Abstract
Objective
Transcranial direct current stimulation (tDCS) has been shown to improve pain symptoms in fibromyalgia (FM), a central pain syndrome; the underlying mechanisms are not well understood. Our objective was to explore the neurochemical action of tDCS in the FM brain using proton magnetic resonance spectroscopy (1H-MRS).
Methods
Twelve patients with FM underwent sham tDCS over the left motor (anode) and contralateral supraorbital cortices (cathode) (M1-SO) for 5 consecutive days, a 7 day washout period, and then active M1-SO tDCS for 5 consecutive days. The subjects had clinical pain assessment and 1H-MRS testing at baseline, the week following post-sham tDCS trial, and the week following post-active tDCS trial.
Results
There was a significant decrease in clinical pain scores between baseline and active tDCS time-points (P=0.04). There was a significant decrease in Glx (glutamate and glutamine) in the anterior cingulate (P=0.013) and a trend towards decreased Glx in the thalami (P=0.056) for the sham-active tDCS comparison. For the baseline-sham tDCS comparison, there was a significant increase in N-acetylaspartate (NAA) levels in the posterior insula (P=0.015). There was a trend towards increased γ-aminobutyric acid (GABA) in the anterior insula for the baseline-active tDCS comparison (P=0.064). There were significant linear regression coefficients between anterior cingulate Glx levels at baseline and the clinical pain scale changes between the baseline-sham tDCS comparison (β1=1.31;P<0.001) and the baseline-active tDCS comparison (β1=1.87;P<0.001).
Conclusion
Our findings suggest that GABA, Glx and NAA play an important role in the pathophysiology of FM and its modulation by tDCS.
Chronic pain impacts approximately 100 million people in the United States with annual costs more than $635 billion [1]. Fibromyalgia (FM) is considered the prototypical central pain syndrome and emerging data suggests that there are central nervous system alterations in FM patients [2-4]. Despite the presence of multiple treatments for this condition, many FM patients still report significant unresolved pain and disability. A significant limitation to evaluating potential interventions for chronic pain syndromes, including FM, is the lack of an objective marker of pain. There has been significant interest in using neuroimaging methods to develop an objective test of pain such as proton magnetic resonance spectroscopy (1H-MRS). 1H-MRS is able to measure brain metabolite levels including γ–aminobutyric acid (GABA), the brain’s major inhibitory neurotransmitter, Glx, a combined marker of glutamine and glutamate (the latter being the brain’s major excitatory neurotransmitter), and N-acetylaspartate (NAA), thought to be a measure of neuronal integrity. Our group has reported increased levels of Glx in FM subjects in the posterior insula which is responsible for the graded sensory processing of pain [5, 6]. Our group has also reported decreased levels of GABA in FM subjects in the anterior insula which is important in the emotional processing and affective aspects of pain [6, 7]. Lower NAA levels within the hippocampus has also been reported in the setting of FM [8].
One potential treatment for FM is transcranial direct current stimulation (tDCS). tDCS is a brain stimulating procedure that uses noninvasive weak direct current applied to the scalp. tDCS in FM, as well as other pain conditions, has been shown to modulate experimental and clinical pain measures. Specifically, tDCS has been shown to improve pain symptomatology in FM [9, 10]. Anodal stimulation from tDCS has been shown to increase cortical excitability which is postulated to mitigate pain symptoms through indirect effects on pain processing regions in the brain [9]. However, the mechanisms underlying tDCS efficacy in chronic pain are not well understood, and chronic pain trials using tDCS have not reported consistent results [11]. Our objective was to explore the underlying neurochemical action of tDCS in the FM brain using 1H-MRS.
Patients and Methods
Trial design
Our longitudinal trial had three phases: 1) baseline period to collect pain levels and a MRI scan, 2) sham tDCS for 5 consecutive days followed by pain assessment and MRI, and 3) active tDCS for 5 consecutive days followed by pain assessment and MRI. Phase 2 and phase 3 were separated by a 7 day wash-out period. Randomization was not performed given the presence of significant carry over effects with active tDCS and small sample size of the study [12].
Patients
Thirteen female subjects (ages 27-64, mean ± SD age 47.6 ± 10.6 years) were recruited for this study. Twelve of the subjects completed the entire protocol; one patient dropped out after the baseline pain assessment/MRI. The first 2 of the 12 subjects did not have GABA data collected at the baseline time-point due to protocol change but did have Glx and NAA data collected. All subjects met the 1990 criteria of the American College of Rheumatology for FM, with duration of symptoms of at least one year, had reported continued presence of pain more than 50% of days, and were willing to not introduce any new medications or treatments for control of FM symptoms during the study. Subjects were female, right-handed, had a BMI of 36 or less, and were capable of giving written informed consent. We excluded FM patients with a history of coexisting autoimmune or chronic inflammatory disease that causes pain (i.e. rheumatoid arthritis, systemic lupus erythematosus or inflammatory bowel disease), with a history of substance abuse or were currently taking opiates, or with a history of severe psychiatric illness (i.e. current major depression and schizophrenia). FM patients that were pregnant, breastfeeding, or participating in other therapeutic clinical trials were also excluded. The study was approved by the University of Michigan Institutional Review Board. All subjects gave informed written consent.
Clinical assessments
The clinical assessments refer to the “average” experienced symptoms perceived by the subjects during three time periods: the week prior to the initial MRI, the period between first-trial initiation (sham tDCS) and second MRI, and the period between second-trial initiation (active tDCS) and final MRI. Pain intensity was measured using the visual analog scale (VAS), which was based on a 10-point numeric scale, with 0 being no pain and 10 the worst possible pain. The subject’s affective state was assessed using the positive and negative affect scores from PANAS. Pain discrimination and subjective pain experience was evaluated using the Long Form - McGill Pain Questionnaire for the baseline visit and the Short Form - McGill Pain Questionnaire for the post-sham and post-tDCS visits [13, 14].
Transcranial Direct Current Stimulation (tDCS)
For both the sham and active tDCS sessions, the anode electrode was placed on the scalp overlying the left motor cortex and the cathode electrode was placed on the scalp overlying the right supraorbital cortex. In summary, during the active tDCS sessions, 2 mA of transcranial direct current stimulation was applied for 20 minutes. For the sham tDCS, the current was applied for only 30 seconds at the beginning and end of the session. A 30 second application of current is considered a method of sham stimulation as sensations arising from tDCS treatment occur mostly at the beginning and end of application [15]. Individual measurements determined the anatomical location for placement of the electrodes using the convention of EEG 10/20 system. Placement of the electrodes was performed by the same operators (A.F.D. and T.D.N.).
Proton Magnetic Resonance Spectroscopy (1H-MRS)
A Philips Ingenia 3T system (Best, Netherlands) with a 15 channel receive head coil was used for imaging acquisition. Voxel placement was performed using a 3D-MPRAGE sequence with 0.9 mm3 isotropic voxel resolution. 1H-MRS spectra were collected with 3.0 cm × 2.0 cm × 3.0 cm volumes from the right anterior insula, right posterior insula, and anterior cingulate and a 2.0 cm × 4.0 cm × 2.2 cm volume from bilateral thalami (Supplemental Figure 1). These regions were chosen a priori given their importance in pain perception processing, based on our prior work which has demonstrated significant alterations of GABA and/or Glx in FM subjects in the right insula and anterior cingulate, and because they have also been shown to be altered in chronic pain using other advanced neuroimaging techniques [3, 5, 7, 16]. Single-voxel point resolved spectroscopy (PRESS) (TR/TE=2000/35 ms) was performed using ‘VAPOR’ water suppression with 32 averages to measure Glx and NAA levels. A MEGA-PRESS experiment, which edits out the overlapping creatine peak at 3.0 ppm was performed to measure GABA levels [17]. The MEGA-PRESS experiment used the following parameters: TE=68 ms (TE1=15ms, TE2=53 ms); TR=1.8s; 256 transients of 2k datapoints; spectral width=2 kHz; frequency selective editing pulses (14 ms) applied at 1.9 ppm (ON) and 7.46 ppm (OFF). Amplitude-modulated pulse ‘GTST1203’ (length=7 ms, bandwidth=1.2 kHz) was used for refocusing. Conventional PRESS spectroscopy data were analyzed with LCModel. MEGA-PRESS spectroscopy data were analyzed using Matlab in-house post-processing software to fit Gaussian curves to the GABA and inverted NAA peaks. The absolute NAA concentration, as generated from LCModel analysis of the PRESS data, was multiplied by the ratio of the GABA/NAA ratio as generated from the Matlab analysis to determine the concentration of GABA (in arbitrary institutional units). There was inadequate signal to noise ratio for the GABA spectra for one to two subjects for the anterior cingulate and anterior insula, and two to three subjects for the thalami at each of the trial phases.
Statistical Analyses
Stata v.11 (College Station, TX) was used for the statistical analysis. Changes in brain metabolite levels for the different brain regions were determined using two-tailed paired sample t-tests between the following time-points: baseline-sham tDCS, baseline-active tDCS, and sham-active tDCS. We also correlated the scores from the VAS scale and the McGill and PANAS questionnaires at each of the time-points with the respective brain metabolite levels from the different voxel locations. Linear regression analysis was performed between the brain metabolite values at baseline and the VAS scale changes at the baseline-sham tDCS and baseline-active tDCS comparisons. The significance threshold was set a priori at a P-value of 0.05.
Results
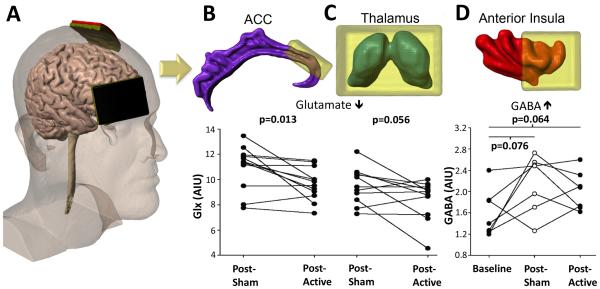
We observed significant longitudinal changes in the clinical pain scores with a significant decreased in the VAS score for the baseline-active tDCS comparison (Table 1; P = 0.04). There was a trend towards a decrease in the VAS score for the baseline-sham tDCS comparison (P = 0.10). There was no significant change in the VAS score for the sham-active tDCS comparison (P = 0.16). There was a significant decrease in the PANAS- score for the baseline-active tDCS comparison (P = 0.02). There were no additional significant changes in the PANAS scores for the different time-point comparisons. There was a significant decrease in Glx levels for the anterior cingulate (P = 0.013) and a trend towards a decrease in Glx levels for the thalamus (P = 0.056) for the sham-active tDCS comparison (Figure 1). For the baseline-active tDCS comparison, there was a trend towards an increase in GABA levels for the anterior insula (P = 0.064). In addition, for the baseline-sham tDCS comparison, there was a significant increase in NAA levels in the posterior insula (baseline: 7.68 ± 0.45 arbitrary institutional units (AIU), sham tDCS: 8.24 ± 0.58 AIU; P = 0.015).
Table 1.
Clinical Scores and Clinical Score - Brain Region Metabolite Correlations
| Baseline | Post-sham tDCS | Post-active tDCS | |
|---|---|---|---|
| VAS Score | 5.1 ± 2.3 | 4.1 ± 2.1 | 3.3 ± 2.81 |
| - ACC GABA correl | r = −0.74a | ns | ns |
| - Post Ins NAA correl | ns | ns | r = −0.60b |
| - Thalamus GABA correl | r = −0.75b | ns | ns |
| - Thalamus NAA correl | ns | r = −0.75a | ns |
| MPQ Score (Long-Form) MPQ Score (Short-Form) | 22.9 ± 14.9 | 18.7 ± 12.5 | 19.3 ± 15.3 |
| PANAS+ Score | 19.8 ± 6.0 | 17.8 ± 5.5 | 16.1 ± 6.4 |
| - Ant Ins Glx correl | r = −0.87a | ns | ns |
| - Ant Ins NAA correl | r = −0.68b | ns | ns |
| - Ant Ins GABA correl | ns | ns | r = 0.71b |
| - Post Ins Glx correl | r = −0.78a | ns | ns |
| - Post Ins NAA correl | ns | ns | r = 0.61b |
| - Thalamus GABA correl | ns | r = 0.78b | ns |
| PANAS- Score | 14.3 ± 3.4 | 15.4 ± 5.5 | 12.7 ± 3.62 |
| - Post Ins GABA correl | ns | ns | r = −0.65b |
| - Post Ins NAA correl | ns | ns | r = −0.63b |
| - Thalamus GABA correl | ns | r = −0.79a | ns |
Abbreviations: ACC = anterior cingulate, Ant Ins = anterior insula, correl = correlation, Glx = combined glutamate + glutamine, GABA = γ-aminobutryic acid, NAA = N-acetylaspartate, ns = not significant. Post Ins = posterior insula.
P = 0.04 for baseline-active tDCS comparison.
P = 0.02 for baseline-active tDCS comparison.
P≤0.01.
P≤0.05
Figure 1.
tDCS electrode placement and 1H-MRS results. tDCS montage with the anode placed over the primary motor cortex (M1) and the cathode over the contralateral supra-orbital cortex (A). Levels of Glx (glutamate + glutamine) are decreased in the anterior cingulate following active tDCS treatment (B) with a trend towards a decrease in levels of Glx for thalami (C). Following post-sham and post-active treatments there is a trend towards increasing levels of GABA (AIU) in the anterior insula as compared to baseline levels (D). ACC = anterior cingulate. AIU = arbitrary institutional units.
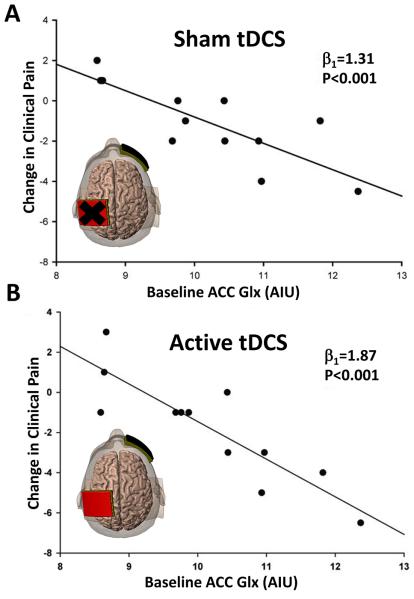
We found a number of moderate to strong correlations between the brain metabolites and the clinical rating scales. Higher levels of GABA and NAA and lower levels of Glx from the prescribed brain region voxels were associated with better clinical pain and affect scores (Table 1). There were significant linear regression coefficients between the anterior cingulate Glx levels at baseline and the VAS scale changes between the baseline-sham tDCS comparison (β1 = 1.31; P < 0.001) and the baseline-active tDCS comparison (β1 = 1.87; P < 0.001) (Figure 2). Greater Glx levels in the anterior cingulate at baseline were associated with stronger reductions in clinical pain following both the sham and active phases.
Figure 2.
Pre-treatment anterior cingulate Glx levels are predictive of subsequent changes in clinical pain. (A) Scatterplot of baseline levels of Glx (AIU) versus change in clinical pain (post-pre) as measured by the Visual Analog Scale (VAS) for FM patients following sham tDCS (β1 = 1.31, p <0.001). (B) Scatterplot of baseline levels of Glx (AIU) versus change in clinical pain (post-pre) as measured by the VAS for FM patients following active tDCS (β1 = 1.87, p <0.001). Individuals that have higher baseline Glx within the anterior cingulate, display greater reductions in clinical pain following sham and active tDCS. ACC = anterior cingulate. AIU = arbitrary institutional units.
Discussion
Our longitudinal trial demonstrated that the anterior cingulate was the most affected by the tDCS treatment with a significant decrease in Glx. Furthermore, we found that subjects with higher levels of Glx in the anterior cingulate at baseline tended to show a greater degree of clinical pain improvement from both the sham and the tDCS treatments. The anterior cingulate is a key component in the brain’s pain modulating regions and is also involved in the emotional processing of pain [6]. The anterior cingulate has been shown to have altered structure and function in chronic pain states; specifically focal atrophy and reduced connectivity have been described in the rostral anterior cingulate [12, 16]. Furthermore, the rostral anterior cingulate has been shown to be an important region for descending inhibition of pain [12]. Our findings suggest that “normalization” of Glx in the anterior cingulate may be an important part of the mechanisms of tDCS therapy for fibromyalgia. In addition, the strength of the regression coefficient and high level of significance between Glx levels in the anterior cingulate and clinical pain improvement suggests that 1H-MRS may prove to be a clinically useful predictor of tDCS efficacy in FM. We speculate that the higher regression coefficient from the baseline Glx-VAS change for the baseline-active tDCS comparison (β1 = 1.87) relative to the baseline-sham tDCS comparison (β1 = 1.31) may reflect additive placebo response to the tDCS treatment. Our findings complement our pregabalin treatment results, which demonstrated that baseline Glx levels were associated with pain response, albeit in a different brain region [3]. Increases in Glx and NAA following tDCS stimulation have been observed in the parietal cortex underneath the anode suggesting that tDCS locally increases glutamatergic activity and neuronal metabolism [18].
There was a trend towards decreased Glx in the thalamus following tDCS treatment. The thalamus which is a critical pain relay and processing center, has been shown to have alterations in blood flow, white matter structure and connectivity in FM [19]. We also found a trend towards increased GABA in the anterior insula following treatment complementing our prior findings of reduced levels of GABA in FM subjects as compared to healthy controls [7]. The pain processing regions of the brain also demonstrated significant correlations with GABA and Glx levels and clinical self-report pain scores: namely subjects with higher levels of GABA and lower Glx displayed lower levels of pain intensity (as measured by VAS scale) and negative affect (as measured by PANAS-) and higher levels of positive affect (as measured by PANAS+). These findings are consistent with the notion that there is an excitatory/inhibitory ratio imbalance within the FM brain resulting in upregulation of pain response/experience as we have previously proposed [5, 7]. Similar results were demonstrated with NAA levels from the anterior insula, posterior insula and thalami: namely, patients with lower pain scores, lower levels of negative affect and higher levels of positive affect tended to have higher levels of NAA. These findings, in addition to the increase in NAA levels that we observed in the posterior insula following sham tDCS treatment, suggest that neuronal integrity, as measured by NAA, is important in chronic pain [8].
Limitations for the study include the relatively small number of subjects and lack of randomization. The large number of statistical comparisons raises the possibility of Type I error. Furthermore, we were unable to demonstrate a significant improvement in clinical pain scores between the sham-active tDCS time-point which may in part be due to placebo response and our small sample size. We suggest that given the exploratory nature of this study, subsequent efforts will be required to confirm our results. In addition, the GABA editing acquisition includes contributions from macromolecules. GABA editing requires relatively large voxel sizes and long time acquisitions limiting number of potential brain regions to interrogate. For future pain studies, it would be interesting to measure the local 1H-MRS changes underneath the anode.
In conclusion, our findings suggest that GABA, Glx, and NAA play an important role in the pathophysiology of chronic pain and its modulation by tDCS. Both the sham and active tDCS phases of the trial resulted in significant alterations in the brain metabolites for various pain centers in the brain. Furthermore, baseline Glx levels in the anterior cingulate predicted response to treatment. These findings encourage further work to pursue targeted therapy with tDCS and other non-invasive brain stimulation modalities in other chronic pain conditions.
Supplementary Material
Supplemental Figure 1. Voxel placement and resulting spectrum. Axial (a,b) and sagittal (c,d) T1-weighted images showing single-voxel placements for right anterior insula (ant Ins), right posterior insula (post Ins), anterior cingulate (ACC), and thalami (Thal). Representative proton magnetic resonance spectroscopy spectrum (e) from the posterior insula using MEGA-PRESS editing technique. Combined measure of glutamine and glutamate (Glx) is resolved at 3.8 ppm, γ-aminobutyric acid (GABA) at 3.0 ppm with an inverted N-acetylaspartate (NAA) peak at 2.0 ppm.
Acknowledgements
This study was funded by a MICHR Clinical Trial Planning Program and CTSA high-tech funding grant, University of Michigan. This study applies application and processing tools developed under NIH grants P41 EB015909 and R01 EB016089. The authors would like to thank Dr. Richard Edden for his help with data acquisition and data post-processing.
This study was funded by a MICHR Clinical Trial Planning Program and CTSA high-tech funding grant, University of Michigan.
References
- [1].Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–24. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- [2].Petrou M, Harris RE, Foerster BR, McLean SA, Sen A, Clauw DJ, et al. Proton MR spectroscopy in the evaluation of cerebral metabolism in patients with fibromyalgia: comparison with healthy controls and correlation with symptom severity. AJNR Am J Neuroradiol. 2008;29:913–8. doi: 10.3174/ajnr.A0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119:1453–64. doi: 10.1097/ALN.0000000000000017. [DOI] [PubMed] [Google Scholar]
- [4].Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- [5].Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–52. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- [7].Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579–83. doi: 10.1002/art.33339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aoki Y, Inokuchi R, Suwa H. Reduced N-acetylaspartate in the hippocampus in patients with fibromyalgia: a meta-analysis. Psychiatry Res. 2013;213:242–8. doi: 10.1016/j.pscychresns.2013.03.008. [DOI] [PubMed] [Google Scholar]
- [9].Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54:3988–98. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- [10].Marlow NM, Bonilha HS, Short EB. Efficacy of transcranial direct current stimulation and repetitive transcranial magnetic stimulation for treating fibromyalgia syndrome: a systematic review. Pain Pract. 2013;13:131–45. doi: 10.1111/j.1533-2500.2012.00562.x. [DOI] [PubMed] [Google Scholar]
- [11].O'Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD008208.pub2. CD008208. [DOI] [PubMed] [Google Scholar]
- [12].Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, et al. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum. 2013;65:3293–303. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–99. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- [14].Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- [15].Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- [16].Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- [17].Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–72. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- [18].Clark VP, Coffman BA, Trumbo MC, Gasparovic C. Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: a (1)H magnetic resonance spectroscopy study. Neurosci Lett. 2011;500:67–71. doi: 10.1016/j.neulet.2011.05.244. [DOI] [PubMed] [Google Scholar]
- [19].Bellato E, Marini E, Castoldi F, Barbasetti N, Mattei L, Bonasia DE, et al. Fibromyalgia syndrome: etiology, pathogenesis, diagnosis, and treatment. Pain Res Treat. 2012 doi: 10.1155/2012/426130. 2012:426130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Voxel placement and resulting spectrum. Axial (a,b) and sagittal (c,d) T1-weighted images showing single-voxel placements for right anterior insula (ant Ins), right posterior insula (post Ins), anterior cingulate (ACC), and thalami (Thal). Representative proton magnetic resonance spectroscopy spectrum (e) from the posterior insula using MEGA-PRESS editing technique. Combined measure of glutamine and glutamate (Glx) is resolved at 3.8 ppm, γ-aminobutyric acid (GABA) at 3.0 ppm with an inverted N-acetylaspartate (NAA) peak at 2.0 ppm.