen R, van Vliet AK, van Gulik TM. Severe steatosis increases hepatocellular injury and impairs liver regeneration in a rat model of partial hepatectomy. Annals of surgery. 2007;245:44–50. doi: 10.1097/01.sla.0000225253.84501.0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Moine O, Louis H, Stordeur P, et al. Role of reactive oxygen intermediates in interleukin 10 release after cold liver ischemia and reperfusion in mice. Gastroenterology. 1997;113:1701–6. doi: 10.1053/gast.1997.v113.pm9352875. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Wang M, Xie HY, et al. Role of reactive oxygen species in mediating hepatic ischemia-reperfusion injury and its therapeutic applications in liver transplantation. Transplantation proceedings. 2007;39:1332–7. doi: 10.1016/j.transproceed.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Ogiku M, Kono H, Hara M, Tsuchiya M, Fujii H. Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by kupffer cells after ischemia-reperfusion in rats. J Pharmacol Exp Ther. 2011;339:93–8. doi: 10.1124/jpet.111.182592. [DOI] [PubMed] [Google Scholar]
- 25.Hardonk MJ, Dijkhuis FW, Hulstaert CE, Koudstaal J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J Leukoc Biol. 1992;52:296–302. doi: 10.1002/jlb.52.3.296. [DOI] [PubMed] [Google Scholar]
- 26.Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem Res Toxicol. 2009;22:1376–85. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kono H, Fujii H, Asakawa M, et al. Functional heterogeneity of the kupffer cell population is involved in the mechanism of gadolinium chloride in rats administered endotoxin. J Surg Res. 2002;106:179–87. doi: 10.1006/jsre.2002.6434. [DOI] [PubMed] [Google Scholar]
- 28.Ellett JD, Atkinson C, Evans ZP, et al. Murine Kupffer cells are protective in total hepatic ischemia/reperfusion injury with bowel congestion through IL-10. J Immunol. 2010;184:5849–58. doi: 10.4049/jimmunol.0902024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meijer C, Wiezer MJ, Diehl AM, et al. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66–77. doi: 10.1034/j.1600-0676.2000.020001066.x. [DOI] [PubMed] [Google Scholar]
- 30.Knolle P, Schlaak J, Uhrig A, et al. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide, LPS; challenge. J Hepatol. 1995;22:226–9. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 31.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–6. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–23. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baffy G, Zhang CY, Glickman JN, Lowell BB. Obesity-related fatty liver is unchanged in mice deficient for mitochondrial uncoupling protein 2. Hepatology. 2002;35:753–61. doi: 10.1053/jhep.2002.32028. [DOI] [PubMed] [Google Scholar]
- 34.Sutter AG, Palanisamy AP, Kurtz N, Spyropoulos DD, Chavin KD. Efficient method of genotyping ob/ob mice using high resolution melting analysis. PLoS One. 2013;8:e78840. doi: 10.1371/journal.pone.0078840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiorini RN, Shafizadeh SF, Polito C, et al. Anti-endotoxin monoclonal antibodies are protective against hepatic ischemia/reperfusion injury in steatotic mice. Am J Transplant. 2004;4:1567–73. doi: 10.1111/j.1600-6143.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 36.Van Rooijen N, Sanders A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology. 1996;23:1239–43. doi: 10.1053/jhep.1996.v23.pm0008621159. [DOI] [PubMed] [Google Scholar]
- 37.Wan CD, Wang CY, Liu T, Cheng R, Wang HB. Alleviation of ischemia/reperfusion injury in ob/ob mice by inhibiting UCP-2 expression in fatty liver. World J Gastroenterol. 2008;14:590–4. doi: 10.3748/wjg.14.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans ZP, Palanisamy AP, Sutter AG, et al. Mitochondrial uncoupling protein-2 deficiency protects steatotic mouse hepatocytes from hypoxia/reoxygenation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G336–342. doi: 10.1152/ajpgi.00049.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palanisamy AP, Cheng G, Sutter AG, et al. Mitochondrial Uncoupling Protein 2 Induces Cell Cycle Arrest and Necrotic Cell Death. Metab Syndr Relat Disord. 2013 doi: 10.1089/met.2013.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans ZP, Ellett JD, Schmidt MG, Schnellmann RG, Chavin KD. Mitochondrial uncoupling protein-2 mediates steatotic liver injury following ischemia/reperfusion. J Biol Chem. 2008;283:8573–9. doi: 10.1074/jbc.M706784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diehl AM. Cytokine regulation of liver injury and repair. Immunol Rev. 2000;174:160–71. doi: 10.1034/j.1600-0528.2002.017411.x. [DOI] [PubMed] [Google Scholar]
- 42.Hasegawa T, Ito Y, Wijeweera J, et al. Reduced inflammatory response and increased microcirculatory disturbances during hepatic ischemia-reperfusion injury in steatotic livers of ob/ob mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1385–95. doi: 10.1152/ajpgi.00246.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamimoto Y, Horiuchi S, Tanase S, Morino Y. Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology. 1985;5:367–75. doi: 10.1002/hep.1840050305. [DOI] [PubMed] [Google Scholar]
- 44.Santodomingo-Garzon T, Han J, Le T, Yang Y, Swain MG. Natural killer T cells regulate the homing of chemokine CXC receptor 3-positive regulatory T cells to the liver in mice. Hepatology. 2009;49:1267–76. doi: 10.1002/hep.22761. [DOI] [PubMed] [Google Scholar]
- 45.Huang S, Rutkowsky JM, Snodgrass RG, et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53:2002–13. doi: 10.1194/jlr.D029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang H, Yang PY, Rui YC. Adenovirus viral interleukin-10 inhibits adhesion molecule expressions induced by hypoxia/reoxygenation in cerebrovascular endothelial cells. Acta pharmacologica Sinica. 2008;29:50–6. doi: 10.1111/j.1745-7254.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 47.Chen XL, Grey JY, Thomas S, et al. Sphingosine kinase-1 mediates TNF-alpha-induced MCP-1 gene expression in endothelial cells: upregulation by oscillatory flow. Am J Physiol Heart Circ Physiol. 2004;287:H1452–8. doi: 10.1152/ajpheart.01101.2003. [DOI] [PubMed] [Google Scholar]
- 48.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1–16. vii. doi: 10.1016/j.cld.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Malik SM, Gupte PA, de Vera ME, Ahmad J. Liver transplantation in patients with nonalcoholic steatohepatitis-related hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:800–6. doi: 10.1016/j.cgh.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 50.Jaeschke H, Gores GJ, Cederbaum AI, et al. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–76. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 51.Kato A, Gabay C, Okaya T, Lentsch AB. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol. 2002;161:1797–803. doi: 10.1016/S0002-9440(10)64456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harada H, Wakabayashi G, Takayanagi A, et al. Transfer of the interleukin-1 receptor antagonist gene into rat liver abrogates hepatic ischemia-reperfusion injury. Transplantation. 2002;74:1434–41. doi: 10.1097/00007890-200211270-00016. [DOI] [PubMed] [Google Scholar]
- 53.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10; inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levings MK, Gregori S, Tresoldi E, et al. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–9. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 55.Salvati VM, Mazzarella G, Gianfrani C, et al. Recombinant human interleukin 10 suppresses gliadin dependent T cell activation in ex vivo cultured coeliac intestinal mucosa. Gut. 2005;54:46–53. doi: 10.1136/gut.2003.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang B, Zhang Q, Zhu B, Cui Z, Zhou J. Protective effect of gadolinium chloride on early warm ischemia/reperfusion injury in rat bile duct during liver transplantation. PLoS One. 2013;8:e52743. doi: 10.1371/journal.pone.0052743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart RK, Dangi A, Huang C, et al. A novel mouse model of depletion of stellate cells clarifies their role in ischemia/reperfusion- and endotoxin-induced acute liver injury. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shao B, Kitchens RL, Munford RS, et al. Prolonged hepatomegaly in mice that cannot inactivate bacterial endotoxin. Hepatology. 2011;54:1051–62. doi: 10.1002/hep.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crouser ED, Julian MW, Huff JE, et al. Abnormal permeability of inner and outer mitochondrial membranes contributes independently to mitochondrial dysfunction in the liver during acute endotoxemia. Crit Care Med. 2004;32:478–88. doi: 10.1097/01.CCM.0000109449.99160.81. [DOI] [PubMed] [Google Scholar]